Abstract
Nature-based agriculture that reduces dependency on chemical inputs requires using ecological principles for sustainable agro-ecosystems, aiming to balance ecology, economics and social justice. There is growing evidence that pollinator-dependent crops with high insect, particularly bee, pollination service can give higher yields. However, the interacting effects between insect pollination and agricultural inputs on crop yields and farm economics remain to be established to reconcile food production with biodiversity conservation. We quantified individual and combined effects of pesticides, insect pollination and soil quality on oilseed rape (Brassica napus L.) yield and gross margin, using a total of 294 farmers' fields surveyed between 2013 and 2016. We show that yield and gross margins are greater (15–40%) in fields with higher pollinator abundance than in fields with reduced pollinator abundance. This effect is, however, strongly reduced by pesticide use. Greater yields may be achieved by either increasing agrochemicals or increasing bee abundance, but crop economic returns were only increased by the latter, because pesticides did not increase yields while their costs reduced gross margins.
Keywords: agroecology, pollinators, herbicide, insecticide, nature-based solutions, rapeseed
1. Introduction
Achieving global food production to meet the demands of a growing population while minimizing environmental impacts is a key priority [1]. Modern agriculture may be at a tipping point, with nature's supporting mechanisms failing [2] and artificial inputs such as fertilizers and pesticides being either ineffective or used inefficiently [3]. There is also growing recognition that ecosystem service degradation has substantial economic as well as environmental consequences [4]. A major challenge in western agriculture is, therefore, to stabilize crop yields while decreasing the dependence on agrochemical inputs [5]. Nature-based solutions for agriculture are a key European Union (EU) research target [6] and form the basis of agroecology [5]. This requires using ecological principles for sustainable agro-ecosystems, balancing ecology, economics and social justice [7]. Sustainable agroecology relies on maximizing the replacement of agrochemicals by natural capital and ecosystem functions, while minimizing the reduction in yield and increasing farm profitability.
Insect pollination is a key intermediate ecosystem service as a third of human food production benefits directly or indirectly from it [8]. However, in recent years, the abundance and diversity of insect pollinators have been declining worldwide, affecting pollination services [9]. At the same time, the cultivated area of oilseed rape (OSR, Brassica napus L.) is rapidly increasing, driven by increasing demand, so that OSR production may become limited by the abundance of pollinators such as honeybees [10]. Pesticides are used for intensive farming to mitigate the direct impact of pests or weed abundance on OSR yield [11,12], but these pesticides, and especially insecticides, can increase the mortality rates of pollinators [13] and reduce their efficiency [13,14]. Herbicides, by modifying weed abundance in crops, may positively [15] or negatively [16] influence pollinator abundance.
Although OSR can be self- and wind-pollinated [17], insect pollination itself can increase OSR yield by 20–35% [17,18], with a possible benefit of €2.6 M yr−1 for the whole of Ireland [19]. Estimating the extent to which OSR production relies on insects for pollination services is, however, not trivial [20,21]. Firstly, measuring pollination services by quantifying the reduction in yield when pollinators are excluded also excludes other ecosystem services such as pest control, and may stress the plants [20]. Secondly, the benefits of a pollination service in terms of increased yield are often assumed to be independent of the level of inputs [20,22]. Recent studies have demonstrated that the value of insect pollination depends on farming practices such as the selection of cultivars [23], pest control [21], soil fertility [21] and field size [24]. When pollinators are limited, farmers can change their practices to compensate for poor pollination by, for example, increasing fertilizer applications [25]. Thirdly, pollinator abundance and pollination efficiency vary with the composition of the surrounding landscape [18]. Landscapes with large quantity of pollinator-friendly areas, such as semi-natural habitats (SNH; woodlands, meadows) can increase the abundance of pollinators [18] or attract pollinators away from the OSR fields [16]. Recent research [26] has showed that a higher proportion of OSR in the surrounding landscape may also decrease insect pollination by spatial dilution of the pollinator population. Although OSR is a well-studied crop, the extent to which pollinators together with farming practices interact to increase or limit OSR yields remains little known [21,27].
Presently, regarding the interaction between pollination services and farming practices, very few studies have been performed under real working farm conditions (but see for exceptions Lindström studies in Sweden [28,29], Morandin & Winston in Canada [30] and Perrot et al. in France [17]). Moreover, studies generally investigated the effect of a single farming practice on the contribution of pollinators, such as fertilizer inputs [25,27], insecticide use [29] or pest exclusion [31]. The effect of interactions between pollination and farming practices on farmers' incomes has never, to our knowledge, been investigated despite pollination being one of the most commonly assessed services. Existing studies of the economic value of pollination have been almost exclusively illustrative, with few cost–benefit analyses of the role of pollinators (review in Hanley et al. [32]).
In our study, we model the effect of pollinators on OSR yield and farmers’ economic return, accounting for farming practices and landscape characteristics (figure 1). We then used our model to test the effect of maximizing pest control or bee abundance on yield and gross margin. We predicted that reducing herbicide use would increase weed diversity and abundance, thus increasing the attractiveness of the OSR field and the bee visitation rate, although this increase may be modulated by competition for pollinators between weeds and OSR plants [16]. We also predicted that reducing insecticide use and so decreasing bee mortality rate [13] would increase OSR fruiting success and yield, and increase the gross margin by reducing costs. To quantify the effect of bee visitation on yields and gross margins for OSR, we collected data over 6 years from 294 OSR fields along landscape gradients with varying proportions of arable and SNH, ensuring a wide variation in pollinator abundance and diversity. We used linear models (LMs) fitted to this large dataset to quantify the individual and combined effects of fertilizer and pesticide applications, soil quality and bee abundance (on a subset of data), on OSR yield and gross margin. Our findings provide evidence in support of promotion of biodiversity as a means of increasing yield and farming profit, an essential step for the adoption of nature-based solutions.
Figure 1.
Schematic relationships between soil type, agricultural practices, bees, landscapes and their effect on yield and economic returns. Red arrows indicate negative interactions, whereas green ones show positive ones.
