Abstract
Ecological studies indicate that structurally complex habitats support elevated biodiversity, stability and resilience. The long-term persistence of structured habitats and their importance in maintaining biodiverse hotspots remain underexplored. We combined geohistorical data (dead mollusc assemblages, ‘DA’) and contemporary surveys (live mollusc assemblages, ‘LA’) to assess the persistence of local seagrass habitats over multi-centennial timescales and to evaluate whether they acted as long-term drivers of biodiversity, stability and resilience of associated fauna. We sampled structured seagrass meadows and open sandy bottoms along Florida's Gulf Coast. Results indicated that: (i) LA composition differed significantly between the two habitat types, (ii) LA from seagrass sites were characterized by significantly elevated local biodiversity and significantly higher spatial stability, (iii) DA composition differed significantly between the two habitat types, and (iv) fidelity between LA and DA was significantly greater for seagrass habitats. Contemporary results support the hypotheses that local biodiversity and spatial stability of marine benthos are both elevated in structured seagrass habitats. Geohistorical results suggest that structured habitats persist as local hotspots of elevated biodiversity and faunal stability over centennial-to-millennial timescales; indicating that habitat degradation and concomitant loss within structurally complex marine systems is a key driver of declining biodiversity and resilience.
Keywords: α-diversity, β-diversity, fidelity, ecology, palaeoecology
1. Introduction
Structural complexity of habitats has been predicted to boost biodiversity [1], stability [2,3] and resilience [4,5] of faunal associations. In marine ecosystems, empirical studies of coral reefs, seagrass meadows and other structurally complex habitats yielded multi-pronged support for this hypothesis. First, structured marine habitats represent well-known hotspots of α-diversity [2,6]. Second, increased biodiversity frequently correlates with increased structural complexity [1,7]. Finally, assemblages of marine organisms associated with structured habitats often display reduced β-diversity owing to spatio-temporal stability and elevated resilience [3,4,8,9]. While past empirical efforts advanced our understanding of how structured habitats support diverse and resilient marine communities, the focus has been on spatial patterns examined over short, subdecadal to decadal timescales [8,9]. By contrast, our long-term (centennial to multi-millennial) understanding of the influence of structured habitats on patterns and processes is limited [10–12]. Questions regarding how structured marine habitats functioned during preindustrial times and whether structural complexity played an important role over centennial-to-millennial timescales remain unexplored, including uncertainties regarding spatio-temporal stability of structured habitats [12]. Are they persistent local features or transient phenomena? Ecological studies alone cannot address those questions directly.
Conservation palaeobiology attempts to answer questions regarding long-term dynamics of species assemblages by providing quantitative estimates of biologically relevant information about past ecosystems [13–15]. One of the key strategies is joint sampling of living assemblages (LA) and surficial death assemblages (DA) that archive past populations [14,16–18]. Live–dead comparative studies in marine systems focus mainly on molluscs because they tend to be abundant, ecologically important and dominated by species with durable shells [17–19]; thus, generating time-averaged DA spanning centuries or millennia [19–22]. In addition, bottom-dwelling molluscs and other macrobenthos are sensitive to substrate type and sediment characteristics (e.g. [23]) providing a potential diagnostic tool for delineating seafloor habitats that vary in structural complexity.
Contemporary LA document present-day characteristics of structured and unstructured habitats and assess hypotheses about spatial variation in benthic fauna (e.g. [24]). However, if individual seagrass meadows wax and wane or shift location, then localized hotspots of α-diversity delineated in contemporary studies are neither unique spatially nor is their specific location distinctive over longer time intervals. In addition, if present-day differences in composition and resilience between assemblages in structured and unstructured habitats are largely driven by human stressors, contemporary data may exaggerate the importance of structured habitats when compared with pre-human systems. DA data can help evaluate those uncertainties by providing records of centennial-to-millennial patterns in evenness, α-diversity, β-diversity and faunal composition in the absence of severe anthropogenic changes [25,26]. In addition, drastic changes in DA may elucidate the nature and magnitude of anthropogenic impacts [13]. Live–dead comparisons have been frequently used in palaeobiology to assess the fidelity of DA as archives of living communities [16,17,27,28] and evaluate the magnitude of recent shifts in biological communities that potentially may reflect anthropogenic activities. DA record past communities largely predating human impacts and LA represent the contemporary, impacted communities [13,14,29,30].
Here, we integrated data from contemporary (live) and historical (dead) mollusc assemblages (e.g. [14,18,29,30]) to evaluate the long-term importance of structured seagrass patches and unstructured open sand habitats that form a patchy mosaic along Florida's Gulf Coast. LA data serve to assess the role of structured seagrass habitats in controlling spatial ecology of present-day mollusc assemblages, whereas DA and live–dead comparisons allow us to evaluate long-term stability and ecological importance of structurally complex habitats [14,16–18]. If local faunal assemblages persist relatively unchanged over centennial-to-millennial timescales, DA should be similar to contemporary faunal associations, and spatial variation in faunal composition of DA should parallel that observed today. Conversely, if local faunal assemblages varied notably through time, the faunal composition and spatial variability of DA are not expected to mimic LA patterns (e.g. [14]). Two explanations can be postulated to explain spatio-temporal instabilities and live–dead disagreements. First, seagrass habitats have not been stationary, and thus, DA in the area are a homogenized (time-averaged) mixture of molluscs from seagrass and open sand habitats [20]. Second, structured habitats in preindustrial ecosystems did not play as prominent a role in dictating assemblage composition as they appear to play today, and thus, DA are not distinguishable between seagrass and less structured sandy sites, even if both habitats have been stationary. Using Florida's Gulf Coast as a model system, we assess the contemporary and long-term importance of structurally complex seagrass habitats.
