Version Changes
Revised. Amendments from Version 1
The main aim of this publication is to facilitate sharing of the global mass spectrometry data generated from the skin organoid models with FLG knockdown (described in detail) and functional analyses (described and reported); this main aim has been clarified. In response to the interest from Reviewers 1 and 3, we have provided an explanation as to why knockdown of FLG at mRNA level is demonstrated to a lesser degree than the knockdown at protein level, reflecting the prolonged duration (10 days) of organoid culture. We have emphasised that increased dye penetration was observed in some but not all of the immature organoids, as requested by Reviewer 1. Additional details have been provided to explain how the protein false discovery rate was determined for the removal of false positives, as requested by Reviewer 1. We have included a fuller explanation of the analysis approach, in which we focus on consistent differences observed across the biological replicate experiments and use thresholds for increased and decreased expression to include roughly equal numbers of proteins. We have corrected the use of the terms ‘ratio’ and ‘fold-change’ where necessary. A new Figure 10 is provided, following advice from reviewer 2. The colour-coding has been removed and we have labelled selected data points. During revision of this figure we discovered an error in the plotting of this figure; this detail has been corrected in the revised Methods section and in the re-drawn volcano plot. The title has been modified to include the term ‘full thickness’, to clarify the complexity of our model (as recommended by reviewer 2). The list of main findings has now been removed from the title because it had become too long. Minor typos have been corrected, including two references numbered incorrectly in the previous manuscript.
Abstract
Background: Atopic eczema is an itchy inflammatory disorder characterised by skin barrier dysfunction. Loss-of-function mutations in the gene encoding filaggrin ( FLG) are a major risk factor, but the mechanisms by which filaggrin haploinsufficiency leads to atopic inflammation remain incompletely understood. Skin as an organ that can be modelled using primary cells in vitro provides the opportunity for selected genetic effects to be investigated in detail.
Methods: Primary human keratinocytes and donor-matched primary fibroblasts from healthy individuals were used to create skin organoid models with and without siRNA-mediated knockdown of FLG. Biological replicate sets of organoids were assessed using histological, functional and biochemical measurements.
Results: FLG knockdown leads to subtle changes in histology and ultrastructure including a reduction in thickness of the stratum corneum and smaller, less numerous keratohyalin granules. Immature organoids showed some limited evidence of barrier impairment with FLG knockdown, but the mature organoids showed no difference in transepidermal water loss, water content or dye penetration. There was no difference in epidermal ceramide content. Mass spectrometry proteomic analysis detected >8000 proteins per sample. Gene ontology and pathway analyses identified an increase in transcriptional and translational activity but a reduction in proteins contributing to terminal differentiation, including caspase 14, dermokine, AKT1 and TGF-beta-1. Aspects of innate and adaptive immunity were represented in both the up-regulated and down-regulated protein groups, as was the term ‘axon guidance’.
Conclusions: This work provides further evidence for keratinocyte-specific mechanisms contributing to immune and neurological, as well as structural, aspects of skin barrier dysfunction. Individuals with filaggrin deficiency may derive benefit from future therapies targeting keratinocyte-immune crosstalk and neurogenic pruritus.
Keywords: Axon guidance, atopic dermatitis, eczema, filaggrin, gene ontology, keratinocyte-immune crosstalk, organoid, proteomics
Introduction
Atopic eczema (also termed atopic dermatitis or eczema 1, 2) is an itchy inflammatory skin disorder with complex and multifactorial aetiology 3. The phenotype is heterogeneous and pathogenic mechanisms vary between affected individuals 4. The pathogenesis may include differential contributions of: immune activation and skin barrier dysfunction; local and systemic effects; multiple genetic and environmental factors 5. Loss-of-function mutations in FLG, encoding filaggrin, cause the common Mendelian disorder of keratinization, ichthyosis vulgaris (OMIM # 146700). FLG null mutations are also strongly associated with increased risk of atopic eczema 6, 7 and multiple other atopic traits 8– 10.
Skin is an organ that can be modelled in vitro to effectively recapitulate the multi-layered structure and gene expression patterns of human skin in vivo 11. This offers the opportunity for selected molecular mechanisms to be investigated in relative isolation from a complex disease state, using relevant primary cells. Replicate experiments can be performed using primary cells with the same genetic background or cells from different donors 12. Detailed functional and biochemical analyses may then be applied to define potential therapeutic targets 12, 13.
