Abstract
Capturing wild animals is common for conservation, economic or research purposes. Understanding how capture itself affects lifetime fitness measures is often difficult because wild and captive populations live in very different environments and there is a need for long-term life-history data. Here, we show how wild capture influences reproduction in 2685 female Asian elephants (Elephas maximus) used in the timber industry in Myanmar. Wild-caught females demonstrated a consistent reduction in breeding success relative to captive-born females, with significantly lower lifetime reproduction probabilities, lower breeding probabilities at peak reproductive ages and a later age of first reproduction. Furthermore, these negative effects lasted for over a decade, and there was a significant influence on the next generation: wild-caught females had calves with reduced survival to age 5. Our results suggest that wild capture has long-term consequences for reproduction, which is important not only for elephants, but also for other species in captivity.
Keywords: human contact, confinement, long-term stress, fertility, wild-born, birth origin
1. Introduction
Every year millions of animals, including many species of birds, reptiles and mammals, are captured from the wild for study and conservational purposes, or to be involved in the illegal wildlife trade [1]. Wild capture can have a negative impact on individual life history, at least for some species, by reducing immediate or subsequent survival (e.g. [2,3]), fertility rates (e.g. [2,4,5]) or offspring survival (e.g. [2]). Generally, however, studies have focused on relatively short time scales, covering only a few months or years after capture, and have not assessed the long-term impacts of capture or how its effects change with an increasing time in captivity or for individuals of different ages. This is important because it is currently unclear how long capture effects persist, and which age groups are the most vulnerable. The long-term effects of capture can be explored by comparing wild-caught and captive-born individuals living in similar conditions, but such studies have produced mixed results. For example, a recent meta-analysis from 44 species reported that wild-caught animals have a 74% increased likelihood of reproductive success compared with their captive-born counterparts in captive environments [6]. However, several studies present contradictory results, with lower reproductive success among wild-caught individuals in captivity. These studies are often from animals with slow life histories and longer lifespans: gorillas [7,8], chimpanzees [8], woolly monkeys [9] and polar bears [10]. Some of the differences between wild-caught and captive-born animals can be attributed to the length of time wild-caught individuals have been in captivity [11] (time since capture has generally been omitted, but see [12]), selective survival [12], inbreeding depression and adaptation to captivity [1], early maternal effects [1], or differences between the captive and wild environments. Conversely, benefits in captivity may arise because animals can receive more resources and veterinary care, and are not exposed to the mortality risks in the natural environment [13].
Capture can influence an individual's life history in the long term through behavioural, physiological and immunological mechanisms [12]. Reproduction may be affected by capture in several ways, both directly or indirectly. For example, drugs used in the capture process to sedate larger mammals can have potentially harmful effects on an animal's reproduction [4] if they are used at an incorrect dose. The capture of pregnant females can also lead to injuries and changes in fetal development or even abortion of the fetus [14]. Stress can impair all aspects of fertility, from implantation of the ovum to permanent anoestrus [4]. Furthermore, interactions with humans (handling/breaking/taming), changes in social system, living conditions, and intra- and interspecies competition can also influence any potential effects of capture on reproductive function [15].
Elephants have been a target of large-scale wild capture for centuries [16,17]. Today, elephants are caught and held in zoos or other captive facilities under CITES, for working purposes [16], or via illegal trade (mostly for tourism) in Asia [17]. Elephants are slow reproducers, with long inter-birth intervals, gestation periods and offspring dependency [18]. Zoo elephants are also known to have several difficulties in breeding: many reproductive-aged females do not experience normal oestrus cycles and are infertile [19]. In addition, abnormal deliveries and stillbirths are major issues in captive elephant populations [20]. However, these findings probably reflect the vast differences between zoos and wild environments [19], and it is largely unknown whether capture itself has an impact on reproductive success in elephants. Specifically, to determine capture effects, we need long-term data and a detailed comparison of individuals of different birth origins that live in a similar environment, with shared food and disease sources, similar social interactions and similar breeding opportunities.
Here, we use an exceptionally detailed longitudinal, multi-generational data set of timber elephants from Myanmar to study the effects of capture on lifetime reproductive success in Asian elephants. Myanmar has the largest captive population of Asian elephants in the world, with approximately 2700 government-owned individuals used in the timber logging industry. Historically, approximately half of the timber elephant population has been wild-caught. Wild-caught and captive-born elephants live, forage and work side by side in the same environment, and are governed by the same regulations and practices concerning data recording, workload and rest periods. The data set includes longitudinal information of reproductive events for 2685 females over 64 823 elephant-year observations, with 1362 wild-caught females (captured between 1951 and 2002) and 1323 captive-born females (born between 1942 and 2011). Specifically, we compare lifetime reproduction probabilities, age-specific reproduction rates, age at first reproduction and calf survival between wild-caught and captive-born individuals. Importantly, we investigate whether the effect of wild capture on reproduction depends on an individual's age at capture and the time spent in captivity. Given the recent findings that both birth rates and population growth rates declined between 1960 and 2014 with declines in wild capture, and that without wild capture the population may not be sustainable [21], detailing any long-term consequences of capture on female reproduction is timely and important. More generally, there is currently a great need for animal welfare specialists, veterinarians and ecologists to identify the potential effects of capture from the wild, especially in endangered species, for the success of the individuals and consequently populations.
2. Methods
(a). Study population
The MTE (Myanma Timber Enterprise) elephants are used in the timber logging industry and work in forest camps as riding, transport and draft animals [22]. Traditionally, the elephants have not been provisioned (except for occasional seasonal fruit and rice when travelling), and at night or during other non-working periods all elephants forage in the forest unsupervised. Breeding rates are natural and not managed by humans, with many captive-born calves (at least in the past) thought to be sired by wild bulls, and calves born in captivity are cared for by their biological and allo-mothers. Thus, timber elephants (i) do not receive the foraging benefits that are present in zoos [13,19], (ii) show comparable growth between wild-caught and captive-born animals [23], and (iii) do not display artificial changes to reproduction such as an unusually early reproductive onset, which is observed in zoo elephants compared with elephants born in the wild [19]. Our data set has been collated from elephant log-books (monitoring life-history information for each individual, e.g. [12]) and annual extraction reports archived and maintained by the MTE. While the ages of captive-born elephants are known from precise dates of birth, wild-caught elephants are aged by comparing their height, body condition and physical features with captive-born elephants of known age. The error in these estimates is unknown but is likely to be within a couple of years for young animals that continue to grow (under 20), which form the majority of those captured [12].
