Abstract
Background
Ingestion of the calorically dense compound alcohol may cause metabolic disturbances including hypoglycaemia, hepatic steatosis and insulin resistance, but the underlying mechanisms are uncertain. The gastrointestinal tract is well recognised as a major influencer on glucose, protein and lipid metabolism, but its role in alcohol metabolism remains unclear.
Objective
To examine the effects of oral and intravenous alcohol, respectively, on plasma concentrations of several gluco-regulatory hormones including serum/plasma insulin, C-peptide, glucagon, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide 1 (GLP-1) and fibroblast growth factor 21 (FGF21).
Design and methods
In a double-blinded, randomised, crossover design, we subjected 12 healthy men to intragastric ethanol infusion (IGEI) and an isoethanolaemic intravenous ethanol infusion (IVEI) (0.7 g alcohol per kg body weight), respectively, on two separate experimental days.
Results
Isoethanolaemia during the two alcohol administration forms was obtained (P = 0.38). During both interventions, plasma glucose peaked after ~30 min and thereafter fell below baseline concentrations. GIP and GLP-1 concentrations were unaffected by the two interventions. Insulin concentrations were unaffected by IGEI but decreased during IVEI. C-peptide, insulin secretion rate and glucagon concentrations were lowered similarly during IGEI and IVEI. FGF21 concentrations increased dramatically (nine-fold) and similarly during IGEI and IVEI.
Conclusions
Alcohol does not seem to affect the secretion of incretin hormones but decreased insulin and glucagon secretion independently of gut-derived factors. IGEI as well as IVEI potently stimulate FGF21 secretion indicating a gut-independent effect of alcohol on FGF21 secretion in humans.
Keywords: alcohol, FGF21, glucose, glucagon, incretin hormones, insulin
Introduction
The majority of the world’s population consumes alcohol occasionally and alcohol-related diseases represent a major healthcare burden worldwide (1). Consumption of alcohol is related to several metabolic processes affecting glucose metabolism (2). It is well known that acute alcohol ingestion increases the risk of hypoglycaemia; most likely due to inhibitory effects of alcohol on hepatic gluconeogenesis (3). In addition, it is well known that chronic alcohol consumption may cause hepatic steatosis which in some individuals leads to insulin resistance and impaired glucose tolerance (4, 5). However, the exact mechanisms underlying these pathophysiological processes have not been identified. Alcohol has been found both to increase and decrease serum insulin concentrations under various conditions and administration forms (6, 7, 8), and results from studies investigating the effect of alcohol on plasma glucagon concentrations are ambiguous as well (9, 10). Likewise, the effect of alcohol on the secretion of the gut-derived insulinotropic incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) remains unclear (6, 11, 12). Recently, alcohol was shown to constitute a potent stimulator of the liver-derived hormone fibroblast growth factor 21 (FGF21) in humans (13, 14). In rodents, FGF21 has been shown to increase insulin sensitivity (15) and decrease hepatic glucose production, and thus, play an important role in the regulation of plasma glucose concentrations. In humans, FGF21 is mainly secreted from the liver in response to fructose (16), high-carbohydrate diet (17) and oral alcohol ingestion (13, 14). It is not known how alcohol mediates FGF21 secretion and it remains obscure whether pancreatic or gut-derived hormones are involved in alcohol-induced secretion of FGF21.
The gastrointestinal tract plays a major role in glucose, protein and lipid metabolism; and energy-rich macronutrients, that is, carbohydrate, protein and lipid, stimulate insulin secretion to a larger extent when administered orally compared to intravenously (18, 19, 20, 21). This phenomenon is called the incretin effect and is due to nutrient-induced secretion of GIP and GLP-1 from enteroendocrine K and L cells, respectively. It remains unknown whether the calorically dense compound alcohol, which can be considered a macronutrient, induces an incretin effect, and the effects of intragastric ethanol infusion (IGEI) and isoethanolaemic intravenous ethanol infusion (IVEI), respectively, have never been evaluated.
In this study we investigated (1) if alcohol elicits an incretin effect, (2) the potential role of the gut in alcohol metabolism and (3) alcohol’s effect on glucose metabolism.
Materials and methods
Approval and ethics
The study was approved by the Scientific-Ethical Committee of the Capital Region of Denmark (identification no. H-16026085) and the Danish Data Protection Agency and registered on Clinicaltrials.gov (clinical trial Identifier: NCT03348371). The study was conducted according to the Helsinki Declaration II and written informed consent was obtained from all participants.
Subjects
We included 12 healthy Caucasian men between 20 and 50 years of age (characteristics in Table 1). Key inclusion criteria were body mass index (BMI) between 19 and 25 kg/m2, weekly alcohol intake of less than 14 units of alcohol (1 unit = 12 g alcohol), fasting plasma glucose <6 mmol/L, haemoglobin A1c <6% (<42 mmol/mol), normal haemoglobin and written informed consent. Key exclusion criteria were liver disease or other alcohol-related disease and nephropathy and first-degree relatives with type 1 diabetes, type 2 diabetes and/or liver disease.
Table 1.
Characteristics of participants.
| Participants (n = 12) | Mean ± s.d. |
|---|---|
| Sex (male/female) | 12/0 |
| Age (years) | 25 ± 3.9 |
| Body weight (kg) | 77 ± 7.9 |
| Height (m) | 1.85 ± 0.1 |
| Body mass index (kg/m2) | 23 ± 2.6 |
| Fasting plasma glucose (mmol/L) | 5.2 ± 0.4 |
| Haemoglobin A1c (%) | 5.3 ± 0.4 |
| Haemoglobin A1c (mmol/mol) | 30 ± 2.8 |
| HOMA2-IR | 0.7 ± 0.2 |
| ALAT (U/L) | 28 ± 11 |
| Creatinine (μmol/L) | 87 ± 12 |
ALAT, alanine aminotransferase; HOMAR2-IR, homeostasis model assessment 2 of insulin resistance (44); s.d., standard deviation.