2. Methods
(a). Area of study
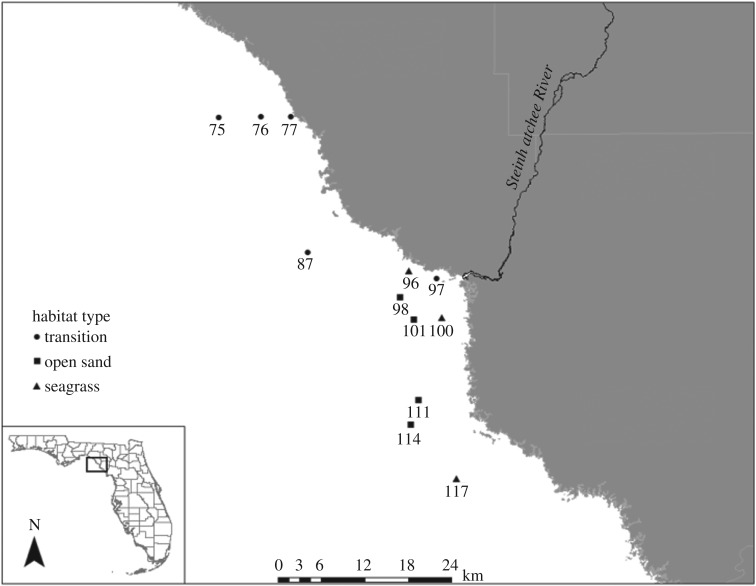
The Big Bend region of Florida, with the second largest contiguous seagrass meadow (ca 3000 km2) in the eastern Gulf of Mexico [31], is among the least disturbed coastal ecosystems in the USA [32]. The extensive size of this ecosystem, which serves as a nursery habitat and haven for a broad suite of marine organisms, underlines the ecological and economic importance of the Big Bend seagrass meadows [32,33]. Detailed historical data on spatial distribution of seagrass habitats are available thanks to two broad-scale surveys. The first study, conducted annually between 1974 and 1980, examined macrophytes at 300 stations between the northern Big Bend and Florida Bay [31]. In 2000, a second survey re-examined 188 of the original 300 stations to quantify changes in seagrass [34]. Based on these surveys, we identified 12 sampling sites in shallow (1.0–4.0 m) coastal waters near the Steinhatchee River: three seagrass sites, four open sand sites and five transition sites (figure 1). Seagrass sites were localities with seagrass present in prior surveys and 2016. Open sand sites were localities with seagrass absent in prior surveys and 2016. Transition sites were localities with seagrass present in 1980, absent in 2000 and either present or absent in 2016.
Figure 1.
Map of the area of study. Individual sites are differentiated by habitat type.
(b). Sampling methods
Between July and August 2016, samples of LA and DA were collected at four stations (northeast, northwest, southeast, southwest; relative to station coordinates at each of the 12 sites (48 LA and 48 DA samples total). Each sample was collected using a 10 cm diameter PVC suction pipe attached to a sampling pump [35]. The pipe was moved continuously for 2 min over a constant surface area (approx. 0.3 m2). Immediately prior to suction sampling, all aboveground macrophyte tissue was collected and frozen for determination of dry weights. Additionally, three quadrats were deployed at each station to quantify macrophytic per cent area cover (PAC). Cover estimates were averaged to yield one PAC for each of the 48 stations.
Individual samples were stepwise sieved. Coarser size fractions (8+ and 4–8 mm) were picked exhaustively, while 2–4 and 1–2 mm size fractions were subsampled (100 ml for LA, 25 ml for DA) to reduce processing time. Live individuals were restricted to only those that retained soft tissue. Dead specimens were defined as shells devoid of any soft tissue. Only shells and valves retaining more than 85% of the original shell were counted. Disarticulated valves of bivalves were counted as 0.5 of an individual. All specimens were identified to the lowest possible taxonomic level using authoritative taxonomic compendia [36–38].
(c). Radiocarbon dating
As indicated by previous dating studies, surficial shell accumulations in shallow subtidal settings typically represent multi-centennial to multi-millennial records (e.g. [21,39,40], and references therein). To confirm those expectations, we performed radiocarbon dating of 90 individual valves of mollusc bivalves. Specimens were dated using carbonate-target radiocarbon dating, an efficient and affordable method [41] successfully tested for marine bivalve molluscs [40].
(d). Statistical analyses
Non-metric multidimensional scaling (NMDS), an indirect ordination technique based on a heuristic search in a low-dimensional space [42–44], was employed to ordinate samples in terms of their similarity in faunal composition. The Bray–Curtis dissimilarity was used to estimate pairwise distances between samples in multivariate space. Raw data were double standardized (maximum standardization and relative standardization based on maximum and total number of individuals, respectively) prior to analysis [45]. Initial ordinations were performed for both k = 2 and k = 3 dimensions. The resulting ordinations produced qualitatively comparable sample configurations, but three-dimensional NMDS was used in the final analyses because of more acceptable stress values.
To test whether the centroids of sample groups were statistically distinguishable, we employed permutational multivariate analysis of variance (PERMANOVA) based on the Bray–Curtis distance for log10-transformed relative abundance data. The model treated assemblage (death or life) and habitat (seagrass, sand or transition) as fixed factors and site (three seagrass, four sand and five transition) as a nested factor in habitat. Although PERMANOVA tends to be an effective test for evaluating differences in location, it may perform poorly in the case of unbalanced sampling design when dispersion is heterogeneous across the sample groups [46]. However, the number of sites per habitat was only slightly unbalanced. Moreover, the largest group (five transition sites) was characterized by the highest dispersion, which made the test more conservative [42]. For both those reasons, PERMANOVA was applied to the complete dataset. In addition, pairwise comparisons were made among levels of significant terms involving fixed factors. The software Primer [47] was used to carry out PERMANOVA.
Station-level (α) diversity was calculated separately for LA and DA using Shannon's H diversity index for sample-standardized data (n = 18 per sample) (table 1). Other measures of diversity/evenness (not reported here) yielded comparable outcomes. Custom-designed resampling (randomization) protocols were used to assess differences in α-diversity between habitats, separately for LA and DA data. β-Diversity was calculated at two spatial scales. Within-site comparisons among habitats estimated small-scale variation in faunal composition. Within-habitat comparisons across sites provided a broad-scale assessment of β-diversity. Because different measures of β-diversity can yield variable outcomes and produce non-monotonically related estimates (e.g. [28]), we used two approaches to ensure that the results were robust. Both involved mean pairwise Bray–Curtis distances with statistical significance estimated using custom-designed randomizations and multivariate homogeneity of dispersion [48] implemented using the ‘betadisper’ function in R package ‘vegan’ [49]. In addition, the relationship between macrophyte PAC and the faunal composition of mollusc LA and DA (estimated by NMDS1 axis scores) was estimated using simple linear regression. Transition sites were used in this analysis owing to their variable aboveground macrophyte cover, which completed the gradient between 0% (open sand) and nearly 100% (seagrass).
Table 1.
Results of comparisons of α-diversity, within-site β-diversity, within-habitat β-diversity, faunal composition and fidelity between LAs and DAs associated with seagrass and open sand habitats. (—, not significant. The p-values are indicated by asterisks; *p < 0.05; **p < 0.01; ***p < 0.001.)
