Abstract
Conspicuous mating signals attract mates but also expose signallers to predators and parasites. Signal evolution, therefore, is driven by conflicting selective pressures from multiple receivers, both target and non-target. Synchronization of mating signals, for example, is an evolutionary puzzle, given the assumed high cost of reduced female attraction when signals overlap. Synchronization may be beneficial, however, if overlapping signals reduce attraction of non-target receivers. We investigate how signal synchronization is shaped by the trade-off between natural and sexual selection in two anuran species: pug-nosed tree frogs (Smilisca sila), in which males produce mating calls in near-perfect synchrony, and túngara frogs (Engystomops pustulosus), in which males alternate their calls. To examine the trade-off imposed by signal synchronization, we conducted field and laboratory playback experiments on eavesdropping enemies (bats and midges) and target receivers (female frogs). Our results suggest that, while synchronization can be a general strategy for signallers to reduce their exposure to eavesdroppers, relaxed selection by females for unsynchronized calls is key to the evolution and maintenance of signal synchrony. This study highlights the role of relaxed selection in our understanding of the origin of mating signals and displays.
Keywords: acoustic communication, communication network, eavesdroppers, relaxed selection, synchrony
1. Introduction
Most communication occurs in a network, where signals are detected and used by multiple receivers, both target and non-target [1,2]. This is true across scales, systems and sensory modalities, from the molecules exchanged between bacteria [3] to the complex multimodal courtship displays exhibited by many birds, fishes, mammals and insects [4]. Despite the pervasive nature of communicating in networks, communication is most often investigated as if occurring in a signaller–receiver dyad. In that framework, a sender produces a signal that is transmitted through the environment and detected by a single target receiver. Empirical studies on signal evolution, for example, have predominantly focused on the selective pressures imposed by mates [5], given that females are the main target receiver of mating signals. Other receivers have also been considered independently such as competitors [2] and non-target receivers such as predatory or parasitic eavesdroppers, the unintended recipients of signals [1,6]. A dyadic approach, however, ignores the conflicting influence of both target and non-target receivers on signals, and the signalling trade-offs that might ensue. In this study, we use a communication network perspective [6] that considers multiple target and non-target receivers to examine signal timing strategies in anuran choruses. We investigate how selective pressure from different receivers has resulted in an unexpected outcome, signal synchronization.
In dense mating aggregations, such as insect and anuran choruses, males of many species use signal timing strategies to avoid signal overlap with neighbouring conspecifics (the ‘cocktail party problem’) [7]. Overlapped mating signals have two main disadvantages compared to non-overlapped signals. For one, signals produced at the same time can interfere, reducing the ability of the females to recognize individual signals [8] and discriminate between males displaying at aggregations [9,10]. Additionally, when signals overlap but are offset, females of many species prefer the initial ‘leading’ signal to the second ‘following’ signal, commonly referred to as the ‘precedence effect’ or ‘leader–follower preferences' [11–16]. For these reasons, competing males often offset the timing of their signals, in a pattern of signal alternation. An alternation signal timing strategy is commonly observed across taxa (insects and anurans [17], birds [18]) and signal modalities (acoustic and visual [19,20]). Signals produced in alternation do not interfere with one another and are therefore more conspicuous, increasing the likelihood of female attraction. More conspicuous signals, however, may also increase the likelihood of attracting non-target eavesdroppers. Inversely, signals that do overlap are less conspicuous, and potentially less attractive to eavesdroppers [21].
Although overlapping signals are not as common as alternating signals, males of some species do deliberately overlap, or ‘synchronize’, their mating signals with neighbouring males (insects and anurans [22], birds [23], mammals [24], crustaceans [25]). A synchronized timing strategy has been proposed to function in evading detection by predatory or parasitic eavesdroppers [21]. By effectively masking their own signals with those of neighbouring competitors, male signallers can reduce the attraction of unwanted receivers. This hypothesis has rarely been tested, however, and a crucial, unresolved piece of this puzzle is how signal synchronization affects female attraction. Given the selective pressure imposed by both mates and eavesdroppers, a comprehensive understanding of the evolution of mating signals requires considering both types of receivers. Here, we use a communication network approach to examine receiver attraction to synchronized mating signals. In particular, we investigate the costs and benefits imposed by mates and multiple eavesdroppers on signal timing strategies in the pug-nosed tree frog (Smilisca sila) and the túngara frog (Engystomops pustulosus).
Chorusing male pug-nosed tree frogs synchronize their calls with those of neighbouring males at extremely short latencies, with a minimum delay of 5 ms and an average delay of 79 ms [26]. While pug-nose tree frogs call during the dry season, in the same habitat, male túngara frogs form dense choruses during the rainy season. Unlike pug-nosed tree frogs, neighbouring male túngara frogs alternate the timing of their mating calls [27]. Both pug-nosed tree frogs and túngara frogs are preyed upon by frog-eating bats (Trachops cirrhosis [21,28]) and frog-biting midges (Corethrella spp. [29]). These bats and midges use the mating calls of the frogs as a cue to localize calling males [30]. Attracting bats has a direct fitness cost to a calling male frog: predation. While frog-biting midges only take small blood meals, a single calling male túngara frog can attract hundreds of midges in half an hour [31]. For male túngara frogs, such high attack rates can potentially contribute to high costs from blood loss, which may be equivalent to about 10% of their blood volume in a night of calling (unpublished calculations by X.E. Bernal based on estimates of the amount of blood collected by a single fly [32]). Additionally, frog-biting midges are themselves vectors for blood parasites [33,34]. We consider these two anuran species, pug-nosed tree frogs and túngara frogs, that share their main eavesdroppers but have distinctly different call timing strategies. For each species, we examine three receivers from the communication network: female frogs, frog-eating bats and frog-biting midges.
We investigate the effectiveness of signal synchronization as a strategy for reducing attraction to multiple eavesdroppers and address the conundrum of how females select mates in a synchronized chorus. Specifically, we examine the potential costs of reduced attractiveness to females and the benefits of reduced risk of attacks by predators associated with producing calls in synchrony versus alternating calls with those of neighbouring males. Typically, female choice is expected to prevent signal synchronization, given that females are considered the primary driver of courtship signal evolution [35,36], and they prefer non-overlapping calls to avoid localization and discrimination challenges [7,37]. For synchronization to evolve or be maintained, natural selection from predators is expected to outweigh sexual selection against signal synchrony. Considering this trade-off, it is possible that eavesdropping predators impose high selective pressure that results in signal display strategies that are suboptimal for female attraction (extreme predator selection hypothesis). Pug-nosed tree frogs, for example, are one of the few anuran species that breed in the dry season [38], making them an important source of food for frog-eating predators during this time. Thus, pug-nosed tree frogs could experience higher selective pressure from eavesdropping predators than other frogs in the community. It is also possible, however, that relaxed selection from females allows the production of signal display strategies that minimize exploitation by eavesdropping predators (relaxed sexual selection hypothesis). These hypotheses are not mutually exclusive. To our knowledge, this is the first study to integrate the effects of target and multiple non-target receivers to understand signalling strategies. We conduct both field and laboratory phonotaxis experiments to assess the preferences of female frogs and eavesdropping predators for synchronized versus alternating call timing strategies. We discuss our results in the context of the role of natural enemies and constraints imposed by mates on the evolution of ornament signals.