Filaggrin is a marker of keratinocyte differentiation; it is expressed in the granular layer of the upper epidermis as a polymer – profilaggrin – which undergoes post-translational modification and stepwise proteolysis to release monomeric filaggrin 14. Profilaggrin and filaggrin have multiple functions, each of which have been described as contributing to the mechanical, biochemical, immunological and microbiological aspects of skin barrier function 15. Clinical studies have shown that filaggrin haploinsufficiency is associated with xerosis and ichthyosis (dry and scaly skin), keratosis pilaris (increased keratin within skin follicles), palmar hyperlinearity 16 and increased transepidermal water loss 17. Transcriptomic analysis of full thickness skin biopsies have shown evidence of an abnormal defence response in FLG haploinsufficient atopic skin 18; proteomic analysis to assess the effect of FLG knockdown in an epidermal organoid has also shown features of inflammation and stress protease activity 19. However, in vitro studies have not shown consistent histological or functional effects of filaggrin deficiency in the various different skin organoid models published to date 20 and the multiple mechanisms by which filaggrin deficiency contributes to atopic disease remain incompletely understood 5.
We have optimised a skin organoid model, with dermal and epidermal compartments cultured using donor-matched primary cells, for functional assessments and global mass spectrometry proteomic analysis. The work aims to investigate in more detail the effect of FLG siRNA-mediated knockdown on one cell type - the keratinocyte - to increase understanding of the filaggrin-deficient phenotype and to further define molecular mechanisms predisposing to atopic skin inflammation.
Methods
Source of primary human cells
Primary keratinocytes and primary fibroblasts were isolated from human skin tissue samples obtained, with written informed consent and Ethical Committee approval (East of Scotland Research Ethics Service reference 17/ES/0130 renewal 12/ES/0083) under governance of the Tayside Biorepository. Surgical surplus samples of clinically normal skin from four adult donors (all females aged 29–65 years; one abdominal and three breast skin reductions) were used for the organoid cultures. Similar samples (n=5) were used for independent biological replicates to test for reproducibility of the functional effects of FLG knockdown.
Organoid culture methods
Primary keratinocytes and dermal fibroblasts were isolated from human skin by sequential trypsin EDTA and collagenase D digestion 21. Using our previously reported methods 12, the keratinocytes were co-cultured with mitomycin C inactivated 3T3 feeder cells in RM media (3:1 DMEM : Hams F12, 10% FCS, 0.4µg/ml hydrocortisone, 5µg/ml insulin, 10ng/ml EGF, 5µg/ml transferrin, 8.4ng/ml cholera toxin and 13ng/ml liothyronine) (Sigma Aldrich, Gillingham, Dorset, UK) 22. Epidermal growth factor (EGF) was omitted for the first day of culture. The fibroblasts were cultured in DMEM supplemented with 10% FCS under standard conditions.
Fibrin gel dermal equivalents 12 were prepared using 0.5ml fibrinogen (35 mg/ml in NaCl) (Sigma Aldrich, Gillingham, Dorset, UK) and 0.5ml thrombin (3U/ml in 2 mM CaCl 2 / 1.1% NaCl) (Sigma Aldrich, Gillingham, Dorset, UK) combined on ice, with 200,000 fibroblasts and aprotinin (0.1 U/ml) (Sigma Aldrich, Gillingham, Dorset, UK) then transferred to a 12-well plate. After 30 minutes incubation at 37°C, the gels were covered in medium (DMEM, 10% FCS, 0.1 U/ml Aprotinin) and cultured overnight (day 1). On day 2, the medium was replaced with RM excluding EGF, 0.1U/ml aprotinin and 2 × 10 6 suspended keratinocytes. Culture medium was refreshed daily on days 3 and 4 using RM containing 0.1ng/ml EGF and 0.1U/ml aprotinin. On day 5 the gels were carefully removed from wells and lifted onto custom-made steel grids lined with nylon gauze (Millipore, Livingston, Scotland, UK). RM medium supplemented with 0.1ng/ml EGF and 0.1U/ml aprotinin was added up to the base of the dermal equivalent so that the epidermis remained at the air-liquid interface. Medium was refreshed on alternate days sand the cultures were used for analysis up to day 12 (representing three, five and seven days after lifting to the air-liquid interface).
