Abstract
Due to the highly mobile nature of the cervical spine, and the fact that most magnetic resonance imagings (MRIs) and computed tomography scans are obtained only in one single position, dynamic cord compression can be an elusive diagnosis that is often missed and not well-understood. In this context, dynamic MRI (dMRI) has been utilized to improve the diagnostic accuracy of cervical stenosis. We performed a literature review on dynamic cord compression in the context of cervical spondylotic myelopathy (CSM), with particular emphasis on the role of dMRI. Cadaveric studies report that the spinal cord lengthens in flexion and the spinal canal dimension increases, whereas the spinal cord relaxes and shortens in extension and the spinal canal decreases. These changes may lead to biomechanical stress in the spinal cord with movement, especially in patients with critical cervical stenosis. The majority of the studies using dMRI in CSM reported that this imaging modality is more sensitive at detecting cervical cord compression compared to routine MRIs done in a neutral position, especially with the neck in extension. Dynamic MRI was also useful to diagnose dynamic cervical cord compression after laminectomies in patients with clinical deterioration without evident cord compression on neutral static MRI. Finally, dMRI is more sensitive in detecting stenosis in patients with CSM than in those with ossification of the posterior longitudinal ligament (OPLL), likely because OPLL patients often have a more limited range of motion than CSM patients. Thus, dMRI is a promising new tool that can help spine surgeons in diagnosing and treating CSM.
Keywords: Cervical cord, Myelopathy, Spinal cord compression
INTRODUCTION
Cervical spondylosis occurs ubiquitously with aging and is comprised of degenerative changes of the cervical discs, facet joints, ligaments, and adjacent structures [1]. These changes may lead to symptoms of spinal cord compression resulting in cervical spondylotic myelopathy (CSM) – the most common cause of spinal cord dysfunction in patients who are 55 years or older [1].
Clinical symptoms are generally chronic with insidious onset, even though traumatic events may precipitate acute exacerbation of a spinal cord dysfunction [2,3]. Clinical presentation may include motor dysfunction, spasticity, sensory disturbance, sphincter, and balance problems, as well as axial neck pain and cervical radiculopathy. The diagnosis of CSM is made from clinical signs and symptoms and confirmed with a cervical magnetic resonance imaging (MRI) or computed tomography (CT) myelogram demonstrating the spinal cord compression [1]. MRI scans, however, are generally completed with the neck in a neutral position. This may be useful in the vast majority of the cases to document stenosis, but may not demonstrate dynamic or “occult” cord compression. We aim to review the literature regarding the evaluation of dynamic cord compression in the context of CSM, with special emphasis on the use of dynamic MRI (dMRI).
NORMAL CERVICAL SPINE RANGE OF MOTION
The cervical spine is extremely mobile. The normal cervical spine range of motion (ROM) includes approximately 80°–90° of flexion, 70° of extension, 20°–45° of lateral bending and up to 90° of rotation on both sides. Cervical ROM can vary due to various factors, including age (which decreases the ROM) and specific individual characteristics (race, soft tissues, training, among many others) [4]. The atlanto-occipital joint ranges from an average of 15° to 20° in flexion and extension, but almost no rotation or lateral flexion is possible at these joints due to the depth of the sockets where the occipital condyles rest and the thick atlanto-occipital joint capsule [5]. The atlanto-axial joints have great potential for rotation due to the ligamentous structures (transverse, alar, and apical) and the unique anatomy that contains the dens enabling the atlas to rotate almost 50° to each side [4]. Van Mameren et al. [6] reported that flexion in the cervical spine initiates at the lower cervical spine (C4–7), followed by Occiput–C2, then C2–3, and finally at C3–4. Similarly, extension also initiates at C4–7, followed by Occiput–C2.
Holmes et al. [7] performed a radiological study in 50 normal subjects (32 males [age range, 21–58 years] and 18 females [age range, 20–55 years]). Patients had lateral radiographs in full flexion to full extension and they measured the angular ROM from C2 to C7. The lowest ROM from C2 to C7 in the 50 subjects was 50° and the greatest exceeded 90° (mean value, 67°). The greater ROM occurred at C4–5 (mean, 17.9°) as well as the greater proportion of ROM (mean, 27.1%). A group of 50 patients with CSM (35 males [age range, 31–63 years] and 15 females [age range, 35–67 years]) had the average ROM of 53°, which was significantly lower than the normal group (67°) (p<0.001). Of note, the age of the CSM group was higher than in the normal subject group. The authors reported that the mean cervical ROM in the normal Chinese was lower than in the Western population, suggesting differences according to races. This wide ROM in the cervical spine may put the spinal cord at risk in individuals with some degree of stenosis when motion occurs.