| parameter | test | result | |
|---|---|---|---|
| life assemblage | α-diversity | two-way randomization | seagrass > open sand* |
| within-site β-diversity | two-way randomization | seagrass < open sand** | |
| within-habitat β-diversity | two-way randomization | seagrass < open sand*** | |
| faunal composition | PERMANOVA | seagrass ≠ open sand*** | |
| death assemblage | α-diversity | two-way randomization | seagrass ≈ open sand — |
| within-site β-diversity | two-way randomization | seagrass < open sand — | |
| within-habitat β-diversity | two-way randomization | seagrass < open sand** | |
| faunal composition | PERMANOVA | seagrass ≠ open sand*** | |
| life versus death assemblages | fidelity (Spearman's ρ) | Wilcoxon test | seagrass < open sand*** |
| fidelity (Jaccard–Chao) | Wilcoxon test | seagrass < open sand*** | |
| fidelity (Spearman's ρ) | two-way randomization | seagrass < open sand*** | |
| fidelity (Jaccard–Chao) | two-way randomization | seagrass < open sand*** |
We quantified live–dead fidelity (i.e. faunal agreement between sympatric LA and DA samples) using: (i) Spearman's rank-order correlation coefficient (ρ) that assesses the within-site LA–DA agreement in species rank order; and (ii) the Jaccard–Chao similarity index that also assesses live–dead faunal agreement but includes abundances and adjusts for under-sampling of rare species [14,50]. The 1–2 mm size fraction was excluded because their inclusion can unduly reduce fidelity [25,41]. Because the fidelity measures are sensitive to sample size for zero-inflated counts (e.g. species-level counts of specimens), fidelity estimates may be increasingly underestimated when either a LA or DA sample used in a given pairwise comparison contains few specimens. To ensure statistically standardized comparisons across groups, LA and DA samples were ordered from highest to lowest ni independently, where ni is the total number of live specimens recorded at a given station. Subsequently, the higher of the two ni was subsampled to the lower of the two ni. This strategy resulted in two series of samples with the same ni, while maximizing the number of specimens retained. The subsampling was repeated 10 000 times to estimate statistical uncertainty for each habitat type (pilot analyses suggested that estimates stabilize well below 10 000 iterations).
To assess the influence of differential post-mortem sorting of shells by currents and storms, we compared the relative contributions of less than 2 mm, less than 4 mm and less than 8 mm size fractions to the total number of shells in each DA sample from seagrass and sand habitats using Welch two-sample t-tests and correlation with NMDS axes.
3. Results
(a). Temporal structure of death assemblages
Valves of small bivalves (Transenella spp. and Codakia orbicularis) were selected for radiocarbon dating owing to their ubiquitous presence. These specimens (n = 90) represented six samples (with the number of specimens per site ranging from n = 15 to n = 19) collected at six different sites along the western coast of Florida. Selected sites varied in density and composition of seagrass. Consistent with previous dating efforts in other regions [40,51], radiocarbon ages spanned two millennia, with the oldest shell age estimated at 1873 years BP. The mean shell age was 426 years BP, and the median shell age was 157 years BP. Moreover, 60% of dated shells were more than a century old, and 40% were more than 500 years old.
(b). Summary for life and death assemblages
The 12 sites yielded 50 619 specimens representing 154 taxa of molluscs. LA included 7224 individuals representing 110 taxa, whereas DA included 43 395 specimens representing 146 taxa. Three species of seagrass (Thalassia testudinum, Syringodium filiforme and Halodule wrightii) and several species of drift algae were recorded. Molluscs, Schwartziella catesbyana and Bittiolum varium, were the most abundant species at seagrass sites for both LA and DA. For open sand sites, the most abundant species in LA were Olivella spp. and Parvanachis obesa, whereas Parvilucina crenella and Chione elevata were the dominant species in DA.
(c). Life assemblages
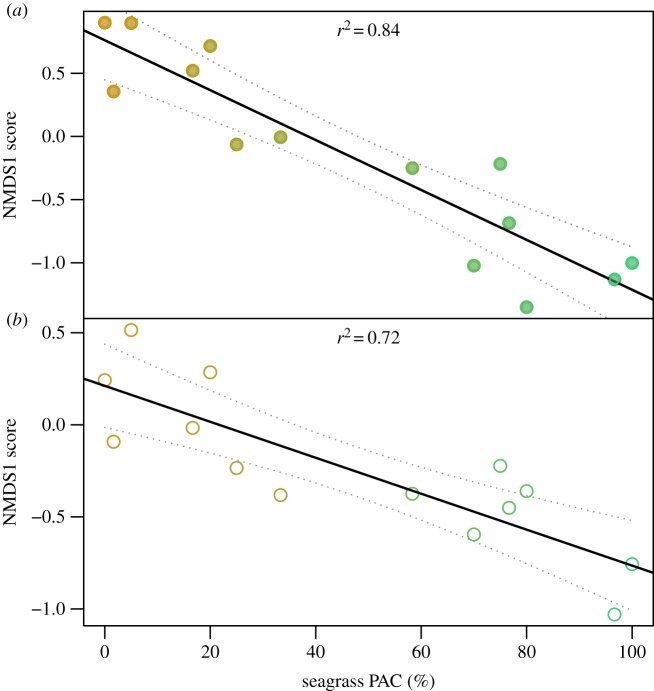
Seagrass and open sand sites differed in their contemporary faunal compositions, with LA from seagrass and open sand forming two non-overlapping clusters in the NMDS ordination space (figure 2). The two-sample groups separated along axis 1 of the ordination; with all seagrass samples exhibiting negative NMDS1 scores (less than −0.5) and all open sand samples exhibiting positive NMDS1 scores (greater than 0.5). Open sand samples were more dispersed in the ordination space (figure 2). Live samples from five transitional sites formed a very broad cluster, including samples that plotted between seagrass and open sand samples and samples that overlapped either seagrass or sand clusters (electronic supplementary material, figure S1). Transition samples exhibited the greatest dispersion (electronic supplementary material, figure S1). Results of PERMANOVA indicated that all factors and interactions represented significant effects and were generally consistent with the visual patterns observed in NMDS (electronic supplementary material, tables S1 and S2). To determine if these differences in community composition correlated with vegetation cover, the NMDS1 axis was compared to seagrass PAC. NMDS1 scores of live mollusc samples displayed a strong positive relation with seagrass PAC (r2 = 0.67, p = 0.001). The correlation increased further when NMDS1 scores were averaged across sample sets binned by PAC values (r2 = 0.83, p < 0.001; figure 3a).
Figure 2.
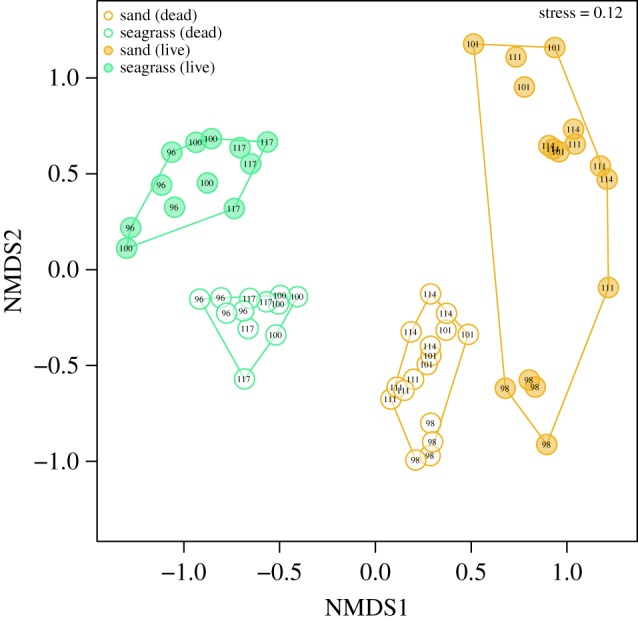
NMDS of 28 samples representing seven sites; three seagrass and four open sand sites, respectively. The ordination (k = 3 dimensions) was derived using the Bray–Curtis dissimilarities applied to relative abundance counts. Filled symbols represent LA and open symbols represent DA. Site numbers, i.e. unique spatial identifiers, are indicated for each sample.