(b). The capture and taming of elephants
The capture of wild elephants to supplement the timber elephant population has been controlled by the government. The estimates differ, and one stated that nearly 17 000 elephants were captured from 1911 to 1982 in Myanmar [16]. Capture was formally banned in Myanmar during the 1990s [16], but smaller-scale capture continues, primarily focusing on elephants involved in human–elephant conflict, yet also from illegal captures. Capture was usually practised in the cool season by three alternative methods [16]: by stockades (kheddah) for whole groups, or immobilization by sedation, and lassoing (milarshikar) for specific individuals (more details in [12]). Government figures estimate that the mortality rates for all methods is between 5 and 30%, with most of these deaths happening during the months following capture [16]. All captured elephants undergo an initial taming or ‘breaking’ procedure immediately after capture that lasts 4–12 weeks, depending on the temperament of each elephant. Older elephants generally require a longer period of taming than animals caught from the wild at younger ages or captive-born individuals, which are tamed using similar methods [24]. The taming undoubtedly incorporates stress and compromises welfare, especially during the first few days. Elephants commonly resist training and reject food/water for the first few days, but are referred to as ‘broken’ when they begin to accept food, water and human contact later in taming. Captive-born elephants are also tamed around the age of 4–5 [24], but their training is thought to be easier and less stressful [16,22]. The government enforces strict workloads and rest periods for all individuals (same regulations for working hours per week, working days per year and tonnage to extract per elephant according to their size and condition). Elephants ‘retire’ by 55. Working females are given rest from mid-pregnancy until the calves reach their first birthday. Mothers are then used for light duties (allowed to nurse their calves on demand until the calves are tamed).
(c). Lifetime reproduction probability
We first investigated whether captive-born and wild-caught elephants differed in their lifetime reproduction probability using a linear mixed-effects model. We only included females (both wild-caught and captive-born) that lived beyond the mean age at first reproduction (19.38 ± 5.59) and wild-caught females that were caught before this age, which resulted in 1678 females (wild-caught = 766, captive-born = 912). Lifetime reproduction was scored as a time-invariant binomial trait (0, did not produce any calves during lifetime; 1, produced at least one calf during lifetime, including stillborns), and analysed using a generalized linear mixed-effects model (GLMM) with a binomial error structure and a logit link function in the R package lme4 [25,26]. The main effects of interest were the birth origin, included as a two-level factor (wild-caught versus captive-born) and the age at capture (integer, range = 0–19 years for wild-caught females), which was included as an interaction term only with birth origin. We set the age at capture as 0 for all captive-born elephants, ensuring that this effect did not exert any influence on the parameter estimation. We also controlled for other covariates as fixed effects, namely, whether the individual was censored (1, died before the end of the study; 0, was censored, e.g. [27]), lifespan at death/censoring (mean 39.3 ± 11.8, range 19–76) and birth cohort (factor, each decade between 1930 and 1990), and we included an intercept-only random effect of regional division in Myanmar (see electronic supplementary material for further details). Here, and in all subsequent reproduction models (excluding calf survival analysis), we assessed the significance of the terms using likelihood-ratio tests (LRTs) with the χ2 distribution.
(d). Age-specific reproduction probability
Using the records of 2685 females, we then examined whether captive-born and wild-caught females differed in their age-specific annual reproduction probability between ages 5 and 64 within a logistic regression model selection framework. The maximum age limit was selected because there were few births for females older than 64, and few ages of death or ages at censoring exceeding 64 years (max. captive-born = 68, max. wild-caught = 76). To avoid the possibility of including birth events and pregnancies from wild-caught females before they entered the captive population (which would over-estimate their reproduction in captivity), we only included reproductive data from wild-caught elephants from 2 or more years after their year of capture (minimum time since capture = 2 years; based on an elephant pregnancy length of 22 months). Both captive-born and wild-caught individuals exited the analysis at their last known age alive, or at an age of censoring less than or equal to 64. We constructed a data set where the annual reproduction of each female was scored as a binomial trait (0, did not produce a calf in a given year of life; 1, produced at least one offspring). We assessed age-specific reproduction probability using GLMMs with a binomial error structure and a logit link function in lme4. The data used in this analysis comprised 64 823 age-event data points from the 2685 females (1362 wild-caught females).
First, we built a ‘base’ model, in which the main effect of interest was birth origin to study the difference in age-specific reproduction between wild-caught and captive-born females. We explicitly tested how the time since capture influenced reproduction probability. We included an interaction term between birth origin and log-transformed years since capture (but no main effect; range = 2–54 for wild-caught females) following Lahdenperä et al. [12] to investigate whether the effect of capture changes with time since capture, but only for wild-caught females. Because time since capture was a continuous covariate, setting the time since capture as 0 for all captive-born elephants ensured that this term did not exert any influence on parameter estimation for the interaction term. We also controlled for other covariates, namely, whether or not an individual was censored, lifespan (mean 35.4 ± 16.5, range 5–76), birth cohort (each decade between 1920 and 2010) to control for temporal variation in keeping practices, survival and reproduction [21], and average age (average of all ages the individual was included in the data) to control for selective (dis)appearance of individuals due to different entry and exit ages [28]. The individual identification number and the regional division in Myanmar were included as intercept-only random-effects terms in the final models to control for non-independent data points from the same individuals and spatial variation in reproduction probability (see electronic supplementary material for further details).
We then explored the effect of age on age-specific reproduction using a model selection framework, first including age as a linear term, a quadratic term or as a factor, and then using threshold or piecewise regression [29] models. Threshold models enabled us to capture complex nonlinear relationships with age using the combination of more simple linear changes between threshold ages. We explored the fit of one-, two- and three-threshold models, where annual reproduction probability changed as a linear function of age in two, three or four age groups, respectively (following Hayward et al. [27]). We used all combinations of ages between 6 and 63 for the locations of the thresholds (21 089 combinations in total), which were selected at each 1-year interval. The change in reproduction either side of (and between) threshold ages was captured in all models using an interaction between the linear age term and the age group. Interactions between the birth origin and both age and age group were also included in all models to allow the effect of capture to change with age according to the thresholds. We then performed model selection (see electronic supplementary material, S2 for further details) and compared the predictive performance of all models using the Akaike information criterion (AIC) [30], where the best model had the lowest AIC value (see electronic supplementary material for details). Finally, to test whether differences in lifetime reproduction probability influenced age-specific patterns, we repeated the age-specific reproduction model selection process, but only including females that reproduced at least once in their lifetime. This data set included 38 492 time-event data points from 1175 reproducing females (wild-caught = 595, captive-born = 580).
