Abstract
Extranodal natural killer (NK)/T-cell lymphoma, nasal type, is a rare peripheral T-cell lymphoma associated with Epstein-Barr virus. It most often presents as limited-stage disease in patients of East Asian descent with a palatal deformity caused by erosion of the tumor through the hard palate. Limited-stage disease is often curable with the use of l-asparaginase–based chemotherapy and high-dose radiation therapy. Obtaining an accurate diagnosis is essential, because treatment with standard lymphoma regimens and omission of radiation severely compromise the likelihood of long-term survival. Conversely, patients with advanced disease have a poor prognosis and are recommended for asparaginase-based chemotherapy followed by consolidation with autologous transplantation as a potentially curative approach. Progress often has been hampered by the rarity of this disease. However, discovery of common genetic alterations in pathways that promote growth and inhibit apoptosis, and actionable markers such as CD30 (among others), have begun to broaden the availability of novel drugs (eg, targeted therapies). There is also cautious optimism about immunotherapies, such as checkpoint blockade and novel cellular therapies that target Epstein-Barr virus. Advances in treatment and understanding of the genetic landscape of this disease offer hope for improved treatment outcomes.
INTRODUCTION
Extranodal natural killer (NK) cell/T-cell lymphoma, nasal type, (ENKL) is an aggressive peripheral T-cell lymphoma with an historic median survival of less than 2 years.1,2 ENKL accounts for approximately 10% of all peripheral T-cell lymphoma occurrences in North America and has an increased incidence among patients of South American and Eastern Asian descent.3 An analysis of the SEER database from 2001 to 2014 demonstrated 797 occurrences in the United States with a male predominance and a median age at diagnosis of 53 years.2 The majority of patients in this data set had early-stage disease (58%, stage I; 19%, stage II; and 17%, stage IV). The median survival was 20 months across groups, but it varied greatly by stage. The median survival was more than 7 years for early-stage disease compared with only 7 months for stage IV disease. However, survival has improved in the modern era.1-3
CLINICAL PRESENTATION
ENKL is almost exclusively extranodal in presentation. The classic presentation involves a palatal perforation. Primary lymph node involvement in the absence of extranodal involvement is exceedingly rare but has been reported.3 At presentation, the disease is often localized to the upper aerodigestive tract. Patients with advanced disease may present with skin, GI tract, bone marrow, and peripheral blood involvement. Survival for disease that occurs outside the nasal cavity is poor.3,4 A prognostic model for the modern era has identified four risk factors notably associated with overall survival (OS) and progression-free survival (PFS): (1) age older than 60 years, (2) stage III or IV disease, (3) distant lymph node involvement, and (4) non–nasal type disease. This prognostic index, called the prognostic index of NK lymphoma, stratifies patient into low-risk (no risk factors), intermediate-risk (one risk factor), or high-risk (two or more risk factors) groups. The 3-year OS rates associated with the respective categories are 81%, 62%, and 25%. Elevated Epstein-Barr virus (EBV) DNA in the peripheral blood was independently associated with inferior survival in the same analysis; therefore, peripheral EBV DNA was added to the model.4
DIAGNOSIS/WORK-UP
Work-up of ENKL includes dedicated imaging of the nasal sinuses by magnetic resonance imaging or computed tomography. Direct visualization with nasal endoscopy and biopsy is critical for the diagnosis of ENKL, whether it presents as localized or extranasal disease.3 The diagnosis may be obscured by extensive necrosis; therefore, multiple biopsies may be necessary for definitive diagnosis. Classically, lesions demonstrate extensive angioinvasion and necrosis as well as positive staining for CD2, CD56, cytoplasmic CD3 (but not surface CD3), and cytotoxic markers. Rarely, cells may be of T-cell origin and express CD4, CD8 and/or CD7. It is essential to demonstrate the presence of EBV-encoded RNA by in situ hybridization for diagnosis. ENKL is a fluorodeoxyglucose-avid lymphoma, so staging with positron emission tomography/computed tomography is recommended.5 Bone marrow biopsy typically is negative for extensive involvement but, when involved, may present as single cells or lymphocyte aggregates. Therefore, we recommend staining for EBV-encoded RNA to positively identify malignant cells. Peripheral-blood EBV polymerase chain reaction is recommended at diagnosis given its role for prognosis and surveillance.4,6
TREATMENT OF NEWLY DIAGNOSED ENKL
Limited-Stage Disease
Limited-stage ENKL is defined practically by its restriction to a single radiation port and typically encompasses stages I to IIE. There are three major approaches to therapy: (1) sequential therapy with chemotherapy followed by consolidative radiation; (2) concurrent radiation and chemotherapy; or, in select situations, (3) radiation therapy (RT) alone. RT alone should be prescribed cautiously in patients with favorable-risk features and truly limited-stage disease, because relapse rates may reach as high as 40% to 50%.7,8
Radiation.