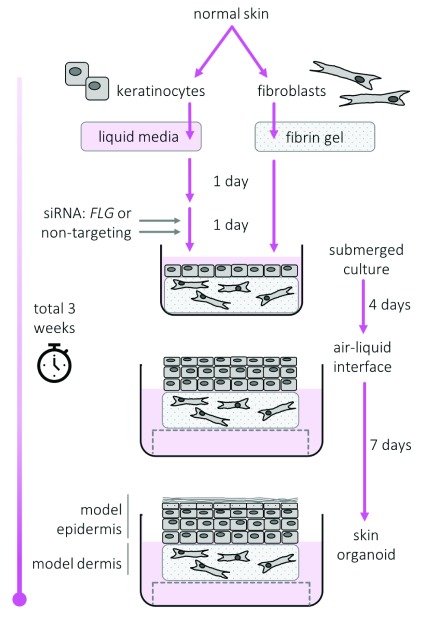
A diagrammatic summary of the process of skin organoid culture is shown in Figure 1.
Figure 1. Diagrammatic summary of skin organoid culture.
Dermal and epidermal equivalents produced using our published methodology optimised for skin barrier assessment 12.
Epidermis was separated from the fibrin gel using hypertonic saline-induced split (4 hours, 1M NaCl, 4°C) to obtain a keratinocyte-only tissue sample for biochemical analyses.
FLG genotyping
DNA was extracted from donor skin samples using standard techniques and genotyping was performed for the four most prevalent loss-of-function mutations in FLG in this white European population (R501X, 2282del4, R2447X and S3247X) 23 using validated KASP TM technology (LGC Genomics, Hoddesdon, Hertfordshire, UK), as previously reported 4, 24. Briefly, KASP TM SNP genotyping consists of two competitive, allele-specific forward primers and one common reverse primer. Each forward primer incorporates an additional tail sequence that corresponds with one of two universal FRET (fluorescent resonance energy transfer) cassettes (FAM and HEX). ROX™ passive reference dye, Taq polymerase, free nucleotides and MgCl2 in an optimised buffer solution make up the reaction master mix.
siRNA mediated knockdown
Keratinocytes were reverse transfected immediately prior to inclusion in the organoid cultures using RNAiMax transfection reagent (Life Technologies, Carlsbad, California, USA) according to manufacturer’s instructions. Briefly, siRNA complexes were formed in OPTIMEM medium (20uM siRNA, 5µl RNAiMAX) and following 20 minutes incubation, combined with 2 × 10 6 suspended normal human keratinocytes and transferred to the pre-prepared dermal substrate. A pool of four siRNA duplexes was used [FLG: LQ-021718-00-0002, Control: ON-TARGETplus non-targeting siRNA #4 D.001810-04-20] (Dharmacon, Lafayette, Colorado, USA).
Quantitative PCR
RNA was extracted from organoid epidermis using the Direct-zol RNA kit (R2071, Zymo Research, Irvine, California, USA) following homogenisation in RNA-Bee (CS-104B, Amsbio, Abingdon, Oxfordhire, UK) using the TissueLyser LT (Qiagen, Manchester, UK) 5 mins at 50 Hz. cDNA was prepared from 1μg total RNA using random priming (2.5µM final) (Integrated DNA Technologies, Coralville, Iowa, USA) in combination with Moloney murine leukemia virus (MMLV) enzyme (100 units) and buffer systems (28025013, Life Technologies, Carlsbad, California, USA). qPCR reactions were performed using exon spanning probe-based assays ( FLG: HS.PT.58.24292320FAM, EF1A: HS.PT.58.24345862FAM) [Integrated DNA Technologies, California, USA]) with TaqMan gene expression Mastermix (4369016, Life Technologies, Carlsbad, California, USA). Reactions were prepared and run using the Qiagility robot in combination with the Rotor-Gene Q (Qiagen, Manchester, UK). EF1A was used as a reference gene. PCR cycling conditions were: 95°C for 10 mins, [95°C for 15 seconds, 60°C for 60 seconds] × 40 cycles. Fold changes were derived via the 2(-Delta Delta C[T]) method.