(e). Age at first reproduction
We assessed whether wild-caught females differed in the onset of reproduction by investigating the age at first reproduction. To ensure that we captured the true age at first reproduction of wild-caught females, we only included wild-caught females caught before the age of 13 (onset of reproduction, mean reproduction probability = 0.002), and also only those captive-born females with an age at first reproduction after 13 (97% of all reproductive captive-born females). We tested the effect of capture on the log-transformed age at first reproduction for 843 females (wild-caught = 283, captive-born = 560), using a linear mixed-effects model in lme4. The main fixed effect of interest was birth origin. We also included censoring, lifespan and birth cohort, and we included regional division as an intercept-only random effect.
(f). Calf survival and mother's birth origin
To investigate the life-history implications of capture from the wild on subsequent generations, we investigated the survival of 2423 calves (born between 1960 and 2016) to 5 years (calves from captive-born mothers = 1290, calves from wild-caught mothers = 1133). Survival to age 5 was selected because age-specific mortality is highest in the first 5 years of life and calves are separated from their mothers for training at this point [31]. We constructed a time-event data set, where the annual survival of each calf (with exact or censored lifespan) from birth to age 5 was scored as a binomial trait (0, died during the focal year, and 1, survived the focal year), resulting in 10 192 data points (calves from captive-born mothers = 5411, calves from wild-caught mothers = 4781). We assessed age-specific mortality using GLMMs with binomial errors and a logit link function with a GLIMMIX procedure in SAS (SAS Institute Inc., release 9.4, 2014).
The main variables of interest were included similarly to previous analysis: the mother's birth origin (binary, captive-born versus wild-caught) and the log-transformed time since the capture of the mother at each calf age (i.e. each focal year; interaction with mother's birth origin only). For calves born to wild-caught mothers, the time since mother's capture varied from 0 to 52 years at the time of the calf's birth. Several terms (e.g. calf age, maternal age and presence, calf sex and inter-birth interval) were controlled for in the analysis that are known to have an influence on calf survival probability in the population [18,31] (for more details, see electronic supplementary material).
3. Results
(a). Differences in reproductive intensity and timing between captive-born and wild-caught females
First, we found that wild-caught females had a significant reduction in their lifetime reproduction probability, even when captured before the mean age of first reproduction (19 years) (figure 1; electronic supplementary material, table S1a). Wild-caught females had a lifetime reproduction odds ratio of 0.72 relative to captive-born females, such that they were 28% less likely to ever reproduce, taking into account known differences in lifespan [12]. Furthermore, females that were older at the time of capture were significantly less likely to reproduce during their lifetime (electronic supplementary material, table S1a).
Figure 1.
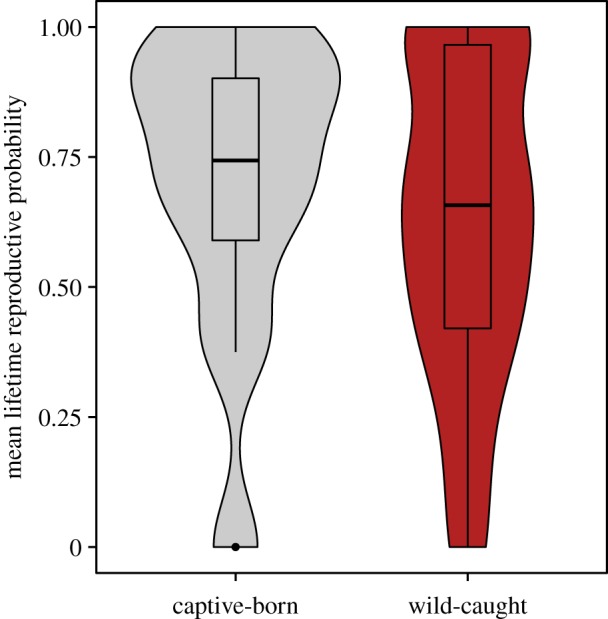
Wild-caught females had a lower probability to reproduce during their lifetime than captive-born females. Box plots indicate the median and interquartile range; violin plots indicate the density of raw (mean) data at different trait values. The mean lifetime reproduction probability averaged for each birth cohort (decade), lifespan (grouped in to 20-year bins) and censoring group (dead or censored) between captive-born (n = 912) and wild-caught (n = 766) females are shown. (Online version in colour.)
Second, we found striking evidence of a reduction in the annual reproductive probability of wild-caught females at almost all ages, compared to females born in captivity that are not subjected to capture stress (table 1). The raw mean annual birth rate for all females across the study period was 0.043 ± 0.20 for all ages and birth origins, such that approximately 4% of females were breeding at a given year. We found that the age-specific reproduction probability for all females was best described by a three-threshold model, such that reproduction probability changed as a linear function of age across four age groups (electronic supplementary material, table S2 and figure S1; figure 2). First, there was an initial period of negligible reproduction probability (mean = 0.002 ± 0.043) with a small increase between the ages of 5 and 12. Second, a rapid exponential increase in female reproduction probability occurred from the age of 13 to the age of 19. The peak in reproduction occurred between the ages of 19 and 20, at which the raw mean annual birth rate was 0.061 ± 0.24 for all females. Third, between the ages of 20 and 44, the age-specific reproduction probability declined slowly, and finally, there was a rapid decline between ages 45 and 64.
Table 1.
Parameter estimates from the best model of age-specific reproduction for all females (n = 2685; 64 823 elephant-year observations), fit using binomial GLMMs. Estimates and s.e. are present on the logit scale. The colon (:) depicts interaction terms. LRT denotes likelihood-ratio test statistics.