2. Material and methods
(a). Risk of predation by frog-eating bats
Field experiments assessing eavesdropper attraction were conducted at eight locations in the forest around Gamboa, Republic of Panama (9°07.0′ N, 79°41.9′ W) during the breeding seasons of pug-nosed tree frogs (January–February 2015) and túngara frogs (June–August 2014). All locations were at least 1 km apart to minimize the chances that the same bats would be sampled at different locations as T. cirrhosus are known to have home range sizes of less than 1 km2 [39]. All locations were also within 0.5 km of a water source with breeding frogs, but also at a distance from where sounds produced by the water source or calling frogs was not audible.
At each of the eight field locations, three speaker stations were positioned 10 m apart from each other in a triangle formation to ensure equidistance between treatments. Speaker stations were placed at a relative distance that resembles the spatial distribution of male frogs calling in a chorus but at distances and broadcasting patterns that minimized acoustic interference. At each station, two Pignose portable amplifier speakers (Model 7–100; Pignose-Gorilla, North Las Vegas, Nevada) were placed 1 m apart from each other, facing upwards. Each pair of speakers broadcast a pair of pre-recorded natural male calls with one of three degrees of temporal overlap: (i) near-perfect synchrony (5 ms of latency), (ii) natural average synchrony (79 ms of latency), or (iii) out of synchrony (alternating calls). These three treatments (5, 79 ms, alternating) were thus presented simultaneously within a location. A diagram of this experimental set-up is included in the electronic supplementary material, figure S1. One, out of the eight locations, was tested in this way per night. The eight locations were rotated through three times for a total sampling period of 24 nights, such that all three of the call timing treatments were tested at each of the three stations within each location.
For all treatments, frog calls were broadcast at a rate of one call every 2 s, at an amplitude of 82 dB sound pressure level (SPL) re. 20 µP at 1 m from the speaker measured at ground level using a digital SPL meter (Radio Shack catalogue number 33-2055; C-weighting, fast root mean square response). We presented the calls of both frog species at a single standardized call rate and amplitude as our question was solely about differences in eavesdropper attraction resulting from the relative timing between calls. Additionally, only playbacks of simple, single note, calls for both pug-nosed tree frogs and túngara frogs were used to control for any confounding effect from signal complexity (figure 1a,b). Calls for both species were randomly selected from a pre-recorded library of 10 different individual males. For each treatment within a night, two calls were drawn from the library without replacement, and specific call combinations were not repeated for a receiver. A diagram of how pre-recorded calls were pooled to build the stimuli is included in the electronic supplementary material, figure S2.
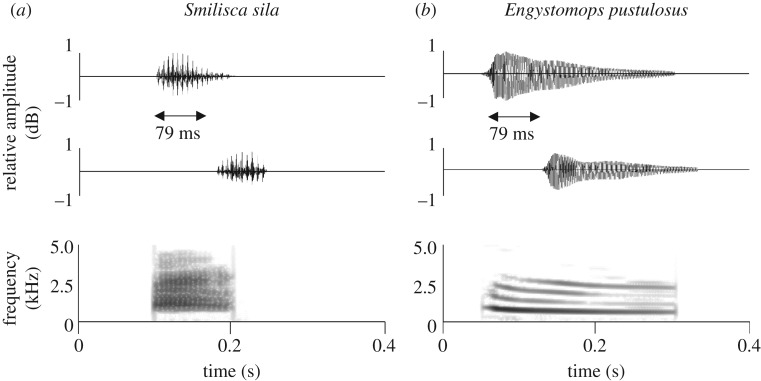
Figure 1.
Oscillogram (top) and spectrogram (bottom) of the mating calls of the pug-nosed tree frog (a) and túngara frog (b). The oscillogram shows two calls synchronized with a latency of 79 ms, the average natural synchrony of pug-nosed tree frogs. The spectrogram shows only the first call. Although both pug-nosed tree frogs and túngara frogs can produce a more complex, multi-note call, only playbacks of simple calls (as shown) were used in this study.
To compare the selective pressure imposed by frog-eating bats on different signal timing strategies, we video recorded bat attraction to the speakers in the field. At each of the three speaker stations, an infrared video camera (Bell and Howell model DNV16HDZ) was positioned 2 m from the two-speaker set and focused on the speaker broadcasting ‘following’ calls. The behaviour of the following male (calling in response to the ‘leader’) is responsible for call synchronization. As such, we were interested in the benefits that males may enjoy by producing following calls of different latencies. For the speaker set producing alternating calls, a focal speaker was chosen randomly from the two. A plastic model frog was placed on each speaker. For 2 h immediately following sunset [40], bat attraction to the speakers was video recorded. Following the protocol of other phonotaxis experiments with wild bats, videos were analysed blind to treatment [40,41]. We quantified the attractiveness of a treatment by counting the number of bat ‘attacks’ in a video, consisting of downwards flight towards the speaker in attempted prey capture. While we could not identify individual bats in the video, if certain treatments were more attractive to bats than others, the relative difference between treatments in the number of attacks should be maintained regardless of multiple visitations [40,41]. Additionally, as our collection methods between the pug-nosed tree frog and túngara frog breeding seasons were identical, we also compared the number of bat attacks between the dry and rainy season to determine differences in selective pressure from bats between the two frog species. Finally, even though other mammalian predators were occasionally recorded in the area around the speakers (e.g. opossums and ocelots), bat attacks were the only recurrent ‘predation events’ recorded.
(b). Risk of attack by frog-biting midges
Immediately following each 2 h recording session quantifying predatory bat attraction, we assessed the attraction of frog-biting midges using the same field set-up and speaker station arrangement. We placed an acoustic trap [42] over the focal speaker at each station for a period of 45 min. These acoustic traps use a small fan that collects any small insects attracted to a speaker broadcasting calls. Following our bat experiment, all three signal timing treatments were presented within a location simultaneously and each of the eight locations was tested three times for a total sampling period of 24 nights. After the insects were collected, they were euthanized in the freezer overnight. Frog-biting midges were counted, identified to genus and then preserved in 75% ethanol. We used the difference in the number of frog-biting midges attracted to the focal speaker between the call timing treatments as an indicator of acoustic preference. We also counted the number of individual mosquitoes (Culicidae) collected by the sound traps to identify possible additional eavesdroppers. Mosquitoes of the genus Uranotaenia, for example, are also known to use frog calls to find their hosts [43]. As with the bat attraction experiment, we also compared the number of midges collected between the pug-nosed tree frog and túngara frog breeding seasons to determine differences in selective pressure from midges between the two frog species.
