Abstract
Offspring produced by older parents often have reduced longevity, termed the Lansing effect. Because adults usually have similar-aged mates, it is difficult to separate effects of maternal and paternal age, and environmental circumstances are also likely to influence offspring outcomes. The mechanisms underlying the Lansing effect are poorly understood. Variation in telomere length and loss, particularly in early life, is linked to longevity in many vertebrates, and therefore changes in offspring telomere dynamics could be very important in this context. We examined the effect of maternal age and environment on offspring telomere length in zebra finches. We kept mothers under either control (ad libitum food) or more challenging (unpredictable food) circumstances and experimentally minimized paternal age and mate choice effects. Irrespective of the maternal environment, there was a substantial negative effect of maternal age on offspring telomere length, evident in longitudinal and cross-sectional comparisons (average of 39% shorter). Furthermore, in young mothers, sons reared by challenged mothers had significantly shorter telomere lengths than sons reared by control mothers. This effect disappeared when the mothers were old, and was absent in daughters. These findings highlight the importance of telomere dynamics as inter-generational mediators of the evolutionary processes determining optimal age-specific reproductive effort and sex allocation.
Keywords: maternal age, maternal effects, stress, telomere length, Lansing effect
1. Introduction
The conditions under which offspring are produced can have profound effects on their subsequent health and life histories [1]. In long-lived, iteroparous species with parental care, key aspects of this are likely to be parental age and the prevailing environmental conditions. The age at which offspring are produced is a fundamental factor in the evolution of reproductive scheduling as the temporal pattern of investment is expected to be tailored to maximize individuals' lifetime fitness [2,3]. This is because both the success of a breeding event and the quality of the offspring produced can be influenced by parental age at reproduction. The general relationship between age and measures of reproductive performance tends to be an inverted U-shape, showing improvement with age early in reproductive life and a decline in old age [3–6]. The late-life decline in offspring production has been attributed to parental senescence [7,8]. However, in addition to a decline in fertility with age, there is substantial evidence that parental age at reproduction also has consequences for the health, pattern of ageing and longevity of those offspring that are produced, with offspring of older parents often showing reduced probability of survival and impaired health, termed the Lansing effect [9–14]. Thus, reduced offspring production in later life could be an evolved strategy to reduce investment in less fit offspring. The stronger such late-life effects, the greater the impact this will have on the evolution of reproductive schedules.
There has recently been substantial work investigating and modelling the evolutionary consequences of parental, and particularly maternal, age effects on offspring performance [15,16]. However, the processes by which offspring are adversely influenced by the age of their parents are not well understood, and are likely to involve both environmental and genetic effects. The quality of both the prenatal and post-natal environment provided by parents is likely to be very important. The genetic inheritance of the offspring can be influenced by parental age via, for example, increased likelihood of their inheriting adverse germ-line mutations with advancing parental age, changes in the genome stability of germ cells or via changes in the epigenome with age [17–21].
Prevailing environmental conditions are also an important component influencing offspring fitness, and potentially also the magnitude of parental age effects, which could be masked or exaggerated under environmentally induced stress. In line with the disposable soma theory of ageing, models have proposed the presence of interactive trade-offs between the optimal allocation of maternal investment in somatic maintenance and investment allocated to the production and rearing of the offspring [16]. Such trade-offs would result in old mothers or mothers living under poor environmental conditions having offspring with altered biological age at birth and long-term fitness consequences, such as reduced lifespans [16,22]. However, empirical evidence in support of such predictions is limited (but see [22,23]). Furthermore, because rearing male versus female offspring could be associated with different costs and benefits, parental age effects on offspring might be sex-specific and vary with environmental conditions [24].
One key mechanism that could have an important inter- and trans-generational effect on offspring performance, and potentially vary with parental age and environmental conditions, is effects on offspring telomere dynamics; this could affect both the telomere length that offspring inherit from their parents and the subsequent pattern of telomere loss in offspring during the period of parental dependence. Telomeres are highly conserved, protective structures that occur at the ends of the linear eukaryotic chromosomes, involving tandem repeats of DNA. Together with shelterin proteins, telomeres play a key role in genome stability, shielding genes from loss of coding sequences as cells divide and preventing end-to-end joining of chromosomes by the DNA repair machinery [25,26]. Across many studied species, telomere length decreases with age in most somatic tissues, and such a decline is especially pronounced during early development [27–30]. Telomere length has been associated with organismal fitness proxies as individuals with shorter telomeres have shorter lifespans [28,31–33] and can have an increased susceptibility to disease [34–37]. Telomeres are thought to be integrative markers of exposure to stress [38]. Stress exposure, induced either via direct experimental elevations of glucocorticoid stress hormones or via exposure to various stressors, including poor parental care or immune challenges, has been shown to increase telomere shortening, especially in developing individuals [39,40]. As recently reviewed [41], accumulating evidence from studies in birds and mammals highlights that stress exposure in the parental generation, occurring primarily via the maternal route during the pre- or post-natal stages, can have a long-lasting impact on offspring telomere dynamics (e.g. [42–44]).
We still know relatively little about parental age effects on offspring telomere dynamics, or the impact that any such effects have for offspring fitness, and we know even less about the extent to which parental age effects vary depending on differing environmental circumstances. The majority of the studies of parental age effects on telomeres carried out to date have focused on testing the association between paternal age and offspring telomere length [45]. While across human populations older fathers have offspring with longer telomere lengths (reviews: [45,46]), in most non-human species, this pattern is either reversed [13,14,47–49] or absent [46,50]. Most studies of the association between maternal age and offspring telomere length have been performed in humans and found no association between these two factors when statistically controlling for the age of the fathers (review: [46]). The limited work in other vertebrate species reports variable results, with some species showing a negative association of maternal age with offspring telomere length [51] and others showing no maternal age effect [13,46,49,50]. However, a multitude of factors, in addition to study design, are likely to be important in this context, including variation in maternal health status [32], the age of offspring at telomere measurement, the age of fathers [46] and the differential survival of parents with differing telomere lengths [28]. It remains therefore unclear to what extent maternal ageing influences offspring telomere length.
