Abstract
Explanations of how organisms might adapt to urban environments have mostly focused on divergent natural selection and adaptive plasticity. However, differential habitat choice has been suggested as an alternative. Here, we test for habitat choice in enhancing crypsis in ground-perching grasshoppers colonizing an urbanized environment, composed of a mosaic of four distinctly coloured substrates (asphalt roads and adjacent pavements). Additionally, we determine its relative importance compared to present-day natural selection and phenotypic plasticity. We found that grasshoppers are very mobile, but nevertheless approximately match the colour of their local substrate. By manipulating grasshopper colour, we confirm that grasshoppers increase the usage of those urban substrates that resemble their own colours. This selective movement actively improves crypsis. Colour divergence between grasshoppers on different substrates is not or hardly owing to present-day natural selection, because observed mortality rates are too low to counteract random substrate use. Additional experiments also show negligible contributions from plasticity in colour. Our results confirm that matching habitat choice can be an important driver of adaptation to urban environments. In general, studies should more fully incorporate that individuals are not only selective targets (i.e. selected on by the environment), but also selective agents (i.e. selecting their own environments).
Keywords: matching habitat choice, local adaptation, phenotype–environment correlation, camouflage, directed gene flow, urban colonization
1. Introduction
Improving the match between individual traits and environmental characteristics is a central challenge to all life, as it increases ecological performance and thereby fitness [1,2]. This challenge is increasing as natural populations face contemporary human-induced rapid environmental change [3,4]. A good example of this is urbanization, a severe form of habitat change [3,5,6]. Nonetheless, some species have been able to cope with urbanization and the associated changes in abiotic conditions, resources and natural enemies [5,7].
Several mechanisms have been proposed to explain the success of urban colonists [4,8,9]. First, when natural selection acts on heritable traits, populations colonizing urban sites may rapidly evolve local adaptation [8,10–12]. Alternatively, past selection in a population's original (non-urban) habitat may have favoured the evolution of adaptive plasticity, by which individuals may adjust their phenotype to better match novel environments including urban habitat [13]. Such plasticity can help colonizing populations to habituate to and persist in novel urban environments [8,9,14,15]. Evidence for natural selection and plasticity in aiding the colonization of urban habitats has been extensively reported [4,8,10,11,15].
Yet another pre-adaptation is adaptive habitat choice. Like plasticity, this can also evolve in a natural setting, but subsequently facilitate urban colonization. Habitat choice is the logical mirror image of adaptive plasticity [16], as it involves individuals changing their environment (via movement) to better match their phenotype. By contrast, plasticity entails individuals changing their phenotype to better match their environment. Especially when genotypes have the capacity to choose among available habitats based on a comparison of local performance [17–22], habitat choice could contribute to adaptation to novel environments [9,22–24]. Performance-based habitat choice is nowadays mostly called ‘matching habitat choice’ [16,25]. It has been hypothesized that matching habitat choice could also contribute to adaptation to urban environments [9,26,27], for instance, if genotypes that perform well in urban environments are particularly likely to settle there. However, the data published so far to support this hypothesis are not definitive, because studies have not carefully excluded the effects of alternative mechanisms that can lead to the same observed patterns of appearing locally adapted [9,28]. Hence, we still have little data on the relative importance of habitat choice, plasticity and contemporary natural selection in driving adaptation to urban habitats (and more is needed for natural systems).
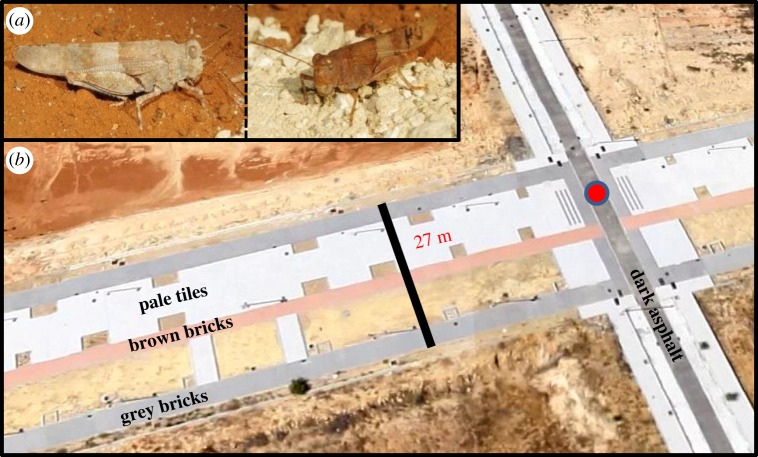
In this article, we test for the occurrence of habitat choice in the colonization and adaptation to four distinctly coloured urban environments by the cryptic and ground-perching azure sand grasshopper (Sphingonotus azurescens, figure 1a): sidewalks, foot paths, bike paths and even asphalt roads. In our study area, these diverse substrates are arranged in a fine-grained mixture of narrow adjoining patches (figure 1b; electronic supplementary material, figure S1). As a second objective, we also compare the relative importance of habitat choice to that of natural selection and plasticity with respect to any match between colour of grasshoppers and colour of the substrates.
Figure 1.
Study species and study area. (a) Azure sand grasshoppers: the left individual was captured on the pale grey soil; the right individual was captured on the brown soil. Dark grey individuals (and all sorts of intermediates) also exist (figure 3a). (b) Aerial view of a small part of the study area, showing the proximity of the four different linear urban habitats (pavements) in-between large square areas containing natural brown, pale grey and some dark grey soils. (Online version in colour.)