SPINAL CORD CHANGES IN FLEXION AND EXTENSION OF THE NECK
Breig et al. [8] performed a cadaveric study to evaluate the role of flexion, extension and neutral position in the cervical spinal cord. They demonstrated after fixing 42 cervical specimens in different positions that the cervical spinal canal elongates in flexion causing the spinal cord and the dentate ligaments to stretch and lengthen (with a decreased anteroposterior diameter in histologic cuts). When the neck is extended, the spinal cord relaxes and shortens, as do the nerve roots and dentate ligaments. The lateral columns and the anterior horns were deformed by mechanical stress produced by spondylotic bars during flexion. Because of this, mechanical stresses with excessive flexion and extension may lead to disturbances in conduction in overstretched or compressed axons [8].
SPINAL CANAL CHANGES IN FLEXION AND EXTENSION
Muhle et al. [9] proposed a classification system based on kinematic MRI in CSM. They evaluated 81 patients with different stages of cervical degenerative diseases who underwent an MRI in neutral, maximum flexion, and extension position. The degree of spinal stenosis was classified as 0=normal, 1=partial obliteration of the anterior or posterior subarachnoid space, 2=complete obliteration of the anterior or posterior subarachnoid space, and 3=cervical cord compression or displacement. They reported that the prevalence of spinal stenosis increased with flexion and extension compared with a neutral position. Patients with more advanced spondylosis had significantly more stenosis at dynamic positions than those with less advanced disease. Finally, significant increase in cord impingement was seen in extension (22 of 81 patients, 27%) versus in flexion (4 of 81 patients, 5%). They concluded that regardless of the degree of cervical spondylosis and an initial amount of cervical stenosis, cervical spinal motion contributes to the development of CSM.
Dalbayrak et al. [10] performed dMRI in 258 patients with CSM and evaluated this imaging modality compared with conventional MRI and X-rays – they had measured the canal diameter on lateral X-rays and axial CT scans for all levels. They reported that dynamic MRI was more accurate than CT and X-rays in detecting canal changes in flexion and extension. On average, the canal underwent 14.9% of expansion (1.05 mm) in flexion and 13.4% of reduction (0.94 mm) in extension. Additionally, instability was inferred in 25.6% of the CSM cases with dynamic X-rays, but this increased to 54.3% when using dMRI.
Of note, although the spinal canal diameter may increase in flexion, some studies reported that in this position, the tension forces applied to the spinal cord causes ventral spinal cord compression against osteophytes and discs – worsening an eventual ventral compression [11]. For this reason, it is not accurate to assume that in all patients with CSM, flexion is a protective position. On the other hand, extension shortens the cervical cord, decreasing the space available for it, with ligamentum flavum (LF) buckling which could potentially aggravate posterior compression, especially in patients with pre-existing stenosis [8,11].
DYNAMIC MRI STUDIES
1. General Considerations
Dynamic MRI studies are not performed routinely due to the additional time, cost, and resources required. In clinical practice, they are often not covered by health insurance plans [11]. Additionally, there is not yet a standardized technique for obtaining the dynamic exams. Some authors recommended maximum flexion and extension until pain tolerance or new a neurological deficits, but this may result in patient discomfort or even risk neurological deterioration in patients with unstable spines or severe cord compression [12]. Stamm et al. [11] suggested to place the patients on a wedge of about 5-in high to flex the head and then put the same wedge under the shoulders to extend the neck. These differences in the position to obtain the exam may change the image and consequently are confounding factors in dMRI evaluation. We review the advantages and disadvantages in Table 1.
Table 1.
Comparison between image modalities for evaluation cervical spondylotic myelopathy
| Dynamic cervical plain radiographs | Dynamic CT scan | Static MRI | Dynamic MRI | |
|---|---|---|---|---|
| Advantages | Highly available | Provides good visualization of bone anatomy | Gold standard for diagnosis Cervical spondylotic myelopathy | Especially useful for cases where the compression is not clear on static MRI |
| Low cost | Useful for detailed evaluation of spinal instability especially in the cranial cervical junction and cervicothoracic area | |||
| Gold Standard for evaluating cervical alignment in neutral position | Highly available Reliable | Diagnosis of dynamic spinal cord compression | ||
| Good visualization of subaxial cervical spine | Low artifact with previous spinal instrumentation | Good visualization of the spinal cord and nerve roots | ||
| Useful for evaluating instability | Provides prognostic information | |||
| Disadvantages | Poor visualization of the craniocervical junction and cervicothoracic area | Poor visualization of the spinal cord (requires an invasive myelogram for better evaluation of spinal cord compression). | Poor bone anatomy visualization compared with CT scan | Different methods of image acquisition are published |
| Does not provide direct visualization of the spinal cord | Myelogram must be obtained with the patient prone and neck extended, as flexing the neck makes the dye flow out of the cervical spine. | Additional time and cost for the MRI exam | ||
| May overexpressed radiological findings |
CT, computed tomography; MRI, magnetic resonance imaging.