Here, we used an experimental manipulation (i) to examine the effect of maternal age at reproduction on offspring telomere length at the time of parental independence and (ii) to assess to what degree challenging environmental conditions experienced by the mothers alter any such maternal age effects. We used zebra finches (Taeniopygia guttata), which begin to show signs of reproductive senescence between 2 and 3 years of age [52,53]. We manipulated the quality of the environment by exposing our study females to unpredictable episodes of food withdrawal throughout adulthood and experimentally controlled the breeding opportunities of the birds. When the females were young, and when they were old, they were paired with a randomly assigned, relatively young adult male. Thus, we experimentally minimized the association between male and female age, and the effect of assortative mating via mate choice often occurring in correlative studies.
2. Material and methods
(a). Study subjects and housing conditions
All females used in this study (n = 180) were produced from the breeding stock at the University of Glasgow. We conducted two replicates of the experiment; replicate 1 females were produced in April–June 2011 and replicate 2 females were produced in August–September 2011. The environmental manipulations started when the focal females were fully grown, sexually mature, young adults (approx. five months old; mean ± s.e.: 152 ± 1 days). Prior to the start of the study, birds were kept in single-sex groups under standard housing and feeding conditions, with ad libitum supply of mixed seeds (common millet, yellow millet and canary seed in a ratio of 3 : 1 : 1; Johnson and Jeff, UK), oyster shell grit, cuttlefish and ad libitum water and treatment-specific cages (n = 7–10 females per 120 × 50 × 50 cm cage). The photoperiod was always maintained at 14 h : 10 h light : dark cycle and the temperature was between 20 and 24°C. All procedures were carried out under UK Home Office Project Licence 60/4109.
(b). Environmental manipulation
When the females were approximately five months of age, they were randomly allocated to one of the two experimental groups: a challenging (n = 89) or control environment (n = 91). In the challenging environment, food was made unavailable for a continuous period of approximately one-third of the daylight period (4.9 h), 4 days per week on a random time schedule. For the remaining two-thirds of the day and on the remaining 3 days per week, challenged females received ad libitum food. Challenged females always experienced this food regime except during breeding when they were given ad libitum access to food from the time they were paired with a male or shortly afterwards until after they completed breeding (approx. two months for each breeding event). The treatment had no detectable effect on female body mass [53]. Control females were always provided with ad libitum food and experienced exactly the same breeding scheduling as the challenged birds. As previously shown, the simulated challenged environmental conditions led to increases in corticosterone secretion, the primary avian glucocorticoid stress hormone. At the end of each food withdrawal exposure, challenged females had higher corticosterone than controls (on average 1.6-fold increase and within the baseline range of variation for our study species), and this physiological response was consistent over a very prolonged exposure periods (up to 3 years), indicating no habituation of the birds to the environmental manipulation [6,53].
(c). Adult female breeding timeline and offspring sampling
We examined the telomere length of offspring produced by mothers that bred at two time points: (i) during young adulthood at six months (i.e. young mother breeding event: mean age ± s.e., 187.6 ± 1.0 days; range: 156–207 days, n = 172 mothers) and (ii) in old age at 3.5 years old (i.e. old mother breeding event: mean age ± s.e., 1269.3 ± 1.3 days; range: 1259–1293 days, n = 52 mothers). When not breeding, the females were kept in single-sex groups and thus did not form long-term pair bonds with particular males. The reduced number of mothers in the old mother breeding event was due to natural maternal mortality and/or breeding failure (i.e. no fledglings produced); offspring telomere length data from the same mothers in both the young and old mother breeding events were available for 44 females (18 controls and 26 challenged). During these two breeding events, females were paired with a different, unrelated, randomly assigned male of prime breeding age. These males had always been kept in control environmental conditions (see above). While the males were similar in age to the females during the first breeding event, when the females themselves were young (age of the males at the young-mother breeding event—mean ± s.e.: 185.7 ± 1.2 days, range: 142–204 days), the experimental design ensured that this was not the case when the females were old; the males with which the females were paired in their old age were still relatively young, on average, just over 1.2 years (age of the males at the old mother breeding event—mean ± s.e.: 464.1 ± 23.9 days, range: 212–699 days). The age of the father in the old mother breeding event, where males were substantially younger than females (t-test: t = −53.72, d.f. = 125, p < 0.0001), had no effect on offspring telomere length (GLMM: p ≤ 0.85; full statistics in electronic supplementary material, table S1).
Each pair was placed in individual breeding cages (60 × 50 × 50 cm) equipped with an external nest-box and nest material (coconut fibres and jute, Haiths Ltd). Breeding birds were provided with a commercial seed mix (Johnson and Jeff, UK), oyster shell grit, cuttlefish and water. Once a week, the birds were also provided with Calcivet calcium supplement (Vetafarm, Wagga Wagga, New South Wales, Australia), a protein conditioning supplement (J. E. Haith, Cleethorpes, UK) and fresh vegetables. Between the young and old mother breeding events, females in both replicate groups experienced the same breeding schedules, with two breeding events in the intervening years. They were allowed to lay, but not rear, a clutch of eggs at 1.1 years and to rear their biological or foster brood when they were 1.8 years old. Both these breeding events involved pairing with similarly young males as in the young- and old-mother breeding events, but the fact that the breeding regime varied from that in the young and old breeding events precludes comparison of the effects on offspring. The actual number of eggs laid and young reared to fledging prior to the 3.5 years breeding event were included in the analysis to check whether variation among females in prior breeding effort was associated with variation in offspring telomere length produced during the old-mother breeding event. Neither of these estimates of breeding effort had any effect on offspring telomere length, thus excluding the possibility of potential confounds between differences in prior maternal reproductive effort and chronological age (electronic supplementary material, table S1).