2. Methods
(a). Overview
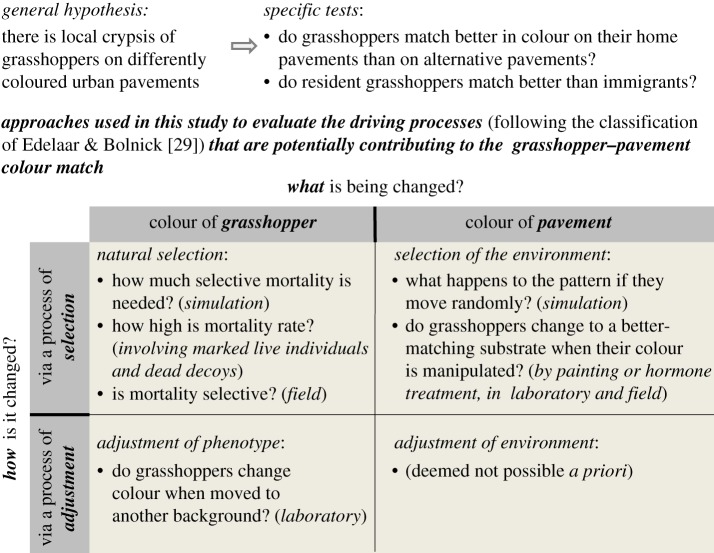
Figure 2 gives an overview of how we determined whether grasshoppers matched their pavement in colour, and which processes might have driven this pattern. In brief, we performed regular captures and recaptures of wild, unmanipulated grasshoppers across our entire study area. New captures were individually marked and photographed to measure grasshopper colour, and to determine crypsis on the local and alternative substrates. Recaptures provided movement distances, which enabled us to simulate how any local crypsis would deteriorate under random movement of various distances. Recaptures also allowed calculation of field mortality rates, and thereby, the upper limit for natural selection to maintain local crypsis if movement among substrates was random. The potential for selective predation was additionally tested by measuring survival of decoy grasshoppers in the study area. These mortality measures were compared to estimates of necessary selective mortality to attain crypsis using simulations. To test for matching habitat choice, we manipulated the colour of grasshoppers and determined their response in use of more matching habitats both in the laboratory and the field. To determine the capacity and rate of plasticity, we measured change in adult colour in response to a manipulation of substrate colour. Below we elaborate on each of the steps listed above. A more detailed description of the used methods and materials is found in the electronic supplementary material, appendix SI.
Figure 2.
Outline of the study. By comparing the colour of grasshoppers and the urban pavements they are found on (or not), we test in two complementary ways if grasshoppers indeed resembled their pavements more than expected by random. We next tested using various approaches which processes might be contributing to such a pattern. We subdivide these processes according to the classification in [29] of the processes driving increased performance, based on what component of the phenotype–environment match is being changed, and how it is being changed. For each process, we list the different measurements and experiments we did to test its contribution. (We could think of no way by which grasshoppers could adjust the colour of the pavement they use (bottom-right) without moving to another pavement.) (More details on methods, measurements and analyses in the Methods and in the electronic supplementary material, appendix SI.) (Online version in colour.)
(b). Study area
We studied the colonization of urban pavements by grasshoppers in a deserted housing development site in the province of Seville (Spain). Here, large blocks of little-vegetated natural soils are subdivided by roads composed of four different types of pavement (figure 1b; electronic supplementary material, figure S1). Roads are closed off to traffic, enabling colonization. Grasshoppers are relatively common on these pavements: adult males are displaying, we have seen copulations and egg deposition, and nymphs are present in spring. Nonetheless, grasshoppers are also common on the natural soils in-between the roads, and undoubtedly, there is frequent and continuous interchange between both types of habitat during the entire season, i.e. the colonization of the urban pavements is ongoing.
(c). Study species
Azure sand grasshoppers (S. azurescens) are ground-perching grasshoppers that normally live on open natural soils, where they are exposed to a diversity of visual predators (wasps, spiders, lizards, small mammals, birds). They are about 2.5–4 cm long (females about 20% larger than males). They show an apparent disruptive colour pattern (figure 1a), and a shadow-preventing flattened body shape, presumably all to decrease detectability and reduce their risk of predation. Individuals typically also match the colour of their natural local substrate, partly because nymphs can change their colour (in nature ranging from very pale to nearly black, and from bluish-grey to reddish-brown) to match that of the soil during successive moults [30,31] (figure 1a). Biologists typically assume for many kinds of organisms that such a phenotype–environment colour match acts to further enhance crypsis and reduce predation risk; for grasshoppers in general, see [30,32]. In the absence of actual fitness measures for our own grasshoppers, we follow this assumption that the degree of colour matching between grasshopper and substrate is positively related to survival and therefore to fitness. Further support for this assumption is provided by the positive effect of crypsis on survival in a virtual predation experiment [33], by the finding that nymphs employ greater plasticity to match the colour of the substrate better when they are exposed to a treatment mimicking predation risk [31], and that less-matching individuals are more fearful when approached and employ alternative behaviour to avoid detection [33].
(d). Sampling and monitoring of populations on urban pavements
We systematically searched for grasshoppers on all the pavements included in our study area by sweeping a net from left to right close to the surface of the pavement while walking slowly forward, which induces easily detected escape behaviours (virtually independent of degree of crypsis or observer). After capture, individuals were photographed and released at the location of the first encounter. We recorded sex, date of capture, type of substrate on which it was found and GPS location. Upon first capture, grasshoppers were individually marked with three letters on the posterior part of both fore wings using a black permanent marker pen (Staedtler permanent Lumocolour, resistant to water and ultraviolet (UV) light) for subsequent visual tracking (without actual recapturing). Even though markings can reduce survival (e.g. by reducing crypsis), our markings resembled natural dark marking in the wing tip (and any reduction in survival rate would have conservative effects on our conclusions). The entire study area was revisited for marked and new, unmarked adult grasshoppers 10 times from June to October.
(e). Measurement of colour and colour distances
We digitally photographed grasshoppers and pavements in situ under fixed conditions, and images included an 18% grey standard card. Ideally, chromatic differences between grasshoppers and pavements should be calculated for the visual system of the relevant predator. However, our grasshoppers might be predated on by a wide range of visual predators with very different and unknown visual systems, and in unknown proportions. We therefore used basic red, green, blue (RGB) reflectance data to quantify colour (as neither grasshoppers nor natural and urban substrates reflect UV; own spectrophotometric data). Aligned and normalized mean RGB values were extracted from the RAW files using the software ImageJ [34] and the Mica Toolbox v. 1.49, following [35]. We calculated chromatic differences between a fixed, representative area of the thorax of each grasshopper and the average values (based on five images) for each of the four pavements by measuring their Euclidean distances.
(f). Testing for local crypsis
Following [36], we first tested for local crypsis from the view point of the individuals (home versus away). We tested this by randomization. We first calculated the observed average RGB difference across all individuals. By simulation, we then randomly distributed them across the four available pavements, in proportion to the surface area of each pavement in the study area, and again calculated the average RGB difference. This process was repeated 10 000 times, and we calculated the proportion of times that the random distribution produced an RGB difference that was as small or smaller as the observed one (i.e. a one-sided test). The same procedure was used for the four subsets of grasshoppers from each pavement separately.