2. Material and methods
(a). Study area
The study took place from October 2011 to August 2016 in the LTSER ‘Zone Atelier Plaine & Val de Sèvre’, a long-term social–ecological research site covering 450 km2 [33] in central western France (46.23° N, 0.41 W). It is an agricultural landscape dominated by intensive cereal production, with 8–12% OSR of arable land area, and average field size of 4–5 ha. The site is also used by professional or amateur beekeepers who own several hundreds of hives, though none of them contract or are paid by farmers for crop pollination. Information about crop yields and farming practices (pesticide and fertilizer use, tillage and mechanical weed control) and general information about the farm (number of crops, agricultural equipment) were collected by farm surveys after harvest. The sample comprised 142 farmers with 294 OSR fields of which 273 fields were sown with hybrid OSR and 21 with pure line OSR (further details on field selection in the electronic supplementary material, Methods S1). Fields with OSR were sown with 66 cultivars in total, from which DK Ekstrom, DK Expertise and Aviator represented 38.4% of the fields. The majority of farmers (n = 103) managed two fields (2.1 ± 1.4 (s.d.) fields per farmer), and 19 farmers managed four or more fields. The field size ranged from 0.4 to 28.5 ha (mean 6.9 ± 5.0 ha). The soil type varied from very poor dry soil 20 cm deep or less, to 50 cm silt and was classified in four categories: three highly calcareous soils, with depths of 20, 30 and 40 cm, and one with red silt over limestone.
(b). Insect pollinator surveys
Between 2013 and 2016, the abundance and diversity of the major groups of flower-visiting insects, including bees (Hymenoptera, Apoidea, Apiformes) and hoverflies (Diptera, Syrphidae) were surveyed [34]. A full list of bee species present in the study site is available in the appendix of Perrot et al. [17]. A total of 85 fields (10, 19, 24 and 32 in 2013, 2014, 2015 and 2016, respectively) were sampled using both pan traps and sweep nets to get local estimates of the pollinator abundance and richness. The counts of four groups of pollinators (honeybees, bumblebees, other wild bees and hoverflies) in each field obtained by these samples were combined to provide a pollinator abundance index (further details in the electronic supplementary material, Methods S2). Owing to their limited effect in the study area as demonstrated in [17], hoverflies were excluded from the calculation of pollinator abundance. For each of the three remaining groups of pollinators and for each field, we averaged the counts for each trapping method. Then, we standardized the values using z-scores [35] across the whole sample size per trapping method. The z-scores for pan traps corresponded to the total abundance catch per field which were centred (mean of total abundance was removed to each value of total abundance) and reduced (each total abundance value was divided by the standard deviation of total abundance). The final total abundance for each of the three groups of pollinators was the sum of z-scores for sweep net and pan traps counts in 2013 and 2014, and for visual counts and pan traps in 2015 and 2016. Combining both sampling methods was possible because pollinator species abundance was uncorrelated between the two sampling methods (honeybee: Spearman correlation test: rs = 0.0034, p = 0.9754; Lassioglossum sp.: rs = −0.098, p = 0.3722), i.e. most of species were either sampled with sweep net/visual counts or pan traps. This first metric was called total pollinator abundance. A second metric was further derived, as in our study area the main bee pollinators in OSR fields are Lasioglossum spp. (a wild bee) and honeybees [17]. We thus used the sum of the reduced-scores values of Lasioglossum spp. and honeybees, and excluding hoverflies, as a bee index (electronic supplementary material, table S1).
(c). Farm surveys
The general farm statistics obtained from the survey questionnaires during interviews are given in the electronic supplementary material, table S2. From these surveys, we derived the treatment frequency indicator (TFI) as a pesticide use indicator in order to compare pesticide use intensity between fields. Being a standard quantitative index, TFI measures the intensity of applications as the dosage applied per unit of cropped area in relation to the recommended dosage per crop type regarding environmental risks of active substances [36]. It measures farmers' pesticide use relative to the recommended dose necessary to control pests, allowing us to rank fields along a gradient of pesticide use intensity (e.g. [3,37]). We selected this standard quantitative index as we aimed to disentangle the relative contribution of pesticides (agricultural practices) and bee abundance (nature-based solutions) on OSR production and farmers’ economic returns, rather than aiming to evaluate the effects of pesticides on bees.
The index TFI can be broken down per group of pesticides (herbicide, insecticide and fungicide) or aggregated for all pesticides. Per hectare, per field and per year, TFI is expressed as
where Di is the dose in application i, Dhj is the national recommended dose for pesticide j and Si is the surface area treated in application i and St is the total field area. This includes all the pesticide treatments applied in a given crop field. The recommended dose is defined for each combination of pesticide product and crop type, according to the product's marketing authorization to fulfil safety requirements. We computed for each field a global TFI and a TFI for each group of pesticides. For our sample of farms, the global TFI varied from 0.6 to 11.3 (mean: 4.9 ± 1.8, n = 294).
The quantity of inorganic nitrogen used was directly calculated from the fertilizer composition and the quantity applied, while the quantity of nitrogen mineralized in organic fertilizers was calculated using the method described by Jeuffroy & Recous [38].
(d). Statistical analyses
Using the complete dataset (294 fields), we first analysed with a linear mixed model (LMM), the effects of agrochemical applications and soil type (four classes) on yield and gross margin (GM; for further details on GM calculation, see the electronic supplementary material, Methods S3) accounting for direct and interacting effects. We included interactions between fertilizers and soil types to account for farmers adapting their practices to soil quality. We also included farmer identity (ID) as a random factor to account for varying number of fields per farmer, and present results in the proportion of variance explained by the fixed factors (marginal R2, ), and by both the fixed and random factors (conditional R2, ). To estimate the effect of pollinators, we added bee abundance index and its two-way interactions with agrochemical applications. The effect of pollinators was studied for the years 2013–2016 with a sample size of 85 fields, as bees were not sampled before 2013. In this dataset, because 80% of farmers managed only one field, farmer ID was not included as a random factor. The larger field sizes and the presence of other OSR fields nearby may either attract bees or dilute the honeybee population, while Lasioglossum spp. may depend on nearby SNH. In the last step, we therefore tested whether including the field size, percentage of OSR (%OSR) and of SNH (%SNH; i.e. the sum of the area of meadows, woodland and hedges, considering a hedge to have a width of 2 m) in the surrounding landscape improved the model. The landscape metrics were estimated outside the focal field, to account for differences in field size, at eight buffer sizes (250–2000 m). The model with buffer width with the highest explanatory power was kept (see below). All models were checked for normality and homoscedasticity. Collinearity was low in all models, with variance inflation factors less than 3.1.