Radiation is an essential component of limited-stage ENKL treatment planning; its omission is associated with a notable detriment to survival.7,8 Both radiation dose and volume must be considered during initial treatment planning. Doses of 50 Gy or greater are recommended; doses less than 50 Gy are associated with inferior survival, with the exception of patients who receive intensive concurrent therapy, who may be considered for a slightly lower dose of 40 to 54 Gy.9 Given the limitations in ascertaining extent of disease in many patients, the Lymphoma Radiation Oncology Group proposes the use of extended clinical target volumes to improve locoregional control.9 In additional support of larger radiation fields, patients who received treatment of macroscopic disease only, compared with a larger radiation field, had a severe detriment in survival: only half as many patients were alive at 5 years.9 The timing of radiation is not clearly defined. A recent meta-analysis demonstrated that there was a survival advantage for patients with limited-stage disease who were treated with radiation therapy first, as opposed to after chemotherapy (hazard ratio, 0.70; P = .002), with no evidence of impact by chemotherapy regimens (anthracycline-based or non–anthracycline-based regimens), concurrent chemoradiotherapy or not, or RT dose (≥ 45 or < 45 Gy).10 Nevertheless, concurrent and sequential treatments remain reasonable approaches for limited-stage disease.
Sequential therapy.
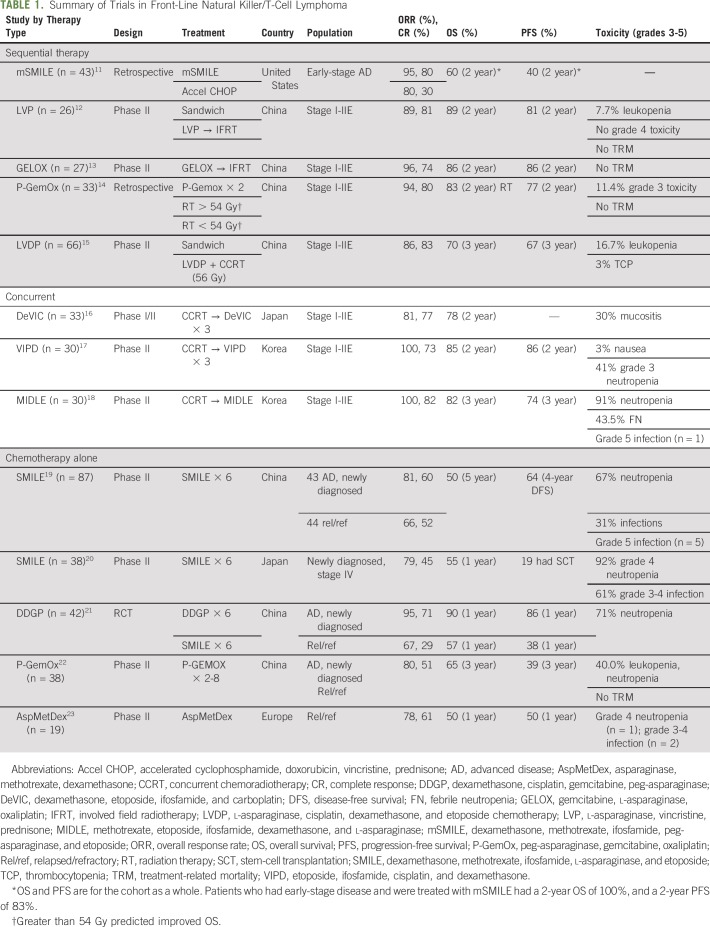
Sequential therapy may involve chemotherapy followed by radiation, or a “sandwich” approach. Table 1 lists front-line therapies. Dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) showed early promise in studies of relapsed and advanced-stage ENKL, and it resulted in superior response rates and relapse-free survival compared with traditional cyclophosphamide, doxorubicin, vincristine, and prednisone–based therapy.15,24-26 Retrospective analyses demonstrated that SMILE and RT combined was superior to cyclophosphamide, doxorubicin, vincristine, and prednisone; the respective 2-year survival rates were 60% and 40%.11,24 Modified SMILE contains peg-asparaginase, the pegylated derivative of l-asparaginase. Peg-asparaginase is substituted in because of its lower rates of infusion reactions and its longer half-life, which result in a more favorable toxicity profile and ease of administration compared with l-asparaginase.14,24 A phase I study of this combination, albeit with a lower dose of RT (45 Gy), demonstrated similar results in American non-Asian patients compared with Asians and supported its use in Western countries.24 However, SMILE is associated with high amounts of toxicity.20 In early-phase clinical trials, grade 4 neutropenia was observed in more than 90% of patients; three treatment deaths in phase I and II trials were attributed to neutropenic infections.20,26 Consequently, there is mandatory growth factor support, prophylaxis with trimethoprim-sulfamethoxazole, and count recovery between cycles.20