(d). Blood sampling and telomere length analysis
Chicks were weighed and small blood samples (approx. 70 µl) were collected by venipuncture of the alar vein when they were approximately 30 days old and feeding independently of their parents (fledgling mean age ± s.e.: 29.6 ± 0.05 days, range: 25–32 days) during both the young mother and old mother breeding events. Chick age was estimated from the first chick hatched within each clutch; hatching order within each nest was also recorded since this can influence telomere length [54]. Blood samples were immediately placed on ice after sampling. Within 4 h, the blood samples were spun to separate plasma from red blood cells, and the latter were stored at −80°C until later telomere analysis. Our cohort of experimental females was also periodically sampled for telomere analysis. However, the blood sampling was never performed in close proximity to or during the breeding events to minimize disturbance and potential additional stress associated with handling. We found no correlations between maternal and offspring telomere lengths (to be published in a separate manuscript).
During the first breeding event, we measured telomere length in two randomly selected chicks per nest (brood sizes reaching the sampling age for mothers that produced a clutch: 3.15 ± 0.09 chicks, mean ± s.e.); there was no sampling bias in the hatching order of the selected chicks among the differing clutch sizes (χ2 = 47.15, d.f. = 40, p = 0.20). At the old mother breeding event, when brood sizes were smaller, all chicks were measured (brood sizes reaching the sampling age for mothers that produced a clutch: 2.50 ± 0.17 chicks, mean ± s.e.) to ensure an adequate sample size per brood similar to that during the young mother breeding event. DNA from red blood cells was extracted using commercial kits and following the manufacture's protocol (Macherey-Nagel, USA). Relative telomere length (RTL) was quantified in the red blood cell DNA by using qPCR as described elsewhere [55]; this correlates well with measurements using TRF method [55]. Briefly, the RTL of each sample was measured by determining the ratio (T : S) of telomere repeat copy number (T) to a single copy control gene (S), relative to the same DNA reference sample run on each plate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the single-copy control gene. The telomere and GAPDH reactions were carried out on separate plates, and in both reactions, the number of PCR cycles (Ct) required for the products to accumulate enough fluorescent signal to cross a threshold was determined. Reaction efficiencies were always within the acceptable range (i.e. 100 ± 10%). All samples fell within the bounds of the standard curve run on every plate (6 standard dilutions, from 40 to 125 ng of DNA). All telomere assays were run between October 2015 and February 2016 and samples were randomly spread across the different plates; each plate contained a standard curve and all standards and samples were always run in triplicate. The intra-plate coefficient of variation for the telomere and GAPDH assays for the raw Ct values were 0.65% and 0.97%, respectively; the inter-plate coefficient of variation calculated using the standard dilutions that were run across each plate for both the telomere and GAPDH assays were 1.63% and 1.96%, respectively.
The raw qPCR data were analysed using the software Qbase+ [56]. The mean Ct values were used to calculate a relative measure of telomere length as a T : S ratio of telomere repeat copy number to a control, single copy gene number (GAPDH). The Qbase+ software provides the advantage of adjusting for differences in amplification efficiencies among plates (as described in [57]) and correcting for further inter-run variation by including three inter-run calibrators (i.e. the reference sample and two points from the standard curve—10 and 5 ng of DNA). For each sample, the software produced a calibrated normalized relative telomere measurement, which is similar to the T : S ratio described by Cawthon [31] but offers a greater control of inter-plate stochastic variation. The inter-assay coefficient of variation for the calibrated normalized T : S ratios calculated using the standard dilutions run across each plate was 15.25%.
(e). Data analysis
Analyses were performed in R (v. 3.5.1; R Core Team, 2014). We used generalized linear mixed models with a Gaussian distribution—GLMMs R package ‘lme4’ [58] and ‘lmerTest’ [59]—to examine whether maternal age and/or the maternal environmental treatment influenced offspring body mass or offspring telomere length at fledgling, upon nutritional independence of the chicks from their parents. Telomere data were ln-transformed to improve the normality of model residuals. One offspring produced during the first breeding event with a telomere length value of 4.56 was excluded from telomere analyses because this value was an extreme statistical outlier as suggested by inspection of model residuals and as exceeds the upper quartile by more than three times the interquartile range [60].
All final models included the effects of experimental design factors expected to influence the response variables either as parameters of interest integral to the questions being investigated or for the purpose of adjustment (i.e. to control for potentially confounding variables). These relevant factors were always retained in the main models rather than tested using selection procedures to avoid overfitting and inflating the type I error. Unless otherwise specified, final models always included the following main factors: maternal age (young mother breeding event or old mother breeding event), maternal treatment (control environment or challenging environment), replicate, offspring sex (determined by colour plumage when the chicks were approximately 50 days old), brood size (i.e. number of chicks reared) at the time of sampling and the hatching order within the clutch to control for the slight variation in age of the chicks at the time of sampling [54]. We also entered the two- and three-way interactions among maternal age, maternal treatment and offspring sex in order to test whether the potential effect of maternal age and/or treatment on offspring body mass or offspring telomere length differed between male and female offspring; non-significant interactions (p > 0.05) were sequentially removed using backward selection starting from the three-way interaction. In initial models of the telomere length data, we also examined whether body mass of the offspring at the time of sampling (values available for 441 out of 444 chicks) and the two-way interaction between offspring body mass and maternal age influenced offspring telomere length; but neither of these factors were significant (p ≥ 0.5) and were consequently removed from the final models. The identities of the mothers were included as random factor to account for non-independence of offspring from the same mother. In order to assess within-mother age and treatment effects and to exclude bias in the results associated with the loss of specific individuals from the female population due to death or non-breeding, we also performed analyses using only those offspring telomere data from females that reared chicks during both the young- and old-mother breeding events (185 out of 444 chicks and 44 out of 180 mothers). We used the R package ‘lsmeans’ [61] to perform pairwise post hoc comparisons for significant outcomes in the main models (Tukey's p-value adjustment). Multi-collinearity was examined in all models by calculating variance inflation factors; these ranged from 1.0 to 1.3 indicating acceptable degrees of multi-collinearity among the explanatory variables. All models met the assumption of normality and homogeneity, which was assessed via graphical diagnostics of the residuals [62]. Unless otherwise specified, descriptive statistics are provided as mean ± s.e.