Western blotting
Organoid epidermis was homogenized in RIPA buffer (Cell Signalling Technologies, London, UK) using the TissueLyser LT (Qiagen, Manchester, UK) 5 mins at 50 Hz. Protein lysates were normalized by Pierce BCA assay (23225, Thermo Fisher, Waltham, Massachussets, USA) and resolved under reducing conditions using the NuPage® gel electrophoresis system (Life Technologies, Carlsbad, California, USA). Primary antibodies (monoclonal mouse filaggrin [RRID: AB_1122916, catalogue # sc66192, Santa Cruz Biotechnology, Texas, USA] and monoclonal mouse GAPDH [RRID: AB_2756824, catalogue # 97166, Cell Signalling Technologies, Boston, USA]) were prepared in 5% bovine serum albumin (BSA) and incubated overnight at 4°C with agitation. Immunoblots were developed using either goat anti rabbit (P0448, RRID: AB_2617138) or goat anti mouse (P0447, RRID: AB_2617137) peroxidase conjugated secondary antibodies (DAKO, Glostrup, Denmark, 1:5000) in combination with chemiluminescent HRP substrate (Immobilon, Millipore, Billerica, Massachussets, USA) onto photographic film. Densitometry was performed using Fuji ImageJ (May 2017).
Structural assessments
(i) Histology
Formalin-fixed, paraffin embedded full thickness organoids were sectioned and stained using haematoxylin and eosin and imaged using Visiscope LT384P (VWR International, Lutterworth, UK).
(ii) Transmission electron microscopy (TEM)
Organoid skin samples were fixed in 4% paraformaldehyde, 2.5% gluteraldehyde in 0.1M sodium cacodylate buffer (pH 7.2) for one hour then cut into small pieces, washed in buffer and post-fixed in 1% osmium tetroxide in cacodylate buffer for one hour or 1% ruthenium tetroxide in cacodylate buffer for one hour. The pieces were dehydrated through alcohol series, into propylene oxide and embedded in Durcupan resin. Ultrathin sections were stained with 3% aqueous uranyl acetate and Reynold’s lead citrate and examined on a JEOL 1200EX electron microscope. TEM images were collected on a SIS Megaview III camera.
Functional assessments
(i) Trans-epidermal water loss (TEWL)
Organoid cultures were equilibrated at room temperature and atmospheric conditions for 30 minutes before TEWL was measured at two locations on the epidermal surface using an Aquaflux AF200 instrument (Biox Systems Ltd, London, UK) with a closed chamber and a custom (5mm diameter) probe head. TEWL measurements were taken every second for a minimum of 60 seconds until a stable reading, as determined by the software, was obtained. TEWL directly measures water evaporation from the skin surface but it may be considered as a measurement of ‘inside-outside’ barrier function 25.
(ii) Capacitance
To inform the interpretation of TEWL, we measured electrical capacitance; this is directly proportional to water content of the uppermost 10–20µm of tissue. Organoid cultures were equilibrated at room temperature and atmospheric conditions for 30 minutes prior to measurement of epidermal surface capacitance, using a Corneometer TM and multiprobe adapter (CM825 and MPA2, Courage and Khazaka, Cologne, Germany). Three measurements were recorded from each organoid and the mean was calculated.
(iii) Fluorescent dye penetration
50μl of 1mM lucifer yellow dye (Sigma Aldrich) was added to the epidermal surface of the organoid and incubated at 37°C for 4 hours. Metal cloning rings were used to control uniform dosing on the epidermal surface. The lucifer yellow was removed and the organoids washed in PBS before formalin fix-paraffin embedding under standard conditions. 4µM sections were deparaffinized, counterstained with DAPI (1μg/ml for 10 mins) (Life Technologies, Carlsbad, California, USA) and imaged by confocal Zeiss LSM710 microscope. Quantification of dye penetration in the upper dermis (average intensity in upper 40μm) was performed using Zeiss Zen Blue lite version 2.3 software and compared using paired t-tests. Dye penetration represents a measurement of the ‘outside-inside’ barrier function.