At each step, we selected the LMs and LMMs with the highest explanatory power, using a multi-model Akaike information criterion (AIC) method and model averaging using the ‘dredge’ function in MuMln R package [39]. We kept all models with AIC less than 2.0 greater than the best model [40]. The resulting average model was subsequently used (electronic supplementary material, table S3). Consequently, although similar sets of variables were included in the models for yield and GMs, after the model selection procedure, a different set of variables was retained. The total amount of variance explained (R2) was calculated using the model with the smallest AIC among all models in which the parameter was retained. Agrochemical applications were also standardized per year using z-scores. This transformation does not constrain the variability found in the raw data and allows focusing on each effect independently of the year effect.
Based on our empirical data, we finally explored whether the losses owing to reducing herbicide and insecticide use could be balanced by an increase in the yield and/or GM owing to an increase in bee abundance. We used an LM including TFI pesticide (sum of herbicide, fungicide and insecticide TFIs), bee abundance and the interaction between bee abundance and TFI pesticide. Annual variation in yield was taken into account by subtracting the average yield of the studied year. We varied TFI and bee abundance within the observed range of values assuming that pest pressure was not increased by the reduction in insecticide. To test the robustness of this assumption, we assessed the relationship between OSR yield, insecticide use and insect pest abundance, using an LM fitted to a third dataset with 74 data points over 3 years (18 in 2014, 24 in 2015 and 32 in 2016) for which insect pest abundance scores were available. The effect of pesticides on insect pest abundance was tested using an LM with insecticides, herbicides and fungicides as explanatory variables. We also analysed the relationship between bee abundance and pest abundance to investigate whether they were correlated; this allowed us to test whether any benefit to crop yield through an increase in pollination when pesticide use was reduced would overcome potential yield losses from higher pest abundance. Pest abundance was obtained from the pan trap surveys which give good predictions of pest abundance in plant inflorescences [41] (see the electronic supplementary material, Methods S4 and table S4).
3. Results
(a). Effect of agrochemical applications on oilseed rape yields and gross margins
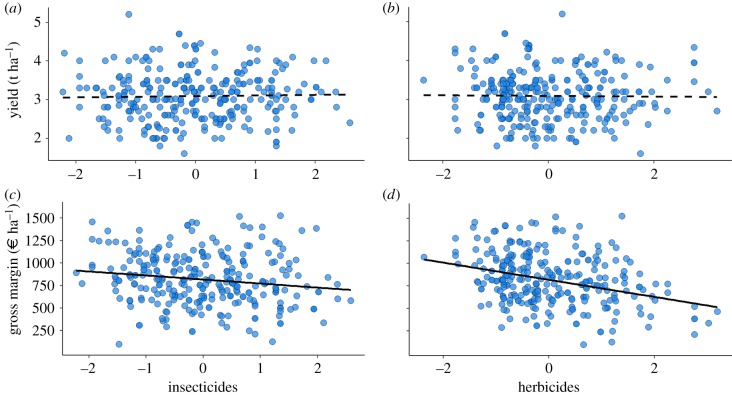
Overall, OSR crop yield averaged 3.1 t ha−1 (±0.6, range 1.6–5.4, n = 294), with greatest yields on red soils (ca 16% on average). The best model (explaining of the variance and ) showed that fungicide significantly increased yield (table 1a). For GM, all inputs were kept in the selected model, as well as all interactions between fertilizer and the soil type (explaining of the variance ; table 1b). The practices most affecting yield and GM were quite different. But, most importantly, except phosphorus, all inputs kept in the final model negatively affected GM (table 1b), including the significant negative effect of nitrogen and herbicides. The soil type and its interaction with nitrogen also had significant effects, with more effect for red soils. Keeping only the variables selected for the yield model (table 1a) resulted in a model with slightly poorer fit and fewer explanatory variables (ΔAIC = 163.17, and ). Our results further suggested that neither insecticides nor herbicides had a direct significant effect on yield (figure 2a,b), but both strongly reduced GM (figure 2c,d).
Table 1.
Models of yield (a) and gross margin (b) as a function of agrochemical applications and soil type and their interaction. (Weight (w), estimated coefficient (β), 95% confidence intervals (CI) and p-value are given for each explanatory variable for the average yield and GM models. β and CI are not given for categorical variables. Significant terms with CIs not including zero are in italics. All explanatory variables were centred/reduced before analysis.)
| w | β | lower CI | upper CI | p-value | |
|---|---|---|---|---|---|
| (a) yield | |||||
| fungicides | 0.34 | 0.081 | 0.0143 | 0.1479 | 0.0173 |
| soil type | 1.00 | <0.0001 | |||
| (b) gross margin | |||||
| nitrogen | 1.00 | −132.02 | −278.77 | −136.84 | 0.0026 |
| potassium | 1.00 | −65.75 | −139.36 | 37.10 | 0.2051 |
| phosphorus | 1.00 | 20.093 | −84.49 | 108.72 | 0.7402 |
| herbicides | 1.00 | −79.61 | −126.27 | −66.69 | <0.0001 |
| insecticides | 1.00 | −30.52 | −63.85 | −6.97 | 0.0620 |
| fungicides | 1.00 | −17.86 | −30.93 | 23.03 | 0.2577 |
| soil type | 1.00 | <0.0001 | |||
| nitrogen × soil type | 1.00 | >0.075 | |||
| potassium × soil type | 1.00 | >0.40 | |||
| phosphorus × soil type | 1.00 | >0.35 | |||
Figure 2.
Relationship between insecticides, herbicides on yield (a,b) and gross margins (c,d), n = 294. Solid lines show significant regressions and dashed lines non-significant regressions. Values for both herbicides and insecticides were centred/reduced.