TABLE 1.
Summary of Trials in Front-Line Natural Killer/T-Cell Lymphoma
Less toxic alternatives include peg-asparaginase, gemcitabine, oxaliplatin (P-GemOx) and asparaginase, vincristine, and prednisone.12,14 Two cycles of P-GemOx before and after RT resulted in a complete response (CR) rate of 50% and 2-year OS of 83% in a phase II clinical trial.14 Likewise, asparaginase, vincristine, and prednisone resulted in high efficacy and low toxicity in a small, phase II study, in which the occurrence of grade 3 leukopenia was less than 10% and no grade 4 events were reported.12
Concurrent therapy.
Concurrent therapy allows for earlier exposure to RT, and early RT is associated with improved survival.10 Two concurrent regimens have been prospectively studied: (1) dexamethasone, etoposide, ifosfamide, and carboplatin and (2) etoposide, ifosfamide, cisplatin, and dexamethasone.16,17 Dexamethasone, etoposide, ifosfamide, and carboplatin given for three courses concurrently with 50 Gy RT in limited-stage ENKL (n = 33) resulted in a 2-year OS of 78%, which compared favorably with the historical control of RT alone (45%).16 Concurrent therapy was associated with notable mucositis related to RT (30% rate of grade 3 toxicity), but no treatment-related deaths occurred in the study.16 Similarly, concurrent cisplatin and RT followed by three cycles of etoposide, ifosfamide, cisplatin, and dexamethasone resulted in a 100% overall response rate (ORR). Only one patient experienced grade 3 toxicity during concurrent chemoradiotherapy (nausea), whereas 12 of 29 patients experienced grade 4 neutropenia. The estimated 3-year PFS and OS rates were 85.19% and 86.28%, respectively.17
Advanced-Stage Disease
Typically, 20% to 30% of patients with ENKL present in advanced stage. Prognosis is poor, in particular for stage IV disease, and the median survival is 4 to 7 months.2,4 Treatment response varies greatly according to the available literature: response rates are as low as 15%.2,4,27 Treatment with an asparaginase-containing regimen improves response rates to 56.5% compared with 32.5% for nonasparaginase treatment.27 Conversely, clinical trials performed in Asian countries report ORRs that range from 60% to 95%, and CRs of 27% to 66% as well as a longer median survival of 1 to 5 years.19,22,23 Treatment with chemotherapy alone is standard, but consolidation with RT or autologous stem-cell transplantation (auto-SCT) may provide a benefit for some. Chemotherapy regimens include modified SMILE; asparaginase, methotrexate, and dexamethasone (AspaMetDex); dexamethasone, cisplatin, gemcitabine, and peg-asparaginase (DDGP); and P-GemOx.
Chemotherapy.