Mass spectrometry proteomic analysis
Frozen organoid epidermal samples were ground on dry ice, solubilized in 200μl of Cellular and Organelle Membrane Solubilizing Reagent (C0356-4BTL, also called Protein Extraction Reagent Type 4, Sigma-Aldrich, Gillingham, Dorset, UK), 7.0 M urea, 2.0 M thiourea, 40 mM Trizma® base (Sigma Aldrich, Gillingham, Dorset, UK) and 1.0% C7BzO, pH 10.4 buffer with protease inhibitors. The lysate was acetone precipitated for 1 hour at -20°C and spun for 20 mins at 2°C at 15,000 rpm. The pellet was re-suspended in 200μl of 50mM ammonium bicarbonate. 100μl was taken for in-solution digest. 22μl of dithiothreitol was added and samples heated to 50°C for 15mins. 24μl of iodoacetamide was added and the samples incubated at 22°C for 30 mins. 1μl of RapiGest TM (186001860, Waters, Herts, UK) was added, followed by 2.5μl of trypsin, for a 12-hour digest. A further 1μl trypsin was added for an additional 4-hour digest. The peptide samples were fractionated into 24 fractions with high pH Reversed Phase C18 chromatography, run on a Q Exactive Classic or Plus for 160 mins and the top 15 ions selected for sequencing. Mass spectrometry resolution was 70,000 and tandem mass spectrometry (MS/MS) resolution 17,500. Data were processed using MaxQuant (v1.6.0.13) and Human UniProt Database (Dec 2017) with a protein and peptide false discovery rate of 0.01.
Proteins identified as contaminants (trypsin and other lab originating proteins) and contained within the MaxQuant database were removed. To account for the possibility of keratin as a contaminant, we analysed the blank runs for keratin peptide intensity and subtracted this from the measured keratin intensities. A reversed database was used to identify false positives. The peptide and protein false discovery rate was set at 1%, and these were removed from further analysis.
Total intensities of the samples were normalized to total protein abundance, with adjustment to the lowest total protein yield. All samples were run in quadruplicate and reproducibility was assessed by Pearson correlation.
Mass spectrometry lipidomic analysis
Organoid samples were sonicated in phosphate buffered saline (pH7.4) and then extracted according to the method of Folch et al. 26. The samples were analysed by liquid chromatography-mass spectrometry (LC-MS) on a Thermo Exactive Orbitrap mass spectrometer (Thermo Scientific, Hemel Hempsted, UK) coupled to a Thermo Accela 1250 ultra high pressure liquid chromatography (UHPLC) system. Samples were injected on to C18 column (Thermo Hypersil Gold, 2.1 mm x 100 mm, 1.9 μm) and separated using a water/acetonitrile/isopropanol gradient 27. Ceramide 17:0 (Avanti Polar Lipids, Alabaster, AL, USA) was included as an internal standard. Ion signals corresponding to individual ceramide molecular species were extracted from raw LC-MS data sets. Concentration of ceramide was expressed as pmol/mg after normalisation to mg of wet weight tissue.
Data analysis of qPCR, immunoblotting and functional assessments
Measurements were compared between non-targeting control-treated organoid models and FLG-siRNA-treated models, using paired t-test.
Histological sections of 10 replicate skin organoid experiments were measured using a standardized technique to minimize bias, as follows: three points were measured on each slide, at the right side, middle and left of each sample, across viable cell layers and stratum corneum; measurements were generated using Fuji ImageJ (May 2017) and the mean of the three measurements calculated.