2. Dynamic MRI and Cervical Spine Myelopathy
Nigro et al. [13] performed conventional and dynamic MRIs in 38 patients with CSM (many other patients were excluded due to trauma, infection, or tumors in their series). The dMRI was performed with the neck flexed and extended as much as the patient could tolerate. A total of 16 men and 22 women were included with a mean age of 62.3 years. Static MRI reported 156 points of compression according to the Muhle’s classification – 96 anterior (61.54%) and 60 posterior (38.46%). Comparatively, dMRI showed 186 points of compression – 81 anterior (43.5%) and 105 posterior (56.5%). Interestingly, anterior compression was more commonly diagnosed using static MRI and extension MRI was useful for the diagnosis of posterior compression. They concluded that dynamic cervical MRI is more sensitive for stenosis, which may be useful for surgical planning and should be performed together with static MRI.
Lee et al. [14] performed a dMRI study to evaluate changes in severity of cervical stenosis in flexion and extension. They evaluated 92 patients with CSM (mean age, 57.8 years) who had a dMRI with a cushion under the posterior neck for extension and under the posterior head for flexion, using T2-weighted sagittal images in both flexion and extension. To assess the degree of stenosis, a semiquantitative score was used, ranging from 0 (no spinal stenosis) to 18 (severe stenosis) – cervical spinal stenosis (CSS) score. The score was defined as the CSS grade from the vertebral segments from C2/3 to C7/T1 using the following graduation: 0, no spinal stenosis; 1, more than 50% of subarachnoid space obliterated without signs of spinal cord deformity; 2, cord deformity without signal change of the spinal cord, and 3, increased signal intensity on the spinal cord in T2-weighted image. They reported that the CSS score was higher in extension (6.04±2.68) than in a neural position (5.25±2.47) (p<0.001) and also higher in a neural than in flexion position (4.40±2.25) (p<0.001). They concluded that extension increases radiological severity of the stenosis while flexion decreases the severity. This supports the fact that extension narrows the spinal canal and can worsen cervical stenosis. It should be noted that some other studies reported that flexion may worsen the segmental stenosis in patients with ventral compression, such as in the case of discogenic disease [9].
3. Soft Tissue Compression After Laminectomy
Stamm et al. [11] reported that dMRI was useful for revealing soft tissue compression after postlaminectomy patients. Three cases of progressive myelopathy, despite good satisfactory decompression with laminectomy documented in static cervical MRI scans, revealed clear and evident compression in extension by posterior soft tissues buckling into the laminectomy defect. This occurred in one of the patients despite a fusion with instrumentation. An example case is in Figs. 1 and 2. The patients were treated with placement of crosslinks to prevent soft tissue bucking causing compression of the cord. They reported that surgeons should be aware of persistent compression in patients with previous laminectomy for CSM who had worsening their symptoms and dMRI in extension may be a useful tool for additional radiological screening and even for indicating a new surgical procedure.
Fig. 1.