Second, we tested for local crypsis from the view point of the local environment (resident versus immigrant). We proceeded as above, except that for each pavement, we randomly drew individuals from the total pool of individuals until we obtained the same number per pavement as the number of individuals originally observed there.
(g). Calculation of daily grasshopper movement
The GPS positions of recaptured individuals provided information on their net displacements since their last observation. We followed [37] in using mean net squared displacement (MSD) as a synthetic measure of animal movement rate: MSD = D × tα, where D is a diffusion constant, t is the time and α is an exponent. We fitted our data to the linear double-logarithmic form of this function by a linear mixed model (LMM), including individual identity to deal with the repeated measures of individuals. The back-transformed diffusion constant D is then the estimated average daily movement. Note that our observed movements are conservatively biased downwards, because larger movements are more likely to take individuals outside the areas monitored by us.
(h). Simulation of population homogenization with increasing movement
We simulated the spatially explicit effects of random movement (i.e. no habitat choice) on a grid identical to our study site. Each individual for which we had recapture data (n = 72) was simulated as starting at its original position, then moving in a random direction and distance. The random distance was drawn from a uniform distribution ranging from zero to the maximum distance for that simulation. If it did not land on pavement within the study area (i.e. on a block of natural soil, or outside the study area), the initial movement was repeated until accepted. We then calculated the individual's visual distance between its colour and that of its new substrate. This was repeated for all individuals, and the average visual distance after a single bout of movement of the entire population was calculated for 1000 of such independent, uncorrelated repeats. This was done for a range of maximum distances of movement up to 200 m (the largest observed movement). This allowed us to plot how the observed local similarity in colour decreases with increasing movement distances, when these movements are random.
(i). Laboratory habitat use of grasshopper after manipulation of their colour
To test if grasshoppers can increase their crypsis by selecting their environment, we experimentally altered grasshopper colour to see if it affected their habitat choice. Phenotypic manipulation decouples phenotype from genotype and from past experience, and therefore can distinguish performance-based matching habitat choice from genetically determined habitat choice and habitat choice due to imprinting [25]. We changed colour in two ways: (i) we applied pigments externally to field-caught adults by using pale or dark aquarelle paint. These appear to give a very natural final look (at least to a human observer). We painted all areas potentially visible to the grasshopper, taking care not to paint over main receptors (eyes, ocelli, antennae and tarsi) and flexible body parts/joints; and (ii) following [38], we used a micro-syringe to inject laboratory-reared nymphs with the hormone corazonin diluted in purified olive oil to induce the deposition of dark pigments into the cuticle by the individuals themselves. This invariably resulted in adults that were much darker than non-injected control individuals. Control individuals were assigned independent of initial colour. Habitat use as a function of individual coloration was then measured in a small rectangular transparent plastic box (32.0 by 17.3 cm) where each long side was filled with a layer of pale or dark 2–4 mm stones as substrate, thus creating two long rectangular contrasting habitat patches. Boxes were lit by high-performance daylight fluorescent tubes (Philips TL-D 90 De Luxe Master). After habituation for 30 min, we recorded the position of the grasshopper every 15 min, 20 times. Individuals were made to jump after each moment of data collection in order to obtain more independent measures of habitat use (otherwise grasshoppers may sit still for hours). No food or water was provided during the choice trials in order to prevent these from influencing habitat use. For the painted grasshoppers, we modelled the use of the dark habitat as a binomial response variable with a generalized linear mixed model (GLMM), with colour manipulation and substrate of collection in the field as fixed effects, and identity of the individual and rearing box in the laboratory as random effects. Habitat use of grasshoppers injected with corazonin was modelled and tested the same way, except that we modelled corazonin injection (yes/no) and date as fixed effects (all were reared on the same substrate), and the rearing box and experimental box as random effects.
(j). Habitat selection in the field after manipulating grasshopper colour
We released the grasshoppers of the corazonin injection experiment (n = 112) on a 115 m long street. This street had a 7 m wide central area of dark asphalt (similar to the human eye to the colour of grasshoppers made darker by injection with corazonin), and strips of pale pavement of 5.5 m on either side (similar to the colour of control, pale grasshoppers). At four locations in this street, we released mixtures of individuals in the morning, placing them on the border between the two pavements. The next morning we surveyed the entire street, and recorded the type of pavement selected by recaptured individuals. We fitted a GLMM estimating how habitat use (binomial response variable) depended on colour manipulation, sex and year as fixed effects, and identity of rearing box in the laboratory as random effect.
(k). Simulation of mortality rates necessary to obtain observed population divergence
We determined the necessary strength of natural selection to create the observed mean RGB difference between grasshoppers and their background for each substrate, assuming random settlement and no adaptive plasticity. Hence, our entire set of marked and phenotyped grasshoppers (i.e. from across the four pavement types, n = 272) are simultaneously introduced onto a single focal pavement. Next, stabilizing selection is exerted on these individuals, with fitness distributed normally around the optimum (RGB difference = 0), according to the standard fitness function (e.g. [39]: fitness = exp(–(RGB difference)2/(2ω2)), where ω2 is the variance (width) of the Gaussian fitness function and RGB difference is an individual's measure of maladaptation in coloration on the focal pavement. Actual death or survival of each individual was subsequently stochastically determined by a draw from the binomial distribution, with a probability of survival equal to its relative fitness as calculated with the fitness function. We visually determined the range of probable values for selection strength ω that could have resulted in the observed mean RGB difference for the focal habitat (see Results), and then derived which mortality rates this strength of selection would imply.
(l). Measuring predation rate with decoy grasshoppers
One way to assess the presence and impact of predators is to measure the removal rate of food items, assumed to be indicative of relative predation pressure. We used intact, dead grasshoppers (n = 45) that were dried in a natural position. These were placed on the street pavements with the aid of some sticky Blue Tag poster fixing material (not visible after pressing down the grasshoppers onto the material). After about 24 h, we counted how many individuals were removed. We excluded removal by ants (largely non-visual predators) by also sticking a small metal ball to the underside of the grasshopper, which is left behind in the same spot if ants dissemble a dead grasshopper little by little. The experiment was repeated in a natural area (grey limestone rock interspersed with low grassland, and with a high density of grasshoppers and insectivorous lizards and birds; province of Palencia, Spain) to validate that visual predators indeed attack such fixed dead grasshoppers (n = 45).
