Abstract
Summary
A 67-year-old woman with a past history of type 2 diabetes mellitus presented with worsening glycemic control. She had some acromegaly symptoms and magnetic resonance imaging demonstrated a pituitary tumor. Endocrinological examination found the resting growth hormone (GH) level within the normal range, but elevated insulin-like growth factor 1 level. A 75 g oral glucose tolerance test showed inadequate suppression of nadir GH levels. Acromegaly due to GH-secreting pituitary tumor was diagnosed. The patient underwent endoscopic transsphenoidal surgery resulting in gross total removal of the tumor and recovered well postoperatively. Histological examination of the tumor showed coexistence of relatively large gangliocytoma cells and pituitary adenoma cells, suggesting mixed gangliocytoma-pituitary adenoma. In addition, colocalization of GH and GH-releasing hormone (GHRH) in pituitary adenoma cells was revealed, so the adenomatous components were more likely to produce GHRH in our mixed gangliocytoma-pituitary adenoma case. Mixed gangliocytoma-pituitary adenoma is very rare, and the present unique case demonstrated only the adenomatous components associated with GHRH production.
Learning points:
Sellar gangliocytoma coexisting with pituitary adenoma is recognized as a mixed gangliocytoma-pituitary adenoma and is very rare.
A proposed developmental mechanism of growth hormone (GH)-secreting mixed gangliocytoma-pituitary adenoma involves GH-releasing hormone (GHRH) produced by the gangliocytic components promoting the growth of tumor including GH-secreting adenomatous components.
Since our present case indicated that the adenomatous components of mixed gangliocytoma-pituitary adenoma could secrete both GH and GHRH simultaneously, progression of GH-secreting mixed gangliocytoma and pituitary adenoma may involve exposure to spontaneously produced GHRH due to the adenomatous components.
Background
Gangliocytoma occurs in all regions of the central nervous system but rarely in the sellar region. Sellar gangliocytoma coexisting with pituitary adenoma is recognized as a mixed gangliocytoma-pituitary adenoma. Mixed gangliocytoma-pituitary adenoma is extremely rare, accounting for 0.14–0.52% of all sellar tumors, and mostly occurs in women (1, 2). Since mixed gangliocytoma-pituitary adenoma has no specific clinical symptoms, endocrine findings, or brain imaging appearance, it is usually only diagnosed by postoperative histopathology (3). The adenomatous components of mixed gangliocytoma-pituitary adenoma are known to frequently secrete excess growth hormone (GH) and are typically characterized as pituitary somatotroph adenoma (2, 4). The gangliocytic components of mixed gangliocytoma-pituitary adenoma showed immunoreactivity for hypothalamic releasing hormones other than the neuroendocrine markers (2, 5, 6). A proposed but controversial developmental mechanism of GH-secreting mixed gangliocytoma-pituitary adenoma involves GH-releasing hormone (GHRH) produced by the gangliocytic components promoting the growth of tumor including GH-secreting adenomatous components (2, 6).
We report a rare case of mixed gangliocytoma-pituitary adenoma in which the GH-secreting adenoma cells, but not the ganglion cells, produced GHRH.
Case presentation
A 67-year-old woman with a past history of type 2 diabetes mellitus presented with worsening glycemic control. She had some physical abnormalities such as face with enlarged forehead, nose, and chin, increased bone size of the extremities, and mild enlargement of the tongue.
Investigation
Magnetic resonance (MR) imaging revealed a pituitary tumor of 1.9 cm maximum length with Knosp grade 0 (7), appearing with less contrast enhancement than the normal pituitary gland on gadolinium-enhanced T1-weighted MR imaging and isointense on T2-weighted MR imaging. Endocrinological examination found resting GH level (3.321 ng/mL; normal: 0.010–3.607 ng/mL) within the normal range, but elevated insulin-like growth factor 1 (IGF-1) level (381 ng/mL; normal: 61–183 ng/mL). A 75 g oral glucose tolerance test (OGTT) achieved inadequate suppression of nadir GH level (3.190 ng/mL). Acromegaly due to GH-secreting pituitary tumor was diagnosed according to these findings.