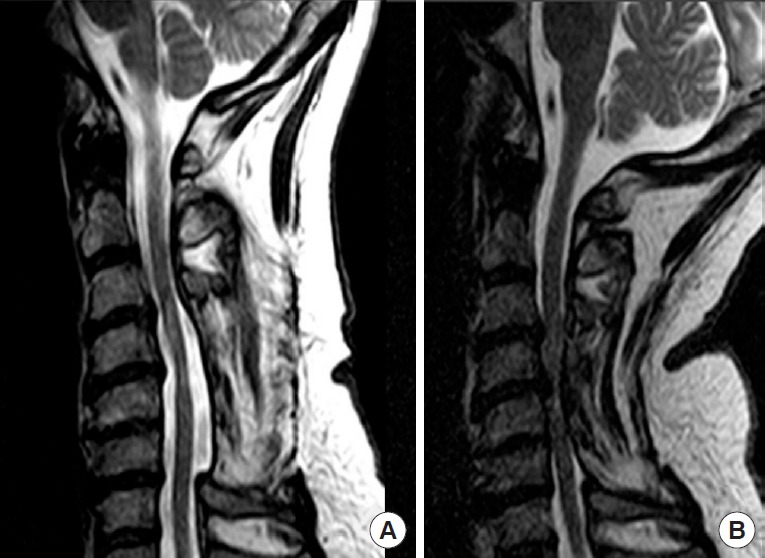
Sagittal T2 sequence cervical spine magnetic resonance imaging of an adult man who had a previous C3–6 laminectomy and late neurological deterioration after full recovery. In panel A, the neck is in neutral position and no evidence of spinal cord compression. In panel B, with the neck in extension, there is severe infolding of the posterior muscles and soft tissues into the spinal canal [11].
Fig. 2.
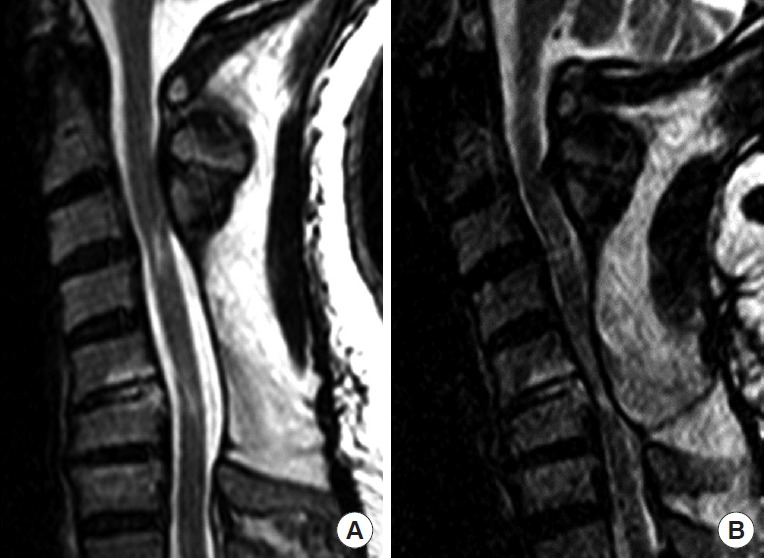
Sagittal T2 sequence cervical spine magnetic resonance imaging of a 56-year-old man who had a previous C4–6 laminectomy with fusion from C3–7 after rolling a vehicle. After surgery, he had no symptoms. Some months after the index surgery, he presented with increasing cervical pain and progression of cervical myelopathic symptoms when extending the neck. In panel A, the neck is neutral and no spinal cord is visualized. When the neck is extended, in panel B, there is severe spinal cord compression by the soft tissues in the back [11].
4. Dynamic MRI and Ossification of the Posterior Longitudinal Ligament
Lee et al. [15] performed dMRI in 66 patients with CSM and 22 patients with ossification of the posterior longitudinal ligament (OPLL). They evaluated the cervical spine according to the Muhle’s grade of stenosis as well as canal diameters and LF thickness in standard and dMRI. The mean ages in the CSM group were 68.2±12.27 years vs. 63.1±9.36 years in the OPLL group. Extension decreases the mean canal diameters at all measured levels, especially at C3/4 and C6/7 in patients with CSM. The LF was also significantly thicker in extension than in neutral or flexion position in the CSM group, but not in the OPLL group. Finally, positional changes in Muhle’s grade were greater in the CSM compared with the OPLL group (p=0.042). They concluded that dMRI morphological changes were more significant in CSM than in OPLL patients.
5. Risk Factors for Spinal Cord Compression Not Present in the Neutral Position
Hayashi et al. [16] evaluated the risk factors for spinal cord compression in 435 symptomatic patients with CSM (2,610 cervical segments) not diagnosed in static and conventional MRI but that was seen in dMRI. After excluding segments with compression in a neutral position, multivariate logistic regression was performed to evaluate the risks for dynamic compression. In a neutral position, 5.3% of the segments (139 of 2,160) have spinal cord compression. Missed stenosis in static MRI was additionally found in 8.3% (204 of 2,471) of segments in extension and 1.6% (40 of 2,471) in flexion. The most frequent level with missed compression was C5/6. The risk factors for missed diagnosis of stenosis on static MRI were: Extension: disc bulge greater than 2.4 mm, angular motion greater than 4.8°, moderate and severe disc degeneration, segmental kyphosis and developmental stenosis; Flexion: disc bulge greater than 1.9 mm, moderate to severe disc degeneration and segmental kyphosis.