| fixed effects | estimate | s.e. | LRT χ2 | p-value |
|---|---|---|---|---|
| intercept | −7.20 | 1.00 | ||
| age | 0.20 | 0.10 | 273.3 | <0.001 |
| age group | 1061 | <0.001 | ||
| ages 13–19 | 1.53 | 1.03 | ||
| ages 20–44 | 6.37 | 0.94 | ||
| ages 45–64 | 9.32 | 1.22 | ||
| birth origin | 179.9 | <0.001 | ||
| wild-caught | −2.41 | 0.53 | ||
| lifespan | −0.04 | 0.01 | 11.79 | <0.001 |
| average age | 0.10 | 0.02 | 17.59 | <0.001 |
| birth cohort | 74.27 | <0.001 | ||
| 1930 | −0.51 | 0.29 | ||
| 1940 | −0.55 | 0.29 | ||
| 1950 | −0.96 | 0.30 | ||
| 1960 | −1.24 | 0.31 | ||
| 1970 | −1.56 | 0.32 | ||
| 1980 | −1.85 | 0.34 | ||
| 1990 | −1.46 | 0.35 | ||
| 2000 | −2.21 | 1.00 | ||
| 2010 | −7.30 | 11.89 | ||
| censored | 1.50 | 0.221 | ||
| dead (1) | 0.08 | 0.07 | ||
| age : age group | 143.1 | <0.001 | ||
| age : ages 13–19 | 0.01 | 0.10 | ||
| age : ages 20–44 | −0.23 | 0.10 | ||
| age : ages 45–64 | −0.31 | 0.10 | ||
| age : birth origin | 26.70 | <0.001 | ||
| age : wild-caught | −0.06 | 0.01 | ||
| age group : birth origin | 24.06 | <0.001 | ||
| ages 13–19 : wild-caught | −1.61 | 0.47 | ||
| ages 20–44 : wild-caught | −1.69 | 0.47 | ||
| ages 45–64 : wild-caught | −1.01 | 0.54 | ||
| birth origin: ln years since capture | 95.35 | <0.001 | ||
| wild-caught : ln years since capture | 1.66 | 0.18 |
| random effects | variance | s.d. | ||
|---|---|---|---|---|
| individual ID | 0.42 | 0.65 | ||
| regional division group | 0.06 | 0.23 |
Figure 2.
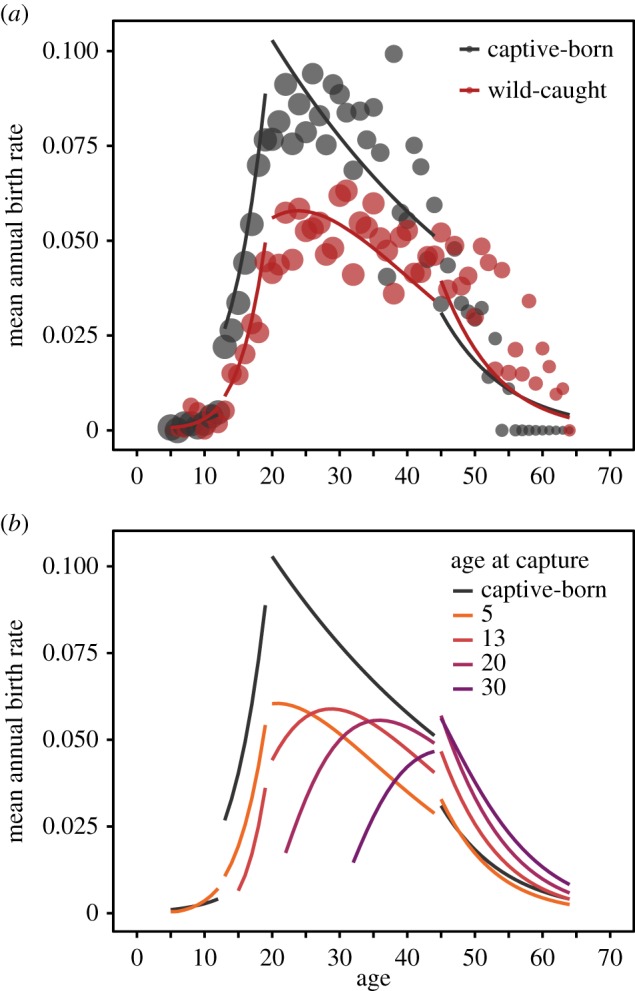
Wild-caught females had reduced age-specific reproduction probabilities compared with (a) captive-born females and (b) females captured at different ages had different age-specific reproduction. Age-specific patterns of reproduction from the best threshold regression model (age groups: 5–12, 13–19, 20–44, 45–64) for all females (1323 captive-born females and 1362 wild-caught females) are shown. (a) Points are the raw mean annual predicted birth rates at each age for all females, with the size of the points denoting the square root of the sample size at each age (range = 11–1323 time-event data points). Lines are the mean predicted values for an extended data set (observed females but extended to span all ages) of the observed females in the 1960 birth cohort, which were most similar to raw mean birth rates. (b) Mean predicted values from an extended data set of observed females in the 1960 birth cohort for captive-born females (grey), and example predicted values from wild-caught females captured at the ages of 5, 13, 20 and 30 (coloured lines).
The significant reduction in age-specific reproduction probability for wild-caught individuals depended on the age of the female (table 1 and figure 2a). There was little discernible difference between captive-born and wild-caught females between the ages of 5 and 12 when reproduction probability was low for all animals (raw mean annual birth rates of 0.002 ± 0.043 and 0.003 ± 0.053, respectively). At the onset of reproduction between 13 and 19, captive-born females had a significantly larger mean reproduction probability of 0.046 ± 0.21 relative to 0.022 ± 0.15 for wild-caught females. At the age of 13, a wild-caught female had an odds ratio of 0.36 with respect to a captive-born female, such that a wild-caught female was approximately 65% less likely to reproduce (electronic supplementary material, figure S2). At peak reproductive age (19 years), captive-born females from the 1960 birth cohort (closest to mean age-specific birth rates, electronic supplementary material, figure S3) had a mean predicted reproduction probability of 0.105 ± 0.042 relative to 0.057 ± 0.031 for wild-caught females, who were 42% less likely to reproduce (odds ratio = 0.58). Then, there were general declines in annual reproduction probabilities between the ages of 20 and 44. These were more pronounced in captive-born females (figure 2a), though their annual reproduction probability (raw mean rate of 0.078 ± 0.27) remained above that of wild-caught females (0.050 ± 0.22) (table 1). The mean annual reproduction probability in the oldest age group (between ages 45 and 64) was 0.032 ± 0.18. Interestingly, wild-caught females had a higher annual reproductive probability with respect to captive-born females at these advanced ages (raw mean annual reproduction rates of 0.034 ± 0.18 and 0.027 ± 0.16, respectively; figure 2a). Given that wild-caught females have previously been found to have increased mortality [12], selective disappearance (i.e. most robust wild-captured animals or those more adapted to semi-captivity contribute to older ages) probably plays a role in this finding. The average odds ratio for wild-caught females aged 45–64 ranged between 1.63 and 1.95, meaning that, on average, wild-caught females were between 63% and 95% more likely to reproduce than captive-born females between 45 and 64.
The difference in age-specific reproduction between captive-born and wild-caught females also depended on the length of time spent in captivity, with a significant positive effect of the interaction between birth origin and log-transformed years since capture (table 1 and figure 3). Specifically, there was a large reduction in annual reproduction probability compared to captive-born females immediately (2 years) after capture, which then increased slowly. It took approximately 12 years before wild-caught females reached the mean annual reproductive probability of captive-born females (figure 3). Furthermore, there were differences in age-specific reproduction probabilities between females captured at different ages (figure 2b), and the decrease in reproduction immediately after capture was the most pronounced in wild-caught females that were captured at older ages (electronic supplementary material, figure S2). Females caught at the ages of 13, 20 and 30 had reproductive odds ratios of 0.18, 0.07 and 0.08 2 years after capture, relative to captive-born females (electronic supplementary material, figure S2).