3. Results
(a). Effects of maternal age and environmental conditions on offspring body mass
There was no effect of the maternal treatment, replicate, offspring sex and hatching order on offspring body mass as main factors; there were no interacting effects among maternal age, maternal treatment and offspring sex on the response variable (full statistics in electronic supplementary material, table S2a). Regardless of the maternal environment and offspring sex, fledglings produced during the old mother breeding event were lighter when compared with the fledglings produced during the young mother breeding event (maternal age: p = 0.001; electronic supplementary material, figure S1a). We also found that lighter offspring were those reared in larger broods (p = 0.001, electronic supplementary material, table S2a). However, when restricting the analysis to the subset of offspring produced by the mothers that bred during both the young and old mother breeding events, the significant effects of maternal age and brood size on offspring body mass disappeared (electronic supplementary material, table S2b and figure S1b).
(b). Effects of maternal age and environmental conditions on offspring telomere length
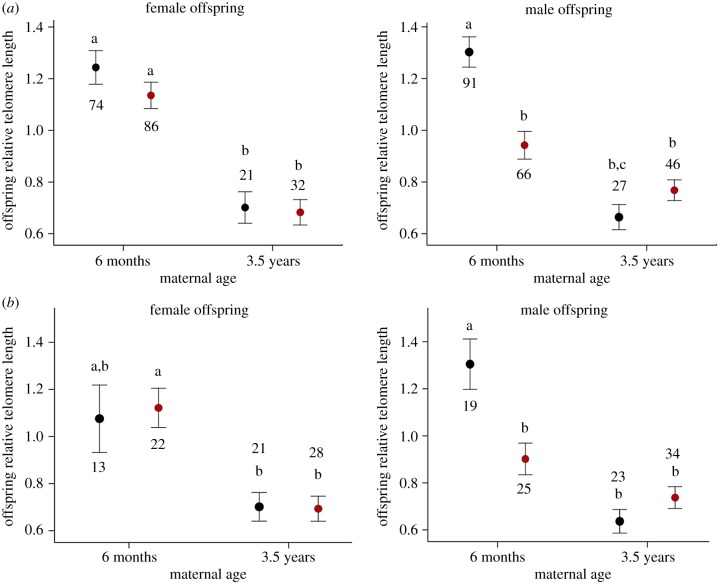
The strongest main effect on offspring telomere length was maternal age (p < 0.001, full statistics in electronic supplementary material, table S3a) with offspring produced in the old-mother breeding event having substantially shorter (39% on average) telomere lengths compared to offspring produced during the young mother breeding event (figure 1a). However, the effect of the maternal environment on offspring telomere length differed with offspring sex and with maternal age (maternal treatment × maternal age × offspring sex: p = 0.02; electronic supplementary material, table S3a; figure 1). For mothers living in the control conditions, the effect of maternal age was consistent in both sons and daughters; daughter telomere lengths were 43.5% shorter when their mothers were old compared with when mothers were young, and 49.2% shorter in sons (p ≤ 0.003 for both). There was no effect of the maternal environment on the telomere length of daughters either when their mothers were young or old (p ≥ 0.8 for both). Telomere length of sons however was reduced in young mothers living in the challenging environment compared with the sons produced by the young control mothers (by 27.7%, p = 0.0001). This resulted in daughters produced by young mothers living in challenging conditions having longer telomeres than equivalent sons (by 20.7%, p = 0.048). By contrast, when mothers were old, telomere length in their sons did not differ between the two maternal treatment groups (p = 0.8), and the same was true in their daughters (p = 0.9). Telomere lengths of sons produced by young mothers in the challenging environment were similar to those of the sons of old challenged mothers (p = 0.2), but slightly longer compared to the telomere lengths of sons of the old control mothers (p = 0.04). We found no effect of replicate, hatching order or brood size as main factors on offspring telomere length (electronic supplementary material, table S3a). Results were qualitatively similar (36% on average telomere shortening with maternal age) when we performed the same analysis on the subset of offspring reared by the same mothers during both the young- and old-mother breeding event (figure 1b; electronic supplementary material, table S3b).
Figure 1.
(a) Early-life RTL (approx. 30 days of age—values were adjusted for plate amplification efficiencies and inter-run calibration using the software Qbase+, see ‘Material and methods' for full details) of offspring produced by mothers that bred during the young-mother breeding event at six months of age (89 control and 83 challenged mothers) and/or during the old-mother breeding event at 3.5 years of age (20 control and 32 challenged mothers). In (b), data are shown only from the subset of females that produced offspring during both the young and old mother breeding event (18 control and 26 challenged mothers). Data are shown as means ± s.e.; black circles indicate offspring produced by control mothers and red circles indicate offspring produced by challenged mothers, numbers indicate offspring sample sizes separately by maternal treatment, maternal age and offspring sex; different letters indicate significant post hoc pairwise contrasts after Tukey's multiple comparison adjustment.