(m). Measuring survival with multi-state capture–recapture modelling
Capture–recapture models provide estimates of survival which take into account that detection probability for surviving individuals may not be 100% (here: because grasshoppers may leave the studied pavements temporarily and return later). We fitted multi-state capture–recapture models to our data on the live grasshoppers (n = 272) using the program Mark [40,41]. We used as state variable whether a grasshopper used the pavement on which it was most cryptic (lowest RGB difference) or not. For each of the three parameters (recapture probability, probability to switch states, survival probability), we fitted a model where the parameter was either state-dependent or not, yielding eight possible models. Based on preliminary analyses, we also included sex-dependence for recapture probability, giving 16 possible models. We corrected for the unequal number of days between each capture occasion. We calculated the model-weighted average and lower and upper 95% confidence limits for each parameter. Note that our estimated survival probabilities are conservatively biased downwards, owing to permanent emigration outside the areas monitored by us.
(n). Measurement of rate of phenotypic plasticity in adult coloration
Young adults were randomly assigned to boxes which were either painted black (n = 20) or white (n = 20) on the inside. It is known that in nymphs this treatment results in the development of dark respectively pale matching colours [30,31] (see also the electronic supplementary material, figure S2). Also, older adults have been found to respond to manipulation of colour of substrates [42]. (For both nymphs and adults having colour plasticity is beneficial in coarse-grained natural habitats, when mostly a single substrate is experienced.) Boxes were lit from above with high-performance daylight fluorescent tubes (Philips TL-D 90 De Luxe Master), and heat was provided from below using heat mats. We took pictures of each adult at intervals (every few days to weeks) until it died, up to 162 days later. From these pictures, we measured its visual distance to the type of box it occupied (its RGB difference): if there is adaptive phenotypic plasticity, its RGB difference should diminish over time. To test this, we fitted the effect of time and its interaction with colour treatment, while allowing for random intercepts and random slopes for each individual.
3. Results
(a). Support for local divergence in colour on diverse urban pavements despite large scope for dispersal-mediated homogenization
The grasshoppers are highly mobile in the field. Analysis of mark-resighting data (72 observations on 50 individuals; see [43] for all data and R code files of this paper) showed that the grasshoppers move on average 12.3 m d−1 (LMM, 95% confidence interval (CI) 6.1–24.9 m d−1; electronic supplementary material, figure S3). Spatially explicit simulations confirm that at this rate, random movement across the heterogeneous urban landscape should prevent or rapidly erode local cryptic coloration (electronic supplementary material, figure S4). In contrast with this prediction, we observed significant spatial structure in grasshopper coloration, leading to enhanced local crypsis (figure 3a). Grasshoppers overall were more cryptic on their home pavement than they would be on other pavements (figure 3b; average RGB difference is 13.3 points less nindividuals = 272, p < 10−4). The same is true when tested separately for populations on asphalt (−55.6 points), brown bricks (−25.4 points) and grey bricks (−47.9 points; all p < 10−4) (but not for pale tiles where grasshoppers were actually less cryptic: p = 1.00). (Similar results were obtained the year before, and when dividing the data in early and late season: results not shown.) Additionally, resident grasshoppers overall were more cryptic than potential immigrant grasshoppers from other substrates would be (figure 3c; average RGB difference is 5.59 points less, nindividuals = 272, p < 10−4). This resident advantage is most pronounced for grasshoppers from asphalt (nindividuals = 41, p < 10−4) but also true for grasshoppers from pale tiles (nindividuals = 152, p = 0.0002).
Figure 3.
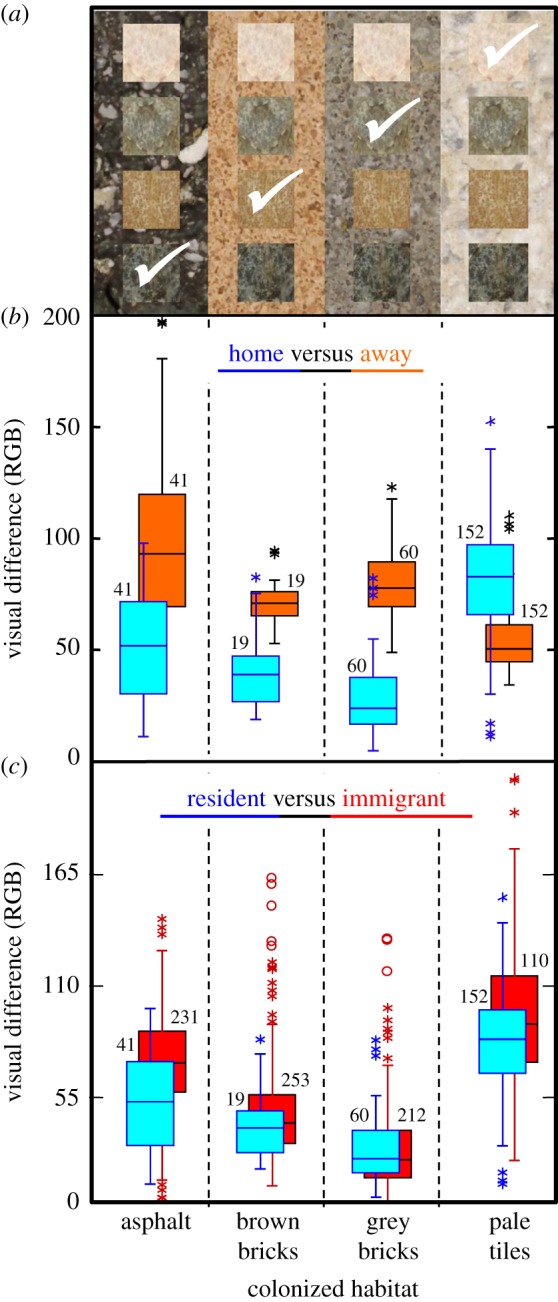
Grasshoppers are more cryptic than expected by chance as they colonize novel, urban habitats. (a) Background images are representative of each of the four street habitats, with representative parts of four grasshopper individuals positioned on top (small square images of the thorax). For each individual (same individual per row), the white tick-mark indicates in which habitat it is presumably (this depends on the predator's visual system) most cryptic (i.e. lowest visual distance). (b) Comparing crypsis in own versus other habitats. Pale blue boxes: observed visual differences between colour of the grasshoppers and colour of their local habitats (home). Dark orange boxes: predicted visual differences if those same individuals were using the other three habitats in proportion to their availability (away). Visual differences are expressed as RGB differences (a quantification of colour differences, see Methods). (c) Comparing crypsis between resident versus potential immigrant grasshoppers. Pale blue boxes: observed visual differences (local residents). Dark red boxes: predicted visual differences if all the grasshoppers from the other three habitats would use the focal habitat (potential immigrants). Sample sizes are given for each box plot; ntotal = 272. All comparisons of (b,c) are significant at p < 0.001, except home versus away for pale tiles (p = 1.00) and resident versus immigrant for brown bricks (p = 0.096) and grey bricks (p = 0.377). Tukey-type box plots: middle line = median; box = central 50% of values = interquartile range; whiskers = highest and lowest value within 1.5 × interquartile range; stars = values within 3 × interquartile range; circles = values outside 3 × interquartile range. (Online version in colour.)