(c). Attractiveness to female frogs
We collected frog pairs in amplexus from naturally occurring choruses during the breeding season for each species (n = 23 pug-nosed tree frogs in the dry season, January–March 2017, and n = 40 túngara frogs in the rainy season, October 2017). Female preference for conspecific calls broadcast at natural average synchrony (79 ms) or calls broadcast in alternation was tested in a 2 m × 3 m semi-anechoic chamber. Calls were broadcast from two speaker stations, with each station containing two speakers. The speaker stations were spaced 3 m apart, with speakers spaced 1 m apart within the stations. The station playing each acoustic treatment, synchrony or alternation, was randomly selected for each female. Females were gently separated from the male and positioned in the centre of the chamber at 1.5 m from each of the two speaker stations under an acoustically transparent plastic cup. To ensure that female movement did not consist of escape behaviour, females were given 1 min to adjust to the chamber before being remotely released. Mirroring the eavesdropper experiments, calls for both species were broadcast at 82 dB SPL re. 20 µP at a rate of one call every 2 s. Following standard decision rules used in phonotaxis experiments with anurans [44,45], a choice was scored when the female approached a speaker within 10 cm without following the walls of the arena. Females were tested in each treatment once and both males and females were released together at the end of the night at their exact capture location.
To avoid retesting, we toe-clipped female túngara frogs prior to releasing them. Toe-clipping is a standard and efficient method to mark anurans [46]. We avoided toe-clipping pug-nosed tree frogs, however, given that tree frogs heavily depend on their toepads for climbing. To identify female pug-nosed tree frogs, we built a photo library of all females captured over the course of the experiment. Both male and female pug-nosed tree frogs have pigmentation patterns on their backs that are individually distinctive [47], allowing for the successful implementation of this photo-based identification method.
(d). Statistical analysis
All statistical analyses were conducted using program R 3.5.2 [48]. To investigate the predation pressure imposed by bats and midges on different call timing strategies, we compared eavesdropper attraction to calls produced in near-perfect synchrony (5 ms of latency), average synchrony (79 ms of latency) and out of synchrony (alternating calls). We used generalized linear mixed effect model (GLMM) functions in the glmmTMB package [49] with a negative binomial error structure and a log link function [41]. Treatment was included as a fixed factor, site as a random factor and date as a random factor nested within site. To determine differences among treatments, we performed a Tukey contrast test and calculated least-squares means using the emmeans R package [50]. Effect sizes, Cohn's d, were calculated using the lsr R package [51]. We performed this analysis on the number of attacks for bats and number of midges or mosquitoes collected. These analyses were used to examine the effect of signal overlap in the calls of pug-nosed tree frogs and túngara frogs independently. As comparisons of eavesdropper attraction were performed within each night, those nights without any bat attacks or no midges captured are uninformative were thus removed from the respective analyses [41]. The number of bat attacks and the number of midges collected were compared between the pug-nosed tree frog and túngara frog breeding seasons using a permutation test in the coin R package [52], with bat attacks or number of midges grouped within the eight sampling locations. We analysed female preference for either calls broadcast at natural average synchrony or alternating calls using a two-tailed binomial test.
3. Results
(a). Risk of predation by frog-eating bats
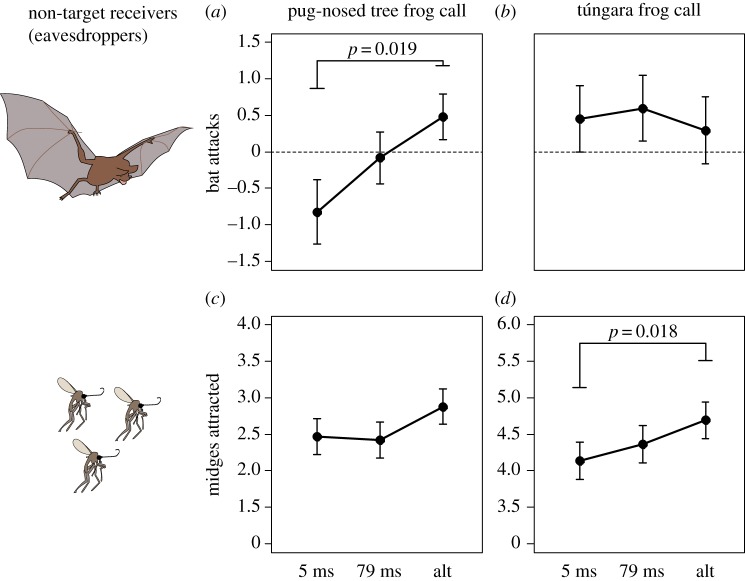
Field playback experiments using both pug-nosed tree frog and túngara frog calls were successful at attracting bats. Across all three treatments, a total of 343 general bat sightings were recorded during the playbacks of pug-nosed tree frog calls. The majority of those sightings involved bats passing by the area recorded by the camera and 54 were attacks (an average attack rate of 2.25 ± 0.65 per night). For túngara frog calls, a total of 398 general bat sightings were recorded, of which 65 were attacks (an average of 2.71 ± 1.14 per night). Between these two frog species, during their respective breeding seasons, the number of attacks by bats did not significantly differ (Z = −0.52, p = 0.602). The rate of bat observations reported here is similar to what has been observed in other studies examining bat phonotaxis in the wild [41].
For pug-nosed tree frog calls, bat attacks were observed on 15 of the 24 nights. When cueing on pug-nosed tree frog calls, bats were more likely to attack speakers broadcasting alternating calls, as calls broadcast at near-perfect synchrony (5 ms latency) received on average about a quarter of the attacks of calls broadcast in alternation (0.53 ± 0.27 versus 1.93 ± 0.49 attacks per night, t39 = −2.85, p = 0.019, d = 0.73; figure 2a). Other comparisons between call timings were not significantly different (p > 0.05). For túngara frog calls, bat attacks were observed on 11 of the 24 nights, and bats were equally likely to attack speakers of any of the three treatments (p > 0.05 for all combinations; figure 2b).
Figure 2.
Eavesdropper preferences for synchronized and unsynchronized calls, measured for both pug-nosed tree frog (left) and túngara frog (right) calls during each species' respective breeding season. Number of bat attacks per night (a,b) and number of midges captured per night (c,d) for calls were compared for different call timings: near-perfect synchrony (5 ms of latency), average pug-nosed tree frog call synchrony (79 ms of latency) and alternating calls (alt). Values are the least squared means and bars show standard error. Note that for midge attraction, the y-axis range for pug-nosed tree frogs (c) is different than túngara frogs (d).