Figure 3.
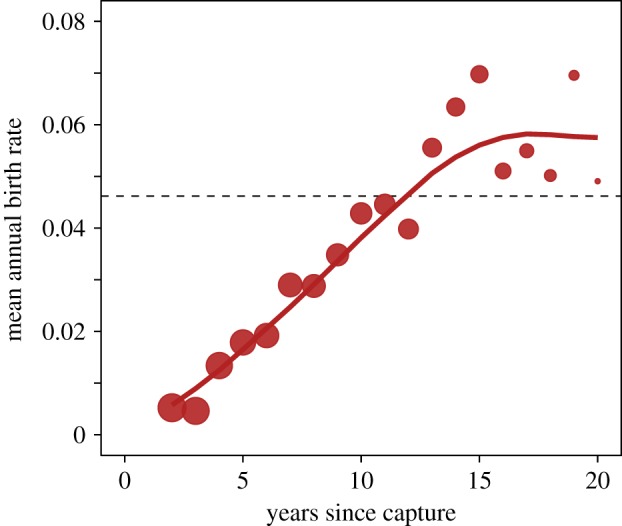
Age-specific reproduction for wild-caught females was lowest immediately after capture, and it took approximately 12 years before wild-caught females reached the mean annual reproductive rate of captive-born females (1362 wild-caught females and 1323 captive-born females—dashed line). The mean annual birth rates for wild-caught females with the number of years since capture across all ages are shown. Points are raw mean annual birth rates, with the size indicating the sample size (range = 856–1338). Lines are the mean fitted values from the best-fit model including all females. The dashed line indicates the raw mean annual birth rate for captive-born females across all ages. (Online version in colour.)
Third, the lower age-specific reproduction of wild-caught females was not only due to a lower lifetime reproduction probability, because wild-caught elephants that reproduced at least once also had lower age-specific reproduction probabilities. When restricting the data set to include only reproductive females, wild-caught females had reduced age-specific reproduction, particularly at peak reproductive ages (electronic supplementary material, figure S4 and table S3), consistent with the previous analysis. There were slight differences in the thresholds for the best model for reproducers, which included four threshold age groups at ages 5–12, 13–20, 21–51 and 52–64 (electronic supplementary material, figure S4 and table S3).
Fourth, we found that wild-caught females started to reproduce later than captive-born females. The age at first reproduction for wild-caught females captured before the age of 13 was significantly later than for captive-born females (figure 4; electronic supplementary material, table S1b): the mean age at first reproduction for captive-born females was 2 years earlier (21.2 ± 6.1) than for wild-caught females (23.3 ± 7.25 years).
Figure 4.
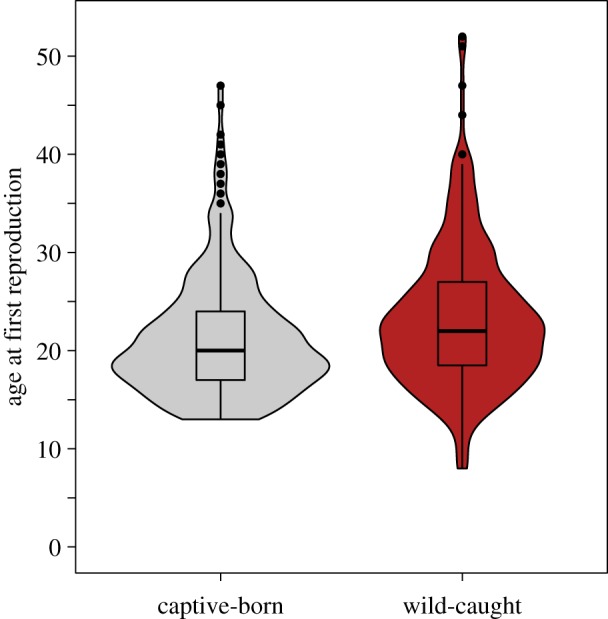
Wild-caught females had a later age at first reproduction than captive-born females. Box plots indicate the median and interquartile range; violin plots indicate the density of raw age at first reproduction data for captive-born (n = 560) and wild-caught females (n = 283). (Online version in colour.)
(b). Cross-generational effects of wild capture
Finally, we found evidence that wild capture was also associated with the life history of the following generations living in captivity. Elephants suffer from high infant mortality [31], with 23.5% of calves born in captivity in our sample dying before the age of 5. We found that the calves of wild-caught mothers had an increased mortality before the age 5 when compared with the calves of captive-born mothers. However, this effect depended on the number of years since the capture of the mother (electronic supplementary material, table S4). Calf mortality was highest in the year immediately after their mother's capture, with an annual mortality rate of 0.134 ± 0.076 for the calves of wild-caught females compared to 0.058 ± 0.029 for the calves of captive-born mothers, decreasing thereafter (figure 5). The odds ratio of calf death in the first year was 2.50 compared with calves from captive-born mothers, declining exponentially with increased time since capture (electronic supplementary material, figure S5). An equal odds ratio of calf mortality between wild-caught and captive-born females was only achieved 16 years after the mother's capture from wild.
Figure 5.
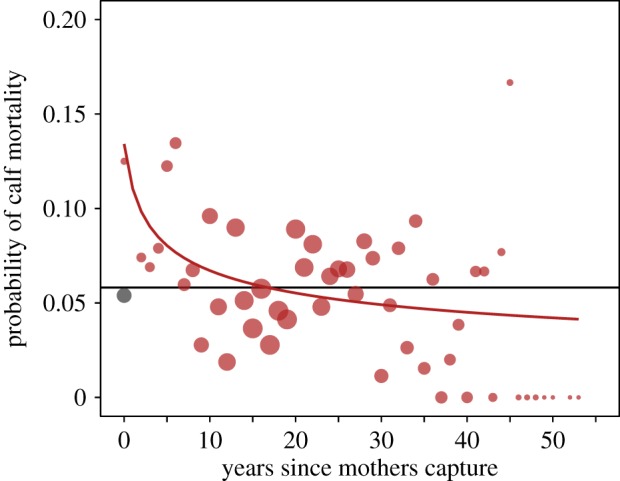
Calves of wild-caught mothers had an increased mortality to age 5 compared with the calves of captive-born mothers, the effect being strongest during the first years after the mother's capture from the wild but lasting on average 16 years (n = 10 192 observations, 2423 calves, 1030 mothers). The red points are raw mean mortalities for calves from wild-caught mothers, with the size indicating the sample size (range = 1–226). The red line shows predicted values for calves born to wild-caught mothers as a function of the years since the mother's capture. The grey dot indicates the raw mean mortality of calves from captive-born mothers (n = 5411). The black line shows the predicted value for calves born to captive-born mothers. The predicted values are mean values from birth to age 5. One raw mortality value for calves of wild-caught mothers is not displayed (mortality of 0.28 1 year after capture).