(b). Support for matching habitat choice
Adult grasshoppers that were painted darker made a greater use of dark habitat in the laboratory than grasshoppers painted paler (figure 4a; GLMM, z = 2.58, n = 30, p = 0.0098). The same effect was observed in grasshoppers darkened via corazonin hormone injection (on average 54% darker; figure 4b), which also used the dark habitat more (figure 4b; GLMM, χ2 = 26.0, n = 52, p < 10−6). Importantly, this effect was also seen in the field (figure 4c): after release, corazonin-darkened grasshoppers predominantly used the dark asphalt habitat (70.4%, n = 27), whereas pale control grasshoppers mostly avoided it (28.6%, n = 14; GLMM, χ2 = 9.84, p < 0.002).
Figure 4.
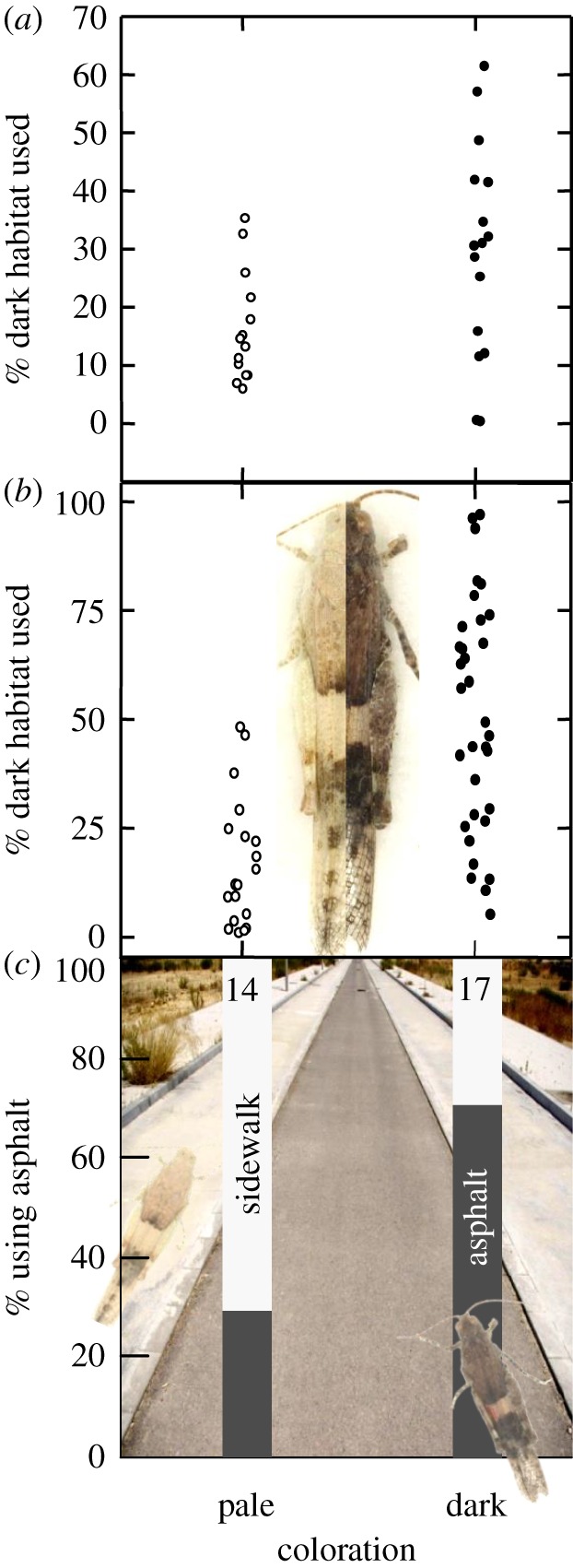
Manipulating the phenotype of grasshoppers causes them to change habitat use in the expected direction. (a) Grasshoppers (n = 30) painted dark (dark dots, right) are found more often on the dark laboratory habitat than grasshoppers painted pale (pale dots, left). Dots are individual means based on 20 observations. (b) Grasshoppers (n = 52) injected with the hormone corazonin become darker (right half of image) and are found on the dark laboratory habitat more often (dark dots) than untreated, pale grasshoppers (left half of the image and pale dots). Dots are individual means based on 20 observations. (c) Grasshoppers darkened by corazonin (right individual, n = 17) are mostly recaptured on dark asphalt, whereas pale control individuals (left individual, n = 14) are mostly recaptured on pale parking spaces and sidewalks. Background image: the dark asphalt road bordered by pale parking spaces and sidewalks. (Online version in colour.)
(c). Little to no support for natural selection and plasticity as current drivers of colour divergence between urban habitat patches
Simulations showed that mortality owing to natural selection on grasshopper colour would have to be unrealistically strong to create and maintain the observed colour divergence between pavements if settlement were random with respect to colour and substrate: mortality must be 58–72% d−1 for asphalt (electronic supplementary material, figure S5), and more than 20% for pale tiles and brown bricks (not shown). Such very strong selection is inconsistent with population maintenance, and with the low observed 3.8% daily mortality rate in the field (state-dependent capture–recapture model, n = 272, 95% CI = 2.9–5.2%). Moreover, these estimated mortality rates did not differ between substrates conferring higher versus lower crypsis (CIs were narrow but virtually identical). Lastly, we found a negligible daily removal rate of dead grasshoppers that we placed on the urban substrates, in contrast with a high removal rate (presumably by predators) of those at a natural site (4.4 versus 40.0%, respectively; n = 90, Fisher's exact test, p = 0.00007).