(b). Risk of attack by frog-biting midges
A total of 1253 and 9052 frog-biting midges were attracted to pug-nosed tree frog and túngara frog calls, respectively. Thus, between these two species, significantly more midges were attracted to túngara frog calls than pug-nosed tree frog calls during each species' breeding season (Z = −0.52, p < 0.001, d = 0.94). For pug-nosed tree frog calls, midges were captured on 23 out of the 24 nights, but there were no significant differences in the number of midges captured between any of the treatments (p > 0.05 for all combinations; figure 2c). For túngara frog calls, midges were captured on all 24 nights, and calls broadcast in alternation attracted significantly more midges per night compared to calls broadcast at near-perfect synchrony (149.08 ± 35.70 versus 104.25 ± 33.12 midges, t66 = −2.81, p = 0.018, d = 0.23; figure 2d). No other comparisons between traps broadcasting calls with different timing were significant (p > 0.05). A full list of the Tukey comparisons for the eavesdropper experiments is included in the electronic supplementary material, table S1.
Additionally, an average of 4.29 and 16.67 mosquitoes (Culicidae) were collected per night for pug-nosed tree frog and túngara frog calls, respectively. A small proportion (less than 2%) of the mosquitoes in the acoustic traps was Uranotaenia lowii, a species known to acoustically orient to frog calls [43]. There was, however, no significant difference in the number of mosquitoes attracted to different treatments (p > 0.05 for all combinations) for the calls of either frog species.
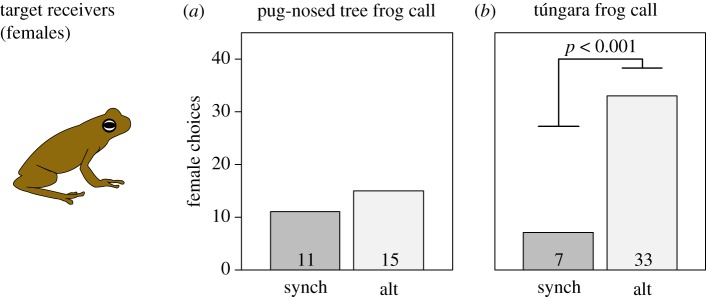
(c). Attractiveness to female frogs
Female pug-nosed tree frogs were indifferent to speakers broadcasting alternating calls versus synchronized ones, with 15 of the 26 tested females choosing alternating calls (two-tailed binomial test: p = 0.557; figure 3a). By contrast, female túngara frogs displayed a strong preference, with a higher proportion of females choosing alternating calls (33 of 40, two-tailed binomial test: p < 0.001; figure 3b). Using the effect size of the female túngara choice experiment for comparison, the pug-nosed tree frog experiment had adequate statistical power (1 − β = 0.93, α = 0.05, effect size = 0.325; using G*Power 3.1 [53]).
Figure 3.
Female preference for synchronized and unsynchronized calls, measured for both pug-nosed tree frog (a) and túngara frog (b) calls during each species' respective breeding season. Female choice was compared between calls broadcast in synchrony with 79 ms of latency between calls (synch), or broadcast in alternation (alt). Each choice represents a single female.
4. Discussion
Synchronization of mating signals is an evolutionary puzzle, given the assumed high cost of reduced female attraction when signals overlap. Synchronization may be beneficial, however, if overlapping signals reduce attraction of non-target receivers. We found that synchronized pug-nosed tree frog calls attract fewer frog-eating bats than unsynchronized signals. Similarly, synchronized túngara frog calls attract fewer frog-biting midges than calls produced in alternation. These findings support the eavesdropper avoidance function of synchronized signals (first proposed by Tuttle & Ryan [21]). Furthermore, by testing the calls of two frog species, one that naturally synchronizes its calls (pug-nosed tree frogs) and one that calls antiphonally (túngara frogs), we find that the eavesdropper avoidance benefit of synchronization is not limited only to synchronizing species.
(a). Signal synchronization in the context of multiple eavesdroppers
While we found decreased eavesdropper attack rates in response to synchronous calling, bats and midges responded differently to call timing for each frog species. Synchronized pug-nosed tree frog calls attracted fewer bats but did not affect midge attraction, while synchronized túngara frog calls attracted fewer midges but did not affect bat attraction. We propose that the species-specific differences in eavesdropper attraction are probably owing to differences in spectral and temporal properties of each frog species’ calls [21,27] (figure 1). In addition, we expect such diverse eavesdroppers to greatly differ in the way acoustic signals are received and processed. How each receiver perceives such signals, however, is still a mystery [30]. Further studies that investigate the physiology of the auditory systems of these eavesdroppers are necessary to confirm the relationship between call timing and other call properties in reducing eavesdropper attraction.
In both eavesdropper experiments, only calls presented in near-perfect synchrony significantly reduced the attraction of bats or midges, with no difference between calls broadcast at average synchrony and antiphonally. It is likely that eavesdropper preference decreases as a function of the degree of synchrony. To better understand the ecological relevance of the delay between signals of neighbouring males, further studies assessing eavesdropper preference along a finer gradient of latencies are needed. Given that males of many frog species, including those in this study [54,55], can alter their calling behaviour in response to perceived increased predation risk, it is possible that frogs plastically increase the degree of signal overlap to further decrease signal conspicuousness. Studies that examine the plasticity of fine timing responses of males signalling in choruses and how they are modulated by perceived predation risk would provide valuable insights to further understand the evolution of signal synchronization.
(b). Implications for the origin and maintenance of signal synchronization
While our results suggest that call synchronization can reduce eavesdropper attraction, we found no evidence of greater predation risk from bats on pug-nosed tree frogs compared to túngara frogs. In both species, calling males are attacked at similar rates by frog-eating bats. As mentioned above, however, male pug-nosed tree frogs calling in synchrony benefit from a reduction in bat attacks, a benefit absent for túngara frogs. By contrast, frog-biting midges attacked túngara frogs in much greater numbers than pug-nosed tree frogs. The immediate lethal effects of bat attacks compared to the slower, additive effects of midge attacks suggest that pug-nosed tree frogs enjoy a larger benefit than túngara frogs when their calls are produced in synchrony. It is thus possible that a larger benefit of obscuring the calls by synchronizing them with neighbouring males could have favoured call synchronization in pug-nosed tree frogs. Overall, however, given the rates of bat and midge attraction across seasons, our results suggest that male pug-nosed tree frogs synchronize even though the general selective pressure imposed by eavesdroppers is similar to the levels experienced by other frog species in the community. That is, despite being one of the few anuran species calling in the dry season [38], pug-nose tree frogs are not attacked more by bats and are even attacked less by midges, than túngara frogs. Therefore, higher predation pressure is unlikely to have been a major driver of call synchronization, and the extreme predator selection hypothesis is not supported. Instead, the key to signal synchronization may be in the selective preferences of a different receiver in the communication network, the target receiver of mating calls, conspecific females.