(b). Effect of bees on yield and gross margin
Lasioglossum spp. ranged from 0 to 16 individuals per field (mean 2.49 ± 3.37), while honeybees ranged from 0 to 29 individuals per field (mean 3.68 ± 5.92). For yield, adding Lasioglossum spp. plus honeybees (i.e. the bee index) improved the model (table 2). The yield of OSR increased with bee abundance (est. = 0.068, p = 0.026), with a significant negative interaction between insecticides and bees (est. = 0.129, p = 0.039). The model explained 20.6% of the variance (p < 0.01; table 2a). Bee abundance and its interaction with insecticide accounted for 70.4% of the total variance explained in the yield. Using total pollinator abundance (i.e. including wild bees plus honeybees) did not change the general pattern (electronic supplementary material, table S5a). When including field size, %OSR and %SNH in the surrounding landscape, the model that best fitted the data (R2 = 22.1%; table 2b) had a 250 m buffer width. Within this buffer, %OSR and %SNH had a positive effect on yield, although non-significant. All other buffers resulted in lower AIC (data not shown).
Table 2.
Models of yield (a), as a function of agrochemical applications, soil type, bee index and interactions, and (b) including landscape variables; and gross margins (c) as a function of agrochemical applications, soil type, bee index and interactions. (Weight (w), estimated coefficient (β), 95% confidence intervals (CI) and p-value are given for each explanatory variable for the averaged yield and GM. β and CI are not given for the categorical variables. Significant terms with CIs not including zero are in italics. All explanatory variables were centred/reduced before analysis. Bees represent the bee index, i.e. sum of honeybee and Lasioglossum spp. abundances.)
| w | β | lower CI | upper CI | p-value | |
|---|---|---|---|---|---|
| (a) yield | |||||
| bees | 1.00 | 0.068 | 0.0081 | 0.1288 | 0.0262 |
| nitrogen | 0.07 | 0.038 | −0.0824 | 0.1576 | 0.5388 |
| phosphorus | 0.43 | 0.102 | −0.0322 | 0.2363 | 0.1363 |
| potassium | 0.24 | −0.070 | −0.1913 | 0.0522 | 0.2625 |
| fungicides | 0.84 | 0.129 | 0.0063 | 0.2525 | 0.0394 |
| insecticides | 0.55 | 0.047 | −0.0682 | 0.1626 | 0.4231 |
| bees × insecticides | 0.55 | −0.054 | −0.1035 | −0.0047 | 0.0318 |
| bees × fungicides | 0.11 | 0.028 | −0.0215 | 0.0776 | 0.2665 |
| (b) yield, including landscape variables | |||||
| bees | 1.00 | 0.077 | −0.020 | 0.215 | 0.0061 |
| phosphorus | 0.37 | 0.103 | −0.026 | 0.231 | 0.1172 |
| potassium | 0.12 | −0.073 | −0.070 | 0.161 | 0.2296 |
| fungicides | 0.88 | 0.131 | 0.022 | 0.131 | 0.0305 |
| insecticides | 0.23 | 0.036 | −0.079 | 0.151 | 0.5406 |
| %OSR | 0.95 | 0.097 | 0.012 | 0.250 | 0.1044 |
| %SNH | 0.17 | 0.045 | −0.193 | 0.462 | 0.4424 |
| bees × insecticides | 0.23 | −0.047 | −0.093 | −0.954 | 0.0495 |
| bees × %SNH | 0.05 | −0.038 | −0.089 | 0.014 | 0.1500 |
| bees × %OSR | 0.63 | −0.052 | −0.112 | 0.009 | 0.0937 |
| (c) gross margin | |||||
| bees | 1.00 | 23.95 | 1.319 | 46.582 | 0.0381 |
| nitrogen | 0.16 | −20.60 | −35.714 | 4.619 | 0.4524 |
| potassium | 1.00 | −69.80 | −119.943 | −19.647 | 0.0064 |
| herbicides | 1.00 | −107.58 | −157.332 | −57.836 | <0.0001 |
| insecticides | 0.25 | −20.43 | −12.615 | 39.125 | 0.4310 |
| bees × herbicides | 0.46 | −16.27 | −37.733 | 5.200 | 0.1375 |
| bees × insecticides | 0.09 | −15.55 | −74.317 | 33.124 | 0.1308 |
| bees × potassium | 0.20 | 13.25 | −71.288 | 30.419 | 0.3153 |
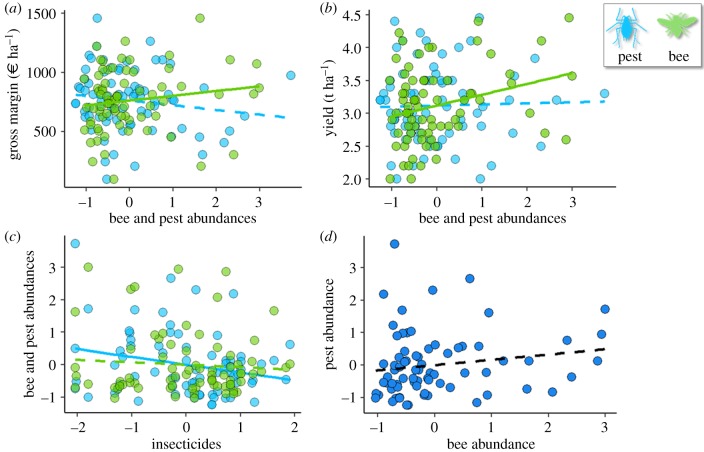
For the GM, bee abundance was the only variable with a positive effect (est. = 23.95, p = 0.0381; figure 3a). Agrochemical applications (potassium and herbicide) had a significantly negative effect, and also interacted with bees (table 2c). Including %OSR and %SNH in the surrounding landscape did not change the effect of pollinators, although the %SNH had a direct significant positive effect for a 250 m buffer (electronic supplementary material, table S6).
Figure 3.
(a) The effect of pest (blue dots) and bee (green dots) abundance on gross margins; (b) the effect on yield; (c) the effect of insecticides on bees and pests; and (d) the relationship between pest and bee abundances. Abundances were centred/reduced. Solid lines show significant regressions and dashed lines non-significant regressions. Bee abundance includes honeybee plus Lasioglossum spp.
For average levels of inputs, yield was 0.31 t ha−1 higher and GM was 111 € ha−1 (i.e. 15%) higher in fields with the high compared to low bee abundance using 0.1–0.9 quantile (figure 3a,c). Keeping extreme values of bee abundance (i.e. the lowest compared to the highest) yielded a much larger increase in OSR yield (figure 3b; 0.77 t ha−1) and GM (figure 3a; 289 € ha−1, i.e. 40%).