Adaptive phenotypic plasticity (colour change to match the used pavement) by adults is also insufficient to explain the observed colour divergence between pavements. Plasticity of colour in adults (0.33 ± 0.064 s.e. units RGB d−1; n = 40, electronic supplementary material, figure S6) is between one and two orders of magnitude too slow/weak to maintain current colour matching if their movements were random with respect to habitat (figure 2). Additionally, plasticity in adults is unidirectional: they only darkened and did not lighten (electronic supplementary material, figure S6), so plasticity cannot generate the observed crypsis on pale tiles (figure 3c).
4. Discussion
We found differences in colour for grasshoppers in the process of colonizing adjacent but distinctly coloured urban habitats (figure 3b,c), presumably in order to improve grasshopper crypsis (figures 1a and 3a; [30,32]; see [31,33] for supporting evidence). This is rather surprising, given the large scope for homogenization owing to movements between urban and natural habitats, and among the distinct urban habitats. We found little to no evidence this divergence was driven by present-day natural selection, as observed mortality was far lower than the selective mortality needed to counteract the high movement rates (assuming random settlement, electronic supplementary material, figure S5). Also, mortality was not higher for mismatched individuals, and attack rates on immobilized grasshoppers were much lower in the urban site than in a natural site, suggesting a lower risk of predation in the urban environment (which is probably unknown to the grasshoppers which keep behaving as to reduce predation). Nor does divergence appear to be caused by colour plasticity, which was slow and unidirectional (electronic supplementary material, figure S6; see also [42]). Instead, grasshopper colour differences between urban substrates are most likely maintained by habitat choice, for which we obtained multiple lines of supporting evidence. Most importantly, when we experimentally altered grasshopper colour, they changed their substrate use accordingly (figure 4). It thus strongly appears that these grasshoppers can evaluate their degree of crypsis on alternative substrates, and spend more time on substrates that provide greater crypsis.
As the average use of dark substrate by darkened individuals was sometimes below parity (figure 4a) or above parity (figure 4c), it does not seem that darkening just resulted in random substrate use. Colour-dependent substrate choice after colour manipulation has been previously demonstrated for some grasshoppers, but only in laboratory settings [44,45]. The continuous variation in colour present in our colonizing grasshoppers can be explained by the diverse soil colours of the nearest natural habitat (figure 1b), and will involve both heritable variation and plasticity by developing nymphs ([31]; electronic supplementary material, figure S2). By coincidence, the colours of the urban substrates newly made available coincided with the range of possible colours of the grasshoppers (figure 3a): if the urban substrates were very differently coloured, we predict that the grasshoppers would have adaptively avoided the urban environments in order to maintain crypsis.
Our results provide observational and experimental evidence for the hypothesis that the colonization of, and adaptation to, urban habitats is enhanced by individuals actively preferring certain urban habitats. Theory has long suggested that biased dispersal can drive population genetic structure and adaptation to different environments in general [16–23,46,47]. While several recent studies have drawn attention to the possible role of biased dispersal in the colonization of urban habitats and the spatial structuring of phenotypes in urban environments [9,26,27], alternative hypotheses have not been tested as extensively as we did here. In doing so, we found negligible support for effects of present-day natural selection and plasticity. This is not to say that these alternative drivers play a minor role during the colonization of urban habitats in general, either by themselves or in interaction with habitat choice, and several studies have found evidence that does support their operation [4,8,10,11,15]. Nonetheless, our study provides some of the best evidence that biased movement can also contribute to colonization and even local divergence in urban environments. Moreover, it shows that its contribution can be large, and even dominant. Our empirical results suggest that pre-existing habitat choice and dispersal behaviour (evolved via previous natural selection favouring this in heterogeneous natural environments) may generally play an important role in the adaptation to changed and new environments, as long anticipated by theory.
Our results also highlight two important aspects of the ecology and evolution of crypsis. First, while traditionally, this research has mostly focused on changes in the phenotype to improve crypsis (via evolution or plasticity), the possibility that organisms choose or manipulate their habitat [29] to improve crypsis is gaining more attention. Our data strongly support this alternative route towards greater crypsis. In addition, until recently, studies on habitat choice as a means to improve crypsis have focused on the average phenotype of species or discrete classes (morph, sex, life-history stage, etc). But, we show that habitat choice can also be specific to individual colour variation, in line with a few recent studies [48–50]. Second, we show that individual habitat choice is responsive to a change in phenotype, indicating that individual grasshoppers are somehow able to assess their degree of crypsis in local habitats. That they have this ability is reinforced by some of our earlier findings: individuals that were better matched in colour to their substrates could be approached more closely before they fled (see [51] for a similar finding in birds, but [52] for a lack of an effect), and even when they fled, they were more likely to stay where they landed instead of moving to a linear object that made detection more difficult [33]. Both observations suggest that more cryptic individuals felt safer.
Whether organisms generally have access to and use information on their degree of local crypsis is unknown [48]. Crypsis-enhancing habitat choice has now been reported for very different organisms, but the mechanism by which they achieve this is poorly known. In general, one could distinguish three kinds of habitat choice [25]: owing to genetic preference alleles, owing to imprinting or owing to a comparison of local performance. With respect to habitat choice as related to individual variation as we found here, the first option seems unlikely as this would require strong genetic components to phenotype (i.e. little to no plasticity) and habitat preference which are moreover mediated by pleiotropy or a strong genetic linkage. Imprinting on natal habitat also seems to be an unlikely route to achieve an adaptive preference for crypsis-enhancing habitats, because phenotypic plasticity and segregation variance would largely uncouple the link between parental and offspring phenotypes, and thereby make parental habitat choice a poor predictor of optimal offspring habitat. Performance-based (=matching) habitat choice is therefore a priori the more likely explanation when habitat choice is linked to individual variation, and in fact, the only explanation when individuals adaptively respond to phenotypic manipulation, as we observed here. For the grasshoppers, this evaluation of local crypsis may be facilitated by their protruding round eyes and mobile heads (figure 1a), allowing them to view, compare and evaluate the colour of their body relative to that of the substrate. This is in line with the findings by Gillis [44], who also painted the areas around the eyes of grasshopper nymphs (two different colour types, possibly owing to plasticity) just as we did, and also observed a change in habitat use that would provide greater crypsis. However, such self-referential habitat choice should not be assumed a priori, for example [53], painted around the eyes of two species of moths and found no effect, and concluded that the (species-specific) crypsis-enhancing habitat choice had a genetic basis. This may well be the norm for species which show little phenotypic variation among individuals.