SMILE treatment was originally evaluated in advanced and relapsed disease and demonstrated favorable efficacy but high toxicity, as noted in the discussion about limited-stage disease.20 In a pivotal phase II trial of SMILE chemotherapy, 39 patients with stage IV disease (n = 20) or relapsed disease (n = 18) were treated with two planned courses of SMILE, which could be followed by additional chemotherapy or transplantation (autologous or allogenic). The ORR and CR rates after two cycles of SMILE chemotherapy in the entire cohort were 79% and 45%, respectively. The 1-year survival in patients with stage IV disease was poor—45%. Importantly, 19 patients were able to later receive allogenic stem-cell transplantation (allo-SCT) or auto-SCT and had improved outcomes compared with those who did not.20 Toxicity was high and included a more than 90% rate of grade 4 neutropenia, tumor lysis syndrome that resulted in intestinal perforation, methotrexate encephalopathy, pancreatitis, and liver function test abnormalities. Three infection-related deaths occurred in patients with lymphocyte counts lower than 500/μL in phase I and II trials of SMILE. Therefore, we require granulocyte colony-stimulating factor treatment that starts on day 6. Because of the risk of morbidity and mortality, we avoid treatment with full-dose SMILE in patients with poor performance status, lymphopenia (< 500/μL lymphocytes), or high tumor burden.
Less toxic alternatives include DDGP, AspaMetDex, and P-GemOx.21 DDGP was compared with SMILE in patients with advanced or relapsed disease, and patients in the DDGP arm experienced fewer instances of grades 3 or 4 allergy. SMILE-treated patients had increased grade 3 or 4 diarrhea, mucositis, heart failure, and arrhythmia that resulted in several treatment-related deaths and that positioned DDGP as a more tolerable regimen in advanced disease.21 AspaMetDex and P-GemOx are similarly tolerable and have ORRs comparable to that of SMILE.22,23 These regimens are all listed in the National Comprehensive Cancer Network guidelines as options for advanced disease but also may be used in relapsed-disease scenarios.
Role of radiation.
The role of radiation in limited-stage disease had been defined by several prospective trials.15-17 However, it may also be beneficial in some patients with advanced disease.27 A retrospective analysis of consolidative RT in patients with stage III to IV disease (n = 71) demonstrated benefit in OS and PFS among patients who received RT compared with those who did not.27 Radiation improved outcomes regardless of initial treatment (asparaginase-containing or nonasparaginase regimens). The advantage was seen primarily in patients who achieved a CR to initial therapy, and the 2-year OS doubled among those who received versus did not receive consolidative radiation (OS rates, 81.5% v 40.2%, respectively; P = .002).
Relapsed Disease
The median survival of patients who experience disease relapse after initial treatment is approximately 6 months.28 Risk factors that predict poor outcome in this group include stage at relapse, elevated lactate dehydrogenase, presence of “B” symptoms, and relapse within 6 months of primary therapy.28 RT should be presented to those who have limited sites of involvement at relapse; otherwise, salvage chemotherapy is given.
AspaMetDex demonstrated a CR rate of 61% in a study of 19 patients with relapsed or refractory disease.23 Similarly, gemcitabine, dexamethasone, and cisplatin demonstrated an ORR of 83%.29 However, these high response rates may be misleading, because many patients in these trials were largely naïve to asparaginase treatment. Patients rechallenged with asparaginase-containing regimens within 6 months of primary therapy have notably inferior responses compared with those who are treated later (0% v 50%).28 Consolidation with allo-SCT or auto-SCT should be considered, because durability of response after salvage therapy often is limited.30 One study showed that long-term survival occurred only in the small portion of patients (6.7%) who were able to undergo allo-SCT while in remission.30
Role of SCT
The role of auto- or allo-SCT in ENKL was initially assessed in a small, retrospective series that enrolled Asian patients.30-32 Because of the rarity of the disease and the infrequent utility of this SCT, these studies included widely heterogeneous populations of those in first remission (CR1), those with relapsed disease, and a conglomerate of results on allo- and auto-SCT; thus, interpretation for individual clinical scenarios was obscured. Overall, these series demonstrated 40% to 50% rates of long-term survival in patients who received transplantation and no difference between SCT received at first or second remission.30-32 Recent larger, registry-based and matched analyses have improved the level of evidence to provide consensus recommendations.30,34,35 A summary of key points from the American Society for Blood and Marrow Transplantation guidelines are as follows: (1) SCT is not recommended in first CR or partial response (PR) for limited-stage disease; (2) auto-SCT is recommended in CR1 for disseminated disease, and consolidative allo-SCT may be considered; (3) auto-SCT in relapsed-sensitive disease is recommended for localized and disseminated ENKL; and (4) Allo-SCT is recommended for advanced NK/T-cell lymphoma after relapse, or if refractory, and in refractory localized disease.30 The data that contributed to these guidelines are detailed in the Auto-SCT and Allo-SCT sections.