Figure 3.
Bivariate plot of NMDS1 ordination scores of samples (from figure 2) and seagrass PAC. Samples with the same PAC and the mean NMDS1 scores are plotted. Transition samples were also used in this analysis owing to their variable aboveground macrophyte cover which completed the gradient between 0% (sand) and nearly 100% (seagrass). Linear regression (solid line) and 95% confidence intervals (dashed lines) are shown for each dataset. Symbol colour reflects PAC values. Symbols: r2, coefficient of determination. (a) LA; (b) DA.
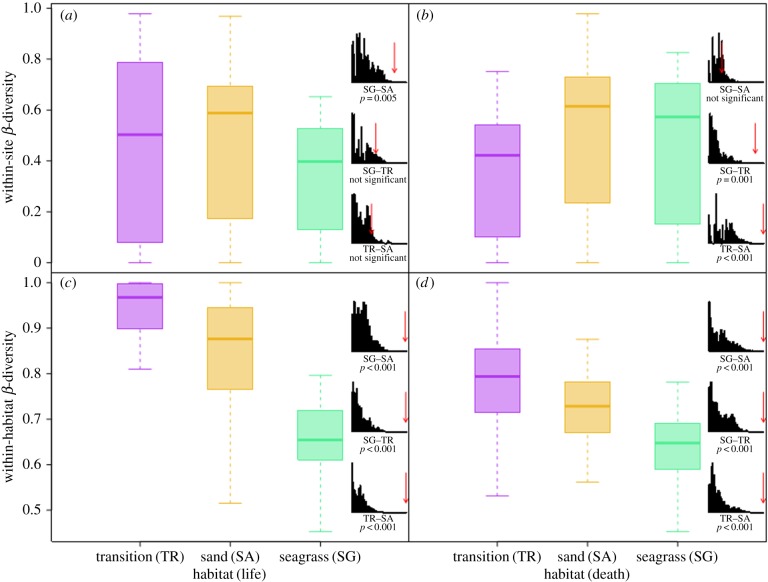
α-Diversity (Shannon's H) for live samples from seagrass sites was significantly greater than for open sand sites (two-sample randomization; p = 0.016; figure 4a). Analysis of pairwise Bray–Curtis dissimilarities among stations within specific sites indicated that seagrass stations were less variable in faunal composition than open sand stations (two-sample randomization; p = 0.001; figure 5a). The same relationship of reduced β-diversity was observed within-habitat types, where samples compared across seagrass sites were more similar in faunal composition than samples compared across open sand sites (two-sample randomization; p < 0.001; figure 5c). This difference also manifested in the ordination plot (figure 2), with samples from seagrass sites clustered more tightly than samples from open sand sites (figure 2) despite seagrass sites being further apart than open sand sites (mean pairwise distance: 17 versus 9 km; figure 1). The median α-diversity scores of live samples associated with transition sites were similar to open sand sites (figure 4a). Transition sites displayed the highest median within-habitat β-diversity (figure 5c), but intermediate within-site β-diversity (figure 5a). Consistent results were obtained when estimating β-diversity using homogeneity of multivariate dispersion [45].
Figure 4.
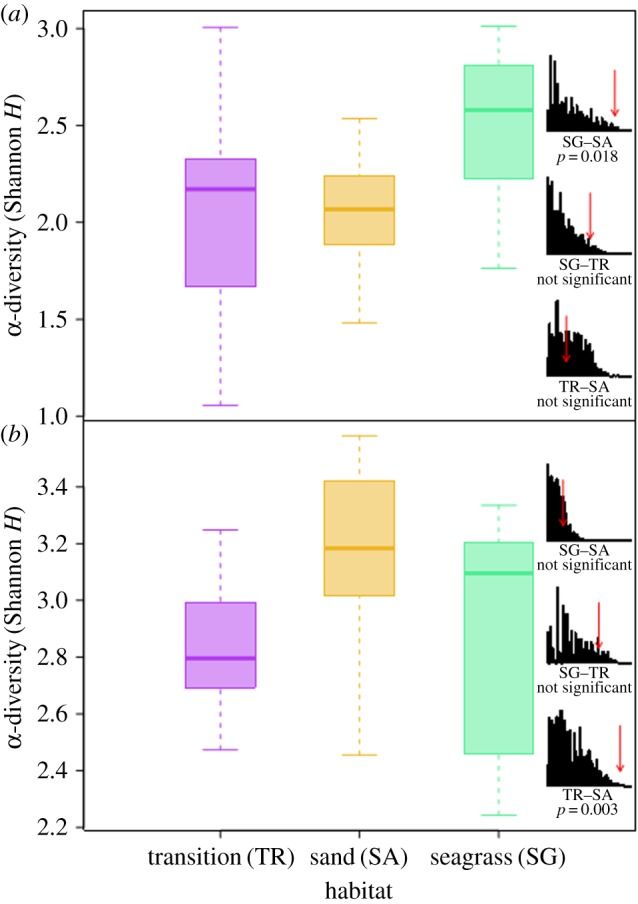
Boxplots depicting α-diversity (Shannon's H) for (a) LA and (b) DA associated with seagrass, open sand and transition habitats. Histograms represent results of two-way randomization tests.
Figure 5.
Boxplots depicting β-diversity (dispersion) for LA and DA samples associated with seagrass, open sand and transition habitats. (a,b) Within-site β-diversity; (c,d) within-habitat β-diversity for LA and DA. Histograms represent results of two-way randomization tests.
(d). Death assemblages
NMDS ordination of DA samples was largely consistent with patterns reported for LA: (i) seagrass samples and open sand samples formed non-overlapping clusters separated along NMDS1 axis (figure 2); (ii) DA samples from seagrass sites were least dispersed and transition samples most dispersed in the ordination space (figure 2; electronic supplementary material, figure S1); (iii) transition samples plotted between seagrass and open sand habitat types (electronic supplementary material, figure S1); (iv) seagrass, open sand and transition samples differed significantly in faunal composition (electronic supplementary material, tables S1 and S2); and (v) NMDS1 scores for DA samples displayed a strong linear relationship with PAC estimates (r2 = 0.71; p < 0.001; figure 3b). However, DA samples differed from LA samples by having a less notable separation in the ordination space along the NMDS1 axis and more tightly grouped clusters of samples relative to LA samples within each habitat (figure 2).