4. Discussion
This is the first long-term, longitudinal study to compare changes in telomere length in offspring produced by females at different ages (i.e. when young at six months of age, and when old at 3.5 years of age) and in which the age of their partners was experimentally standardized to enable maternal effects to be identified. From young adulthood and when not breeding, our focal females were living either under control (ad libitum food) or more challenging environmental circumstances (random withdrawals of food, which produced repeated increases in circulating glucocorticoid stress hormones). Our study therefore also enabled us to examine whether any reduction in telomere length resulting from maternal age was affected by the environmental conditions experienced by the mothers prior to breeding (thus excluding direct effects of the environment on offspring), and whether effects differed between sons and daughters. Our data clearly show that, in non-stressful environments, both sons and daughters produced by mothers in old adulthood have substantially shorter telomeres than those produced by mothers in young adulthood. We also found sex-specific interactive effects between the maternal environment and maternal age with sons produced by the challenged females as young breeders effectively having their telomere lengths equivalent to those sons produced when mothers were old; the decline in telomere length in the sons was of comparable magnitude to the telomere shortening associated with maternal age. These results are consistent with the results we obtain when we restrict the analyses to the subset of mothers that reared chicks during both breeding events, which confirms that these trans-generational effects on offspring telomere length occurred within-individual mothers and were not due to selective mortality or breeding quality of the females. Overall, the mean decline in offspring telomere length with maternal age was marked—approximately 39% over the elapsed maternal age period of approximately 3 years—overriding any potential effect associated with the maternal environmental manipulation. However, we do not know whether this decline was linear, or only occurred after a particular maternal age, which warrants future investigation.
The negative relationship between maternal age and offspring telomere length in both offspring reared by either challenged or control females can be attributed to the change in maternal age; other factors such as variation in previous maternal reproductive effort had no significant effect on offspring telomere length. Experience and resource acquisition by older individuals are also likely to be important factors, especially in the field. Our study was conducted in captivity under controlled environmental conditions, thus making it easier to isolate effect due to changes in maternal age as well as to challenging environmental circumstances. Paternal age, independently of maternal age, has been shown to affect telomere length in zebra finches as early as the embryonic developmental stages [14]. The design of our study aimed at minimizing variation in the father's age; the females grew older and we were therefore able to examine the effect of maternal ageing in the absence of an effect of paternal ageing on offspring telomere length. We do not however know whether the stage at which maternal effects occur differs from that of paternal effects, or indeed whether such maternal and paternal effects are additive. Clearly, the effect of maternal age was also influenced by the maternal environment and offspring sex, which could also contribute to inconsistencies in the effects found in different studies [13,49,51]. The lack of an effect of the maternal treatment during old adulthood in either sons or daughters suggests that the effect of maternal age might have overridden any maternally environmentally derived effects on offspring telomere length. It may be that there is a critical length below which offspring telomere length cannot fall and the offspring remain viable; hence, the absence of an additive effect. All our experimental females were housed in single-sex groups and were paired with a young adult male only during the age-specific breeding events to minimize mate familiarity. We can thus exclude the possibility that the reduction in offspring telomere length during the old mother breeding event could be attributable to increased maternal stress due to the sudden introduction of an unfamiliar male after years being paired with the same male, thereby having formed a long-term pair bond which is broken. That the effect of the maternal treatment was observed only when the mothers were young, and only in their male offspring is also intriguing. There are several possibilities that could explain such sex-dependent sensitivity to maternal effects. For instance, it is plausible that male nestlings were simply more vulnerable to poorer maternal rearing conditions than female nestlings as has been reported in a number of studies in birds including lesser black-backed full (Larus fuscus) [63,64], great tits (Parus major) [65] and collared flycatchers (Ficedula albicollis) [66]. However, studies in the zebra finch suggest that sons are generally over-produced under poor rearing conditions and so daughters may be more vulnerable [67–69]. We note, however, that in our study, we are unable to distinguish among effects that might arise from differential survival of sons and daughters during the prenatal or the very early post-natal stages or by shifts in primary sex ratios linked to maternal condition [63,64]. It is also possible that the sex-specific effect could relate to differences in telomere dynamics in the sex chromosomes, but nothing is known about this in birds.
The reduction in offspring body mass with maternal age probably reflected earlier mortality of females producing heavier offspring during early adulthood, not maternal age-specific variation in offspring body mass within individual mothers. Such effect is interesting as it occurred in the benign conditions of captivity and it might be associated with trade-offs between reproduction and survival. That adults can adopt differing patterns of reproductive investment that are related to their lifespan variation has also been found in other studies. For example, in the red-billed chough, parents that produced high-quality offspring had reduced longevity compared to parents producing lower quality offspring [23].
Studies in humans suggest that maternal effects on offspring telomere length could occur as early as the oocyte. This may be because eggs ovulated in older women enter meiosis at a later point in fetal egg formation than eggs ovulated when women are younger [20]. These late ovulated eggs will therefore have been produced via more cell replications, which could shorten telomeres [20,70]. Increased exposure to ROS-induced oxidative damage with storage time in the ovary may also play a role [21]. Alternatively, the decrease in offspring telomere length with maternal age in birds could occur as a consequence of differences in egg composition, including differences in yolk : albumen ratio content [71], concentrations of hormones and immune antibodies [72], and yolk fatty acid profiles [73]. Such differences could be the result of adaptive age-specific adjustments or could arise because of physiological constraints associated with female reproductive senescence. Similar proposed mechanisms could also explain the shortening of telomere length in the offspring produced by the challenged mothers as mothers exposed to stress deposit higher levels of stress hormones in ovo [74], and this effect has been linked to faster offspring telomere loss in early life [42].
Rearing conditions after hatching could also play a key role in telomere shortening. The latter effect was reported in the European shag (Phalacrocorax aristotelis), where telomere length at hatching was not related to parental age, but at fledging, offspring of older parents had shorter telomeres. This post-hatching effect is presumably attributable to the quality of the rearing environment, which could potentially be poorer and thus more challenging when parents are older, and stress exposure during the rearing developmental stages is associated with faster offspring telomere loss [39,43]. Evidence suggesting that the quality of female parental care might be important comes from a recent experiment in the Alpine swift, in which offspring telomere length at parental independence was negatively related to the age of the cross-fostered mother but not to the age of the cross-fostered father [48]. Potential age-related differences in maternal and paternal care, together with associated offspring fitness consequences, would be important to investigate in future research in our study species.