Auto-SCT.
Auto-SCT has demonstrated benefit in small, retrospective Asian series for advanced disease in CR1 or at the time of relapse33-35 Auto-SCT is not recommended in limited-stage ENKL, because a matched analysis found no improvement in patients with limited-stage disease who underwent auto-SCT compared with those who did not.30,34 Patients with advanced disease who undergo auto-SCT in CR1 have a wide range of outcomes: the median survival ranges from 10 months to 3 years compared with a median survival of 6 to 7 months without transplantation.33-35 In a retrospective, matched, controlled study, the greatest benefit of transplantation was noted in those with high-risk disease; 5-year survival rate were 100% compared with 51.2% in those who did not receive SCT (P = .053).34 Predictors of relapse after transplantation included EBV titer before transplantation.36 However, disease status at the time of transplantation (CR v PR), stage, and transplantation in first CR or PR versus later did not reliably predict outcomes across studies.33-35
Allo-SCT.
An analysis of allo-SCT from the Center for International Blood and Marrow Transplant Research was recently described.37 This analysis included data from the North American cohort; it was largely composed of white patients, and only 18% of those enrolled were Asian. The cohort included patients with newly diagnosed and relapsed disease, all with central pathology review. The 3‐year PFS and OS rates of the entire group were 28% and 34%, respectively, and all relapses occurred before 2 years. Interestingly, there was no benefit of earlier allo-SCT (ie, in CR1) in terms of relapse risk. Prognostic indices and remission status at the time of allo-SCT also did not affect relapse risk. Overall, these data suggest that allo‐SCT provides a curative option in a subset of patients with ENKL and may be considered as a consolidative therapy for advanced disease in CR1 or at the time of relapse.
Novel therapies.
Given the poor long-term outcomes in advanced stage and relapsed disease, more effective therapies are needed. Checkpoint blockade demonstrated impressive single-agent results in early-phase trials. Among seven patients with relapsed disease who were treated with single-agent blockade of programmed death 1, five achieved a CR, and two achieved a PR, which provided a 100% ORR.38 However, follow-up was limited, and subsequent studies have not been able to duplicate these remarkable results.39 Excitement for checkpoint blockade has been tempered more by reports of hyperprogression in some patients with T-cell lymphoma.40 Nevertheless, clinical trials to assess checkpoint blockade are ongoing (ClinicalTrials.gov identifier: NCT03021057). Cellular therapy is also gaining traction. Cytotoxic T lymphocytes (CTLs) have been adapted for use in T-cell lymphomas by identifying novel common targets, such as EBV and latent membrane protein (LMP). A clinical trial of an LMP-CTL in EBV-associated lymphomas in the United States demonstrated that eight of 11 patients survived at least 2 years.41 An EBV-CTL also delivered promising results. Thirteen patients with ENKL treated with an EBV-CTL after transplantation had 4-year OS and PFS rates of 100%, and 90%, respectively.42 These trials represent major progress for ENKL and EBV-associated lymphomas, but longer follow-up and larger trials are needed to confirm these results.