4. Discussion
Large numbers of animals are routinely captured from the wild for many purposes, including research and conservation, but surprisingly little is known about the consequences of capture for the subsequent long-term performance of those individuals. Using unique records of wild-caught and captive-born Asian elephants in Myanmar, we demonstrate for the first time that capture from the wild has lasting adverse effects on lifetime reproduction in a long-lived mammal. An alternative explanation would be that captive-born elephants benefit from human care, boosting their body condition and reproduction when compared with conditions in the wild, and wild-caught elephants only gain better body condition and reproductive ability with time in semi-captivity. However, wild-caught and captive-born females in this population experience the same conditions in captivity (e.g. are not provisioned) and have similar weight and growth patterns [23,32], captive-born elephants have mortality [31,33] and fertility rates [34,35] that are similar to wild elephants, and wild-caught elephants have large reductions in survival immediately after capture and taming, which last for over a decade in captivity [12]. Therefore, the differences in reproduction we observe are most likely to be due to a negative effect of the capture process itself.
These results are timely given that 60–80% of the current captive elephants in zoos are wild-caught [19,36], and the capture of wild elephants continues to supplement waning tourism and working populations worldwide [17,19]. That wild-caught animals take more than 10 years to recover from their experiences, show higher lifetime infertility and transfer the negative effect of capture on to the survival of the next generation in a long-lived social species such as the Asian elephant has implications for captive animal welfare and research. Although some species in captivity are healthier, live longer and have a higher reproductive success than their wild counterparts, other species perform less well in captivity (e.g. [13]). Furthermore, depending on the species, wild-born animals constitute different proportions of the number of animals in captive populations [1,11]. This raises the questions of whether the observed negative effects of captivity are actually due to negative long-term capture effects, and whether captive populations can be used as reference groups for species-typical parameter values [13]. In addition to these long-term effects on captive populations, an increasing human population has seen more animals taken from the wild, imposing a strong negative impact on wild population growth rates [37].
We found a consistent pattern of reduced reproductive success in wild-caught females compared with captive-born females. The adverse effects were stronger in elephants that were caught at older ages and immediately after capture from the wild, and lasted for more than a decade. These findings are probably due to both the immediate and long-term effects of the capture process. First, captures during critical phases of gestation may lead to the abortion of fetuses, premature births or reduced calf survival [4,14]. Calf mortality was highest during the first year after capture, suggesting that mothers who were pregnant during capture may have suffered from physical trauma or complications risking the calf's subsequent survival. Second, reduced calf survival and impaired reproduction may be the result of high acute and chronic stress due to capture [38], also depending on an individual's age [38]. For example, through the action of glucocorticoids, chronic psychological stress can impact reproductive hormone levels, function of the placenta and fetus development [39]. In support of this, we found that wild-caught females were less likely to reproduce during their lifetime, had an increased age at first reproduction and had a reduced age-specific fertility at peak ages. Other studies have found negative impacts of capture on reproduction, for example, in chimpanzees [8], gorillas [7,8], black rhinos [4], polar bears [5], pampas deer [40] and mountain goats [2], though many of these studies were carried out over short time scales or did not account for the length of time in captivity. Third, capture can lead to decreases in activity and feeding [41], lower body condition [42] and social disruption [43], further reducing the long-term reproductive performance of wild-caught animals.
Interestingly, we found that wild-caught females were more likely to reproduce at old ages than captive-born females. This finding can be explained partly by selective (dis)appearance [44], which is known to bias fitness estimates in demographic studies. Previously, we have found that wild-caught Asian elephants had high mortality risk immediately after capture from the wild, which, similar to the results here, lasted for a decade after capture [12]. Therefore, given the increased mortality rates following capture and an initial bias for capturing animals in good condition [12], it is feasible that only the most robust wild-caught animals or those more adapted to semi-captivity contribute to older age reproductive rates by being able to continue reproducing even at such advanced ages. Given that we controlled for individual variation, lifespan, censoring and average age in age-specific analysis, our results present evidence that there are selective disappearance effects.
The delayed (and lower) reproduction earlier in life and conversely increased reproduction later in life suggest that capture from the wild may have caused a shift in reproductive strategy in this population. More broadly, disturbance by humans cannot only have immediate consequences for mortality [12] and reproduction, but long-term evolutionary consequences. Poor early-life conditions have previously been associated with a delay in reproduction [45] and a change in life-history trajectories (e.g. [46]) in a range of species, and our results suggest that similar cross-generational effects may arise from (presumably stressful) wild capture. Human activity such as hunting and poaching has also been found to have long-lasting demographic consequences for wildlife populations [47,48]. A recent study on brown bears (Ursa arctos) found that regulated hunting has resulted in a shift of reproductive strategy, mortality and life expectancy [49]. Along with other recent findings, our results highlight the importance of understanding the long-term evolutionary consequences of human disturbance and wild capture for wildlife populations.
The long-term effects of capture are currently not considered in research and conservation programmes, but our results suggest that the life history of captured individuals may differ substantially compared with those born in captivity. Current evidence suggests that some species, often those with longer lifespan or slow life history, may be more prone to the negative effects of capture [7,9,10,50,51]. We therefore welcome more long-term studies in other animals to identify the species and individuals most at risk from capture. In elephants, although capture might be inevitable sometimes (e.g. for conservation, veterinary and anti-poaching purposes), consistent large-scale wild capture should be avoided to supplement captive populations because it fails to provide a sustainable long-term strategy [21,37] and may have far-reaching evolutionary consequences for captive populations.
Supplementary Material
Acknowledgements
We thank the Ministry of Natural Resources and Environmental Conservation, the Government of the Union of Myanmar for giving permission to work with the Myanma Timber Enterprise (MTE), the MTE, Thu Zar Thwin, Khin Than Win, Mumu Thein and Khyne U. Mar for helping with data collection, as well as the Myanmar Timber Elephant Project members for help and support. We also thank Hannah Mumby and Adam Hayward for help with the analyses, and Jennie Crawley and Simon Chapman, who provided useful comments on the manuscript.