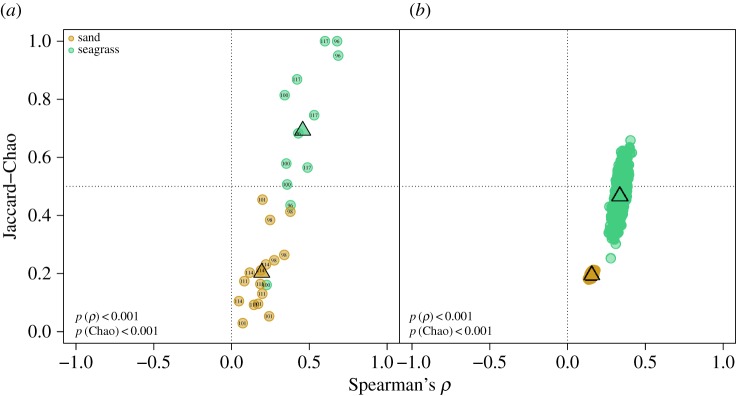
(e). Live–dead fidelity
Live–dead fidelity was high for nearly all samples from seagrass sites, but relatively low for all samples from open sand sites (figure 6a,b). The mean per-habitat fidelity estimates (average fidelity of sites within a given habitat) were statistically distinguishable between seagrass and open sand sites for both Spearman's rank correlation and Jaccard–Chao similarity indices (electronic supplementary material, table S3). Significant differences in fidelity also were observed for sample-standardized comparisons. Transition samples displayed intermediate fidelity (electronic supplementary material, figure S2a,b), which were significantly different from both seagrass and open sand habitats in raw samples for Spearman and Jaccard–Chao metrics (electronic supplementary material, table S3). Similar outcomes were obtained for sample-standardized comparisons, although the mean Spearman ρ estimates were not significantly different when comparing open sand and transition sample sets (electronic supplementary material, table S3).
Figure 6.
Comparison of LA and DA using (a) raw-score sample values and (b) random sample-standardized abundances to control for small-sample bias (10 000 iterations). Indices are (i) Spearman's non-parametric rank-order correlation of species abundances (Spearman's ρ), and (ii) live–dead community similarity using the Jaccard–Chao's similarity index with an adjustment to control for the effects of unseen species [50]. Samples occurring in the top-right quadrat have the highest fidelity, while samples occurring lower have reduced fidelity. Triangles represent the respective group centroids. Respective p-values are superimposed in the lower left corner.
(f). Comparative taphonomy of habitats
No significant differences in relative contribution were found for any of the size fractions examined (electronic supplementary material, table S4). Proportions of fractions also were not correlated with NMDS axis 1 (r2 < 0.15 in all comparisons; median r = 0.03; electronic supplementary material, table S4).
4. Discussion
(a). Overview
Results for LA data indicated (i) seagrass and open sand habitats differed in faunal composition; (ii) faunal assemblages displayed a compositional gradient as a function of vegetation density; and (iii) α-diversity was elevated and β-diversity depressed in seagrass habitats relative to open sand habitats. These results are consistent with the hypothesis that structured habitats enhance local diversity and spatial stability of contemporary communities. Radiocarbon dating indicates that the DAs represent a time-averaged, multi-centennial archive from preindustrial times. Results for DA samples indicated that (i) seagrass and open sand sites differ in faunal composition consistent with LA data; (ii) faunal composition of DA samples varied in concert with changes in present-day vegetation density like LA samples; and (iii) live–dead fidelity was higher in seagrass compared to open sand. These results are consistent with the hypothesis that localized seagrass patches represent persistent features that harbour more stable, resilient communities relative to unstructured habitats.
(b). Long-term spatio-temporal dynamics
Spatial structuring of molluscs archived in time-averaged DA and contrasting fidelity results for the two habitats are consistent with a two-pronged hypothesis that (i) seagrass habitats are persistent and spatially stable features and (ii) structured habitats played an important ecological role in controlling diversity and composition of marine benthos over multiple centuries. Whereas there is no reason to expect that the ecological role of seagrass would have changed over evolutionarily short timescales, a long-term persistence of seagrass patches, including subtle variations in vegetation density, is not intuitively obvious. If seagrass patches had been ephemeral or mobile over longer timescales, DA representing the two habitat types would not have been distinguishable. The statistically significant correlation between ordination scores for the time-averaged DA and PAC of contemporary vegetation would also not be possible if density of vegetation fluctuated notably. This further reinforces the interpretation that small-scale habitat features observed today are not ephemeral.
Outcomes of live–dead fidelity comparisons are also consistent with the notion that seagrass meadows were spatially stable and ecologically important features ([52,53] and references therein). If DA had been an averaged mixture of seagrass and non-seagrass mollusc associations, high live–dead fidelity would not have been likely for seagrass sites. Lower live–dead fidelity for less structured open sand sites suggest this habitat is less stable. The intermediate location of samples of DA from transition sites in ordination space and relatively low fidelity for those sites reaffirms the long-term persistence of seagrass sites as hotspots of elevated faunal stability and resilience relative to adjacent, less structurally complex habitats.
The long-term persistence of local seagrass patches suggested by DA and live–dead analyses are consistent with previous studies that postulated centennial-to-millennial persistence of seagrass meadows in other regions (e.g. [54]) and palaeontological studies based on subsurface cores and across surficial DA from areas where seagrass habitats are present [11,55].
Although stability and importance of seagrass habitats appears clear, the patterns in α and β-diversity in DA are more difficult to interpret. One reason is that time-averaging tends to increase α-diversity and suppress β-diversity [17,25] by transferring β-diversity present in living communities to α-diversity in DA [17]. This confounding effect is particularly important here because time-averaging tends to overestimate α-diversity and suppress β-diversity more substantially when β-diversity is high, as is the case here for open sand habitats. Thus, the absence of a significant difference in α-diversity of DA between habitat types does not necessarily negate the importance of structured habitats in influencing local diversity of faunal assemblages. However, significantly lower β-diversity in DA from seagrass sites, when compared with DA from open sand sites, suggests that time-averaging did not entirely mask stabilizing effects of structured habitats.
(c). Contemporary diversity and spatial stability in faunal composition
LA results are consistent with the current understanding of the ecological importance of seagrass. Elevated α-diversity and depressed β-diversity observed for LA from seagrass indicate that this habitat supports more diverse and spatially stable communities than the neighbouring open sand habitats [52,56]. Similarly, greater spatial variability in LA from open sand habitats, relative to those from structured habitats, is consistent with previous studies [28,47,53]. Suppressed β-diversity estimates at multiple spatial scales (among stations within sites and among sites within habitat) agree with the general notion that physical features of structured habitats foster development of distinct, spatially stable faunal communities [57]. In summary, LA results support the established paradigm that structured seagrass habitats, buffered against both biotic and abiotic fluctuations, harbour distinct, more diverse and more spatially homogeneous mollusc assemblages than adjacent non-structured habitats more readily influenced by local fluctuations [58].