Targeted Therapy
Ongoing studies to assess the genetic basis of ENKL have identified common targetable alterations in the phosphatidylinositol 3-kinase/protein kinase B, Janus kinase–signal transducers and activators of transcription, and nuclear factor κB signaling pathways along with frequent epigenetic changes.43 Histone deacetylase inhibitors and other epigenetic modifiers, such as enhancer of zeste homolog 2, also have demonstrated some activity.44,45 Furthermore, brentuximab vedotin, a CD30 drug-antibody conjugate, may prove efficacious, because CD30 expression is detected in approximately 50% of patients with ENKL.46 Recently, CD38 was discovered to be upregulated in the majority of ENKL and associated with poor prognosis.47 Preclinical data demonstrate efficacy of daratumumab in ENKL and may represent an additional future target.47 Additional active, targeted therapies approved in other malignancies include alemtuzumab and bortezomib.48,49
Our approach.
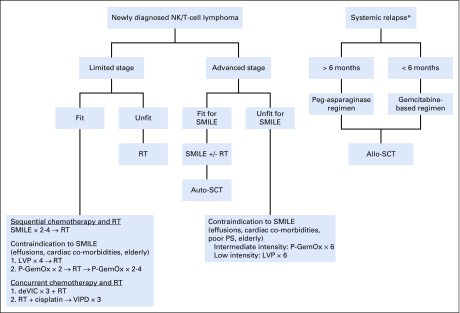
We treat young, fit patients who have limited-stage disease with sequential chemotherapy with modified SMILE for four cycles followed by RT to 50 to 55 Gy (Fig 1). We treat elderly patients and those unfit for modified SMILE with P-GemOx in a sandwich approach of two cycles before and after chemotherapy or with RT alone if they have stage IE disease and no risk factors. As reviewed in this article, patients with advanced and relapsed disease have poor outcomes. Therefore, we strongly encourage participation in clinical trials when available. When clinical trials are not available, we treat these patients with modified SMILE for up to six cycles of therapy. We consider consolidative RT in patients with bulky disease, limited sites or low burden bone marrow involvement, or limited residual disease. Patients with disseminated disease, such as those with leukemia and extensive skin involvement, are treated with chemotherapy alone (modified SMILE or P-GemOx) followed by an auto-SCT in CR1. Patients who experience relapse within 6 months are treated with a gemcitabine-based regimen. Those who experience relapse after 6 months are treated with an asparaginase-based regimen with the plan for allo-SCT if a response is achieved.
Fig 1.
Our treatment schema. (*) Relapse that involved more than one site. If only one site was involved, and if no contraindication existed, RT would be pursued. allo-SCT, allogenic stem-cell transplantation; auto-SCT, autologous stem-cell transplantation; DeVIC, dexamethasone, etoposide, ifosfamide, and carboplatin; LVP, l-asparaginase, vincristine, prednisone, and P-GemOx; NK, natural killer; P-GemOx, peg-asparaginase, gemcitabine, oxaliplatin; PS, performance status; RT, radiation therapy; SMILE, dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide; VIPD, etoposide, ifosfamide, cisplatin, and dexamethasone.
In summary, ENKL is a rare T-cell lymphoma caused by infection with EBV. Patients with limited-stage disease are often cured with asparaginase-based chemotherapy and high-dose RT. Unfortunately, those with advanced-stage and relapsed disease often experience an aggressive disease course, and optimal treatment has not been defined. Recent studies have shown promising activity of agents, such as EBV- and LMP-CTLs and checkpoint blockade. We hope that, as the data and science of these approaches mature, they will provide alternative curative approaches, especially for patients with aggressive disease.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Pamela B. Allen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Management of NK/T-Cell Lymphoma, Nasal Type
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Mary Jo Lechowicz
Consulting or Advisory Role: Kyowa Hakko Kirin
Travel, Accommodations, Expenses: Kyowa Hakko Kirin
No other potential conflicts of interest were reported.