Experimental procedures
The study comprised two separate experimental days performed in randomised order (using a randomisation list generated from www.random.org). On the experimental days, the participants received an infusion of alcohol either via an intravenous catheter or intragastrically through a nasogastric tube (to ensure blinding and avoid the initial metabolism of alcohol in the mucosa of the mouth (22)). The alcohol infusions were designed (see below) to obtain isoethanolaemia on the two study days. The route of alcohol administration was double-blinded using similarly looking saline infusions administered simultaneously via the opposite route of administration, that is, intravenous placebo was given on the day of intragastric alcohol administration and vice versa. A laboratory technician was responsible for preparing the infusions; this person was otherwise not involved in the study. The participants abstained from alcohol intake for 5 days prior to each of the experimental days and met after an overnight fast (10 h). An intravenous catheter was inserted into an antecubital vein in each arm; one for infusion (alcohol or placebo) and one for blood sample collection, and a nasogastric tube was inserted. The hand of the forearm, from which blood samples were drawn, was wrapped in a heating pad (~42ºC) throughout the experiment for arterialisation of the blood. At time point 0 min, the participants received alcohol (0.70 g alcohol per kg body weight (20% alcohol solution (v/w)) mixed in isotonic saline water, equivalent to 20.3 kJ per kg body weight) or placebo (saline) via the gastric tube for 5 min or alcohol (same amount) was infused for 45 min through the intravenous catheter. The time for the intravenous infusion was based on a study made by Christiansen et al. (8) in which a bolus of 0.66 g alcohol/kg was given orally and peaked after 45–60 min. Blood samples were sampled at time points −30, −15, 0, 15, 30, 45, 60, 90, 120, 180 and 240 min. For bedside measurement of plasma glucose, blood was collected in sodium-fluoride tubes and centrifuged (7400 g) immediately for 30 s. For the analyses of insulin and C-peptide in serum, blood was sampled in plain tubes and left to coagulate for 20 min. For analyses of FGF21, GIP, GLP-1 and glucagon in plasma, blood was collected in chilled EDTA tubes containing EDTA and a specific dipeptidyl peptidase 4 (DPP-4) inhibitor (valine pyrrolidide, 0.01 mmol/L, Novo Nordisk). For the analysis of alcohol, blood was collected in lithium-heparin tubes. All tubes were centrifuged for 15 min at 2000 g and 4°C. Plasma and serum samples were stored at −20 and −80°C, respectively, until analyses.
Analyses
Glucose was analysed on a glucose analyzer (YSI 2300 STAT glucose analyzer, Xylem Inc., Yellow Springs, OH, USA). Plasma alcohol was analysed by reflectance photometry at 340 nm (Vitros, Ortho-Clinical Diagnostics). Serum insulin and C-peptide were analysed with two-sided electrochemiluminescence assays (Roche/Hitachi Modular Analytics; Roche Diagnostics). Total plasma GLP-1 (23), total plasma GIP (21) and glucagon (24) were measured by RIAs as previously described. Intact (i.e. full-length and active) plasma FGF21 was analysed by ELISA using detection and capture antibodies targeted to the N and C-termini of the full-length human protein (EagleBiosciences, Nashua NH, USA Cat#: F21K31-K01) (13).
Calculations and statistical analysis
Baseline values for each endpoint were calculated as a mean of time points −30, −15 and 0 min for the plasma/serum samples. Area under the curve (AUC) was calculated by the trapezoid rule and compared with paired t tests. Changes over time were calculated using two-way repeated-measures ANOVA and Tukey's multiple comparison was used to test for differences over time, between alcohol administration forms and for the interaction between intervention and time. Differences resulting in P values <0.05 were accepted as statistically significant. Statistical analyses and graphs were made using GraphPad Prism 7.02 (GraphPad Software, Inc.). Data are presented as mean ± s.d. unless otherwise stated.
Insulin secretion rates (ISRs) were calculated using EasyISEC 1.01 software based on C-peptide concentrations, age, height, weight and population-based variables for C-peptide kinetics as previously described (25). Insulin/glucose ratio was calculated for each data point. Insulinogenic index was calculated as the insulin delta value from baseline to 30 min divided by the glucose delta value from baseline to 30 min (Δinsulin0–30 min/Δglucose0–30 min) (26). The presented gluco-metabolic results represent a sub-study of an investigation of hepatic inflammation (unpublished). Therefore, the sample size was calculated to detect a minimal difference of 15% in the inflammation marker CD163.
Results
Characteristics of participants are shown in Table 1. During the experiments, none of the participants reported unpleasant symptoms of intoxication like nausea, headache or vomiting. Three of the participants developed self-limiting superficial phlebitis after the alcohol infusion. One of the participants experienced symptomatic hypoglycaemia (plasma glucose 2.9 mmol/L) 180 min after receiving IVEI after which the participant received a cup of juice to prevent further drop in plasma glucose. Therefore, only 11 participants are included in the two-way repeated-measurement ANOVA.
Alcohol
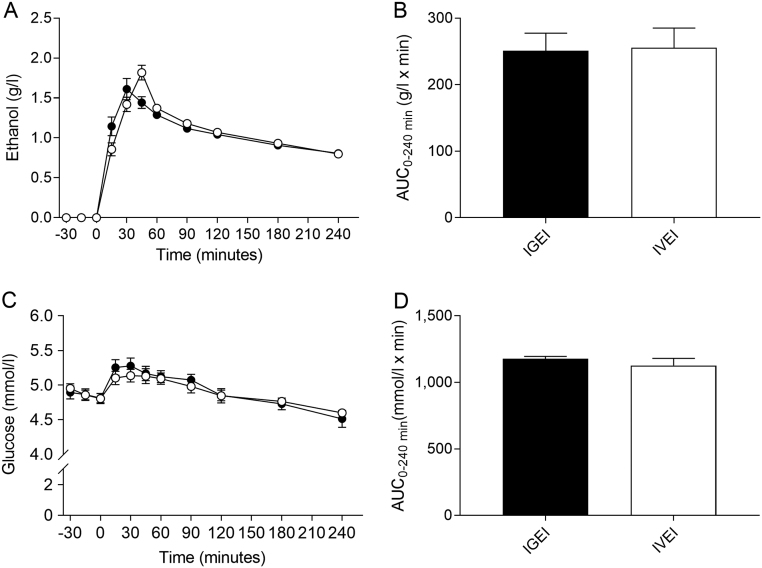
All participants started with a plasma ethanol concentration of 0 g/L on both days. Plasma concentrations increased immediately, rapidly and similarly after the two alcohol administration forms (Fig. 1A, Table 2). There were no significant differences in plasma alcohol concentrations between the two administration forms; therefore, isoethanolaemia was obtained (P = 0.38) (Fig. 1A and B, Table 2).