Proteomic data analysis
The total intensities of the samples were normalised to total protein abundance, with adjustment to the lowest total protein yield. Log2 ratio of protein expression (log2 FLG knockdown – log2 NT control) was visualised as a volcano plot, using GraphPad Prism (version 5) for human proteins detected in (three or all four) of the replicate experiments. P values were calculated by paired t-test applied to log 10 transformed data. Because of the burden of multiple testing in this large dataset, rather than defining arbitrary thresholds for ‘statistical significance’, our analyses focussed on changes that showed consistency across the biological replicate experiments. Approximately 1000 of the >8000 proteins were selected as showing consistently increased or decreased abundance, defined as follows:
Proteins were considered to be reduced in abundance if they showed a reduction in all four biological replicate experiments comparing FLG-knockdown organoid samples to matched non-targeting controls and ratio ≤0.5 in three or four out of four of the replicates and reduced to undetectable levels of protein in a maximum of two replicates. Proteins were considered to be increased in abundance if they showed an increase in all four biological replicate experiments comparing FLG-knockdown organoid samples to matched controls treated with non-targeting controls and ratio ≥1.2 in three or four out of four of the replicates. These magnitudes of change were used to define roughly equal proportions of up- and down-regulated proteins for pathway analysis. The groups were then assessed using gene ontology and network analysis in STRING, version 11 28 and pathway prediction using the Reactome Knowledgebase, version 68 29. This analysis, based on consistency of findings across multiple biological replicate experiments was used in preference to a defined threshold of statistical significance, with the aim to identify proteins and pathways of importance in the context of a common complex but heterogenous trait.
Lipidomic data analysis
The ratio of ceramide and omega-hydroxy ceramide species were compared between the FLG knockdown organoids and matched control samples in five biological replicate experiments.
Results
All samples are wild-type for the prevalent FLG null mutations and filaggrin shows knockdown at mRNA and protein level
Genotyping of DNA from tissue samples from the four skin donors did not detect any of the prevalent FLG null mutations 30; this does not, however, exclude the possibility of rare (<0.01 allele frequency) null mutations.
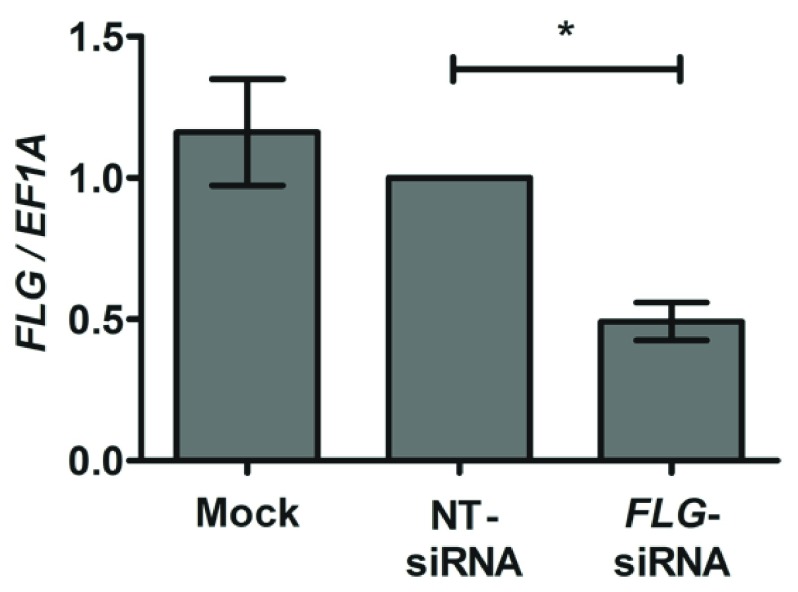
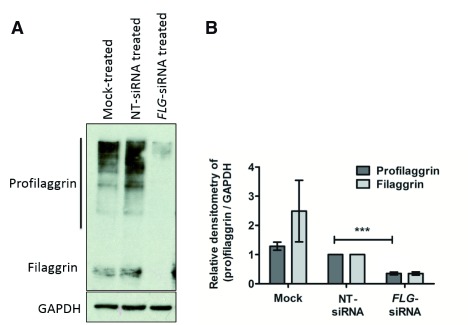
FLG knockdown in the skin organoids persisted to day 10 after siRNA transfection, as demonstrated by qPCR ( Figure 2) and western blotting ( Figure 3). We note the greater magnitude of knockdown quantified at mRNA than protein level in the organoid epidermis 10-days after transfection. This is likely to reflect the inherent differences in mRNA and protein stabilities: FLG mRNA has a half-life of ~2.2 hours 31 whereas the half-lives of profilaggrin and filaggrin are approximately 6 and 24 hours respectively 32.
Figure 2. qPCR analysis of epidermis from skin organoids with and without FLG siRNA-mediated knockdown.
Probes and primers are listed in Methods; n=8 biological replicates; error bars show SEM; *paired t-test p<0.05.
Figure 3. Quantification of filaggrin in organoid epidermis.