(c). Trade-offs between pollinators, pesticides and pests to improve gross margins
Because bee abundance had a consistently positive effect on yield and GM, and there was a negative interaction between bee abundance and pesticide use, we explored whether higher yields and GM could be obtained by reducing the use of agrochemicals to increase bee abundance and their contribution to yield. All variables kept in the yield and GM models, except bee abundance, insecticides, herbicides and fungicides were set to their mean values (electronic supplementary material, table S2). The interactions were visualized using three-dimensional plots with the TFI pesticide on the x-axis, bee abundance on the y-axis and yield or GM on the z-axis. This revealed antagonism between pesticide use and bee abundance, with the latter having a greater positive effect when the use of pesticides was low (figure 4). Assuming that the pest pressure remains constant, this antagonism between pesticide use and bee abundance shows that farmers could maximize yield through two opposite strategies: maximizing either pesticide use or bee abundance (figure 4a). These strategies, however, had a different effect on GM which was always higher when bee abundance was maximized (figure 4b). Additionally, although the use of insecticides reduced the abundance of insect pests (F1,70 = 5.40, p = 0.023; figure 3c), a greater abundance of pests would not significantly affect yield (F1,70 = 0.08, p = 0.78; figure 3b). On the other hand, greater abundance of bees had a strong positive effect on yield (figure 3b) and GM (figure 3a). As bees and pests were positively related (though not significantly: rs = 0.23, p = 0.23; figure 3d), the increase in yield because of the greater bee abundance when insecticide use is reduced was greater than the loss of yield because of the increased abundance of pests.
Figure 4.
Effect of interaction between bee abundance and the combined herbicide and insecticide TFI on yield (a) and gross margins (b). The green surface shows regions where the yield or gross margin is higher and blue where it is lower. Coloured points represent the raw data points and the black ones predicted values from the model. Positive and negative differences between raw data and predicted values are indicated in blue and green. Bee abundance includes honeybee and Lasioglossum spp., and pesticide TFI is the sum of insecticide and herbicide TFIs. Both explanatory variables were centred/reduced before analysis.
4. Discussion
Ecological intensification appears to be a promising alternative to conventional agriculture (e.g. [42]), yet there is no consensus on whether it is possible to reduce agrochemicals by leveraging natural capital and ecological functions without major reductions in yields [43,44]. Bee pollination has been shown to increase OSR yields both in experimental [23,25] and on-farm studies [17,28], but the effect of interactions between pollinators and agricultural practices on yield and income remains largely unknown. Nevertheless, the practical implications for farmers and for policy-makers are critical [45]. Based on a very large dataset spanning 4 and 6 years, this study provides a comprehensive analysis of the synergy and antagonism between agrochemical applications and biodiversity, and their effects on yield and income.
Although agrochemical applications overall accounted for about 24% of the variance of the yield, few practices showed significant positive effects. Fungicides [46] were the only inputs with a significant positive effect on OSR yield. Simultaneously, bee insect pollination was a strong determinant of OSR yield, supporting previous experimental studies [17,18,25]. Indeed, accounting for bees in the model removed the effect of soil type on OSR yield. Although this elimination might be owing to the smaller sample size (85 versus 294 fields), the removal of the soil type was probably because of the higher bee abundance in red soils (about 47% higher, although the difference was not significant, data not shown). Red soils have been shown to have higher weed richness [47], which may explain the higher bee abundance. Taking into account agrochemical applications, pollinator abundance explained 50% of the variance of the yield, increasing yields by 0.77 t ha−1 from the lowest abundance to the highest. This is consistent with previous studies that found increases in yield from 0.4 to 1.0 t ha−1 [17,48]. Fertilizer, especially nitrogen, is a recognized driver of yield, but we failed to detect any direct effect of nitrogen fertilizer on OSR yield. The absence of an increase in yield with nitrogen input has already been reported [49,50], and other studies have even reported negative effects [51,52]. This is possibly explained by the ability of modern cultivars to achieve higher yields with lower nitrogen inputs [49]; indeed, 93% of the farmers in our study used modern hybrid seed varieties. Our results suggest that, for the farms studied, OSR yield is limited by pollinators rather than nutrient availability [27].
Agrochemical applications had little effect on yield which meant that the GM was significantly reduced by nitrogen fertilizer and herbicide applications, as their costs were not recovered in the form of higher yields. Bee abundance was positively correlated with yield, and GM was 15% higher with the highest abundance when compared with the lowest. This increase in GM assumes that no costs were associated, especially with the presence of hives in the landscapes (i.e. honeybees were the dominant pollinator). In some regions, hive rental costs are supported by the farmers. For example, apple pollination fees are about €40 per hive [53]. Assuming similar fees per hive for OSR pollination, GM would still be 4–25% higher with two hives per hectare. Very few experimental OSR studies have assessed the economic benefits of pollinators at the field level [20]. Accounting for average production costs per hectare, Stanley et al. [19] estimated the effect of pollinators on yield in four experimental fields, and then extrapolated to the whole of Ireland to achieve an estimated benefit of €2.6 M yr−1. Bommarco et al. [18], in a pollination exclusion experiment in 10 fields along a landscape gradient, found a 20% increase in the market value of OSR. Our study is, to our knowledge, the first to assess the financial benefits from pollinators in real farming conditions over 85 fields located along a gradient of pollinator abundance.
The benefits of ecosystem services for crop yield may be affected by agricultural practices such as agrochemical inputs [21,27]. In our study, we focused on the interactions between bee pollination and pesticides. These agrochemicals increase crop yield through decreased insect pests, fungi and weed pressure. However, they can also reduce the benefits of pollination by reducing bee abundance or efficiency, and decreasing the reserves of flowers. With constant insect pest pressure, our analysis showed that higher yields may be achieved by two opposite strategies: increasing agrochemicals (reducing pests) or increasing bee abundance (increasing fruiting success, [17]). But GM was only increased by increasing bee abundance, because insecticides reduced bee abundance and neither insecticides nor herbicides increased yields while their costs reduced GMs. This result contradicts the dominant arguments about trade-offs between food production and conservation of biodiversity ([54], but see Pywell et al. [42]) and shows that nature-based solutions can be a win–win strategy.