Figure 1.
Plasma ethanol concentrations (A) and corresponding area under the curve (AUC) values (B) and plasma glucose concentrations (C) and corresponding AUCs (D) during intragastric alcohol infusion (IGEI) (black circles) and intravenous alcohol infusion (IVEI) (white circles). Data are mean ± s.e.m.
Table 2.
Alcohol, glucose, insulin, C-peptide, glucagon, GIP, GLP-1 and FGF21.
| Administration form: | IGEI | IVEI | T test (P values) |
|---|---|---|---|
| Alcohol | |||
| Baseline (g/L) | 0 ± 0 | 0 ± 0 | |
| AUC0–240 min (g/L × min) | 251 ± 26 | 256 ± 30 | 0.38 |
| Peak plasma concentration (g/L) | 1.7 ± 0.3 | 1.8 ± 0.3 | 0.22 |
| Time to peak (min) | 38 ± 18 | 44 ± 4.3 | 0.24 |
| Glucose | |||
| Baseline (mmol/L) | 4.9 ± 0.3 | 4.9 ± 0.2 | 0.54 |
| AUC0-240 min (mmol/L × min) | 1177 ± 65 | 1127 ± 186 | 0.30 |
| Peak plasma concentration (mmol/L) | 5.3 ± 0.4 | 5.3 ± 0.3 | 0.34 |
| Time to peak (min) | 36 ± 53 | 54 ± 60 | 0.65 |
| Insulin | |||
| Baseline (pmol/L) | 53 ± 19 | 50 ± 18 | 0.67 |
| AUC0-240 min (pmol/L × min) | 12 ± 4.0 | 11 ± 3.7 | 0.18 |
| Nadir (pmol/L) | 36 ± 14 | 27 ± 12 | 0.04 |
| Time to nadir (min) | 86 ± 98 | 55 ± 40 | 0.23 |
| C-peptide | |||
| Baseline (pmol/L) | 321 ± 72 | 325 ± 87 | 0.82 |
| AUC0-240 min (pmol/L × min) | 69 ± 17 | 69 ± 21 | 0.95 |
| Nadir (pmol/L) | 242 ± 58 | 235 ± 74 | 0.77 |
| Time to nadir (min) | 151 ± 106 | 105 ± 91 | 0.08 |
| Insulin secretion rate | |||
| Baseline (pmol/kg/min) | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.98 |
| AUC0-240 (pmol/kg/min × min) | 220 ± 56 | 219 ± 53 | 0.97 |
| Nadir (pmol/kg/min) | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.94 |
| Time to nadir (min) | 93 ± 104 | 86 ± 89 | 0.88 |
| Insulin/glucose ratio | |||
| Baseline (pmol/mmol) | 11 ± 2.7 | 10 ± 2.5 | 0.60 |
| AUC0-240 (pmol/mmol × min) | 2504 ± 786 | 2153 ± 687 | 0.20 |
| Nadir (pmol/mmol) | 7.0 ± 2.5 | 5.3 ± 1.8 | 0.04 |
| Time to nadir (min) | 61 ± 88 | 44 ± 4.1 | 0.52 |
| Beta cells | |||
| Insulinogenic index | −29 ± 88 | −127 ± 152 | 0.05 |
| Glucagon | |||
| Baseline (pmol/L) | 6.0 ± 3.8 | 5.1 ± 1.7 | 0.45 |
| AUC0-240 min (pmol/L × min) | 1013 ± 678 | 1227 ± 552 | 0.20 |
| Nadir (pmol/L) | 2.2 ± 1.5 | 2.5 ± 1.6 | 0.37 |
| Time to nadir (min) | 41 ± 34 | 40 ± 27 | 0.91 |
| GIP | |||
| Baseline (pmol/L) | 17 ± 7.7 | 15 ± 4.6 | 0.14 |
| AUC0-240 min (pmol/L × min) | 2479 ± 1071 | 2496 ± 1240 | 0.96 |
| Peak plasma concentration (pmol/L) | 18 ± 5.6 | 18 ± 7.8 | 0.89 |
| Time to peak (min) | 70 ± 81 | 160 ± 73 | 0.01 |
| GLP-1 | |||
| Baseline (pmol/L) | 19 ± 7.9 | 21 ± 9.4 | 0.52 |
| AUC0-240 min (pmol/L × min) | 5093 ± 2103 | 4266 ± 1707 | 0.24 |
| Peak plasma concentration (pmol/L) | 30 ± 9.2 | 30 ± 7.9 | 0.67 |
| Time to peak (min) | 44 ± 34 | 65 ± 113 | 0.56 |
| FGF21 | |||
| Baseline (pg/mL) | 31 ± 35 | 30 ± 35 | 0.89 |
| AUC0-240 min (pg/mL × min) | 37,738 ± 27,855 | 32,592 ± 20,728 | 0.21 |
Differences between groups were compared with paired T test. Data are mean ± s.d.
AUC, area under the curve; FGF21; fibroblast growth factor 21; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; IGEI, intragastric alcohol infusion; IVEI, intravenous alcohol infusion.
Glucose
Baseline plasma glucose concentrations were similar before IGEI and IVEI, respectively (P = 0.54) (Table 2). Plasma glucose concentrations showed significant changes over time (P < 0.0001) but not between interventions (P = 0.66) or for time × intervention (P = 0.21). During both alcohol administration forms, plasma glucose concentrations increased by ~0.4 mmol/L and hereafter slowly decreased to attain significantly lower concentrations compared to baseline after 240 min (4.5 ± 0.4 mmol/L (P = 0.009) and 4.6 ± 0.2 mmol/L (P = 0.002) after IGEI and IVEI, respectively) (Fig. 1C). AUCs for plasma glucose were similar during IGEI and IVEI (P = 0.20) (Fig. 1D, Table 2).