REFERENCES
- 1.Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34:963–971. doi: 10.1200/JCO.2015.63.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kommalapati A, Tella SH, Ganti AK, et al. Natural killer/T-cell neoplasms: Analysis of incidence, patient characteristics, and survival outcomes in the United States. Clin Lymphoma Myeloma Leuk. 2018;18:475–479. doi: 10.1016/j.clml.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: A study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non–anthracycline-based treatment: A multicentre, retrospective analysis. Lancet Oncol. 2016;17:389–400. doi: 10.1016/S1470-2045(15)00533-1. [DOI] [PubMed] [Google Scholar]
- 5.Moon SH, Cho SK, Kim WS, et al. The role of 18F-FDG PET/CT for initial staging of nasal type natural killer/T-cell lymphoma: A comparison with conventional staging methods. J Nucl Med. 2013;54:1039–1044. doi: 10.2967/jnumed.112.113399. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki R, Yamaguchi M, Izutsu K, et al. Prospective measurement of Epstein-Barr virus DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood. 2011;118:6018–6022. doi: 10.1182/blood-2011-05-354142. [DOI] [PubMed] [Google Scholar]
- 7.Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: Analysis from a multicenter study. Blood. 2015;126:1424–1432, quiz 1517. doi: 10.1182/blood-2015-04-639336. [DOI] [PubMed] [Google Scholar]
- 9.Isobe K, Uno T, Tamaru J, et al. Extranodal natural killer/T-cell lymphoma, nasal type: The significance of radiotherapeutic parameters. Cancer. 2006;106:609–615. doi: 10.1002/cncr.21656. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Zhou D, Zhang W. The optimal timing of radiotherapy in the combined modality therapy for limited-stage extranodal NK/T cell lymphoma (ENKTL): A systematic review and meta-analysis. Ann Hematol. 2018;97:2279–2287. doi: 10.1007/s00277-018-3479-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Liu H, Xu XH, et al. Retrospective study of modified SMILE chemotherapy for advanced-stage, relapsed, or refractory extranodal natural killer (NK)/T cell lymphoma, nasal type. Med Oncol. 2013;30:720. doi: 10.1007/s12032-013-0720-7. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of “sandwich” l-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118:3294–3301. doi: 10.1002/cncr.26629. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and l-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119:348–355. doi: 10.1002/cncr.27752. [DOI] [PubMed] [Google Scholar]
- 14.Wei W, Wu P, Li L, et al. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal-type, extranodal natural killer/T-cell lymphoma. Hematology. 2017;22:320–329. doi: 10.1080/10245332.2016.1264163. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Zhang L, Xie L, et al. A phase II prospective study of the “sandwich” protocol, l-asparaginase, cisplatin, dexamethasone and etoposide chemotherapy combined with concurrent radiation and cisplatin, in newly diagnosed, I/II stage, nasal type, extranodal natural killer/T-cell lymphoma. Oncotarget. 2017;8:50155–50163. doi: 10.18632/oncotarget.16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27:5594–5600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 18.Yoon DH, Kim SJ, Jeong SH, et al. Phase II trial of concurrent chemoradiotherapy with l-asparaginase and MIDLE chemotherapy for newly diagnosed stage I/II extranodal NK/T-cell lymphoma, nasal type (CISL-1008) Oncotarget. 2016;7:85584–85591. doi: 10.18632/oncotarget.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: Analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–2980. doi: 10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: A randomized controlled, multicenter, open-label study in China. Clin Cancer Res. 2016;22:5223–5228. doi: 10.1158/1078-0432.CCR-16-0153. [DOI] [PubMed] [Google Scholar]
- 22.Wang JH, Wang L, Liu CC, et al. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget. 2016;7:29092–29101. doi: 10.18632/oncotarget.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaccard A, Gachard N, Marin B, et al. Efficacy of l-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma: A phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 24.Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016;57:2575–2583. doi: 10.1080/10428194.2016.1180689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions (ed 3) New York, NY: John Wiley & Sons; 2003. [Google Scholar]
- 26.Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2008;99:1016–1020. doi: 10.1111/j.1349-7006.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi XW, Jiang WQ, Zhang WW, et al. Treatment outcome of patients with advanced stage natural killer/T-cell lymphoma: Elucidating the effects of asparaginase and postchemotherapeutic radiotherapy. Ann Hematol. 2015;94:1175–1184. doi: 10.1007/s00277-015-2336-9. [DOI] [PubMed] [Google Scholar]