The responses of females to calls with different timing relative to calls of their neighbours revealed species-specific differences in their preferences. Our study confirmed that female túngara frogs, the non-synchronous species, prefer calls broadcast antiphonally over calls that are synchronized (see [56]). This preference for calls out of synchrony is assumed to be a general strategy across anuran species, given that females select signals in aggregations where overlapping signals impose a cognitive challenge for localizing and discriminating individual signals [7,37]. It is this preference for unmasked calls that is assumed to drive the use of non-synchronous, alternating calls as a general strategy in frogs [8–10], including the Smilisca clade (electronic supplementary material, figure S3). Female pug-nosed tree frogs, however, have no such preference and deviate from the general strategy of preferring calls produced out of synchrony. Such lack of preference suggests a reduction in the strength of preference for non-overlapping calls in pug-nosed tree frogs, resulting in a shift in the trade-off of selective pressures on synchronization. For male pug-nosed tree frogs, the selective pressure against producing following calls is lower than for túngara frogs. These findings support the idea that relaxed selection by females has provided the opportunity for signalling males to synchronize their calls and thus reduce attacks by eavesdroppers (relaxed sexual selection hypothesis). While traditional models of female preference and the evolution of mating signals have emphasized positive selection (e.g. [57–59]), there is recent increased attention to the role of relaxed selection in the maintenance and evolution of traits in general [60]. Relaxation of selection ultimately shifts the relationship between costs and benefits, potentially shaping trait trade-offs and resulting in trait evolution. Male anuran signal timing is influenced by a trade-off between eavesdropper and female attraction [30]. Yet, despite experiencing similar levels of predation pressure, pug-nosed tree frogs are one of the few anurans to produce near-perfectly synchronized calls in this community. Overall, our results suggest that male pug-nosed tree frogs are released from a cost imposed by reduced female attraction. We, therefore, propose that while avoidance of eavesdroppers is the function of synchronized signalling, relaxation of female preference for unsynchronized signals has allowed for the evolution and maintenance of a synchronized signal timing strategy in pug-nosed tree frogs. It is still unclear, however, if female pug-nose tree frogs struggle with challenges associated with localizing and discriminating between mates as females from many other anuran species do [9,10]. Further studies that examine the ability of female pug-nosed tree frogs to localize and discriminate between preferred males calling in synchrony would provide valuable insights.
(c). Other functions of signal synchronization in anurans and other taxa
Other drivers, in addition to avoidance of eavesdroppers, may select for call synchronization. For instance, synchrony may arise through male–male competition, where a male may try to mask a neighbour's call with his own call reducing his neighbour's attractiveness [22,61]. Given that by masking a neighbour's call, the synchronizing male also masks his own call reducing his own attractiveness, this function seems unlikely [61]. If features at the end of the call increase female attraction, however, a synchronizing male may be able to preserve the attractiveness of his call while still masking his neighbour's call. In hourglass tree frogs (Dendropsophus ebraccatus) and African running frogs (Kassina fusca), for example, males produce complex multi-note calls and females prefer calls with unobstructed ends. In both species, males overlap calls with neighbouring males resulting in following males masking the tail end of the leader's call, while the end of the follower's call remains unobstructed [62,63]. In comparison, the calls of pug-nosed tree frogs lack distinct features towards the end that a following male would benefit from obstructing. Therefore, owing to the simplicity of the calls of pug-nosed tree frogs, synchrony through male–male competition seems unlikely.
Synchronized calling may also benefit males by increasing the peak amplitude of their combined calls through constructive interference. Groups of synchronized males may create a ‘beacon’, increasing the active space of their signals compared to a group of unsynchronized males [64]. First proposed in fireflies, this ‘beacon effect’ hypothesis has been proposed to explain synchronization of acoustic signals in insect [22,65] and anuran [61] choruses but has not been directly tested in the latter. The beacon effect hypothesis, similar to the eavesdropper hypothesis, comes with a potential cost to female attraction. As with almost any trait related to chorusing, the per capita increase in female attraction to the chorus must outweigh the cost of reduced female attraction to an individual male within the chorus [66]. In the context of the beacon effect, it is also unclear how this calling strategy is resistant to cheaters, as a non-synchronous male producing unobstructed calls would enjoy the benefits of increased numbers of females attending the chorus but would be more attractive to females once they reach the chorus. There are, however, particular habitats that may limit the benefits gained by cheating. In habitats with high levels of background noise, such as waterfalls or streams, the calls of a single male may already be acoustically masked. Overcoming high levels of background noise could thus lead to call synchronization as an evolutionary stable strategy that takes advantage of the beacon effect. Consistent with this idea, male pug-nosed tree frogs form choruses around waterfalls and torrents of streams [54] in which the dominant frequency of their call overlaps with the background noise generated by running water [21,54]. This acoustic masking suggests that males of this species could benefit from the beacon effect to attract females. An increased active space of the chorus, however, will also result in increased attraction of eavesdropping predators. Further studies are needed that examine the potential role of the beacon effect on call synchronization, while also considering the effect of eavesdroppers.
Finally, while synchronization is rare in frogs and toads, having only been identified in a handful of anuran species in addition to pug-nosed tree frogs (Kassina senegalensis [67], D. ebraccatus [62], Cochranella granulose [68], K. fusca [63], Kassina kuvangensis [69], Hyla arenicolor (V.T. Marshall and H.C. Gerhardt 2002, unpublished data, reviewed in [17]), Assa darlingtoni [70], Diasporus diastema [71]), this signal timing strategy has been observed across diverse taxonomic groups. Synchronization is a common strategy for many insects, such as crickets and katydids, that form large nocturnal choruses that share many similarities to anuran choruses [72–78]. In such large multispecies choruses, synchrony may benefit males by maintaining a species-specific rhythm, allowing females to more easily identify conspecifics [22,79].
Signal synchronization in groups other than anurans and insects is used in different contexts. Male and female birds, for example, may synchronize mating signals in the form of duets [23]. Unlike insect and anuran choruses, however, synchronization in bird songs function primarily in mate and territory defence rather than as a mate attraction strategy. The synchronized howling of wolves (Canis lupus) and coyotes (Canis latran) also plays a role in territorial maintenance [24,80] through the Beau Geste effect [81], similar to the beacon effect, in which synchronizing masks a pack's size or make it appear larger to distant receivers. Synchronization is also observed in other signal modalities, such as in the luminescent displays produced by some species of fireflies [64] and marine ostracods [20]. Whether these visual displays help mask individuals from eavesdropping predators, however, has yet to be tested.