Insulin and C-peptide
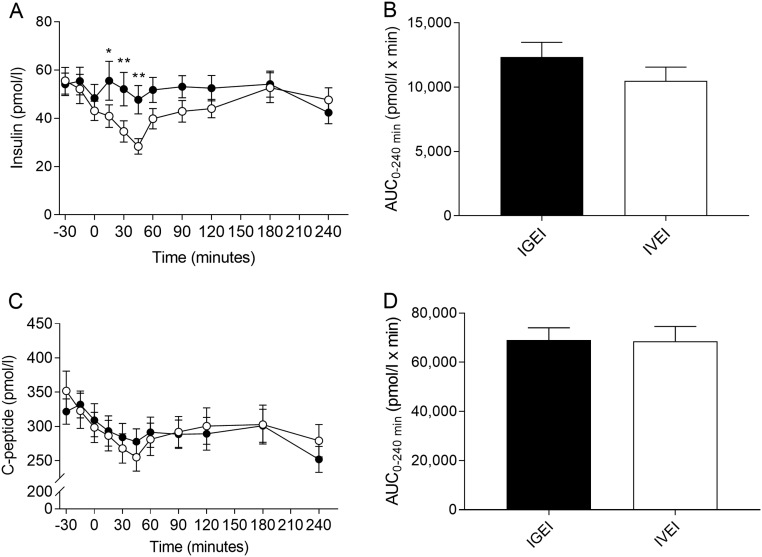
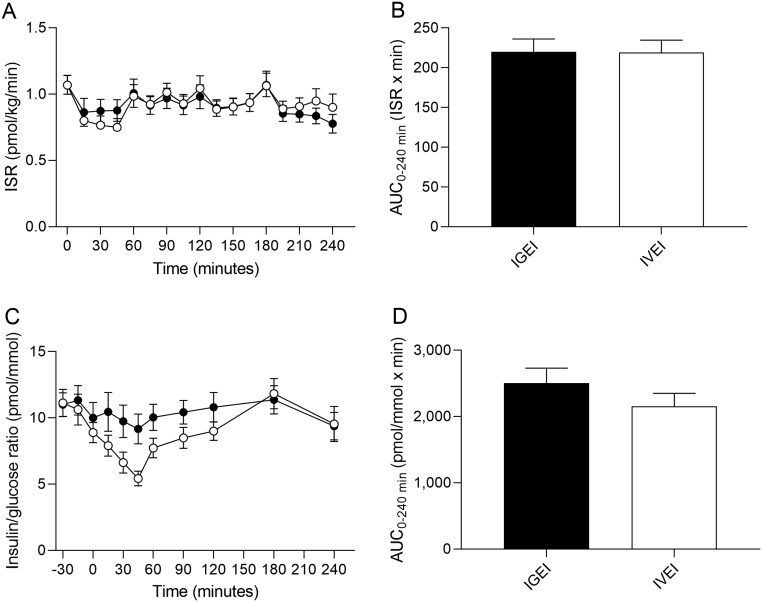
We observed no significant differences between serum insulin concentrations at baseline or between AUCs during the two administration forms (Fig. 2A and B, Table 2). Serum insulin concentrations were unaffected by IGEI, whereas the concentrations declined to a mean nadir of 27 ± 12 pmol/L (P < 0.0001 and P = 0.04 compared to baseline and nadir of IGEI, respectively) at 55 ± 40 min during IVEI after which concentrations increased towards baseline (Fig. 2A). A significant difference was seen over time (P < 0.0001) and for time × intervention (P = 0.0064), but not for intervention (P = 0.18) evaluated using two-way repeated-measures ANOVA. Significant differences between serum insulin concentrations during IGEI and IVEI were observed for time points 15, 30 and 45 min (P = 0.013, P = 0.004 and P = 0.001, respectively) (Fig. 2A). Insulin/glucose ratio fell significantly after alcohol administration compared to baseline (P < 0.0001 after both IGEI and IVEI); however, nadir after IVEI was significantly lower compared to IGEI (P = 0.04) (Fig. 3C and D, Table 2). Serum C-peptide concentrations were similar at baseline and decreased similarly after both alcohol administration forms to reach concentrations below normal range for healthy individuals (27) and significantly below baseline concentrations (P < 0.0001 for both IGEI and IVEI) (Fig. 2C and D, Table 2). There was no difference between baseline values and AUCs for ISR during the two administration forms (Fig. 3A and B, Table 2). ISR fell similarly and significantly after both alcohol administration forms compared to baseline (P < 0.0001 after both IGEI and IVEI). Beta cell function evaluated by insulinogenic index was more reduced after IVEI compared to IGEI (P = 0.05) (Table 2).
Figure 2.
Serum insulin concentrations (A) and corresponding area under the curve (AUC) values (B) and serum C-peptide concentrations (C) and corresponding AUCs (D) during intragastric alcohol infusion (IGEI) (black circles) and intravenous alcohol infusion (IVEI) (white circles). Asterisks indicate statistically significant interactions between intervention and time evaluated by multiple comparisons. *P < 0.05; **P < 0.005. Data are mean ± s.e.m.
Figure 3.
Insulin secretion rate (ISR) (A) and corresponding area under the curve (AUC) values (B) and insulin/glucose ratio (C) and corresponding AUCs (D) during intragastric alcohol infusion (IGEI) (black circles) and intravenous alcohol infusion (IVEI) (white circles). Data are mean ± s.e.m.
Glucagon
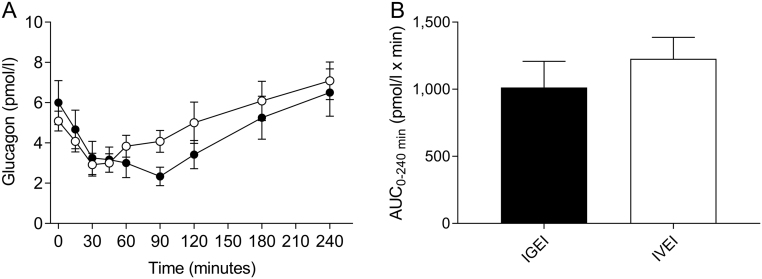
Baseline plasma glucagon concentrations were similar on the two experimental days and decreased significantly (P = 0.0004 and P < 0.0001 for IGEI and IVEI, respectively) and similarly compared to baseline after both forms of alcohol administration, reaching mean nadirs at time point 40 ± 27 min (IVEI) and time point 41 ± 34 min (IGEI), respectively, after which glucagon concentrations returned towards baseline concentrations (Fig. 4A and B, Table 2).