(d). Confounding factors and alternative explanations
Other potential explanations for observed outcomes exist. First, observed differences between habitat types may be an artefact of the spatial distribution of sampling sites. However, the study system is a relatively uniform shallow shelf and all sites are comparable in terms of depth, with which water energy, light penetration, salinity, temperature and other parameters correlate tightly. Nevertheless, with one exception, transition sites were located north of open sand and seagrass sites (figure 1), so intra-regional differences could influence the results. However, our primary findings centre on seagrass and open sand habitats, and results for transition sites tend to be intermediate between open sand and seagrass despite their northward location. In addition, all open sand sites are farther offshore than seagrass sites 100 and 96 (figure 1), potentially suggesting faunal differences may partly reflect distance to the shoreline. However, seagrass site 117 is as far offshore as open sand sites but still clusters tightly with other seagrass sites. Similarly, a minor estuary of Steinhatchee River potentially affects four sites. However, two of those are open sand sites (98 and 101) and two are seagrass sites (96 and 100), and samples from those sites cluster by habitat type in disparate regions of the ordination space (figure 2).
Second, time-averaging could have produced observed patterns if turnover rates of common species were much higher among seagrass-associated molluscs. If seagrass-associated molluscs were dominated by short-lived species with massive spawning/recruitment events, we would expect DA at seagrass sites to be dominated by recently dead molluscs; making those sites more distinct and increasing live–dead fidelity. However, a wide range of radiocarbon dates and median shell ages greater than 100 years suggest our results are unlikely to reflect dominance of recently dead molluscs in the DA.
Third, results could reflect differences in taphonomy and time-averaging between habitats. Several studies based on sediment cores noted stratification of DA beneath seagrass meadows, with shallower cores yielding DA in better agreement with LA [11,27,28]. However, radiocarbon dating attests to extensive time-averaging in the uppermost sediment layer in seagrass settings, and thus, a bias owing to stratification seems unlikely [59].
Finally, taphonomic overprint could have resulted from selective removal and/or entrapment of shell material by currents, storms and hurricanes. Such post-mortem movement of shell material could explain less separation of habitats for DA samples compared to LA samples (figure 2), rather than our preferred explanation of spatial homogenization owing to time-averaging of sympatric, non-contemporaneous assemblages (e.g. [17]). Differential transport could also explain between-habitat difference in faunal composition, elevated live–dead fidelity of seagrass sites and notable correlation between vegetation density and faunal composition. If such processes were important, vegetated and open sand habitats should differ notably in size structure and taphonomic characteristics of local shell accumulations. This is not the case. Comparably small and fragile specimens dominate samples from both habitats and relative contributions of specimens from different size fractions are similar and statistically indistinguishable when comparing the open sand versus seagrass samples (electronic supplementary material, table S4). Also, NMDS1 scores and proportional contributions of size fractions do not correlate notably (electronic supplementary material, table S4): faunal composition does not reflect sample-to-sample differences in proportional representation of specimens from different size classes. These results are consistent with numerous taphonomic studies demonstrating that post-mortem transport is rarely a notable bias in the fossil record of shelled macro-organisms (e.g. [16,22,55,60]).
(e). Concluding remarks
Analysis of LA from Florida's Gulf Coast indicates contemporary seagrass meadows exert a strong influence on spatial structuring of local benthos by harbouring mollusc assemblages with distinct composition, locally elevated biodiversity and increased spatial consistency. Whereas this outcome is not novel on its own, it confirms previous reports from other seagrass systems worldwide. By contrast, the palaeontological dimension of this study provides us with a novel insight into the long-term dynamics of seagrass habitats. The local patchwork of open sandy bottoms and seagrass meadows observed today is not a transient, ever-shifting mosaic of ephemeral habitat spots. Instead, small-scale details of the contemporary ecological landscape originated prior to the industrial revolution and its localized seagrass meadows represent structured habitats with a long legacy.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank Savanna Barry, Joseph Kuehl, Hannah Clements and Riley Cobo for aid in the field and Sahale Casebolt, Kris Kusnerik and Ethan Weber for mollusc taxa identification.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
A.C.H. participated in the design of the study, executed fieldwork, conducted laboratory work, carried out statistical analyses and drafted as well as critically revised the manuscript; T.K.F. assisted with experimental design and drafted as well as critically revised the manuscript; C.A.J. assisted with multivariate statistical analysis and critically revised the manuscript; J.R.F. assisted with experimental design, coordinated and assisted in collecting field data, assisted with laboratory processing and critically revised the manuscript; M.K. assisted with study design, assisted with/conducted all statistical analyses and drafted as well as critically revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Support for this work was provided by the University of Florida's Institute of Food and Agricultural Sciences Seed Grant Program, Thompson Endowment Fund, School of Natural Resources and Environment, and School of Forest Resources and Conservation Fisheries and Aquatic Sciences Program.
References
- 1.Tokeshi M, Arakaki S. 2012. Habitat complexity in aquatic systems: fractals and beyond. Hydrobiologia 685, 27–47. ( 10.1007/s10750-011-0832-z) [DOI] [Google Scholar]
- 2.Kovalenko KE, Thomaz SM, Warfe DM. 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685, 1–17. ( 10.1007/s10750-011-0974-z) [DOI] [Google Scholar]
- 3.Blake RE, Duffy JE, Richardson JP. 2014. Patterns of seagrass community response to local shoreline development. Estuaries Coasts 37, 1549–1561. ( 10.1007/s12237-014-9784-7) [DOI] [Google Scholar]