6. Hirayama Disease
Hirayama disease is also known as nonprogressive juvenile spinal muscular atrophy of the distal upper limbs [17-19]. It is characterized by cervical myelopathy related to flexion of the neck, occurring in young males generally from 15 to 25 years-old [17-19]. On MRI scans, the spinal cord is compressed in flexion between the anterior spinal canal and a restrictive posterior dura. In Fig. 3, we present an example case. The clinical presentation is generally insidious, with unilateral upper extremity weakness and further atrophy, generally with no signs of pyramidal tract injury or sensory changes. The amyotrophy generally affects the C7, C8, and T1 myotomes asymmetrically, although it can also be symmetric [17-19]. The radiological hallmark is demonstrated on dynamic cervical MRI with the posterior dural sac shifting forward, enlarging the posterior epidural space (with enhancement of the epidural space after gadolinium injection). Most of the cases are treated nonsurgically, with conservative management due to its natural tendency to stabilize [20]. However, when surgery is indicated, for patients with severe clinical symptoms or rapid progression, the ideal treatment is questionable. Anterior cervical discectomy and fusion is probably the most accepted treatment but cervical decompressive laminectomy and coagulation of the posterior epidural plexus without fixation has also been reported [21,22].
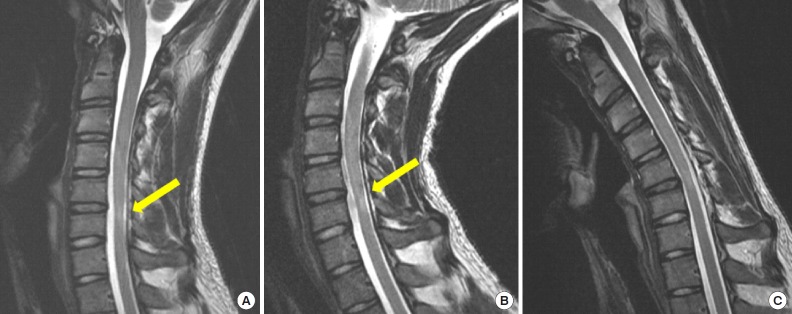
Fig. 3.
Sagittal T2 sequence cervical spine magnetic resonance imaging (MRI) of a young man with progressive upper limbs weakness and no sensorial changes. Hypersignal at C5/6 level was seen in static (A) and extension (B) cervical MRI, without any evidence of compression. In flexion cervical MRI (C), as seen with the yellow arrows the compression of the spinal cord over the posterior aspects of the disc of C5/6 was more evident and also enlargement of the posterior epidural aspect of the sick level – the patient was diagnosed with Hirayama disease.
LIMITATIONS AND FUTURE PERSPECTIVES
The use of dMRI findings for indications for surgery when compared with conventional MRI is not well established in routine cases. Dynamic MRI should be interpreted with caution once, it may “overexpressed” radiological findings. However, especially in cases where there is clinical deterioration or progressive symptoms despite normal conventional cervical spine MRI, dMRI may be a useful tool to detect dynamic cord compression. However, these imaging findings much be correlated with clinical examination to establish the final diagnosis.