Figure 4.
Plasma glucagon concentrations (A) and corresponding area under the curve (AUC) values (B) during intragastric alcohol infusion (IGEI) (black circles) and intravenous alcohol infusion (IVEI) (white circles). Data are mean ± s.e.m.
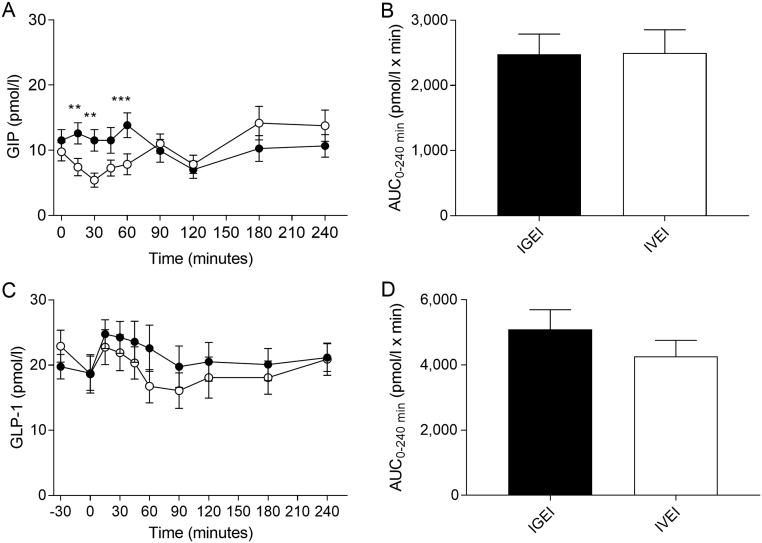
GIP and GLP-1
Baseline plasma concentrations of GIP and GLP-1, respectively, were similar on both experimental days and no significant differences in GIP or GLP-1 responses (as assessed by AUCs) were observed between IGEI and IVEI (Fig. 5A and B, Table 2). Similar peak plasma GLP-1 (P = 0.67) and GIP (P = 0.89) concentrations, respectively, were observed during IGEI and IVEI. We observed a small but significant variation over time in both plasma GIP (P < 0.0001) and plasma GLP-1 (P = 0.01) concentrations during the two experimental days. For plasma GIP a significant difference was seen for time × intervention (P = 0.0001), but not for intervention (P = 0.10) evaluated using two-way repeated-measures ANOVA. A difference was observed between IGEI and IVEI at the time points 15, 30 and 60 min (P = 0.005, P = 0.005 and P = 0.0004, respectively) (Fig. 5A and B).
Figure 5.
Plasma glucose-dependent insulinotropic polypeptide (GIP) concentrations (A) and corresponding area under the curve (AUC) values (B), and plasma glucagon-like peptide 1 (GLP-1) concentrations (C) and corresponding AUCs (D) during intragastric alcohol infusion (IGEI) (black circles) and intravenous alcohol infusion (IVEI) (white circles). Asterisks indicate statistically significant interactions between intervention and time were evaluated by multiple comparisons. **P < 0.005; ***P < 0.0005. Data are mean ± s.e.m.
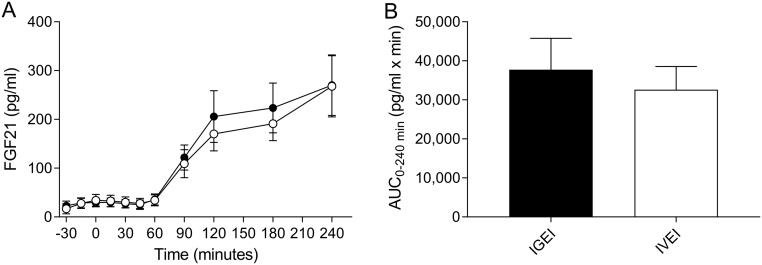
FGF21
There was no significant difference between baseline plasma FGF21 concentrations during the two experimental days (Table 2). Plasma FGF21 concentrations started to increase 60 min after ethanol administration (~20 min after serum ethanol had peaked) and after 240 min FGF21 had increased approximately nine-fold from 31 ± 35 to 270 ± 215 pg/mL for IGEI and from 30 ± 35 to 268 ± 217 pg/mL for IVEI (without signs of peaking and with no difference between the two administration forms (P = 0.97)) (Fig. 6A and B, Table 2).
Figure 6.
Plasma fibroblast growth factor 21 (FGF21) concentrations (A) and corresponding area under the curve (AUC) values (B) during intragastric alcohol infusion (IGEI) (black circles) and intravenous alcohol infusion (IVEI) (white circles). Data are mean ± s.e.m.
Discussion
In this study we provide a detailed description of how acute alcohol (administered intragastrically as well as intravenously) affects circulating concentrations of insulin, glucagon, GIP, GLP-1 and the liver-derived hormone FGF21. Overall, alcohol seems to (1) inhibit the secretion of both insulin and glucagon (independently of administration form), (2) have little or no effect on the secretion of the incretin hormones GIP and GLP-1 and (3) dramatically increase the secretion of FGF21 during both IGEI and IVEI.
Alcohol lowers glucose concentrations regardless of administration form
Alcohol consumption is associated with increased risk of hypoglycaemia (3). However, the mechanism(s) behind this is unclear. We observed an initial increase in plasma glucose concentration ~30–35 min after alcohol administration and a decline to below baseline values after ~3.5 h. The decline could be due to the continued fasting of the participants and not the incretin hormones, insulin or the alcohol infusion itself. We did not include a placebo intervention (intragastric and intravenous placebo administration) which is a limitation of our study. However, previous studies of saline infusions (intraduodenally and intravenous) in healthy subjects observed no changes in the secretion of pancreatic or incretin hormones (28, 29). In addition to the reduced glucagon concentrations we observed after ethanol administration (discussed below), hepatic metabolism of ethanol may contribute to the risk of hypoglycaemia. Alcohol is metabolised in the liver by alcohol dehydrogenase to acetaldehyde and subsequently to acetate. These reactions reduce nicotinamide adenine dinucleotide (NAD+) to NADH and thus facilitate a decline in the NAD+/NADH ratio, which inhibits several dehydrogenases essential for gluconeogenesis (2, 3).