There are two caveats that may limit this interpretation. Firstly, the use of TFI does not allow disentangling of how pesticide use may affect pollinator and pest abundance (i.e. a reduction in floral resources, an increase in bee mortality or both). A deeper understanding of this effect would be of interest for efficiently promoting nature-based solutions. Second, our model assumed constant insect pest and weed pressure, that is, reducing pesticides would not increase pest abundances, whereas a reduction in yield may be expected when reducing pesticides [55]. We found that insect pest abundance was lower in fields with high compared to low insecticide inputs (though we did not measure pest pressure in relation to pest treatment). However, higher insect pest abundances did not translate into reduced yields as there was no relationship between insect pest abundance and OSR yield. It is possible that pest abundance is very low in our study region. For example, with similar trapping methods and effort, more than 20 pests were caught in Germany or Estonia [56,57] while only six were caught in our site. Indeed, other studies have shown that fertilizer [25,49,58] or pesticide use [59] do have positive effects on OSR yield. It is also possible that OSR plants are able to overcompensate for pest damage [60]. However, several recent studies in France have shown that reducing, to a certain extent, pesticides may not reduce yields [3,37]. Moreover, pollinator abundance strongly differs between study sites for the same crop type [61], and our study region is particularly rich with more than 250 bee species [62]. Thus, the benefits depend on the local pollinator population, part of the natural capital. Further research on the effects of variations in pollinators and agrochemical inputs on yields and profits is therefore needed in other agricultural conditions to evaluate the generality of our results. The benefits of pollinators compared to agrochemicals could be assessed directly in farmers' fields by manipulating the amount of pesticides to quantify these benefits while all other conditions are kept constant (see Catarino et al. [37], for example, of such an experiment).
New agricultural strategies must be developed to achieve sustainable crop production and reduce dependency on chemical inputs. Our study suggests that agroecology, by promoting nature-based solutions for agricultural production, can be an alternative to conventional agriculture for both food production and farm income. Based on a large-scale field survey, our results therefore support a ‘win–win–win’ balance between crop production, farm income and the environment. The next challenge will be to assess non-market benefits from pollinators to define the value of this natural capital within a landscape, essential for policy-making and land-use planning.
Supplementary Material
Acknowledgements
We would like to express our thanks to Marilyn Roncoroni, Jean-Luc Gautier, Alexis Saintilan and Anthony Stoquert for their help with pollinator trapping and identification. We sincerely thank the farmers of the LTSER ‘Zone Atelier Plaine & Val de Sèvre’ for their involvement on our research programmes. We thank the two anonymous reviewers and the editor for meaningful comments on a previous manuscript version. We also thank Dr Edward Joy for his proofreading, which greatly improved the manuscript.
Data accessibility
Datasets for the analysis performed in the paper are publicly accessible in the Zenodo data repository at https://doi.org/10.5281/zenodo.3386708.
Authors' contributions
V.B. and S.G. designed the study. F.V. carried out the farm survey. R.C. and S.G. performed the analysis, with help from T.P. and V.B. R.C. and T.P. wrote the first draft of the manuscript, and V.B. and S.G. contributed substantially to revisions and editing. All authors gave their final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by the ANR AGROBIOSPHERE AGROBIOSE (grant no. 2013-AGRO-001), the SUDOE Intereg POLE-OGI project, the French Ministry of Ecology project (2017–2020 ‘Pollinisateurs’) and the 2013–2014 BiodivERsA/FACCE-JPI joint call for research proposals (project ECODEAL), with the national funders ANR, BMBF, FORMAS, FWF, MINECO, NWO and PT-DLR. R.C. was supported by ANR AGROBIOSPHERE AGROBIOSE and SUDOE projects. T.P. was supported by INRA (Meta programme ECOSERV) and ANR AGROBIOSE PhD grant. S.G. and V.B. are funded by INRA and CNRS, respectively.
References
- 1.Godfray HCJ, Garnett T. 2014. Food security and sustainable intensification. Phil. Trans. R. Soc. B 369, 20120273 ( 10.1098/rstb.2012.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnosky AD, et al. 2012. Approaching a state shift in Earth's biosphere. Nature 486, 52–58. ( 10.1038/nature11018) [DOI] [PubMed] [Google Scholar]
- 3.Gaba S, Gabriel E, Chadœuf J, Bonneu F, Bretagnolle V. 2016. Herbicides do not ensure for higher wheat yield, but eliminate rare plant species. Sci. Rep. 6, Article number 30112 ( 10.1038/srep30112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandhu H, Waterhouse B, Boyer S, Wratten S. 2016. Scarcity of ecosystem services: an experimental manipulation of declining pollination rates and its economic consequences for agriculture. PeerJ 4, e2099 ( 10.7717/peerj.2099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tittonell P. 2014. Ecological intensification of agriculture: sustainable by nature. Curr. Opin. Environ. Sustain. 8, 53–61. ( 10.1016/j.cosust.2014.08.006) [DOI] [Google Scholar]
- 6.European Comission. 2015. Towards an EU research and innovation policy agenda for nature-based solutions & re-naturing cities. Final Report of the Horizon 2020 Expert Group on ‘Nature-Based Solutions and Re-Naturing Cities'. Publications Office of the European Union, Brussels, Belgium.