CONCLUSIONS
The cervical spine has a very wide ROM which can have an impact on CSM. Dynamic MRI is a promising tool for surgeons to diagnosis subtle or occult compression on conventional or static MRI. Although the majority of the studies suggest that extension worsens spinal compression, based on the fact that the spinal canal dimension decreases, flexion may also exacerbate some compression, especially in severe degenerative disc disease, disc bulging and segmental kyphosis. Further clinical studies are necessary to determine the cases where dMRI should be used for the diagnosis of cervical stenosis and to determine the clinical validity of these findings.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Joaquim AF, Ghizoni E, Tedeschi H, et al. Management of degenerative cervical myelopathy - an update. Rev Assoc Med Bras (1992) 2016;62:886–94. doi: 10.1590/1806-9282.62.09.886. [DOI] [PubMed] [Google Scholar]
- 2.Sadasivan KK, Reddy RP, Albright JA. The natural history of cervical spondylotic myelopathy. Yale J Biol Med. 1993;66:235–42. [PMC free article] [PubMed] [Google Scholar]
- 3.LaRocca H. Cervical spondylotic myelopathy: natural history. Spine (Phila Pa 1976) 1988;13:854–5. doi: 10.1097/00007632-198807000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Windle WF. The spinal cord and its reaction to traumatic injury: anatomy, physiology, pharmacology, therapeutics. New York: Marcel Dekker; 1980. [Google Scholar]
- 5.Bogduk N, Mercer S. Biomechanics of the cervical spine. I: Normal kinematics. Clin Biomech (Bristol, Avon) 2000;15:633–48. doi: 10.1016/s0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Mameren H, Drukker J, Sanches H, et al. Cervical spine motion in the sagittal plane (I) range of motion of actually performed movements, an X-ray cinematographic study. Eur J Morphol. 1990;28:47–68. [PubMed] [Google Scholar]
- 7.Holmes A, Wang C, Han ZH, et al. The range and nature of flexion-extension motion in the cervical spine. Spine (Phila Pa 1976) 1994;19:2505–10. doi: 10.1097/00007632-199411001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis. A study on fresh cadaver material. J Neurosurg. 1966;25:45–56. doi: 10.3171/jns.1966.25.1.0045. [DOI] [PubMed] [Google Scholar]
- 9.Muhle C, Metzner J, Weinert D, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol. 1998;19:1763–71. [PMC free article] [PubMed] [Google Scholar]
- 10.Dalbayrak S, Yaman O, Firidin MN, et al. The contribution of cervical dynamic magnetic resonance imaging to the surgical treatment of cervical spondylotic myelopathy. Turk Neurosurg. 2015;25:36–42. doi: 10.5137/1019-5149.JTN.9082-13.1. [DOI] [PubMed] [Google Scholar]
- 11.Stamm S, McClellan JW, 3rd, Knierim A, et al. Dynamic MRI reveals soft-tissue compression causing progressive myelopathy in postlaminectomy patients: a report of three cases. JBJS Case Connect. 2013;3:e17. doi: 10.2106/JBJS.CC.L.00174. [DOI] [PubMed] [Google Scholar]
- 12.Kolcun JP, Chieng LO, Madhavan K, et al. The role of dynamic magnetic resonance imaging in cervical spondylotic myelopathy. Asian Spine J. 2017;11:1008–15. doi: 10.4184/asj.2017.11.6.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nigro L, Donnarumma P, Tarantino R, et al. Static and dynamic cervical MRI: two useful exams in cervical myelopathy. J Spine Surg. 2017;3:212–6. doi: 10.21037/jss.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Kim SY, Kim K. A Dynamic magnetic resonance imaging study of changes in severity of cervical spinal stenosis in flexion and extension. Ann Rehabil Med. 2018;42:584–90. doi: 10.5535/arm.2018.42.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JB, Kim IS, Kwon JY, et al. Difference of dynamic morphometric changes between in patients with ossification of posterior longitudinal ligament and patients with cervical spondylosis: assessment by cervical dynamic magnetic resonance imaging. World Neurosurg. 2019;123:e566–73. doi: 10.1016/j.wneu.2018.11.213. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Wang JC, Suzuki A, et al. Risk factors for missed dynamic canal stenosis in the cervical spine. Spine (Phila Pa 1976) 2014;39:812–9. doi: 10.1097/BRS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000;54:1922–6. doi: 10.1212/wnl.54.10.1922. [DOI] [PubMed] [Google Scholar]
- 18.Raval M, Kumari R, Dung AA, et al. MRI findings in Hirayama disease. Indian J Radiol Imaging. 2010;20:245–9. doi: 10.4103/0971-3026.73528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boruah DK, Prakash A, Gogoi BB, et al. The importance of flexion MRI in Hirayama disease with special reference to laminodural space measurements. AJNR Am J Neuroradiol. 2018;39:974–80. doi: 10.3174/ajnr.A5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agundez M, Rouco I, Barcena J, et al. Hirayama disease: is surgery an option? Neurologia. 2015;30:502–9. doi: 10.1016/j.nrl.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Brandicourt P, Sol JC, Aldéa S, et al. Cervical laminectomy and micro resection of the posterior venous plexus in Hirayama disease. Neurochirurgie. 2018;64:303–9. doi: 10.1016/j.neuchi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YH, Kuo CH, Huang WC, et al. Anterior cervical discectomy and fusion for hirayama disease: a case report and literature review. Neurospine. 2019 Jan 4; doi: 10.14245/ns.1836178.089. [Epub]. https://doi.org/10.14245/ns.1836178.089. [DOI] [PMC free article] [PubMed] [Google Scholar]