To conclude, biased dispersal owing to habitat choice can help explain why certain species move into urban environments and others do not, how divergence between urban and rural populations can arise and even how divergence within the urban setting can originate and be maintained. More generally, this study reinforces that improved local performance and adaptive evolution can result from the active and adaptive spatial redistribution by genotypes, even when natural selection is currently not acting. We propose that eco-evolutionary studies more fully incorporate that individuals are not only selected upon by the environment (i.e. selective targets), but also are selectors of the environments to which they expose themselves (i.e. selective agents) (see also [29]).
Supplementary Material
Acknowledgements
We thank Dries Bonte, Carlos Camacho, Bart Kempenaers, Martine Maan, Yoel Stuart, Thor Veen, Jonathan Wright and all people at Axios Review for comments and discussion. Chad Brock provided R code to simulate natural selection.
Data accessibility
The data and R code supporting the results are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.58bb05p [43].
Authors' contributions
P.E. planned and coordinated the study, and wrote the first and final draft of the paper. A.B.-V. and P.E. designed experiments, collected many of the data, and performed the simulations and most data analyses. D.P.Q. and A.J.-A. designed a part of the experiments and collected and analysed data. G.E. helped to design some experiments, took care of animals and logistics and collected part of the data. D.I.B. helped with project design, data interpretation, simulation of selective mortality and writing. All authors participated in improving the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (grant nos RYC-2011-07889, CGL-2012-35232, CGL2013-49460-EXP and CGL2016-79483-P to P.E.; grant no. BES-2013-062905 to A.B.-V.) with support from the European Regional Development Fund, and from the National Science Foundation (grant no. DEB-1456462 to D.I.B.).
References
- 1.Darwin C. 1859. On the origins of species by means of natural selection. London, UK: Murray. [Google Scholar]
- 2.Rose MR, Lauder GV. 1996. Adaptation. San Diego, CA: Academic Press. [Google Scholar]
- 3.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 4.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney ML. 2002. Urbanization, biodiversity, and conservation. Bioscience 52, 883 ( 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2) [DOI] [Google Scholar]
- 6.Ellis EC, Goldewijk KK, Siebert S, Lightman D, Ramankutty N. 2010. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 19, 589–606. ( 10.1111/j.1466-8238.2010.00540.x) [DOI] [Google Scholar]
- 7.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. ( 10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 8.Miranda AC, Schielzeth H, Sonntag T, Partecke J. 2013. Urbanization and its effects on personality traits: a result of microevolution or phenotypic plasticity? Glob. Chang. Biol. 19, 2634–2644. ( 10.1111/gcb.12258) [DOI] [PubMed] [Google Scholar]
- 9.Holtmann B, Santos ESA, Lara CE, Nakagawa S. 2017. Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance. Proc. R. Soc. B 284, 20170943 ( 10.1098/rspb.2017.0943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799. ( 10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti M, Correa C, Marzluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y. 2017. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl Acad. Sci. USA 114, 8951–8956. ( 10.1073/pnas.1606034114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brans KI, Jansen M, Vanoverbeke J, Tüzün N, Stoks R, De Meester L. 2017. The heat is on: genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob. Chang. Biol. 23, 5218–5227. ( 10.1111/gcb.13784) [DOI] [PubMed] [Google Scholar]
- 13.Schlichting CD, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 14.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. ( 10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 15.Lowry H, Lill A, Wong BBM. 2013. Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. ( 10.1111/brv.12012) [DOI] [PubMed] [Google Scholar]
- 16.Edelaar P, Siepielski AM, Clobert J. 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472. ( 10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- 17.Maynard Smith J. 1966. Sympatric speciation. Am. Nat. 100, 637–650. ( 10.1086/282457) [DOI] [Google Scholar]
- 18.Ronce O. 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 38, 231–253. ( 10.1146/annurev.ecolsys.38.091206.095611) [DOI] [Google Scholar]
- 19.Ravigné V, Dieckmann U, Olivieri I. 2014. Live where you thrive: joint evolution of habitat choice and local adaptation facilitates specialization and promotes diversity. Am. Nat. 174, E141–E169. ( 10.1086/605369) [DOI] [PubMed] [Google Scholar]
- 20.Berdahl A, Torney CJ, Schertzer E, Levin SA. 2015. On the evolutionary interplay between dispersal and local adaptation in heterogeneous environments. Evolution 69, 1390–1405. ( 10.1111/evo.12664) [DOI] [PubMed] [Google Scholar]
- 21.Berner D, Thibert-Plante X. 2015. How mechanisms of habitat preference evolve and promote divergence with gene flow. J. Evol. Biol. 28, 1641–1655. ( 10.1111/jeb.12683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelaar P, Bolnick DI. 2012. Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665. ( 10.1016/j.tree.2012.07.009) [DOI] [PubMed] [Google Scholar]
- 23.Bolnick DI, Otto SP. 2013. The magnitude of local adaptation under genotype-dependent dispersal. Ecol. Evol. 3, 4722–4735. ( 10.1002/ece3.850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob S, Legrand D, Chaine AS, Bonte D, Schtickzelle N, Huet M, Clobert J. 2017. Gene flow favours local adaptation under habitat choice in ciliate microcosms. Nat. Ecol. Evol. 1, 1407–1409. ( 10.1038/s41559-017-0269-5) [DOI] [PubMed] [Google Scholar]