That offspring longevity can be adversely affected by parental age, the so-called Lansing effect, has been established in many taxa [9,10]. It is also known that exposure to stressors can accelerate cellular ageing, alter survival trajectories and increase vulnerability to diseases [41,52]. A key question arising from this study is therefore whether the decline in offspring telomere length in relation to maternal age and maternal challenging conditions of the magnitude we observed is sufficient to modulate offspring longevity and life-history trajectories. Noguera et al. [14] recently showed that increasing parental age is associated with a substantial reduction in offspring longevity in zebra finches in captivity, though maternal and paternal effects could not be clearly separated. Heidinger et al. [28] showed that telomere length upon parental independence in zebra finches is predictive of longevity; the relationship observed in that study suggested that the approximately 39% decline that we observed in offspring telomere length with an increase in maternal age of approximately 3 years would be associated with some 25% reduction in offspring lifespan. For mothers living in control conditions, the effect on offspring was more marked—a 44–49% telomere reduction in daughters and sons, respectively. The effect of maternal age on offspring longevity is thus likely to have substantial fitness consequences.
To conclude, our results strongly emphasize the need of more studies to improve our understanding of the role of parental age in determining the optimal timing of breeding and breeding effort across the life course [15]. Such studies should be carried out under a variety of different parental environments for a greater understanding of the dynamics of such induced trans-generational phenotypic plasticity, thus determining the ‘fittest’ genotype depending on the environment. That telomere length is reduced when mothers are old suggests that reduced offspring production at older ages reduces investment in less fit offspring and is not simply a consequence of parental ageing. This study also raises the intriguing question of why zebra finches remain fertile in old adulthood. This could be because some fitness benefits are still accrued from such offspring, provided the effects on parent and offspring survival are not too severe. The potential fitness benefits associated with different scenarios of parental effort, quality of the rearing environment and longevity effects should be further explored in future studies.
Supplementary Material
Acknowledgements
We thank G. Adam, G. Anderson, A. Kirk, J. Laurie, G. Law, G. Grey, R. Philips and A.Magierecka for excellent assistance with animal husbandry; J. C. Noguera, J. Laurie, A. Magierecka and S. Reichert for help in collecting blood samples; A. Magierecka for help with data entry and P. Johnson for advice with the statistical analyses.
Ethics
All procedures were carried out under Home Office Project Licence (60/4109).
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h8c9781 [75].
Authors' contributions
V.M., W.B., B.H. and P.M. designed the experiment; V.M. and P.M. analysed the data and wrote the manuscript; all authors carried out the animal experimental procedures; W.B. and V.M. carried out the laboratory telomere analyses; all authors commented on previous drafts of the manuscript.
Competing interests
We declare no competing interests.
Funding
This work was funded by a European Research Council Advanced Investigator Award (no. 268926) to P.M. V.M. was supported by a Marie Sklodowska-Curie Postdoctoral Fellowship at the time of writing (no. 704582).
References
- 1.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Proc. R. Soc. B 363, 1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 3.Forslund P, Pärt T. 1995. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 10, 374–378. ( 10.1016/s0169-5347(00)89141-7) [DOI] [PubMed] [Google Scholar]
- 4.Stearns SC. 1992. The evolution of life histories Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P. 2003. Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J. Anim. Ecol. 72, 36–46. ( 10.1046/j.1365-2656.2003.00673.x) [DOI] [Google Scholar]
- 6.Marasco V, Boner W, Griffiths K, Heidinger B, Monaghan P. 2018. Environmental conditions shape the temporal pattern of investment in reproduction and survival. Proc. R. Soc. B 285, 1870 ( 10.1098/rspb.2017.2442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monaghan P, Charmantier A, Nussey DH, Ricklefs RE. 2008. The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378. ( 10.1111/j.1365-2435.2008.01418.x) [DOI] [Google Scholar]
- 8.Nussey DH, Froy H, Lemaitre JFO, Gaillard JM, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansing AI. 1947. A transmissible, cumulative, and reversible factor in aging. J. Gerontol. 2, 228–239. ( 10.1093/geronj/2.3.228) [DOI] [PubMed] [Google Scholar]
- 10.Priest NK, Mackowiak B, Promislow DEL. 2002. The role of parental age effects on the evolution of ageing. Evoluation 56, 927–935. ( 10.1111/j.0014-3820.2002.tb01405.x) [DOI] [PubMed] [Google Scholar]
- 11.Schroeder J, Nakagawa S, Rees M, Mannarelli M-E, Burke T. 2015. Reduced fitness in progeny from old parents in a natural population. Proc. Natl Acad. Sci. USA 112, 4021–4025. ( 10.1073/pnas.1422715112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arslan RC, et al. 2017. Older fathers' children have lower evolutionary fitness across four centuries and in four populations. Proc. R. Soc. B 284, 1862 ( 10.1098/rspb.2017.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouwhuis S, Verhulst S, Bauch C, Vedder O. 2018. Reduced telomere length in offspring of old fathers in a long-lived seabird. Biol. Lett. 14, 6 ( 10.1098/rsbl.2018.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguera JC, Metcalfe NB, Monaghan P. 2018. Experimental demonstration that offspring fathered by old males have shorter telomeres and reduced lifespans. Proc. R. Soc. B 285, 20180268 ( 10.1098/rspb.2018.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moorad JA, Nussey DH. 2016. Evolution of maternal effect senescence. Proc. Natl Acad. Sci. USA 113, 362–367. ( 10.1073/pnas.1520494113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Heuvel J, English S, Uller T. 2016. Disposable soma theory and the evolution of maternal effects on ageing. PLoS ONE 11, e0145544 ( 10.1371/journal.pone.0145544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaspina D, et al. 2005. Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatr. Genet. 15, 117–125. ( 10.1097/00041444-200506000-00008) [DOI] [PubMed] [Google Scholar]