Treatment
The patient underwent removal of the pituitary tumor via an endoscopic endonasal transsphenoidal approach without premedication, which was successful in accomplishing gross total removal.
Outcome and follow-up
The postoperative course was uneventful. Postoperative MR imaging demonstrated no obvious residual tumor. Resting GH level (2.467 ng/mL) remained within the normal range, and IGF-1 level (233 ng/mL) decreased but was slightly higher than normal at 1 week postoperatively. Postoperative 75 g OGTT found nadir GH level of 0.880 ng/mL, resulting in no suppression of less than 0.4 ng/mL (8). Since aggravation of diabetic hyperglycemia resolved promptly, the patient was discharged on foot without adjuvant therapy. Random GH level (0.587 ng/mL) remained below 1.0 ng/mL (9) and IGF-1 level (167 ng/mL) was normalized at 3 months postoperatively. Thereafter, the patient received outpatient follow-up with random GH levels <1.0 ng/mL and IGF-1 levels within the normal range.
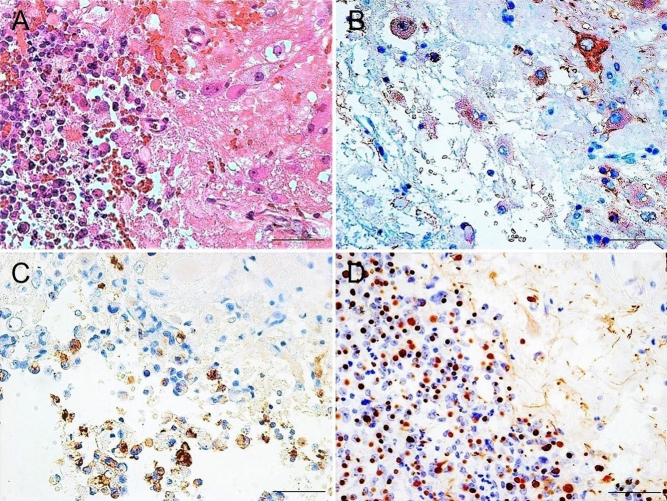
Hematoxylin and eosin staining of the tumor revealed that relatively large cells were adjacent to small round cells corresponding to the pituitary adenoma and were mixed within the boundary area (Fig. 1A). Immunohistochemical staining found the large cells were positive for microtubule-associated protein 2 (MAP-2) (anti-MAP-2 antibody; MAB364, Millipore) as the mature neuron marker, indicating the presence of gangliocytoma (Fig. 1B). Pituitary adenoma cells showed weak immunoreactivity for GH (anti-GH antibody; ab7905, Abcam) (Fig. 1C) and a strong dot-like immunopositive pattern representing the fibrous body for CAM 5.2 (anti-CAM 5.2 antibody; No. 349205, Beckton, Dickinson and Company) as the cytokeratin marker (Fig. 1D), indicating a sparsely granulated subtype of pituitary somatotroph adenoma. The histopathological findings of the gangliocytic and adenomatous components determined the diagnosis as mixed gangliocytoma-pituitary adenoma. Immunohistochemical demonstration of GHRH expression was performed using a pair of mirror section images. No correspondence of MAP-2- and GHRH (anti-GHRH antibody; ab80009, Abcam)-positive cells was found (Fig. 2A and B), but correspondence of GH- and GHRH-positive cells was demonstrated (Fig. 2C and D). Furthermore, double immunofluorescence staining for GH and GHRH revealed cells with colocalization (Fig. 2E, F and G).
Figure 1.
Histopathological examination of the pituitary tumor. (A) Hematoxylin and eosin staining showing relatively large cells and pituitary adenoma cells adjacent and mixed within the boundary area. (B) Immunostaining for microtubule-associated protein 2 demonstrating positivity in large cells, indicating the presence of gangliocytoma. Immunostaining for growth hormone showing positivity but weak reactivity in pituitary adenoma cells (C) and CAM 5.2 immunostaining showing strong dot-like positive pattern (D), indicating the sparsely granulated subtype of pituitary somatotroph adenoma. Scale bar = 50 μm.
Figure 2.