(d). Conclusion
Predators have often been invoked as a driving force that curtails exaggeration of mating signals [36], but evolutionary biologists have devoted less attention to consider how relaxed selection by females can allow signallers to escape eavesdropper exploitation of their communication system. Additionally, to date, most studies on relaxed selection have focused on non-sexual selective pressures, such as predator release or abiotic changes in the environment [60]. To our knowledge, this study provides the first example of how relaxed female choice may result in trait evolution in nature. Our results bring to light the complex nature of trade-offs and the role of relaxed selection at promoting the evolution of unique signalling strategies.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Smithsonian Tropical Research Institute (STRI) for logistical support, as well as Ryan Taylor and Kimberly Hunter for the use of their semi-anechoic sound chamber. We are grateful to Sarah Zlotnik for help with field and laboratory work. We additionally thank Nigel Anderson, Samuel Freedlund, Grace Higginbottom, Tsun Lai ‘Alfa’ Lam, Ryan Madden, Suzette Miller and Carlos Pantoja for their work analysing the videos of the frog-eating bat and female frog experiments. Finally, we appreciate the suggestions from the editors and six anonymous reviewers that greatly improved the quality of the manuscript.
Ethics
This research was approved by Purdue University (IACUC Protocol no. 1504001235), the Smithsonian Tropical Research Institute (IACUC Protocol nos 2015-0104-2018 and 2017-0101-2020-3) and Panamanian Authorities (El Ministerio de Ambiente, MiAmbiente scientific permit no. SE/A-7-15, SEX/A-51-15).
Data accessibility
Data and R code supporting this manuscript are available in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.rd03773 [82].
Authors' contributions
H.D.L., R.A.P. and X.E.B. conceived and designed the study. H.D.L. collected data, performed the statistical analyses and drafted the manuscript. R.A.P. and X.E.B. helped draft and edit the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by an A. Stanley Rand Fellowship from the Smithsonian Tropical Research Institute and an A. A. Lindsey Graduate Fellowship from Purdue University, both to H.D.L. This study was additionally supported by a grant from the National Science Foundation (IOS no. 1433990) to X.E.B.
References
- 1.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438. ( 10.1086/420412) [DOI] [Google Scholar]
- 2.McGregor PK, Peake TM. 2000. Communication networks: social environments for receiving and signalling behaviour. Acta Ethol. 2, 71–81. ( 10.1007/s102110000015) [DOI] [Google Scholar]
- 3.Taga ME, Bassler BL. 2003. Chemical communication among bacteria. Proc. Natl Acad. Sci. USA 100, 14 549–14 554. ( 10.1073/pnas.1934514100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebets EA, Papaj DR. 2004. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. ( 10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 5.McGregor PK. (ed). 2005. Animal communication networks. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Peake TM. 2005. Eavesdropping in communication networks. In Animal communication networks (ed. McGregor PK.), pp. 13–37. Cambridge, UK: Cambridge University Press; ( 10.1017/cbo9780511610363.004) [DOI] [Google Scholar]
- 7.Bee MA, Micheyl C. 2008. The cocktail party problem: what is it? How can it be solved? And why should animal behaviorists study it? J. Comp. Psychol 122, 235–251. ( 10.1037/0735-7036.122.3.235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollerman L. 1999. Acoustic interference limits call detection in a Neotropical frog Hyla ebraccata. Anim. Behav. 57, 529–536. ( 10.1006/anbe.1998.1013) [DOI] [PubMed] [Google Scholar]
- 9.Wollerman L, Wiley H. 2002. Possibilities for error during communication by neotropical frogs in a complex acoustic environment. Behav. Ecol. Sociobiol. 52, 465–473. ( 10.1007/s00265-002-0534-7) [DOI] [Google Scholar]
- 10.Wollerman L, Wiley RH. 2002. Background noise from a natural chorus alters female discrimination of male calls in a Neotropical frog. Anim. Behav. 63, 15–22. ( 10.1006/anbe.2001.1885) [DOI] [Google Scholar]
- 11.Whitney CL, Krebs JR. 1975. Mate selection in Pacific tree frogs. Nature 255, 325–326. ( 10.1038/255325a0) [DOI] [Google Scholar]
- 12.Klump GM, Gerhardt HC. 1992. Mechanisms and function of call-timing in male–male interactions in frogs. In Playback and studies of animal communication (ed. McGregor PK.), pp. 153–174. Boston, MA: Springer; ( 10.1007/978-1-4757-6203-7_11) [DOI] [Google Scholar]
- 13.Grafe TU. 1996. The function of call alternation in the African reed frog (Hyperolius marmoratus): precise call timing prevents auditory masking. Behav. Ecol. Sociobiol. 38, 149–158. ( 10.1007/s002650050227) [DOI] [Google Scholar]
- 14.Greenfield MD, Tourtellot MK, Snedden WA. 1997. Precedence effects and the evolution of chorusing. Proc. R. Soc. B 264, 1355–1361. ( 10.1098/rspb.1997.0188) [DOI] [Google Scholar]
- 15.Bosch J. 2002. Female preference function related to precedence effect in an amphibian anuran (Alytes cisternasii): tests with non-overlapping calls. Behav. Ecol. 13, 149–153. ( 10.1093/beheco/13.2.149) [DOI] [Google Scholar]
- 16.Höbel G. 2010. Interaction between signal timing and signal feature preferences: causes and implications for sexual selection. Anim. Behav. 79, 1257–1266. ( 10.1016/j.anbehav.2010.02.026) [DOI] [Google Scholar]
- 17.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- 18.Farabaugh SM. 1982. The ecological and social significance of duetting. In Acoustic communication in birds, vol. 2, song learning and its consequences (eds Kroodsma DE, Miller EH), pp. 85–124. New York, NY: Academic Press. [Google Scholar]
- 19.Carlson AD, Copeland J. 1985. Flash communication in fireflies. Q. Rev. Biol. 60, 415–436. ( 10.1086/414564) [DOI] [Google Scholar]
- 20.Morin JG. 1986. Firefleas of the sea: luminescent signaling in marine ostracode crustaceans. Fla. Entomol. 69, 105–121. ( 10.2307/3494749) [DOI] [Google Scholar]