Alcohol reduces insulin secretion after IGEI as well as IVEI
High alcohol consumption may cause insulin resistance (30, 31). Traditionally, the mechanism is believed to involve alcohol-induced increase in NADH inhibiting fatty acid oxidation, promoting steatosis and inducing liver inflammation (32, 33). The direct effect of alcohol on insulin secretion has been investigated with ambiguous results (6, 7, 10). Insulin concentrations decreased after intravenous alcohol administration in healthy individuals (7). In contrast, pre-treatment with oral alcohol increased AUCs of insulin and C-peptide during an intravenous glucose tolerance test in healthy individuals (6). Also, glucose and insulin concentrations increased after oral alcohol administration and subsequent continuous intravenous alcohol infusion maintaining constant alcohol concentration of about 2 mmol/L in healthy subjects (10). In the present study, we observed no stimulating effect of alcohol on insulin concentrations after IGEI but a small decrease during IVEI. In line with this, insulin/glucose ratios decreased after alcohol administration reaching a lower nadir after IVEI. This could be due to the relatively higher portal alcohol concentrations after IGEI reducing hepatic insulin extraction more than isoethanolaemic IVEI. In contrast, C-peptide undergoes negligible hepatic extraction which is why circulating C-peptide constitutes a more precise measure of pancreatic insulin secretion than circulating insulin (34). Interestingly, we observed a decline in C-peptide and ISR after both administration forms, which suggests that alcohol suppresses insulin secretion independently of alcohol’s potential effects in the gut. Also, beta cell function evaluated by insulinogenic index appeared to be impaired after IVEI compared to IGEI. Nevertheless, the insulinogenic index was negative after both administration forms, which may indicate an impaired beta cell function after acute alcohol intake. The insulinogenic index is a validated measure of beta cell function after an oral glucose tolerance test and its use during the conditions investigated in the present study may be questioned. Moreover, we used only a single dose of alcohol; repeated dosing may show different results.
Alcohol decreases glucagon concentrations regardless of intestinal stimulation
The effect of alcohol on glucagon secretion has been studied with inconsistent results (9, 10). Intravenous administration of alcohol enhanced alanine-stimulated glucagon release (9), whereas the counter-regulatory glucagon response to insulin-induced hypoglycaemia in young healthy men was delayed and diminished after oral and subsequent maintenance by intravenous alcohol administration (10). In the latter study, alcohol administration had no effect on fasting glucagon concentrations (10) which contrasts to our findings. A direct inhibitory effect of alcohol on the alpha cells or the initially increased glucose concentrations may explain the decline in glucagon concentrations during both administration forms in the present study. Nonetheless, the present results demonstrate that the effect of alcohol on glucagon secretion is not due to mechanisms derived from an intestinal stimulus.
Alcohol does not elicit an incretin effect
The incretin effect is defined as the relative increment in insulin response to oral vs intravenous administration of glucose during similar plasma glucose concentrations (due to insulinotropic actions of the gut-derived incretin hormones) (35). The incretin effect of alcohol has never been investigated properly (comparing incretin concentrations after IGEI vs IVEI). However, the effect of alcohol on circulating concentrations of incretin hormones has previously been studied under various conditions and with inconsistent results (6, 11, 12). Oral alcohol did not have a significant effect on GLP-1 secretion before an intravenous glucose tolerance test in healthy participants (6). In addition, orally administered alcohol did not affect GLP-1 secretion in fasting healthy individuals (11). However, oral alcohol administered during a fat-rich mixed meal suppressed the early postprandial GIP and GLP-1 responses in patients with type 2 diabetes (12). In the present study, we did not observe any physiologically relevant changes in GLP-1 or GIP concentrations during IGEI or IVEI. This suggests that alcohol – unlike other macronutrients – does not stimulate GIP or GLP-1 after intragastric administration, and, combined with the lack of any stimulatory effect of alcohol on insulin secretion (see above), that alcohol intake does not elicit an incretin effect.
Alcohol increases circulating FGF21 concentrations dramatically regardless of administration route
Studies in rodents have shown that exogenous FGF21 decreases the taste preference for sweets and alcohol (14, 36). Also, recent human evidence supports the notion that the FGF21 gene plays a role in taste preference for sweets and alcohol consumption (37, 38, 39, 40) positioning FGF21 as a potential negative regulator of alcohol consumption. Furthermore, FGF21 has been shown to increase insulin sensitivity and decrease hepatic glucose production in mice (15) and an FGF21 analogue has been shown to exert insulinotropic actions in patients with type 2 diabetes (41). In the present study, FGF21 concentrations increased dramatically, which is consistent with previous studies (13, 14). This could point towards FGF21 as a factor linking alcohol intake and glucose metabolism and supports the existence of a liver-brain feedback loop; ingestion of alcohol increases FGF21 secretion which in turn may decrease alcohol intake via the central nervous system (39, 40). Therefore, FGF21 may not only constitute a potential future antidiabetic agent but perhaps also a potential target for patients diagnosed with alcohol use disorder. Interestingly, the temporal secretion of FGF21 was not parallel to the plasma alcohol concentrations. As previously described (14), we observed plasma alcohol to peak ~40 min after alcohol administration, whereas plasma FGF21 rose after ~60 min and kept rising throughout the study period (240 min). Samms et al. (42) found FGF21 secretion to be stimulated by increased insulin concentrations, and others have found acute glucagon administration to cause an increase in FGF21 concentrations in humans (43). However, in the present study we saw no increases in insulin or glucagon; precluding that elevations in insulin and/or glucagon are required for FGF21 secretion. Rather, FGF21 may be secreted as a direct response to alcohol exposure and FGF21 actions could result in the reduced insulin secretion after alcohol administration seen in this study. However, the mechanisms underlying alcohol-induced FGF21 secretion and actions related to insulin secretion remain to be established.