- 18.Jenkins TG, Aston KI, Meyer T, Carrell DT. 2015. The sperm epigenome, male aging, and potential effects on the embryo. Adv. Exp. Med. Biol. 868, 81–93. ( 10.1007/978-3-319-18881-2_4) [DOI] [PubMed] [Google Scholar]
- 19.Wong WSW, et al. 2016. New observations on maternal age effect on germline de novo mutations. Nat. Commun. 7, 10486 ( 10.1038/ncomms10486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keefe DL. 2016. Telomeres, reproductive aging, and genomic instability during early development. Reprod. Sci. 23, 1612–1615. ( 10.1177/1933719116676397) [DOI] [PubMed] [Google Scholar]
- 21.Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. 2018. Impact of maternal age on oocyte and embryo competence. Front. Endocrinol. 9, 327 ( 10.3389/fendo.2018.00327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducatez S, Baguette M, Stevens VM, Legrand D, Fréville H. 2012. Complex interactions between paternal and maternal effects: parental experience and age at reproduction affect fecundity and offspring performance in a butterfly. Evolution 66, 3558–3569. ( 10.1111/j.1558-5646.2012.01704.x) [DOI] [PubMed] [Google Scholar]
- 23.Reid JM, Bignal EM, Bignal S, McCracken DI, Bogdanova MI, Monaghan P. 2010. Parent age, lifespan and offspring survival: structured variation in life history in a wild population. J. Anim. Ecol. 79, 851–862. ( 10.1111/j.1365-2656.2010.01669.x) [DOI] [PubMed] [Google Scholar]
- 24.Garratt M, Lemaître J-F, Douhard M, Bonenfant C, Capron G, Warnant C, Klein F, Brooks Robert C, Gaillard J-M. 2015. High juvenile mortality is associated with sex-specific adult survival and lifespan in wild roe deer. Curr. Biol. 25, 759–763. ( 10.1016/j.cub.2014.11.071) [DOI] [PubMed] [Google Scholar]
- 25.Blackburn EH. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579, 859–862. ( 10.1016/j.febslet.2004.11.036) [DOI] [PubMed] [Google Scholar]
- 26.Aubert G, Lansdorp PM. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579. ( 10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- 27.Monaghan P. 2010. Telomeres and life histories: the long and the short of it. Ann. N Y Acad. Sci. 1206, 130–142. ( 10.1111/j.1749-6632.2010.05705.x) [DOI] [PubMed] [Google Scholar]
- 28.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguera JC, Metcalfe NB, Boner W, Monaghan P. 2015. Sex-dependent effects of nutrition on telomere dynamics in zebra finches (Taeniopygia guttata). Biol. Lett. 11, 2 ( 10.1098/rsbl.2014.0938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monaghan P, Ozanne SE. 2018. Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Phil. Trans. R. Soc. B 373, 1741 ( 10.1098/rstb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthon RM. 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, 10 ( 10.1093/nar/30.10.e47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 33.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 1741 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison BJ, et al. 2016. Divergence of mechanistic pathways mediating cardiovascular aging and developmental programming of cardiovascular disease. FASEB J. 30, 1968–1975. ( 10.1096/fj.201500057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarry-Adkins JL, Fernandez-Twinn DS, Chen JH, Hargreaves IP, Neergheen V, Aiken CE, Ozanne SE. 2016. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats. Dis. Model. Mech. 9, 1221–1229. ( 10.1242/dmm.026591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Entringer S, Buss C, Wadhwa PD. 2012. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci. Signal. 5, 2003580 ( 10.1126/scisignal.2003580) [DOI] [PubMed] [Google Scholar]
- 37.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. ( 10.1016/S0140-6736(03)12384-7) [DOI] [PubMed] [Google Scholar]
- 38.Pepper GV, Bateson M, Nettle D. 2018. Telomeres as integrative markers of exposure to stress and adversity: a systematic review and meta-analysis. R. Soc. open sci. 5, 180744 ( 10.1098/rsos.180744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 1782 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hau M, Haussmann M, Greives T, Matlack C, Costantini D, Quetting M, Adelman J, Miranda A, Partecke J. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front. Zool. 12, 4 ( 10.1186/s12983-015-0095-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haussmann Mark F, Heidinger Britt J. 2015. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett. 11, 20150396 ( 10.1098/rsbl.2015.0396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, Bateson M. 2015. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 282, 20141610 ( 10.1098/rspb.2014.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD. 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl Acad. Sci. USA 108, 3 ( 10.1073/pnas.1107759108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenberg DTA, Kuzawa CW. 2018. The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Phil. Trans. R Soc. B 373, 1741 ( 10.1098/rstb.2016.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Froy H, et al. 2017. No evidence for parental age effects on offspring leukocyte telomere length in free-living Soay sheep. Sci. Rep. 7, 9991 ( 10.1038/s41598-017-09861-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsson M, Pauliny A, Wapstra E, Uller T, Schwartz T, Blomqvist D. 2011. Sex differences in sand lizard telomere inheritance: paternal epigenetic effects increases telomere heritability and offspring survival. PLoS ONE 6, e17473 ( 10.1371/journal.pone.0017473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Criscuolo F, Zahn S, Bize P. 2017. Offspring telomere length in the long lived Alpine swift is negatively related to the age of their biological father and foster mother. Biol. Lett. 13, 9 ( 10.1098/rsbl.2017.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauch C, Boonekamp JJ, Korsten P, Mulder E, Verhulst S. 2019. Epigenetic inheritance of telomere length in wild birds. PLoS Genet. 15, e1007827 ( 10.1371/journal.pgen.1007827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLennan D, Armstrong JD, Stewart DC, McKelvey S, Boner W, Monaghan P, Metcalfe NB. 2018. Links between parental life histories of wild salmon and the telomere lengths of their offspring. Mol. Ecol. 27, 804–814. ( 10.1111/mec.14467) [DOI] [PubMed] [Google Scholar]