- 21.Tuttle MD, Ryan MJ. 1982. The role of synchronized calling, ambient light, and ambient noise, in anti-bat-predator behavior of a treefrog. Behav. Ecol. Sociobiol. 11, 125–131. ( 10.1007/bf00300101) [DOI] [Google Scholar]
- 22.Greenfield MD. 1994. Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am. Zool. 34, 605–615. ( 10.1093/icb/34.6.605) [DOI] [Google Scholar]
- 23.Hall ML. 2009. A review of vocal duetting in birds. Adv. Stud. Behav. 40, 67–121. ( 10.1016/S0065-3454(09)40003-2) [DOI] [Google Scholar]
- 24.Harrington FH, Mech LD. 1979. Wolf howling and its role in territory maintenance. Behaviour 68, 207–249. ( 10.1163/156853979X00322) [DOI] [Google Scholar]
- 25.Reaney LT, Sims RA, Sims SWM, Jennions MD, Backwell PRY. 2008. Experiments with robots explain synchronized courtship in fiddler crabs. Curr. Biol. 18, R62–R63. ( 10.1016/j.cub.2007.11.047) [DOI] [PubMed] [Google Scholar]
- 26.Ryan MJ. 1986. Synchronized calling in a treefrog (Smilisca sila). Brain Behav. Evol. 29, 196–206. ( 10.1159/000118681) [DOI] [PubMed] [Google Scholar]
- 27.Ryan MJ. 1985. The túngara frog: a study in sexual selection and communication. Chicago, IL: University of Chicago Press. [Google Scholar]
- 28.Ryan MJ, Tuttle MD, Rand AS. 1982. Bat predation and sexual advertisement in a neotropical anuran. Am. Nat. 119, 136–139. ( 10.1086/283899) [DOI] [Google Scholar]
- 29.Legett HD, Baranov VA, Bernal XE. 2017. Seasonal variation in abundance and diversity of eavesdropping frog-biting midges (Diptera, Corethrellidae) in a neotropical rainforest. Ecol. Entomol. 43, 226–233. ( 10.1111/een.12492) [DOI] [Google Scholar]
- 30.Page RA, Ryan MJ, Bernal XE. 2014. Be loved, be preyed, be eaten. In Animal behavior case studies: integration and application of animal behavior, vol. 3 (ed. Yasukawa K.), pp. 123–154. New York, NY: Praeger. [Google Scholar]
- 31.Bernal XE, Rand AS, Ryan MJ. 2006. Acoustic preferences and localization performance of blood-sucking flies (Corethrella Coquillett) to túngara frog calls. Behav. Ecol. 17, 709–715. ( 10.1093/beheco/arl003) [DOI] [Google Scholar]
- 32.Camp JV. 2006. Host attraction and host selection in the family Corethrellidae (Wood and Borkent) (Diptera). Master's thesis, Georgia Southern University, GA, USA. [Google Scholar]
- 33.Johnson RN, Young DG, Butler JF. 1993. Trypanosome transmission by Corethrella wirthi (Diptera: Chaoboridae) to the green treefrog, Hyla cinerea (Anura: Hylidae). J. Med. Entomol. 30, 918–921. ( 10.1093/jmedent/30.5.918) [DOI] [PubMed] [Google Scholar]
- 34.Bernal XE, Pinto CM. 2016. Sexual differences in prevalence of a new species of trypanosome infecting túngara frogs. Int. J. Parasitol. Parasites Wildl. 5, 40–47. ( 10.1016/j.ijppaw.2016.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkpatrick M, Ryan MJ. 1991. The evolution of mating preferences and the paradox of the lek. Nature 350, 33–38. ( 10.1038/350033a0) [DOI] [Google Scholar]
- 36.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 37.Schwartz JJ. 1987. The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution 41, 461–471. ( 10.1111/j.1558-5646.1987.tb05818.x) [DOI] [PubMed] [Google Scholar]
- 38.Heyer WR. 1976. Studies in larval amphibian habitat partitioning. Smithson. Contrib. Zool. 242, 1–27. ( 10.5479/si.00810282.242) [DOI] [Google Scholar]
- 39.Jones PL, Hämsch F, Page RA, Kalko EKV, O'Mara MT. 2017. Foraging and roosting behaviour of the fringe-lipped bat, Trachops cirrhosus, on Barro Colorado Island, Panamá. Acta Chiropt. 19, 337–346. ( 10.3161/15081109acc2017.19.2.010) [DOI] [Google Scholar]
- 40.Trillo PA, Athanas KA, Goldhill DH, Hoke KL, Funk WC. 2012. The influence of geographic heterogeneity in predation pressure on sexual signal divergence in an Amazonian frog species complex. J. Evol. Biol. 26, 216–222. ( 10.1111/jeb.12041) [DOI] [PubMed] [Google Scholar]
- 41.Trillo PA, Bernal XE, Caldwell MS, Halfwerk WH, Wessel MO, Page RA. 2016. Collateral damage or a shadow of safety? The effects of signalling heterospecific neighbours on the risks of parasitism and predation. Proc. R. Soc. B 283, 20160343 ( 10.1098/rspb.2016.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKeever S, Hartberg WK. 1980. An effective method for trapping adult female Corethrella (Diptera: Chaoboridae). Mosq. News 20, 111–112. [Google Scholar]
- 43.Borkent A, Belton P. 2006. Attraction of female Uranotaenia lowii (Diptera: Culicidae) to frog calls in Costa Rica. Can. Entomol. 138, 91–94. ( 10.4039/n04-113) [DOI] [Google Scholar]
- 44.Gerhardt HC, Klump GM. 1988. Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim. Behav. 36, 1247–1249. ( 10.1016/s0003-3472(88)80090-3) [DOI] [Google Scholar]
- 45.Ryan MJ, Rand AS. 1990. The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation). Evolution 44, 305–314. ( 10.1111/j.1558-5646.1990.tb05200.x) [DOI] [PubMed] [Google Scholar]
- 46.Donnelly MA, Guyer C, Juterbock EJ, Alford RA. 1994. Techniques for marking amphibians. In Measuring and monitoring biological diversity: standard methods for amphibians (eds Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS), pp. 275–284. Washington, DC: Smithsonian Institution Press; ( 10.1002/zoo.1430140610) [DOI] [Google Scholar]
- 47.Duellman WE. 1970. Hylid frogs of Middle America. Lawrence, KS: University of Kansas Press. [Google Scholar]
- 48.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org. [Google Scholar]
- 49.Brooks ME, Kristensen K, Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. ( 10.32614/rj-2017-066) [DOI] [Google Scholar]
- 50.Searle SR, Speed FM, Milliken GA. 1980. Population marginal means in the linear model: an alternative to least squares means. Am. Stat. 34, 216–221. ( 10.2307/2684063) [DOI] [Google Scholar]
- 51.Navarro D. 2015. Lsr: companion to ‘learning statistics with R’. Adelaide, Australia: University of Adelaide; See https://cran.r-project.org/web/packages/lsr/index.html. [Google Scholar]
- 52.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 53.Faul F, Erdfelder E, Buchner A, Lang A-G. 2009. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. ( 10.3758/brm.41.4.1149) [DOI] [PubMed] [Google Scholar]