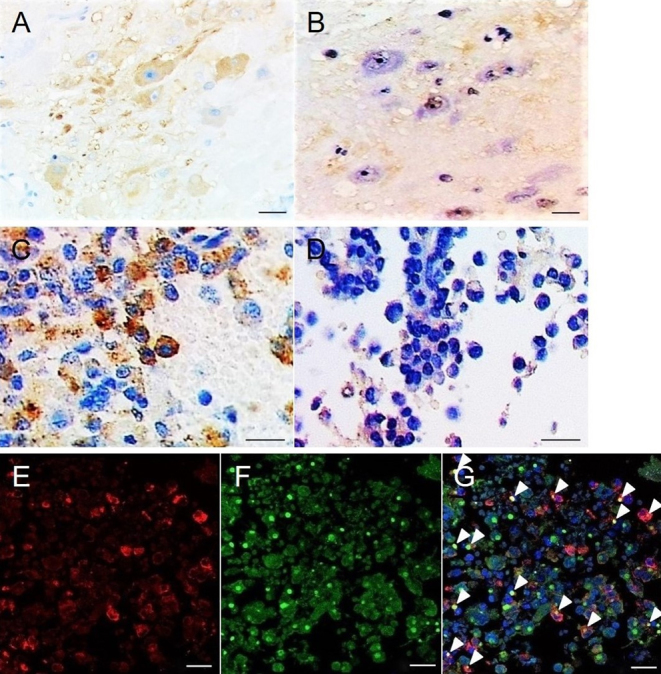
Immunohistochemical demonstration of growth hormone-releasing hormone (GHRH). Immunohistochemical staining using a pair of mirror section images showing no correspondence of microtubule-associated protein 2-positive (A) and GHRH-positive (B) cells, but demonstrating correspondence of growth hormone (GH)-positive (C) and GHRH-positive (D) cells. Double immunofluorescence staining for GH (E) and GHRH (F) revealing cells with colocalization (G). Arrowheads, colocalized cells. Scale bar = 50 μm in A–D; 20 μm in E–G.
Discussion
The gangliocytic components of mixed gangliocytoma-pituitary adenoma are presumed to secrete the hypothalamic releasing hormones including GHRH, gonadotropin-releasing hormone, thyrotropin-releasing hormone, and corticotropin-releasing hormone, and promote the proliferation of the correlated adenomatous components (6, 10, 11). On the other hand, both components of mixed gangliocytoma-pituitary adenoma were demonstrated to produce the hypothalamic releasing hormones (3). All previous reports of mixed gangliocytoma-pituitary adenoma investigating GHRH immunoreactivity have implied GHRH production in the gangliocytic components (Table 1) (1, 5, 6, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19). However, our present case demonstrated that only the adenomatous components may produce GHRH in GH-secreting mixed gangliocytoma-pituitary adenoma. Recently transdifferentiation of neuroendocrine cells to a neuronal phenotype (20) and expression of pituitary transcription factors in gangliocytic components (21) were indicated in mixed gangliocytoma-pituitary adenoma. This indication that the origin of two components in mixed gangliocytoma-pituitary adenoma relates to pituitary lineage supports the possibility that either or both of these components produce GHRH in GH-secreting mixed gangliocytoma-pituitary adenoma.
Table 1.
Summary of literature review reporting mixed gangliocytoma-pituitary adenoma associated with growth hormone-releasing hormone immunoreactivity.