- 7.Altieri MA. 1983. Agroecology: the scientific basis of alternative agriculture. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 20063721 ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potts SG, et al. 2016. Safeguarding pollinators and their values to human well-being. Nature 540, 220–229. ( 10.1038/nature20588) [DOI] [PubMed] [Google Scholar]
- 10.Breeze TD, et al. 2014. Agricultural policies exacerbate honeybee pollination service supply–demand mismatches across Europe. PLoS ONE 9, e82996 ( 10.1371/journal.pone.0082996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Breeze T, Bailey A, Garthwaite D, Harrington R, Potts SG. 2017. Arthropod pest control for UK oilseed rape: comparing insecticide efficacies, side effects and alternatives. PLoS ONE 12, 1–22. ( 10.1371/journal.pone.0169475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Liu Q, Dong X, Liu Y, Lu J. 2019. Herbicide and nitrogen rate effects on weed suppression, N uptake, use efficiency and yield in winter oilseed rape (Brassica napus L.). Glob. Ecol. Conserv. 17, e00529 ( 10.1016/j.gecco.2019.e00529) [DOI] [Google Scholar]
- 13.Henry M, Béguin M, Requier F, Rollin O, Odoux J, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 14.Stanley DA, Garratt MPD, Wickens JB, Wickens VJ, Potts SG, Raine NE. 2015. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550. ( 10.1038/nature16167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norfolk O, Eichhorn MP, Gilbert F. 2016. Flowering ground vegetation benefits wild pollinators and fruit set of almond within arid smallholder orchards. Insect Conserv. Divers. 9, 236–243. ( 10.1111/icad.12162) [DOI] [Google Scholar]
- 16.Nicholson CC, Ricketts TH, Koh I, Smith HG, Lonsdorf EV, Olsson O. 2019. Flowering resources distract pollinators from crops: model predictions from landscape simulations. J. Appl. Ecol. 56, 618–628. ( 10.1111/1365-2664.13333) [DOI] [Google Scholar]
- 17.Perrot T, Gaba S, Roncoroni M, Gautier J-L, Bretagnolle V. 2018. Bees increase oilseed rape yield under real field conditions. Agric. Ecosyst. Environ. 266, 39–48. ( 10.1016/j.agee.2018.07.020) [DOI] [Google Scholar]
- 18.Bommarco R, Marini L, Vaissière BE. 2012. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 169, 1025–1032. ( 10.1007/s00442-012-2271-6) [DOI] [PubMed] [Google Scholar]
- 19.Stanley DA, Gunning D, Stout JC. 2013. Pollinators and pollination of oilseed rape crops (Brassica napus L.) in Ireland: ecological and economic incentives for pollinator conservation. J. Insect Conserv. 17, 1181–1189. ( 10.1007/s10841-013-9599-z) [DOI] [Google Scholar]
- 20.Breeze TD, Gallai N, Garibaldi LA, Li XS. 2016. Economic measures of pollination services: shortcomings and future directions. Trends Ecol. Evol. 31, 927–939. ( 10.1016/j.tree.2016.09.002) [DOI] [PubMed] [Google Scholar]
- 21.Tamburini G, Bommarco R, Kleijn D, van der Putten WH, Marini L.. 2019. Pollination contribution to crop yield is often context-dependent: a review of experimental evidence. Agric. Ecosyst. Environ. 280, 16–23. ( 10.1016/j.agee.2019.04.022) [DOI] [Google Scholar]
- 22.Bartomeus I, et al. 2014. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ 2, e328 ( 10.7717/peerj.328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudewenz A, Pufal G, Bögeholz A-L, Klein A-M. 2014. Cross-pollination benefits differ among oilseed rape varieties. J. Agric. Sci. 152, 770–778. ( 10.1017/S0021859613000440) [DOI] [Google Scholar]
- 24.Isaacs R, Kirk AK. 2010. Pollination services provided to small and large highbush blueberry fields by wild and managed bees. J. Appl. Ecol. 47, 841–849. ( 10.1111/j.1365-2664.2010.01823.x) [DOI] [Google Scholar]
- 25.Marini L, Tamburini G, Petrucco-Toffolo E, Lindström SAM, Zanetti F, Mosca G, Bommarco R. 2015. Crop management modifies the benefits of insect pollination in oilseed rape. Agric. Ecosyst. Environ. 207, 61–66. ( 10.1016/j.agee.2015.03.027) [DOI] [Google Scholar]
- 26.Holzschuh A, et al. 2016. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 19, 1228–1236. ( 10.1111/ele.12657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garratt MPD, Bishop J, Degani E, Potts SG, Shaw RF, Shi A, Roy S. 2018. Insect pollination as an agronomic input: strategies for oilseed rape production. J. Appl. Ecol. 55, 2834–2842. ( 10.1111/1365-2664.13153) [DOI] [Google Scholar]
- 28.Lindström SAM, Herbertsson L, Rundlöf M, Smith HG, Bommarco R. 2016. Large-scale pollination experiment demonstrates the importance of insect pollination in winter oilseed rape. Oecologia 180, 759–769. ( 10.1007/s00442-015-3517-x) [DOI] [PubMed] [Google Scholar]
- 29.Lindström SAM, Klatt BK, Smith HG, Bommarco R. 2018. Crop management affects pollinator attractiveness and visitation in oilseed rape. Basic Appl. Ecol. 26, 82–88. ( 10.1016/j.baae.2017.09.005) [DOI] [Google Scholar]
- 30.Morandin LA, Winston ML. 2005. Wild bee abundance and seed production in conventional, organic, and genetically modified canola. Ecol. Appl. 15, 871–881. ( 10.1890/03-5271) [DOI] [Google Scholar]
- 31.Sutter L, Albrecht M. 2016. Synergistic interactions of ecosystem services: florivorous pest control boosts crop yield increase through insect pollination. Proc. R. Soc. B 283, 20152529 ( 10.1098/rspb.2015.2529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanley N, Breeze TD, Ellis C, Goulson D. 2015. Measuring the economic value of pollination services: principles, evidence and knowledge gaps. Ecosyst. Serv. 14, 124–132. ( 10.1016/j.ecoser.2014.09.013) [DOI] [Google Scholar]
- 33.Bretagnolle V, et al. 2018. Description of long-term monitoring of farmland biodiversity in a LTSER. Data Brief 19, 1310–1313. ( 10.1016/j.dib.2018.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bretagnolle V, et al. 2018. Towards sustainable and multifunctional agriculture in farmland landscapes: lessons from the integrative approach of a French LTSER platform. Sci. Total Environ. 627, 822–834. ( 10.1016/j.scitotenv.2018.01.142) [DOI] [PubMed] [Google Scholar]
- 35.Witte RS, Witte JS. 2010. Statistics, 9th edn. San Francisco, CA: J. Wiley & Sons. [Google Scholar]
- 36.Coll M, Wajnberg E. 2017. Environmental pest management: challenges for agronomists, ecologists, economists and policymakers. Oxford, UK: John Wiley & Sons. [Google Scholar]
- 37.Catarino R, Gaba S, Bretagnolle V. 2019. Experimental and empirical evidence shows that reducing weed control in winter cereal fields is a viable strategy for farmers. Sci. Rep. 9, 9004 ( 10.1038/s41598-019-45315-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeuffroy M, Recous S. 1999. Azodyn: a simple model simulating the date of nitrogen deficiency for decision support in wheat fertilization. Eur. J. Agron. 10, 129–144. ( 10.1016/S1161-0301(98)00059-8) [DOI] [Google Scholar]
- 39.Barton K. 2018. Package ‘MuMIn’. CRAN. R-project, 1–73. See ftp://155.232.191.229/cran/web/packages/MuMIn/MuMIn.pdf (accessed on 2 February 2018).