Data accessibility
The data and code supporting our results are archived in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q0m0d84 [52].
Authors' contributions
M.L., J.J. and V.L. conceived and designed the paper. W.H. contributed to data collection. J.J. and M.L. performed the analyses. M.L., J.J. and V.L. wrote the paper. All authors read and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the European Research Council (V.L.), the Academy of Finland (V.L., M.L.), the Kone Foundation (M.L.) and the Natural Environment Research Council (J.J.).
References
- 1.Mason G, Burn CC, Dallaire JA, Kroshko J, McDonald Kinkaid H, Jeschke JM. 2013. Plastic animals in cages: behavioural flexibility and responses to captivity. Anim. Behav. 85, 1113–1126. ( 10.1016/j.anbehav.2013.02.002) [DOI] [Google Scholar]
- 2.Côté SD, Festa-Bianchet M, Fournier F. 1998. Life-history effects of chemical immobilization and radiocollars on mountain goats. J. Wildl. Manage. 62, 745–752. ( 10.2307/3802351) [DOI] [Google Scholar]
- 3.Arnemo JM, Ahlqvist P, Andersen R, Berntsen F, Ericsson G, Odden J, Brunberg S, Segerström P, Swenson JE. 2006. Risk of capture-related mortality in large free-ranging mammals: experiences from Scandinavia. Wildl. Biol. 12, 109–113. ( 10.2981/0909-6396(2006)12[109:ROCMIL]2.0.CO;2) [DOI] [Google Scholar]
- 4.Alibhai SK, Jewell ZC, Towindo SS. 2001. Effects of immobilization on fertility in female black rhino (Diceros bicornis). J. Zool. Lond. 253, 333–345. ( 10.1017/S0952836901000309) [DOI] [Google Scholar]
- 5.Ramsay MA, Stirling I. 1986. Long-term effects of drugging and handling free-ranging polar bears. J. Wildl. Manage. 50, 619–626. ( 10.2307/3800972) [DOI] [Google Scholar]
- 6.Farquharson KA, Hogg CJ, Grueber CE. 2018. A meta-analysis of birth-origin effects on reproduction in diverse captive environments. Nat. Commun. 9, 1055 ( 10.1038/s41467-018-03500-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan S, Thompson SD, Roth AM, Gold KC. 2002. Effects of hand-rearing on the reproductive success of western lowland gorillas in North America. Zoo Biol. 21, 389–401. ( 10.1002/zoo.10045) [DOI] [Google Scholar]
- 8.Bolton RL, Lea RG, Masters NJ, Milham P. 2012. Environment and reproductive dysfunction in captive female great apes (Hominidae). Vet. Rec. 170, 676 ( 10.1136/vr.100701) [DOI] [PubMed] [Google Scholar]
- 9.Mooney JC, Lee PC. 1999. Reproductive parameters in captive woolly monkeys (Lagothrix lagotricha). Zoo Biol. 18, 421–427. () [DOI] [Google Scholar]
- 10.Curry E, Safayi S, Meyerson R, Roth TL. 2015. Reproductive trends of captive polar bears in North American zoos: a historical analysis. J. Zoo Aquarium Res. 3, 99–106. [Google Scholar]
- 11.Kohler IV, Preston SH, Lackey LB. 2006. Comparative mortality levels among selected species of captive animals. Demogr. Res. 15, 413–434. ( 10.4054/DemRes.2006.15.14) [DOI] [Google Scholar]
- 12.Lahdenperä M, Mar KU, Courtiol A, Lummaa V. 2018. Differences in age-specific mortality between wild-caught and captive-born Asian elephants. Nat. Commun. 9, 3023 ( 10.1038/s41467-018-05515-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason GJ. 2010. Species differences in responses to captivity: stress, welfare and the comparative method. Trends Ecol. Evol. 25, 713–721. ( 10.1016/j.tree.2010.08.011) [DOI] [PubMed] [Google Scholar]
- 14.Adams KR, Fetterplace LC, Davis AR, Taylor MD, Knott NA. 2017. Sharks, rays and abortion: the prevalence of capture-induced parturition in elasmobranchs. Biol. Conserv. 217, 11–27. ( 10.1016/j.biocon.2017.10.010) [DOI] [Google Scholar]
- 15.Morgan KN, Tromborg CT. 2007. Sources of stress in captivity. Appl. Anim. Behav. Sci. 102, 262–302. ( 10.1016/j.applanim.2006.05.032) [DOI] [Google Scholar]
- 16.Lair RC. 1997. Gone astray: the care and management of the Asian elephant in domesticity. Bangkok, Thailand: FAO. [Google Scholar]
- 17.Nijman V. 2014. An assessment of the live elephant trade in Thailand. Cambridge, UK: TRAFFIC International. [Google Scholar]
- 18.Lahdenperä M, Mar KU, Lummaa V. 2016. Short-term and delayed effects of mother death on calf mortality in Asian elephants. Behav. Ecol. 27, 166–174. ( 10.1093/beheco/arv136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clubb R, Mason G. 2002. A review of the welfare of zoo elephants in Europe Horsham, UK: RSPCA. [Google Scholar]
- 20.Hermes R, Saragusty J, Schaftenaar W, Göritz F, Schmitt DL, Hildebrandt TB. 2008. Obstetrics in elephants. Theriogenology 70, 131–144. ( 10.1016/j.theriogenology.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 21.Jackson J, Childs DZ, Mar KU, Htut W, Lummaa V. 2019. Long-term trends in wild-capture and population dynamics point to an uncertain future for captive elephants. Proc. R. Soc. B 286, 20182810 ( 10.1098/rspb.2018.2810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaw K. 1997. Utilization of elephants in timber harvesting in Myanmar. Gajah 17, 9–22. [Google Scholar]
- 23.Mumby HS, Chapman SN, Crawley JAH, Mar KU, Htut W, Thura Soe A, Aung HH, Lummaa V. 2015. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evol. Biol. 15, 214 ( 10.1186/s12862-015-0487-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min-Oo Z. 2010. The training methods used in Myanma timber enterprise MTE. Gajah 33, 58–61. [Google Scholar]
- 25.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 26.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org/. [Google Scholar]
- 27.Hayward AD, Mar KU, Lahdenperä M, Lummaa V. 2014. Early reproductive investment, senescence and lifetime reproductive success in female Asian elephants. J. Evol. Biol. 27, 772–783. ( 10.1111/jeb.12350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhulst S, Geerdink M, Salomons HM, Boonekamp JJ. 2014. Social life histories: jackdaw dominance increases with age, terminally declines and shortens lifespan. Proc. R. Soc. B 281, 20141045 ( 10.1098/rspb.2014.1045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toms JD, Lesperance ML. 2003. Piecewiese regression: a tool for identifying ecological thresholds. Ecology 84, 2034–2041. ( 10.1890/02-0472) [DOI] [Google Scholar]