| No. | Author | Year | Number of cases | Hormone produced | Immunoreactivity of GHRH | |
|---|---|---|---|---|---|---|
| Gangliocytic component | Adenomatous component | |||||
| 1 | Asa et al. (10) | 1984 | 2 | GH | + | − |
| GH | + | − | ||||
| 2 | Bevan et al. (13) | 1989 | 1 | GH, PRL | + | − |
| 3 | Li et al. (15) | 1989 | 1 | GH | + | − |
| 4 | Asada et al. (12) | 1990 | 1 | GH, PRL | + | − |
| 5 | Slowik et al. (19) | 1990 | 1 | GH, PRL | + | − |
| 6 | Horvath et al. (5) | 1994 | A few cases | GH (all cases) | + | − |
| 7 | Saeger et al. (11) | 1994 | 1 | GH, PRL | + | − |
| 8 | Iwase et al. (14) | 1995 | 1 | GH | + | − |
| 9 | Morikawa et al. (17) | 1997 | 1 | GH | + | − |
| 10 | Saeger et al. (18) | 1997 | 5 | GH | + | − |
| GH | + | − | ||||
| GH | + | − | ||||
| GH | + | − | ||||
| ACTH | + | − | ||||
| 11 | Luna et al. (16) | 2001 | 1 | GH | + | − |
| 12 | Kurosaki et al. (6) | 2002 | 5 | GH, PRL | + | − |
| GH | + | − | ||||
| GH | + | − | ||||
| GH | + | − | ||||
| GH | + | − | ||||
| 13 | Kontogeorgos et al. (1) | 2006 | 3 | GH | + | − |
| GH | + | − | ||||
| GH | + | − | ||||
ACTH, adrenocorticotropic hormone; GH, growth hormone; GHRH, growth hormone-releasing hormone; PRL, prolactin.
We present a unique case of mixed gangliocytoma-pituitary adenoma with the adenomatous components which could secrete both GH and GHRH simultaneously. This finding suggested that progression of GH-secreting mixed gangliocytoma-pituitary adenoma may involve exposure to spontaneously produced GHRH due to the adenomatous components.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent was obtained from the patient/patient’s mother for publication of this case report.
Author contribution statement
ST, YT, HG and HA contributed to the conception and design of the study. HI and YT treated the patient and collected the data. IO performed immunohistochemical staining and prepared the pathological images.
References
- 1.Kontogeorgos G, Mourouti G, Kyrodimou E, Liapi-Avgeri G, Parasi E. Ganglion cell containing pituitary adenomas: signs of neuronal differentiation in adenoma cells. Acta Neuropathology 2006. 21–28. ( 10.1007/s00401-006-0055-y) [DOI] [PubMed] [Google Scholar]
- 2.Puchner MJ, Ludecke DK, Saeger W, Riedel M, Asa SL. Gangliocytomas of the sellar region–a review. Experimental and Clinical Endocrinology & Diabetes 1995. 129–149. ( 10.1055/s-0029-1211342) [DOI] [PubMed] [Google Scholar]
- 3.Yang B, Yang C, Sun Y, Du J, Liu P, Jia G, Jia W, Zhang Y, Wang J, Xu Y, et al. Mixed gangliocytoma-pituitary adenoma in the sellar region: a large-scale single-center experience. Acta Neurochirurgica 2018. 1989–1999. ( 10.1007/s00701-018-3632-7) [DOI] [PubMed] [Google Scholar]
- 4.Towfighi J, Salam MM, McLendon RE, Powers S, Page RB. Ganglion cell-containing tumors of the pituitary gland. Archives of Pathology & Laboratory Medicine 1996. 369–377. [PubMed] [Google Scholar]
- 5.Horvath E, Kovacs K, Scheithauer BW, Lloyd RV, Smyth HS. Pituitary adenoma with neuronal choristoma (PANCH): composite lesion or lineage infidelity? Ultrastructural Pathology 1994. 565–574. ( 10.3109/01913129409021900) [DOI] [PubMed] [Google Scholar]
- 6.Kurosaki M, Saeger W, Ludecke DK. Intrasellar gangliocytomas associated with acromegaly. Brain Tumor Pathology 2002. 63–67. ( 10.1007/BF02478929) [DOI] [PubMed] [Google Scholar]
- 7.Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993. 610–617; discussion 617–618. ( 10.1227/00006123-199310000-00008) [DOI] [PubMed] [Google Scholar]
- 8.Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S, et al. A consensus on criteria for cure of acromegaly. Journal of Clinical Endocrinology & Metabolism 2010. 3141–3148. ( 10.1210/jc.2009-2670) [DOI] [PubMed] [Google Scholar]