- 28.Lim SH, Hong JY, Lim ST, et al. Beyond first-line non–anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: Clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017;28:2199–2205. doi: 10.1093/annonc/mdx316. [DOI] [PubMed] [Google Scholar]
- 29.Park BB, Kim WS, Suh C, et al. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: A consortium for improving survival of lymphoma (CISL) trial. Ann Hematol. 2015;94:1845–1851. doi: 10.1007/s00277-015-2468-y. [DOI] [PubMed] [Google Scholar]
- 30.Yhim HY, Kim JS, Mun YC, et al. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural killer/T-cell lymphoma. Biol Blood Marrow Transplant. 2015;21:1597–1604. doi: 10.1016/j.bbmt.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T-cell and NK/T-cell lymphomas: An international collaborative effort on behalf of the guidelines committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23:1826–1838. doi: 10.1016/j.bbmt.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz N, Wu HS, Glass B. Allogeneic transplantation in T-cell lymphomas. Semin Hematol. 2014;51:67–72. doi: 10.1053/j.seminhematol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Park S, Kang ES, et al. Induction treatment with SMILE and consolidation with autologous stem-cell transplantation for newly diagnosed stage IV extranodal natural killer/T-cell lymphoma patients. Ann Hematol. 2015;94:71–78. doi: 10.1007/s00277-014-2171-4. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Au WY, Park MJ, et al. Autologous hematopoietic stem-cell transplantation in extranodal natural killer/T cell lymphoma: A multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant. 2008;14:1356–1364. doi: 10.1016/j.bbmt.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Fox CP, Boumendil A, Schmitz N, et al. High-dose therapy and autologous stem-cell transplantation for extra-nodal NK/T lymphoma in patients from the Western hemisphere: A study from the European Society for Blood and Marrow Transplantation. Leuk Lymphoma. 2015;56:3295–3300. doi: 10.3109/10428194.2015.1037764. [DOI] [PubMed] [Google Scholar]
- 36.Lim SH, Hyun SH, Kim HS, et al. Prognostic relevance of pretransplant Deauville score on PET-CT and presence of EBV DNA in patients who underwent autologous stem cell transplantation for ENKTL. Bone Marrow Transplant. 2016;51:807–812. doi: 10.1038/bmt.2016.6. [DOI] [PubMed] [Google Scholar]
- 37.Kanate AS, DiGilio A, Ahn KW, et al. Allogeneic haematopoietic cell transplantation for extranodal natural killer/T-cell lymphoma, nasal type: A CIBMTR analysis. Br J Haematol. 2018;182:916–920. doi: 10.1111/bjh.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong YL, Chan TSY, Tan D, et al. PD-1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11:15. doi: 10.1186/s13045-018-0559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratner L, Waldmann TA, Janakiram M, et al. Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N Engl J Med. 2018;378:1947–1948. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]
- 41.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho SG, Kim N, Sohn HJ, et al. Long-term outcome of extranodal NK/T-cell lymphoma patients treated with postremission therapy using EBV LMP1- and LMP2a-specific CTLs. Mol Ther. 2015;23:1401–1409. doi: 10.1038/mt.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, de Reyniès A, de Leval L, et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood. 2010;115:1226–1237. doi: 10.1182/blood-2009-05-221275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766–1771. doi: 10.1093/annonc/mdv237. [DOI] [PubMed] [Google Scholar]
- 45.McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 46.Feng Y, Rao H, Lei Y, et al. CD30 expression in extranodal natural killer/T-cell lymphoma, nasal type among 622 cases of mature T-cell and natural killer-cell lymphoma at a single institution in South China. Chin J Cancer. 2017;36:43. doi: 10.1186/s40880-017-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Wang H, Li PF, et al. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann Hematol. 2015;94:1381–1388. doi: 10.1007/s00277-015-2359-2. [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Suh C, Kang HJ, et al. Phase I study of proteasome inhibitor bortezomib plus CHOP in patients with advanced, aggressive T-cell or NK/T-cell lymphoma. Ann Oncol. 2008;19:2079–2083. doi: 10.1093/annonc/mdn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang ST, Lu CL, Chuang SS. CD52 expression in non-mycotic T- and NK/T-cell lymphomas. Leuk Lymphoma. 2007;48:117–121. doi: 10.1080/10428190601016167. [DOI] [PubMed] [Google Scholar]