- 30.Burnham KP, Anderson DR. 2003. Model selection and multimodel inference: a practical information-theoretic approach. Berlin, Germany: Springer. [Google Scholar]
- 31.Mar KU, Lahdenperä M, Lummaa V. 2012. Causes and correlates of calf mortality in captive Asian elephants (Elephas maximus). PLoS ONE 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawley JAH, Mumby HS, Chapman SN, Lahdenperä M, Mar KU, Htut W, Thura Soe A, Aung HH, Lummaa V. 2017. Is bigger better? The relationship between size and reproduction in female Asian elephants. J. Evol. Biol. 30, 1836–1845. ( 10.1111/jeb.13143) [DOI] [PubMed] [Google Scholar]
- 33.Clubb R, Rowcliffe M, Lee P, Mar KU, Moss C, Mason GJ. 2008. Compromised survivorship in zoo elephants. Science 322, 1649 ( 10.1126/science.1164298) [DOI] [PubMed] [Google Scholar]
- 34.De Silva S, Elizabeth Webber C, Weerathunga US, Pushpakumara TV, Weerakoon DK, Wittemyer G. 2013. Demographic variables for wild Asian elephants using longitudinal observations. PLoS ONE 8, e82788 ( 10.1371/journal.pone.0082788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clubb R, Rowcliffe M, Lee P, Mar KU, Moss C, Mason GJ. 2009. Fecundity and population viability in female zoo elephants: problems and possible solutions. Anim. Welf. 18, 237–247. [Google Scholar]
- 36.Prado-Oviedo NA, Bonaparte-Saller MK, Malloy EJ, Meehan CL, Mench JA, Carlstead K, Brown JL. 2016. Evaluation of demographics and social life events of Asian (Elephas maximus) and African elephants (Loxodonta africana) in North American zoos. PLoS ONE 11, e0154750 ( 10.1371/journal.pone.0154750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leimgruber P, Senior B, Uga AM, Songer MA, Mueller T, Wemmer C, Ballou JD. 2008. Modeling population viability of captive elephants in Myanmar (Burma): implications for wild populations. Anim. Conserv. 11, 198–205. ( 10.1111/j.1469-1795.2008.00172.x) [DOI] [Google Scholar]
- 38.Baker MR, Gobush KS, Vynne CH. 2013. Review of factors influencing stress hormones in fish and wildlife. J. Nat. Conserv. 21, 309–318. ( 10.1016/j.jnc.2013.03.003) [DOI] [Google Scholar]
- 39.Joseph DN, Whirledge S. 2017. Stress and the HPA axis: balancing homeostasis and fertility. Int. J. Mol. Sci. 18, E2224 ( 10.3390/ijms18102224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungerfeld R, Gonzalez-Sierra UT, Piaggio J. 2008. Reproduction in a semi-captive herd of pampas deer Ozotoceros bezoarticus. Wildl. Biol. 14, 350–357. ( 10.2981/0909-6396(2008)14[350:RIASHO]2.0.CO;2) [DOI] [Google Scholar]
- 41.Morellet N, Verheyden H, Angibault J-M, Cargnelutti B, Lourtet B, Hewison MAJ. 2009. The effect of capture on ranging behaviour and activity of the European roe deer Capreolus capreolus. Wildl. Biol. 15, 278–287. ( 10.2981/08-084) [DOI] [Google Scholar]
- 42.Cattet M, Boulanger J, Stenhouse G, Powell RA, Reynolds-Hogland MJ. 2008. An evaluation of long-term capture effects in ursids: implications for wildlife welfare and research. J. Mammal. 89, 973–990. ( 10.1644/08-MAMM-A-095.1) [DOI] [Google Scholar]
- 43.Shannon G, Slotow R, Durant SM, Sayialel KN, Poole J, Moss C, McComb K. 2013. Effects of social disruption in elephants persist decades after culling. Front. Zool. 10, 1 ( 10.1186/1742-9994-10-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Pol M, Verhulst S.. 2006. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773. ( 10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 45.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 46.Mumby HS, Mar KU, Hayward AD, Htut W, Htut-Aung Y, Lummaa V. 2015. Elephants born in the high stress season have faster reproductive ageing. Sci. Rep. 5, 13946 ( 10.1038/srep13946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milner JM, Nilsen EB, Andreassen HP. 2007. Demographic side effects of selective hunting in ungulates and carnivores: review. Conserv. Biol. 21, 36–47. ( 10.1111/j.1523-1739.2006.00591.x) [DOI] [PubMed] [Google Scholar]
- 48.Sæther BE, Engen S, Lande R, Møller AP, Bensch S, Hasselquist D, Beier J, Leisler B. 2004. Time to extinction in relation to mating system and type of density regulation in populations with two sexes. J. Anim. Ecol. 73, 925–934. ( 10.1111/j.0021-8790.2004.00869.x) [DOI] [Google Scholar]
- 49.Bischof R, Bonenfant C, Rivrud IM, Zedrosser A, Friebe A, Coulson T, Mysterud A, Swenson JE. 2018. Regulated hunting re-shapes the life history of brown bears. Nat. Ecol. Evol. 2, 116–123. ( 10.1038/s41559-017-0400-7) [DOI] [PubMed] [Google Scholar]
- 50.Jett J, Ventre J. 2015. Captive killer whale (Orcinus orca) survival. Mar. Mammal Sci. 31, 1362–1377. ( 10.1111/mms.12225) [DOI] [Google Scholar]
- 51.Tidière M, Gaillard JM, Berger V, Müller DWH, Lackey LB, Gimenez O, Clauss M, Lemaître JF. 2016. Comparative analyses of longevity and senescence reveal variable survival benefits of living in zoos across mammals. Sci. Rep. 6, 36361 ( 10.1038/srep36361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lahdenperä M, Jackson J, Htut W, Lummaa V. 2019. Data from: Capture from the wild has long-term costs on reproductive success in Asian elephants Dryad Digital Repository. ( 10.5061/dryad.q0m0d84) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lahdenperä M, Jackson J, Htut W, Lummaa V. 2019. Data from: Capture from the wild has long-term costs on reproductive success in Asian elephants Dryad Digital Repository. ( 10.5061/dryad.q0m0d84) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and code supporting our results are archived in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q0m0d84 [52].