- 9.Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA. & Endocrine Society. Acromegaly: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism 2014. 3933–3951. ( 10.1210/jc.2014-2700) [DOI] [PubMed] [Google Scholar]
- 10.Asa SL, Scheithauer BW, Bilbao JM, Horvath E, Ryan N, Kovacs K, Randall RV, Laws ER Jr, Singer W, Linfoot JA. A case for hypothalamic acromegaly: a clinicopathological study of six patients with hypothalamic gangliocytomas producing growth hormone-releasing factor. Journal of Clinical Endocrinology & Metabolism 1984. 796–803. ( 10.1210/jcem-58-5-796) [DOI] [PubMed] [Google Scholar]
- 11.Saeger W, Puchner MJ, Ludecke DK. Combined sellar gangliocytoma and pituitary adenoma in acromegaly or Cushing's disease. A report of 3 cases. Virchows Archiv 1994. 93–99. [DOI] [PubMed] [Google Scholar]
- 12.Asada H, Otani M, Furuhata S, Inoue H, Toya S, Ogawa Y. Mixed pituitary adenoma and gangliocytoma associated with acromegaly–case report. Neurologia Medico-Chirurgica 1990. 628–632. ( 10.2176/nmc.30.628) [DOI] [PubMed] [Google Scholar]
- 13.Bevan JS, Asa SL, Rossi ML, Esiri MM, Adams CB, Burke CW. Intrasellar gangliocytoma containing gastrin and growth hormone-releasing hormone associated with a growth hormone-secreting pituitary adenoma. Clinical Endocrinology 1989. 213–224. ( 10.1111/j.1365-2265.1989.tb02229.x) [DOI] [PubMed] [Google Scholar]
- 14.Iwase T, Nishizawa S, Baba S, Hinokuma K, Sugimura H, Nakamura S, Uemura K, Shirasawa H, Kino I. Intrasellar neuronal choristoma associated with growth hormone-producing pituitary adenoma containing amyloid deposits. Human Pathology 1995. 925–928. ( 10.1016/0046-8177(95)90018-7) [DOI] [PubMed] [Google Scholar]
- 15.Li JY, Racadot O, Kujas M, Kouadri M, Peillon F, Racadot J. Immunocytochemistry of four mixed pituitary adenomas and intrasellar gangliocytomas associated with different clinical syndromes: acromegaly, amenorrhea-galactorrhea, Cushing's disease and isolated tumoral syndrome. Acta Neuropathologica 1989. 320–328. ( 10.1007/bf00687585) [DOI] [PubMed] [Google Scholar]
- 16.Luna V, Morales F, Luengo LM, Sanz A, Diaz J. Pituitary gangliocytoma-adenoma presenting with acromegaly: response to treatment. Archives of Internal Medicine 2001. 1010–1011. ( 10.1001/archinte.161.7.1010) [DOI] [PubMed] [Google Scholar]
- 17.Morikawa M, Tamaki N, Kokunai T, Imai Y. Intrasellar pituitary gangliocyto-adenoma presenting with acromegaly: case report. Neurosurgery 1997. 611–614; discussion 614–615. ( 10.1097/00006123-199703000-00036) [DOI] [PubMed] [Google Scholar]
- 18.Saeger W, Lüdecke DK, Losa M. Combined neuronal and endocrine tumors of the sellar region. Der Pathologe 1997. 419–424. ( 10.1007/s002920050236) [DOI] [PubMed] [Google Scholar]
- 19.Slowik F, Fazekas I, Bálint K, Gazsó L, Pásztor E, Czirják S, Lapis K. Intrasellar hamartoma associated with pituitary adenoma. Acta Neuropathologica 1990. 328–333. ( 10.1007/bf00294652) [DOI] [PubMed] [Google Scholar]
- 20.Lopes MB, Sloan E, Polder J. Mixed gangliocytoma-pituitary adenoma: insights on the pathogenesis of a rare sellar tumor. American Journal of Surgical Pathology 2017. 586–595. ( 10.1097/PAS.0000000000000806) [DOI] [PubMed] [Google Scholar]
- 21.Sergeant C, Jublanc C, Leclercq D, Boch AL, Bielle F, Raverot G, Daly AF, Trouillas J, Villa C. Transdifferentiation of neuroendocrine cells: gangliocytoma associated with two pituitary adenomas of different lineage in MEN1. American Journal of Surgical Pathology 2017. 849–853. ( 10.1097/PAS.0000000000000803) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a