Conclusion
Alcohol – unlike other macronutrients (i.e. carbohydrate, lipid and protein) – does not seem to stimulate GIP or GLP-1 after intragastric administration. Moreover, alcohol appears to decrease the secretion of both glucagon and insulin from the pancreas independently of the route of administration (intragastrically vs intravenously). The higher concentrations of circulating FGF21 observed after both alcohol administration forms, suggest alcohol to be a stimulator of FGF21 secretion acting independently of the gut (and gut-derived factors), glucagon and insulin.
Declaration of interest
A R L, L S G, N C B, S B, M M H, M P G, B H and T V have nothing to declare. J J H has served on advisory boards, served as a consultant and/or received research support from AstraZeneca, Hanmi, Intarcia, Merck, MSD, NovoNordisk and Zealand Pharma. F K K has served on scientific advisory panels and/or been part of speaker’s bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi and Zealand Pharma.
Funding
This work was supported by Herlev-Gentofte’s Research Foundation (2016), Brødrene Hartmann’s Foundation (2016) and the A. P. Møller Foundation (2016).
Acknowledgments
The authors are thankful to all study subjects for their participation and lab technicians Sisse M Schmidt and Inass Al-Nachar (Center for Clinical Metabolic Research, Gentofte Hospital) for their invaluable help with experimental procedures. In addition, they want to thank Lene Albæk (Department for Biomedical Sciences, University of Copenhagen) for GIP, GLP-1 and glucagon measurements.
References
- 1.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. Journal of Hepatology 2013. 160–168. ( 10.1016/j.jhep.2013.03.007) [DOI] [PubMed] [Google Scholar]
- 2.Steiner JL, Crowell KT, Lang CH. Impact of alcohol on glycemic control and insulin action. Biomolecules 2015. 2223–2246. ( 10.3390/biom5042223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandi S, deshpande N, rao P, Ramana KV. Alcoholism and its relation to hypoglycemia – an overview. American Journal of Medicine Studies 2014. 46–49. ( 10.12691/ajms-2-3-2) [DOI] [Google Scholar]
- 4.Zhao YY, Yang R, Xiao M, Guan MJ, Zhao N, Zeng T. Kupffer cells activation promoted binge drinking-induced fatty liver by activating lipolysis in white adipose tissues. Toxicology 2017. 53–60. ( 10.1016/j.tox.2017.09.001) [DOI] [PubMed] [Google Scholar]
- 5.Taguchi K, Yamanaka-Okumura H, Mizuno A, Nakamura T, Shimada M, Doi T, Takeda E. Insulin resistance as early sign of hepatic dysfunction in liver cirrhosis. Journal of Medical Investigation 2014. 180–189. ( 10.2152/jmi.61.180) [DOI] [PubMed] [Google Scholar]
- 6.Svartberg J, Holst JJ, Gutniak M, Adner N. The ethanol augmentation of glucose-induced insulin secretion is abolished by calcium antagonism with nifedipine: no evidence for a role of glucagon-like peptide-1 (GLP-1). Pancreas 1998. 66–71. ( 10.1097/00006676-199801000-00011) [DOI] [PubMed] [Google Scholar]
- 7.Leggio L, Schwandt ML, Oot EN, Dias AA & Ramchandani VA. Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within-subject placebo controlled study. Psychoneuroendocrinology 201. 3 38 3085–3091. ( 10.1016/j.psyneuen.2013.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen C, Thomsen C, Rasmussen O, Hansen C, Hermansen K. The acute impact of ethanol on glucose, insulin, triacylglycerol, and free fatty acid responses and insulin sensitivity in type 2 diabetes. British Journal of Nutrition 1996. 669–675. ( 10.1079/bjn19960074) [DOI] [PubMed] [Google Scholar]
- 9.Aagaard NK, Thøgersen T, Grøfte T, Greisen J, Vilstrup H. Alcohol increases glucagon secretion in normal man. Alcoholism, Clinical and Experimental Research 2004. 1643–1647. ( 10.1097/01.alc.0000145780.32355.4f) [DOI] [PubMed] [Google Scholar]
- 10.Kolaczynski JW, Ylikahri R, Härkonen M, Koivisto VA. The acute effect of ethanol on counterregulatory response and recovery from insulin-induced hypoglycemia. Journal of Clinical Endocrinology and Metabolism 1988. 384–388. ( 10.1210/jcem-67-2-384) [DOI] [PubMed] [Google Scholar]
- 11.Calissendorff J, Gustafsson T, Holst JJ, Brismar K, Röjdmark S. Alcohol intake and its effect on some appetite-regulating hormones in man: influence of gastroprotection with sucralfate. Endocrine Research 2012. 154–162. ( 10.3109/07435800.2012.662662) [DOI] [PubMed] [Google Scholar]
- 12.Dalgaard M, Thomsen C, Rasmussen BM, Holst JJ, Hermansen K. Ethanol with a mixed meal decreases the incretin levels early postprandially and increases postprandial lipemia in type 2 diabetic patients. Metabolism: Clinical and Experimental 2004. 77–83. ( 10.1016/j.metabol.2003.08.011) [DOI] [PubMed] [Google Scholar]
- 13.Søberg S, Andersen ES, Dalgaard NB, Jarlhelt I, Hansen NL, Hoffmann N, Vilsbøll T, Chenchar A, Jensen M, Grevengoed TJ, et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Molecular Metabolism 2018. 96–103. ( 10.1016/j.molmet.2018.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher FM, Mather ML, Vardeh HG, Douris N, Adams AC, et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Molecular Metabolism 2017. 1395–1406. ( 10.1016/j.molmet.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014. 4057–4063. ( 10.2337/db14-0595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular Metabolism 2015. 51–57. ( 10.1016/j.molmet.2014.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundsgaard AM, Fritzen AM, Sjøberg KA, Myrmel LS, Madsen L, Wojtaszewski JFP, Richter EA, Kiens B. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Molecular Metabolism 2017. 22–29. ( 10.1016/j.molmet.2016.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauck MA, Homberger E, Siegel EG, Allen R C, Eaton RP,, Ebert R & Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. Journal of Clinical Endocrinology and Metabolism 1986. 492–498. ( 10.1210/jcem-63-2-492) [DOI] [PubMed] [Google Scholar]