- 4.Gartner A, Lavery PS, Lonzano-Montes H. 2015. Trophic implications and faunal resilience following one-off and successive disturbances to an Amphibolis griffithii seagrass system. Mar. Pollut. Bull. 94, 131–143. ( 10.1016/j.marpolbul.2015.03.001) [DOI] [PubMed] [Google Scholar]
- 5.Ferrari R, Bryson M, Bridge T, Hustache J, Williams SB, Byrne M, Figueira W. 2016. Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob. Change Biol. 22, 1965–1975. ( 10.1111/gcb.13197) [DOI] [PubMed] [Google Scholar]
- 6.Davis TR, Harasti D, Kelaher B, Smith SD. 2017. Spatial and temporal variation in subtidal molluscan diversity amongst temperate estuarine habitats. Mar. Ecol. 38, 12428 ( 10.1111/maec.12428) [DOI] [Google Scholar]
- 7.Loke LH, Todd PA. 2016. Structural complexity and component type increase intertidal biodiversity independently of area. Ecology 97, 383–393. ( 10.1890/15-0257.1) [DOI] [PubMed] [Google Scholar]
- 8.Stachowicz JJ. 2001. Mutualism, facilitation, and the structure of ecological communities: positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. AIBS Bull. 51, 235–246. ( 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2) [DOI] [Google Scholar]
- 9.Whanpetch N, Nakaoka M, Mukai H, Suzuki T, Nojima S, Kawai T, Aryuthaka C, 2010. Temporal changes in benthic communities of seagrass beds impacted by a tsunami in the Andaman Sea, Thailand. Estuarine Coastal Shelf Sci. 87, 246–252. ( 10.1016/j.ecss.2010.01.001) [DOI] [Google Scholar]
- 10.Van Tussenbroek BI, et al. 2014. Caribbean-wide, long-term study of seagrass beds reveals local variations, shifts in community structure and occasional collapse. PLoS ONE 9, e90600 ( 10.1371/journal.pone.0090600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feser KM, Miller AI. 2014. Temporal dynamics of shallow seagrass-associated molluscan assemblages in St. Croix, US Virgin Islands: toward the calibration of taphonomic inertia. Palaios 29, 218–230. ( 10.2110/palo.2013.103) [DOI] [Google Scholar]
- 12.Lyons MB, Roelfsema CM, Phinn SR. 2013. Towards understanding temporal and spatial dynamics of seagrass landscapes using time-series remote sensing. Estuarine Coastal Shelf Sci. 120, 42–53. ( 10.1016/j.ecss.2013.01.015) [DOI] [Google Scholar]
- 13.Kowalewski M, Serrano GEA, Flessa KW, Goodfriend GA. 2000. Dead delta's former productivity: two trillion shells at the mouth of the Colorado River. Geology 28, 1059–1062. () [DOI] [Google Scholar]
- 14.Kidwell SM. 2007. Discordance between living and death assemblages as evidence for anthropogenic ecological change. Proc. Natl Acad. Sci. USA 104, 17 701–17 706. ( 10.1073/pnas.0707194104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietl GP, Kidwell SM, Brenner M, Burney DA, Flessa KW, Jackson ST, Koch PL. 2015. Conservation paleobiology: leveraging knowledge of the past to inform conservation and restoration. Annu. Rev. Earth Planet. Sci. 43, 79–103. ( 10.1146/annurev-earth-040610-133349) [DOI] [Google Scholar]
- 16.Kidwell SM. 2008. Ecological fidelity of open marine molluscan death assemblages: effects of post-mortem transportation, shelf health, and taphonomic inertia. Lethaia 41, 199–217. ( 10.1111/j.1502-3931.2007.00050) [DOI] [Google Scholar]
- 17.Tomašových A, Kidwell SM. 2009. Preservation of spatial and environmental gradients by death assemblages. Paleobiology 35, 122–148. ( 10.1666/07081.1) [DOI] [Google Scholar]
- 18.Tomasových A, Kidwell SM. 2009. Fidelity of variation in species composition and diversity partitioning by death assemblages: time-averaging transfers diversity from beta to alpha levels. Paleobiology 35, 94–118. ( 10.1666/08024.1) [DOI] [Google Scholar]
- 19.Kowalewski M. 2009. The youngest fossil record and conservation biology: holocene shells as eco-environmental recorders. In Conservation paleobiology: using the past to manage for the future, vol. 15 (eds Dietl GD, Flessa KW), pp. 1–23. Chicago, IL: University of Chicago Press. [Google Scholar]
- 20.Kowalewski M, Goodfriend GA, Flessa KW. 1998. High-resolution estimates of temporal mixing within shell beds: the evils and virtues of time-averaging. Paleobiology 24, 287–304. ( 10.1666/0094-8373(1998)024[0287:HEOTMW]2.3.CO;2) [DOI] [Google Scholar]
- 21.Kidwell SM, Best MM, Kaufman DS. 2005. Taphonomic trade-offs in tropical marine death assemblages: differential time averaging, shell loss, and probable bias in siliciclastic vs. carbonate facies. Geology 33, 729–732. ( 10.1130/G21607.1) [DOI] [Google Scholar]
- 22.Kidwell SM, Tomasových A. 2013. Implications of time-averaged death assemblages for ecology and conservation biology. Annu. Rev. Ecol. Evol. Syst. 44, 539–563. ( 10.1146/annurev-ecolsys-110512-135838) [DOI] [Google Scholar]
- 23.Veiga P, Redondo W, Sousa-Pinto I, Rubal M. 2017. Relationship between structure of macrobenthic assemblages and environmental variables in shallow sublittoral soft bottoms. Mar. Environ. Res. 129, 396–407. ( 10.1016/j.marenvres.2017.07.002) [DOI] [PubMed] [Google Scholar]
- 24.Dumas P, Jimenez H, Peignon C, Wantiez L, Adjeroud M. 2013. Small-scale habitat structure modulates the effects of no-take marine reserves for coral reef macroinvertebrates. PLoS ONE 8, e58998 ( 10.1371/journal.pone.0058998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olszewski TD, Kidwell SM. 2007. The preservational fidelity of evenness in molluscan death assemblages. Paleobiology 33, 1–23. ( 10.1666/05059.1) [DOI] [Google Scholar]
- 26.Tyler CL, Kowalewski M. 2017. Surrogate taxa and fossils as reliable proxies of spatial biodiversity patterns in marine benthic communities. Proc. R. Soc. B 284, 20162839 ( 10.1098/rspb.2016.2839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons-Hubbard K, Hubbard D, Tems C, Burkett A. 2014. The relationship between modern mollusk assemblages and their expression in subsurface sediment in a carbonate lagoon, St. Croix, US Virgin Islands. In Experimental approaches to understanding fossil organisms (eds Hembree D, Platt B, Smith J), pp. 143–167. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 28.Arkle KM, Miller AI. 2018. Evidence for stratigraphy in molluscan death assemblages preserved in seagrass beds: St. Croix, US Virgin Islands. Paleobiology 44, 155–170. ( 10.1017/pab.2017.26) [DOI] [Google Scholar]
- 29.Gilad E, Kidwell SM, Benayahu Y, Edelman-Furstenberg Y. 2018. Unrecognized loss of seagrass communities based on molluscan death assemblages: historic baseline shift in tropical Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 589, 73–83. ( 10.3354/meps12492) [DOI] [Google Scholar]