- 54.da Silva Nunes V. 1988. Vocalizations of treefrogs (Smilisca sila) in response to bat predation. Herpetologica 44, 8–10. [Google Scholar]
- 55.Page RA, Ryan MJ. 2005. Flexibility in assessment of prey cues: frog-eating bats and frog calls. Proc. R. Soc. B 272, 841–847. ( 10.1098/rspb.2004.2998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz JJ, Rand AS. 2010. The consequences for communication of call overlap in the tungara frog, a neotropical anuran with a frequency-modulated call. Ethology 89, 73–83. ( 10.1111/j.1439-0310.1991.tb00294.x) [DOI] [Google Scholar]
- 57.Haldane JBS. 1932. The causes of evolution (revised edn 1990) Princeton, NJ: Princeton University Press. [Google Scholar]
- 58.Prout T. 1964. Observations on structural reduction in evolution. Am. Nat. 98, 239–249. ( 10.1086/282323) [DOI] [Google Scholar]
- 59.Fong DW, Kane TC, Culver DC. 1995. Vestigialization and loss of nonfunctional characters. Annu. Rev. Ecol. Evol. Syst. 26, 249–268. ( 10.1146/annurev.es.26.110195.001341) [DOI] [Google Scholar]
- 60.Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster SA. 2009. Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496. ( 10.1016/j.tree.2009.03.010) [DOI] [PubMed] [Google Scholar]
- 61.Wells KD. 1977. The social behaviour of anuran amphibians. Anim. Behav. 25, 666–693. ( 10.1016/0003-3472(77)90118-x) [DOI] [Google Scholar]
- 62.Wells KD, Schwartz JJ. 1984. Vocal communication in a neotropical treefrog, Hyla ebraccata: advertisement calls. Anim. Behav. 32, 405–420. ( 10.1016/s0003-3472(84)80277-8) [DOI] [Google Scholar]
- 63.Grafe TU. 1999. A function of synchronous chorusing and a novel female preference shift in an anuran. Proc. R. Soc. B 266, 2331–2336. ( 10.1098/rspb.1999.0927) [DOI] [Google Scholar]
- 64.Buck J, Buck E. 1978. Toward a functional interpretation of synchronous flashing by fireflies. Am. Nat. 112, 471–492. ( 10.1086/283291) [DOI] [Google Scholar]
- 65.Shelly TE, Greenfield MD. 1991. Dominions and desert clickers (Orthoptera: Acrididae): influences of resources and male signaling on female settlement patterns. Behav. Ecol. Sociobiol. 28, 133–140. ( 10.1007/bf00180990) [DOI] [Google Scholar]
- 66.Ryan MJ, Tuttle MD, Taft LK. 1981. The costs and benefits of frog chorusing behavior. Behav. Ecol. Sociobiol. 8, 273–278. ( 10.1007/bf00299526) [DOI] [Google Scholar]
- 67.Wickler W, Seibt U. 1974. Rufen und Antworten bei Kassina senegalensis, Bufo regularis und anderen Anuren. Z. Tierpsychol. 34, 524–537. ( 10.1111/j.1439-0310.1974.tb01819.x) [DOI] [Google Scholar]
- 68.Ibáñez R. 1993. Female phonotaxis and call overlap in the neotropical glassfrog Centrolenella granulosa. Copeia 1993, 846–850. ( 10.2307/1447249) [DOI] [Google Scholar]
- 69.Grafe TU. 2003. Synchronized interdigitated calling in the Kuvangu running frog, Kassina kuvangensis. Anim. Behav. 66, 127–136. ( 10.1006/anbe.2003.2173) [DOI] [Google Scholar]
- 70.Clulow S, Mahony M, Elliott L, Humfeld S, Gerhardt HC. 2016. Near-synchronous calling in the hip-pocket frog Assa darlingtoni. Bioacoustics 26, 249–258. ( 10.1080/09524622.2016.1260054) [DOI] [Google Scholar]
- 71.Capshaw G, Foss-Grant AP, Hartmann K, Sehuanes JF, Moss CF. In press. Timing of the advertisement call of the common tink frog (Diasporus diastema) shifts with the acoustic behaviour of local conspecifics. Bioacoustics. ( 10.1080/09524622.2018.1555715) [DOI] [Google Scholar]
- 72.Walker TJ. 1969. Acoustic synchrony: two mechanisms in the snowy tree cricket. Science 166, 891–894. ( 10.1126/science.166.3907.891) [DOI] [PubMed] [Google Scholar]
- 73.Shaw KC, Galliart PL, Smith B. 1990. Acoustic behavior of Amblycorypha parvipennis (Orthoptera: Tettigoniidae). Ann. Entomol. Soc. Am. 83, 617–625. ( 10.1093/aesa/83.3.617) [DOI] [Google Scholar]
- 74.Sismondo E. 1990. Synchronous, alternating, and phase-locked stridulation by a tropical katydid. Science 249, 55–58. ( 10.1126/science.249.4964.55) [DOI] [PubMed] [Google Scholar]
- 75.Greenfield MD, Roizen I. 1993. Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364, 618–620. ( 10.1038/364618a0) [DOI] [Google Scholar]
- 76.Nityananda V, Balakrishnan R. 2006. Synchrony during acoustic interactions in the bushcricket Mecopoda ‘Chirper’ (Tettigoniidae: Orthoptera) is generated by a combination of chirp-by-chirp resetting and change in intrinsic chirp rate. J. Comp. Physiol. A 193, 51–65. ( 10.1007/s00359-006-0170-1) [DOI] [PubMed] [Google Scholar]
- 77.Greenfield MD, Schul J. 2008. Mechanisms and evolution of synchronous chorusing: emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: Tettigoniidae). J. Comp. Psychol. 122, 289–297. ( 10.1037/0735-7036.122.3.289) [DOI] [PubMed] [Google Scholar]
- 78.Schul J, Bush SL, Frederick KH. 2013. Evolution of call patterns and pattern recognition mechanisms in Neoconocephalus katydids. In Animal signals and communication, vol.1 (ed. Hedwig B.), pp. 167–183. Berlin, Germany: Springer; ( 10.1007/978-3-642-40462-7_10) [DOI] [Google Scholar]
- 79.Moiseff A, Copeland J. 2010. Firefly synchrony: a behavioral strategy to minimize visual clutter. Science 329, 181 ( 10.1126/science.1190421) [DOI] [PubMed] [Google Scholar]
- 80.McCarley H. 1975. Long-distance vocalizations of coyotes (Canis latrans). J. Mammal. 56, 847–856. ( 10.2307/1379656) [DOI] [Google Scholar]
- 81.Harrington FH. 1989. Chorus howling by wolves: acoustic structure, pack size and the Beau Geste effect. Bioacoustics 2, 117–136. ( 10.1080/09524622.1989.9753122) [DOI] [Google Scholar]
- 82.Legett HD, Page RA, Bernal XE. 2019. Data from: Synchronized mating signals in a communication network: the challenge of avoiding predators while attracting mates Dryad Digital Repository. ( 10.5061/dryad.rd03773) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Legett HD, Page RA, Bernal XE. 2019. Data from: Synchronized mating signals in a communication network: the challenge of avoiding predators while attracting mates Dryad Digital Repository. ( 10.5061/dryad.rd03773) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R code supporting this manuscript are available in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.rd03773 [82].