- 19.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahrén B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. American Journal of Physiology-Endocrinology and Metabolism 2008. E779–E784. ( 10.1152/ajpendo.90233.2008) [DOI] [PubMed] [Google Scholar]
- 20.Lindgren O, Pacini G, Tura A, Holst JJ, Deacon CF, Ahrén B. Incretin effect after oral amino acid ingestion in humans. Journal of Clinical Endocrinology and Metabolism 2015. 1172–1176. ( 10.1210/jc.2014-3865) [DOI] [PubMed] [Google Scholar]
- 21.Lindgren O, Carr RD, Deacon CF, Holst JJ, Pacini G, Mari A, Ahrén B. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Journal of Clinical Endocrinology and Metabolism 2011. 2519–2524. ( 10.1210/jc.2011-0266) [DOI] [PubMed] [Google Scholar]
- 22.Dong YJ, Peng TK, Yin SJ. Expression and activities of class IV alcohol dehydrogenase and class III aldehyde dehydrogenase in human mouth. Alcohol 1996. 257–262. ( 10.1016/0741-8329(95)02052-7) [DOI] [PubMed] [Google Scholar]
- 23.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994. 535–539. ( 10.2337/diab.43.4.535) [DOI] [PubMed] [Google Scholar]
- 24.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. Journal of Clinical Investigation 1991. 415–423. ( 10.1172/JCI115012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Computer Methods and Programs in Biomedicine 1996. 253–264. ( 10.1016/0169-2607(96)01755-5) [DOI] [PubMed] [Google Scholar]
- 26.Pacini G, Tura A, Vinzer C, Kautzky-Willer A. The insulinogenic index is a valid marker of beta cell function in different metabolic categories. Diabetes 2005. A370. [Google Scholar]
- 27.Larsen PB, Linneberg A, Hansen T, Friis-Hansen L. Reference intervals for C-peptide and insulin derived from a general adult Danish population. Clinical Biochemistry 2017. 408–413. ( 10.1016/j.clinbiochem.2016.12.004) [DOI] [PubMed] [Google Scholar]
- 28.Vamvini MT, Hamnvik OP, Sahin-Efe A, Gavrieli A, Dincer F, Farr OM, Mantzoros CS. Differential effects of oral and intravenous lipid administration on key molecules related to energy homeostasis. Journal of Clinical Endocrinology and Metabolism 2016. 1989–1997. ( 10.1210/jc.2015-4141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veedfald S, Wu T, Bound M, Grivell J, Hartmann B, Rehfeld JF, Deacon CF, Horowitz M, Holst JJ, Rayner CK. Hyperosmolar duodenal saline infusion lowers circulating ghrelin and stimulates intestinal hormone release in young men. Journal of Clinical Endocrinology and Metabolism 2018. 4409–4418. ( 10.1210/jc.2018-00699) [DOI] [PubMed] [Google Scholar]
- 30.Carlsson S, Hammar N, Efendic S, Persson PG, Ostenson CG, Grill V. Alcohol consumption, type 2 diabetes mellitus and impaired glucose tolerance in middle-aged Swedish men. Diabetic Medicine 2000. 776–781. ( 10.1046/j.1464-5491.2000.00387.x) [DOI] [PubMed] [Google Scholar]
- 31.Carlsson S, Hammar N, Grill V, Kaprio J. Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish twin cohort study. Diabetes Care 2003. 2785–2790. ( 10.2337/diacare.26.10.2785) [DOI] [PubMed] [Google Scholar]
- 32.Carr RM, Correnti J. Insulin resistance in clinical and experimental alcoholic liver disease. Annals of the New York Academy of Sciences 2015. 1–20. ( 10.1111/nyas.12787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004. 9–19. ( 10.1016/j.alcohol.2004.07.008) [DOI] [PubMed] [Google Scholar]
- 34.Rubenstein AH, Pottenger LA, Mako M, Getz GS, Steiner DF. The metabolism of proinsulin and insulin by the liver. Journal of Clinical Investigation 1972. 912–921. ( 10.1172/JCI106886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986. 46–52. ( 10.1007/bf02427280) [DOI] [PubMed] [Google Scholar]
- 36.Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, et al. FGF21 regulates sweet and alcohol preference. Cell Metabolism 2016. 344–349. ( 10.1016/j.cmet.2015.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. American Journal of Clinical Nutrition 2013. 1395–1402. ( 10.3945/ajcn.112.052183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Qi Q, Curhan GC, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Human Molecular Genetics 2013. 1895–1902. ( 10.1093/hmg/ddt032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Søberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WLP, Potthoff MJ, et al. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metabolism 2017. 1045–1053.e6. ( 10.1016/j.cmet.2017.04.009) [DOI] [PubMed] [Google Scholar]
- 40.Schumann G, Liu C, O’Reilly P, Gao H, Song P, Xu B, Ruggeri B, Amin N, Jia T, Preis S, et al. KLB is associated with alcohol drinking, and its gene product β-klotho is necessary for FGF21 regulation of alcohol preference. PNAS 2016. 14372–14377. ( 10.1073/pnas.1611243113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 Analog, in obese human subjects with type 2 diabetes. Cell Metabolism 2013. 333–340. ( 10.1016/j.cmet.2013.08.005) [DOI] [PubMed] [Google Scholar]
- 42.Samms RJ, Lewis JE, Norton L, Stephens FB, Gaffney CJ, Butterfield T, Smith DP, Cheng CC, Perfield JW, Adams AC, et al. FGF21 is an insulin-dependent postprandial hormone in adult humans. Journal of Clinical Endocrinology and Metabolism 2017. 3806–3813. ( 10.1210/jc.2017-01257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habegger KM, Stemmer K, Cheng C, Müller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 2013. 1453–1463. ( 10.2337/db12-1116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geloneze B, Vasques ACJ, Stabe CFC, Pareja JC, Rosado LE, Queiroz EC, Tambascia MA. & BRAMS Investigators. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arquivos Brasileiros de Endocrinologia e Metabologia 2009. 281–287. ( 10.1590/S0004-27302009000200020) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a