- 30.Tweitmann A, Dietl GP. 2018. Live-dead mismatch of molluscan assemblages indicates disturbance from anthropogenic eutrophication in the Barnegat Bay-Little Egg Harbor estuary. J. Shellfish Res. 37, 615–625. ( 10.2983/035.037.0314) [DOI] [Google Scholar]
- 31.Iverson RL, Bittaker HF. 1986. Seagrass distribution and abundance in eastern Gulf of Mexico coastal waters. Estuarine Coastal Shelf Sci. 22, 577–602. ( 10.1016/0272-7714(86)90015-6) [DOI] [Google Scholar]
- 32.Mattson RA, Frazer TK, Hale J, Blitch S, Ahijevych L. 2002. Florida Big Bend. In Seagrass status and trends in the northern Gulf of Mexico (eds Handley L, Altsman D, DeMay R), pp. 171–188. Reston, VA: US Department of the Interior, US Geological Survey. [Google Scholar]
- 33.Costanza R, et al. 1997. The value of the world's ecosystem services and natural capital. Nature 387, 253 ( 10.1038/387253a0) [DOI] [Google Scholar]
- 34.Hale JA, Frazer TK, Tomasko DA, Hall MO. 2004. Changes in the distribution of seagrass species along Florida's central Gulf Coast: Iverson and Bittaker revisited. Estuaries 27, 36 ( 10.1007/BF02803558) [DOI] [Google Scholar]
- 35.Raz-Guzman A, Grizzle RE. 2001. Techniques for quantitative sampling of infauna and small epifauna in seagrass. In Global seagrass research methods (eds Short FT, Coles RG), pp. 237–253. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 36.Redfern C. 2001. Bahamian seashells: a thousand species from Abaco, Bahamas. Boca Raton, FL: Bahamian-seashells.com. [Google Scholar]
- 37.Mikkelsen PM, Bieler R. 2008. Seashells of southern Florida: living marine mollusks of the Florida keys and adjacent regions, bivalves (no. C/594.110975 M5) Princeton, NJ: Princeton University Press. [Google Scholar]
- 38.Tunnell JW. 2010. Encyclopedia of Texas seashells: identification, ecology, distribution, and history. College Station, TX: Texas A&M University Press. [Google Scholar]
- 39.Flessa KW, Kowalewski M. 1994. Shell survival and time-averaging in nearshore and shelf environments: estimates from the radiocarbon literature. Lethaia 27, 153–165. ( 10.1111/j.1502-3931.1994.tb01570.x) [DOI] [Google Scholar]
- 40.Kowalewski M, Casebolt S, Hua Q, Whitacre KE, Kaufman DS, Kosnik MA. 2018. One fossil record, multiple time resolutions: disparate time-averaging of echinoids and mollusks on a Holocene carbonate platform. Geology 46, 51–54. ( 10.1130/G39789.1) [DOI] [Google Scholar]
- 41.Kidwell SM. 2002. Mesh-size effects on the ecological fidelity of death assemblages: a meta-analysis of molluscan live–dead studies. Geobios 35, 107–119. ( 10.1016/S0016-6995(02)00052-9) [DOI] [Google Scholar]
- 42.Kruskal JB. 1964. Nonmetric multidimensional scaling: a numerical method. Psychometrika 29, 115–129. ( 10.1007/BF02289694) [DOI] [Google Scholar]
- 43.Kruskal JB. 1964. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29, 1–27. ( 10.1007/BF02289565) [DOI] [Google Scholar]
- 44.Kenkel NC, Orlóci L. 1986. Applying metric and nonmetric multidimensional scaling to ecological studies: some new results. Ecology 67, 919–928. ( 10.2307/1939814) [DOI] [Google Scholar]
- 45.Anderson MJ, Ellingsen KE, McArdle BH. 2006. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 9, 683–693. ( 10.1111/j.1461-0248.2006.00926.x) [DOI] [PubMed] [Google Scholar]
- 46.Anderson MJ, Walsh DC. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol. Monogr. 83, 557–574. ( 10.1890/12-2010.1) [DOI] [Google Scholar]
- 47.Karp MA, Seitz RD, Fabrizio MC. 2018. Faunal communities on restored oyster reefs: effects of habitat complexity and environmental conditions. Mar. Ecol. Prog. Ser. 590, 35–51. ( 10.3354/meps12470) [DOI] [Google Scholar]
- 48.Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E. [Google Scholar]
- 49.Oksanen J, et al. 2013. Package ‘vegan’. Community ecology package, version, 2. See https://CRAN.R-project.org/package=vegan.
- 50.Chao A, Chazdon RL, Colwell RK, Shen TJ. 2005. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159. ( 10.1111/j.1461-0248.2004.00707.x) [DOI] [Google Scholar]
- 51.Bush SL, Santos GM, Xu X, Southon JR, Thiagarajan N, Hines SK, Adkins JF. 2013. Simple, rapid, cost effective: a screening method for 14C analysis of small carbonate samples. Radiocarbon 55, 631–640. ( 10.1017/S0033822200057787) [DOI] [Google Scholar]
- 52.Seitz RD, Ewers Lewis CJ. 2018. Loss of seagrass results in changes to benthic infaunal community structure and decreased secondary production. Bull. Mar. Sci. 94, 1273–1292. ( 10.5343/bms.2017.1011) [DOI] [Google Scholar]
- 53.Alsaffar Z, Cúrdia J, Borja A, Irigoien X, Carvalho S. 2017. Consistent variability in beta-diversity patterns contrasts with changes in alpha-diversity along an onshore to offshore environmental gradient: the case of Red Sea soft-bottom macrobenthos. Mar. Biodivers. 49, 247–262. ( 10.1007/s12526-017-0791-3) [DOI] [Google Scholar]
- 54.Serrano O, Lavery P, Masque P, Inostroza K, Bongiovanni J, Duarte C. 2016. Seagrass sediments reveal the long-term deterioration of an estuarine ecosystem. Glob. Change Biol. 22, 1523–1531. ( 10.1111/gcb.13195) [DOI] [PubMed] [Google Scholar]
- 55.Casebolt S, Kowalewski M. 2018. Mollusk shell assemblages as archives of spatial structuring of benthic communities around subtropical islands. Estuarine Coastal Shelf Sci. 215, 132–143. ( 10.1016/j.ecss.2018.09.023) [DOI] [Google Scholar]
- 56.Barnes RSK, Hamylton S. 2016. On the very edge: faunal and functional responses to the interface between benthic seagrass and unvegetated sand assemblages. Mar. Ecol. Prog. Ser. 553, 33–48. ( 10.3354/meps11800) [DOI] [Google Scholar]
- 57.Barnes RSK. 2017. Patterns of benthic invertebrate biodiversity in intertidal seagrass in Moreton Bay, Queensland. Reg. Stud. Mar. Sci. 15, 17–25. ( 10.1016/j.rsma.2017.07.003) [DOI] [Google Scholar]
- 58.Pusceddu A, Fraschetti S, Scopa M, Rizzo L, Danovaro R. 2016. Meiofauna communities, nematode diversity and C degradation rates in seagrass (Posidonia oceanica l.) and unvegetated sediments invaded by the algae Caulerpa cylindracea (Sonder). Mar. Environ. Res. 119, 88–99. ( 10.1016/j.marenvres.2016.05.015) [DOI] [PubMed] [Google Scholar]
- 59.Korpanty CA, Kelley PH. 2014. Molluscan live–dead agreement in anthropogenically stressed seagrass habitats: siliciclastic versus carbonate environments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 410, 113–125. ( 10.1016/j.palaeo.2014.05.014) [DOI] [Google Scholar]
- 60.Poirier C, Sauriau P-G, Chaumillon E, Bertin X. 2010. Influence of hydrosedimentary factors on mollusc death assemblages in a temperate mixed tide-and-wave dominated coastal environment: implications for the fossil record. Cont. Shelf Res. 30, 1876–1890. ( 10.1016/j.csr.2010.08.015) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.