( A) Representative western blot. ( B) Approximated quantification by densitometry. NT, non-targeting; n=7; ***p<0.0005 compared to non-targeting control.
FLG knockdown results in morphological changes in the epidermis
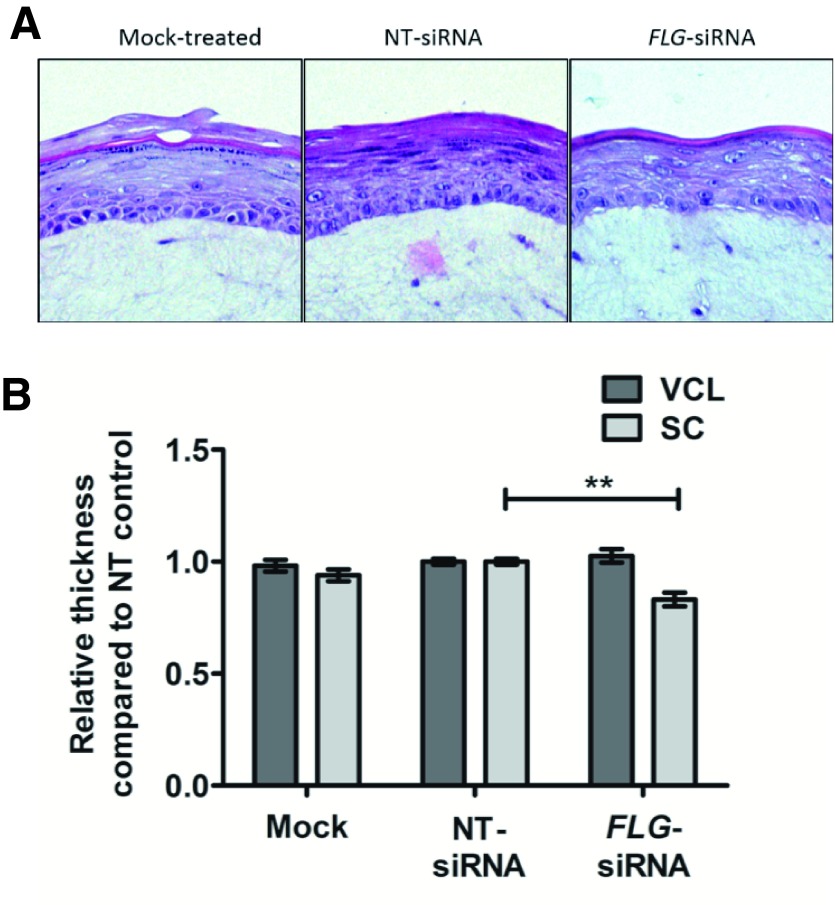
Histological examination confirmed a reduction of the granular layer, in keeping with a reduction in profilaggrin ( Figure 4A). There was also a slight reduction in thickness of the stratum corneum (ratio 0.83 +/- 0.05 (mean +/- SEM), but no difference in thickness of the keratinocyte cell layers ( Figure 4B).
Figure 4. Structural changes in organoid models.
( A) Histological examination (representative images from 8 biological replicates). NT, non-targeting. ( B) Measurement of relative thickness of epidermal layers: VCL, viable cell layer and SC, stratum corneum; n=8; compared using paired t-test; **p<0.005.
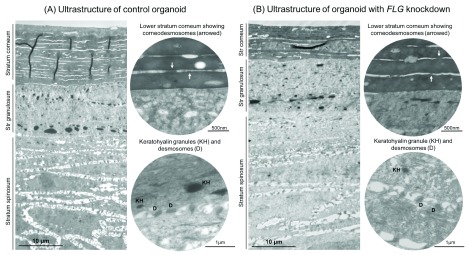
Transmission electron microscopy (TEM) of the skin organoid model shows an effective recapitulation of the physiological layers of keratinocyte differentiation within skin, including a mature, multi-layered stratum corneum with corneodesmosomes and a granular layer with keratohyalin granules and desmosomes ( Figure 5A). TEM of the FLG-knockdown organoid shows a similarly mature epidermis, but the stratum corneum has fewer layers and the granular layer contains keratohyalin granules that are generally smaller and less numerous than in the control organoid ( Figure 5B).