- 25.Akcali CK, Porter CK. 2017. Comment on Van Belleghem et al. 2016: habitat choice mechanisms in speciation and other forms of diversification. Evolution 71, 2754–2761. ( 10.1111/evo.13375) [DOI] [PubMed] [Google Scholar]
- 26.Carrete M, Tella JL. 2010. Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol. Lett. 6, 167–170. ( 10.1098/rsbl.2009.0739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sol D, Lapiedra O, González-Lagos C. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. ( 10.1016/j.anbehav.2013.01.023) [DOI] [Google Scholar]
- 28.Wang IJ, Bradburd GS. 2014. Isolation by environment. Mol. Ecol. 23, 5649–5662. ( 10.1111/mec.12938) [DOI] [PubMed] [Google Scholar]
- 29.Edelaar P, Bolnick DI. 2019. Appreciating the multiple processes increasing individual or population fitness. Trends Ecol. Evol. 34, 435–446. ( 10.1016/j.tree.2019.02.001) [DOI] [PubMed] [Google Scholar]
- 30.Rowell CHF. 1972. The variable coloration of the Acridoid grasshoppers. Adv. Insect Phys. 8, 145–198. ( 10.1016/S0065-2806(08)60197-6) [DOI] [Google Scholar]
- 31.Edelaar P, Baños-Villalba A, Escudero G, Rodríguez-Bernal C. 2017. Background colour matching increases with risk of predation in a colour-changing grasshopper. Behav. Ecol. 20, 65–71. ( 10.1093/beheco/arx016) [DOI] [Google Scholar]
- 32.Forsman A, Karlsson M, Wennersten L, Johansson J, Karpestam E. 2011. Rapid evolution of fire melanism in replicated populations of pygmy grasshoppers. Evolution 65, 2530–2540. ( 10.1111/j.1558-5646.2011.01324.x) [DOI] [PubMed] [Google Scholar]
- 33.Baños-Villalba A, Quevedo DP, Edelaar P. 2018. Positioning behavior according to individual color variation improves camouflage in novel habitats. Behav. Ecol. 29, 404–410. ( 10.1093/beheco/arx181) [DOI] [Google Scholar]
- 34.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troscianko J, Stevens M. 2015. Image calibration and analysis toolbox—a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol. 6, 1320–1331. ( 10.1111/2041-210X.12439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 37.Börger L, Fryxell J. 2012. Quantifying individual differences in dispersal using net squared displacement. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 222–230. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Yerushalmi Y, Pener MP. 2001. The response of a homochrome grasshopper, Oedipoda miniata, to the dark-colour-inducing neurohormone (DCIN) of locusts. J. Insect Physiol. 47, 593–597. ( 10.1016/S0022-1910(00)00156-6) [DOI] [PubMed] [Google Scholar]
- 39.Estes S, Arnold SJ. 2007. Resolving the paradox of stasis: models with stabilizing selection explain evolutionary divergence on all timescales. Am. Nat. 169, 227–244. ( 10.1086/510633) [DOI] [PubMed] [Google Scholar]
- 40.White GC, Burnham KP. 1999. Program mark: survival estimation from populations of marked animals. Bird Study 46, S120–S139. ( 10.1080/00063659909477239) [DOI] [Google Scholar]
- 41.Lebreton J-DD, Pradel R. 2002. Multistate recapture models: modelling incomplete individual histories. J. Appl. Stat. 29, 353–369. ( 10.1080/02664760120108638) [DOI] [Google Scholar]
- 42.Peralta-Rincon JR, Escudero G, Edelaar P. 2017. Phenotypic plasticity in color without molt in adult grasshoppers of the genus Sphingonotus (Acrididae: Oedipodinae). J. Orthoptera Res. 26, 21–27. ( 10.3897/jor.26.14550) [DOI] [Google Scholar]
- 43.Edelaar P, Baños-Villalba A, Quevedo DP, Escudero G, Bolnick DI, Jordán-Andrade A. 2019. Data from: Biased movement drives local cryptic coloration on distinct urban pavements Dryad Digital Repository. ( 10.5061/dryad.58bb05p) [DOI] [PMC free article] [PubMed]
- 44.Gillis JEE. 1982. Substrate colour-matching cues in the cryptic grasshopper Circotettix rabula rabula (Rehn & Hebard). Anim. Behav. 30, 113–116. ( 10.1016/S0003-3472(82)80244-3) [DOI] [Google Scholar]
- 45.Karpestam E, Wennersten L, Forsman A. 2011. Matching habitat choice by experimentally mismatched phenotypes. Evol. Ecol. 26, 893–907. ( 10.1007/s10682-011-9530-6) [DOI] [Google Scholar]
- 46.Armsworth PR. 2009. Conditional dispersal, clines, and the evolution of dispersiveness. Theor. Ecol. 2, 105–117. ( 10.1007/s12080-008-0032-2) [DOI] [Google Scholar]
- 47.Kerr B, Godfrey-Smith P. 2009. Generalization of the Price equation for evolutionary change. Evolution 63, 531–536. ( 10.1111/j.1558-5646.2008.00570.x) [DOI] [PubMed] [Google Scholar]
- 48.Stevens M, Ruxton GD. 2019. The key role of behaviour in animal camouflage. Biol. Rev. 94, 116–134. ( 10.1111/brv.12438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall KLAA, Philpot KE, Stevens M. 2016. Microhabitat choice in island lizards enhances camouflage against avian predators. Sci. Rep. 6, 19815 ( 10.1038/srep19815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens M, Troscianko J, Wilson-Aggarwal JK, Spottiswoode CN. 2017. Improvement of individual camouflage through background choice in ground-nesting birds. Nat. Ecol. Evol. 1, 1325–1333. ( 10.1038/s41559-017-0256-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson-Aggarwal JK, Troscianko JT, Stevens M, Spottiswoode CN. 2016. Escape distance in ground-nesting birds differs with individual level of camouflage. Am. Nat. 188, 231–239. ( 10.1086/687254) [DOI] [PubMed] [Google Scholar]
- 52.Zimova M, Mills LS, Lukacs PM, Mitchell MS. 2014. Snowshoe hares display limited phenotypic plasticity to mismatch in seasonal camouflage. Proc. R. Soc. B 281, 20140029 ( 10.1098/rspb.2014.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sargent TD. 1968. Cryptic moths: effects on background selections of painting the circumocular scales. Science 159, 100–101. ( 10.1126/science.159.3810.100) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Edelaar P, Baños-Villalba A, Quevedo DP, Escudero G, Bolnick DI, Jordán-Andrade A. 2019. Data from: Biased movement drives local cryptic coloration on distinct urban pavements Dryad Digital Repository. ( 10.5061/dryad.58bb05p) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and R code supporting the results are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.58bb05p [43].