- 51.Heidinger BJ, Herborn KA, Granroth-Wilding HMV, Boner W, Burthe S, Newell M, Wanless S, Daunt F, Monaghan P. 2016. Parental age influences offspring telomere loss. Funct. Ecol. 30, 1531–1538. ( 10.1111/1365-2435.12630) [DOI] [Google Scholar]
- 52.Monaghan P, Heidinger BJ, D'Alba L, Evans NP, Spencer KA. 2012. For better or worse: reduced adult lifespan following early-life stress is transmitted to breeding partners. Proc. R. Soc. B 279, 709–714. ( 10.1098/rspb.2011.1291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marasco V, Boner W, Heidinger B, Griffiths K, Monaghan P. 2015. Repeated exposure to stressful conditions can have beneficial effects on survival. Exp. Gerontol. 69, 170–175. ( 10.1016/j.exger.2015.06.011) [DOI] [PubMed] [Google Scholar]
- 54.Noguera JC, Metcalfe NB, Reichert S, Monaghan P. 2016. Embryonic and postnatal telomere length decrease with ovulation order within clutches. Sci. Rep. 6, 25915 ( 10.1038/srep25915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG, Griffiths K, Gault EA, Monaghan P. 2009. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. ( 10.1111/j.1600-048X.2008.04623.x) [DOI] [Google Scholar]
- 56.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 ( 10.1186/gb-2007-8-2-r19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bates D, Machler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 59.Kuznetsova A.2016. Package ‘lmerTest’. See https://cran.r-project.org/web/packages/lmerTest/lmerTest.pdf .
- 60.Tukey JW. 1977. Exploratory data analysis. Reading, MA: Addison-Wesely. [Google Scholar]
- 61.Lenth RV. 2016. Least-squared means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 62.Zuur A, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Chapter 5 ‘Mixed effects modelling for nested data’ In Mixed effects models and extensions in ecology with R (eds Gail M, Krickeberg KS, Tsiatis A, Wong W), pp. 101–142. Berlin, Germany: Springer. [Google Scholar]
- 63.Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R. 1999. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. R. Soc. Lond. B 96, 570–573. ( 10.1073/pnas.96.2.570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nager RG, Monaghan P, Houston DC, Genovart M. 2000. Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus). Behav. Ecol. Sociobiol. 48, 452–457. ( 10.1007/s002650000262) [DOI] [Google Scholar]
- 65.Wilkin TA, Sheldon BC. 2009. Sex differences in the persistence of natal environmental effects on life histories. Curr. Biol. 19, 1998–2002. ( 10.1016/j.cub.2009.09.065) [DOI] [PubMed] [Google Scholar]
- 66.Rosivall B, Szöllősi E, Hasselquist D, Török J. 2010. Males are sensitive—sex-dependent effect of rearing conditions on nestling growth. Behav. Ecol. Sociobiol. 64, 1555–1562. ( 10.1007/s00265-010-0969-1) [DOI] [Google Scholar]
- 67.Bradbury RB, Blakey JK. 1998. Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc. R. Soc. Lond. B 265, 895–899. ( 10.1098/rspb.1998.0375) [DOI] [Google Scholar]
- 68.Kilner R. 1998. Primary and secondary sex ratio manipulation by zebra finches. Anim. Behav. 56, 155–164. ( 10.1006/anbe.1998.0775) [DOI] [PubMed] [Google Scholar]
- 69.Martins TLF. 2004. Sex-specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio adjustment. Behav. Ecol. 15, 174–180. ( 10.1093/beheco/arg094) [DOI] [Google Scholar]
- 70.Polani PE, Crolla JA. 1991. A test of the production line hypothesis of mammalian oogenesis. Hum. Genet. 88, 64–70. ( 10.1007/bf00204931) [DOI] [PubMed] [Google Scholar]
- 71.Guibert F, Richard-Yris M-A, Lumineau S, Kotrschal K, Möstl E, Houdelier C. 2012. Yolk testosterone levels and offspring phenotype correlate with parental age in a precocial bird. Physiol. Behav. 105, 242–250. ( 10.1016/j.physbeh.2011.08.009) [DOI] [PubMed] [Google Scholar]
- 72.Okuliarova M, Skrobanek P, Zeman M. 2009. Variability of yolk testosterone concentrations during the reproductive cycle of Japanese quail. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 154, 530–534. ( 10.1016/j.cbpa.2009.08.012) [DOI] [PubMed] [Google Scholar]
- 73.Latour MA, Peebles ED, Doyle SM, Pansky T, Smith TW, Boyle CR. 1998. Broiler breeder age and dietary fat influence the yolk fatty acid profiles of fresh eggs and newly hatched chicks. Poult. Sci. 77, 47–53. ( 10.1093/ps/77.1.47) [DOI] [PubMed] [Google Scholar]
- 74.Henriksen R, Rettenbacher S, Groothuis TGG. 2011. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci. Biobehav. Rev. 35, 1484–1501. ( 10.1016/j.neubiorev.2011.04.010) [DOI] [PubMed] [Google Scholar]
- 75.Marasco V, Boner W, Griffiths K, Heidinger B, Monaghan P. 2019. Data from: Intergenerational effects on offspring telomere length: interactions among maternal age, stress exposure and offspring sex Dryad Digital Repository. ( 10.5061/dryad.h8c9781) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Marasco V, Boner W, Griffiths K, Heidinger B, Monaghan P. 2019. Data from: Intergenerational effects on offspring telomere length: interactions among maternal age, stress exposure and offspring sex Dryad Digital Repository. ( 10.5061/dryad.h8c9781) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h8c9781 [75].