- 40.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108. ( 10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 41.Lundin O, Rundlöf M, Smith HG, Bommarco R. 2012. Towards integrated pest management in red clover seed production. J. Econ. Entomol. 105, 1620–1628. ( 10.1603/ec12179) [DOI] [PubMed] [Google Scholar]
- 42.Pywell RF, Heard MS, Woodcock BA, Hinsley S, Ridding L, Nowakowski M, Bullock JM. 2015. Wild-life friendly farming increases crop yield: evidence for ecological intensification. Proc. R. Soc. B 282, 20151740 ( 10.1098/rspb.2015.1740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bommarco R, Kleijn D, Potts SG. 2013. Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. ( 10.1016/j.tree.2012.10.012) [DOI] [PubMed] [Google Scholar]
- 44.Maes J, Jacobs S. 2015. Nature-based solutions for Europe's sustainable development. Conserv. Lett. 10, 121–124. ( 10.1111/conl.12216) [DOI] [Google Scholar]
- 45.Tamburini G, Lami F, Marini L. 2017. Pollination benefits are maximized at intermediate nutrient levels. Proc. R. Soc. B 284, 20170729 ( 10.1098/rspb.2017.0729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ijaz M, Mahmood K, Honermeier B. 2015. Interactive role of fungicides and plant growth regulator (trinexapac) on seed yield and oil quality of winter rapeseed. Agronomy 5, 435–446. ( 10.3390/agronomy5030435) [DOI] [Google Scholar]
- 47.Henckel L, Börger L, Meiss H, Gaba S, Bretagnolle B. 2015. Organic fields sustain weed metacommunity dynamics in farmland landscapes. Proc. R. Soc. B 282, 20150002 ( 10.1098/rspb.2015.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodcock BA, Bullock JM, McCracken M, Chapman RE, Ball SL, Edwards ME, Nowakowski M, Pywell RF. 2016. Spill-over of pest control and pollination services into arable crops. Agric. Ecosyst. Environ. 231, 15–23. ( 10.1016/j.agee.2016.06.023) [DOI] [Google Scholar]
- 49.Stahl A, Pfeifer M, Frisch M, Wittkop B, Snowdon RJ. 2017. Recent genetic gains in nitrogen use efficiency in oilseed rape. Front. Plant Sci. 8, 963 ( 10.3389/fpls.2017.00963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colnenne C, Meynard JM, Roche R, Reau R. 2002. Effects of nitrogen deficiencies on autumnal growth of oilseed rape. Eur. J. Agron. 17, 11–28. ( 10.1016/S1161-0301(01)00140-X) [DOI] [Google Scholar]
- 51.Ozturk O. 2010. Effects of source and rate of nitrogen fertilizer on yield, yield components and quality of winter rapeseed (Brassica napus L.). Chil. J. Agric. Res. 70, 132–141. ( 10.4067/S0718-58392010000100014) [DOI] [Google Scholar]
- 52.Cheema MA, Malik MA, Hussain A, Shah SH, Basra SMA. 2001. Effects of time and rate of nitrogen and phosphorus application on the growth and the seed and oil yields of canola (Brassica napus L.). J. Agron. Crop Sci. 186, 103–110. ( 10.1046/j.1439-037X.2001.00463.x) [DOI] [Google Scholar]
- 53.Rucker RR, Thurman WN, Burgett M. 2012. Honey bee pollination markets and the internalization of reciprocal benefits. Am. J. Agric. Econ. 94, 956–977. ( 10.1093/ajae/aas031) [DOI] [Google Scholar]
- 54.Glamann J, Hanspach J, Abson DJ, Collier N, Fischer J. 2017. The intersection of food security and biodiversity conservation: a review. Reg. Environ. Chang. 17, 1303–1313. ( 10.1007/s10113-015-0873-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popp J, Pető K, Nagy J. 2013. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 33, 243–255. ( 10.1007/s13593-012-0105-x) [DOI] [Google Scholar]
- 56.Hiiesaar K, Metspalu L, Lääniste P, Jõgar K, Kuusik A, Jõudu J. 2003. Insect pests on winter oilseed rape studied by different catching methods. Agron. Res. 1, 17–29. [Google Scholar]
- 57.Veromann E, Tarang T, Kevväi R, Luik A, Williams I. 2006. Insect pests and their natural enemies on spring oilseed rape in Estonia: impact of cropping systems. Agric. Food Sci. 15, 61–72. ( 10.2137/145960606777245579) [DOI] [Google Scholar]
- 58.Rose TJ, Rengel Z, Ma Q, Bowden JW. 2008. Post-flowering supply of P, but not K, is required for maximum canola seed yields. Eur. J. Agron. 28, 371–379. ( 10.1016/j.eja.2007.11.003) [DOI] [Google Scholar]
- 59.Sutter L, Albrecht M, Jeanneret P. 2017. Landscape greening and local creation of wildflower strips and hedgerows promote multiple ecosystem services. J. Appl. Ecol. 55, 612–620. ( 10.1111/1365-2664.12977) [DOI] [Google Scholar]
- 60.Gagic V, Riggi LGA, Ekbom B, Malsher G, Rusch A, Bommarco R. 2016. Interactive effects of pests increase seed yield. Ecol. Evol. 6, 2149–2157. ( 10.1002/ece3.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rader R, et al. 2015. Non-bee insects are important contributors to global crop pollination. Proc. Natl Acad. Sci. USA 113, 146–151. ( 10.1073/pnas.1517092112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rollin O, Bretagnolle V, Fortel L, Guilbaud L, Henry M. 2015. Habitat, spatial and temporal drivers of diversity patterns in a wild bee assemblage. Biodivers. Conserv. 24, 1195–1214. ( 10.1007/s10531-014-0852-x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets for the analysis performed in the paper are publicly accessible in the Zenodo data repository at https://doi.org/10.5281/zenodo.3386708.