Figure 5. Ultrastructural features of control and FLG knockdown organoids.
( A) Control organoid sample, showing elements of normal human skin structure including stratified differentiation. ( B) Organoid with FLG siRNA-mediated knockdown, showing thinner stratum corneum, reduced prominence if the corneodesmosomes and desmosomes, and smaller keratohyalin granules (organelles containing profilaggrin).
FLG knockdown shows some evidence of a barrier defect in the immature organoids but no functional effect on skin barrier in the mature organoid model
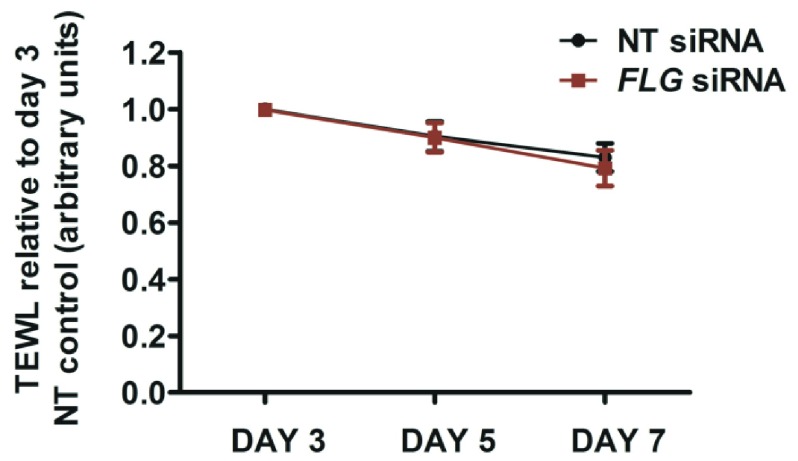
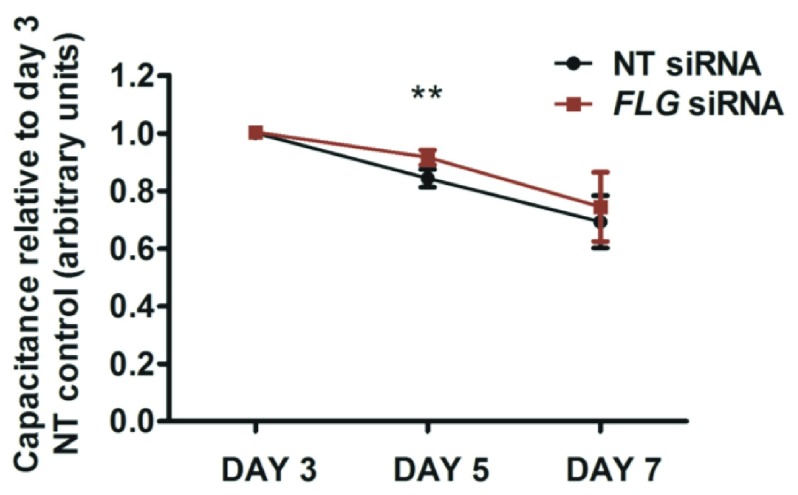
The organoid model described here shows progressive development of skin barrier function after lifting to the air-liquid interface: there is a progressive reduction in TEWL ( Figure 6), progressive reduction in capacitance ( Figure 7) and the hydrophilic dye, lucifer yellow, is excluded from the epidermis by day 7 in both the control and FLG-knockdown organoids ( Figure 8).
Figure 6. Transepidermal water loss (TEWL) measurements.
TEWL measured under standard conditions at days 3, 5 and 7 after the organoid is lifted to the air-liquid interface, using an Aquaflux AF200 instrument (Biox Systems Ltd, London, UK) with a 5mm diameter probe head. NT, non-targeting; N=5; error bars show SEM.
Figure 7. Epidermal capacitance measurements.
Electrical capacitance, directly proportional to water content of the uppermost 10–20µm of tissue, was measured under standard conditions at days 3, 5 and 7 after the organoid is lifted to the air-liquid interface, using a Corneometer TM (Courage and Khazaka, Cologne, Germany); the mean of three measurements is recorded. NT, non-targeting; N=5; error bars show SEM; paired t test **p<0.005.
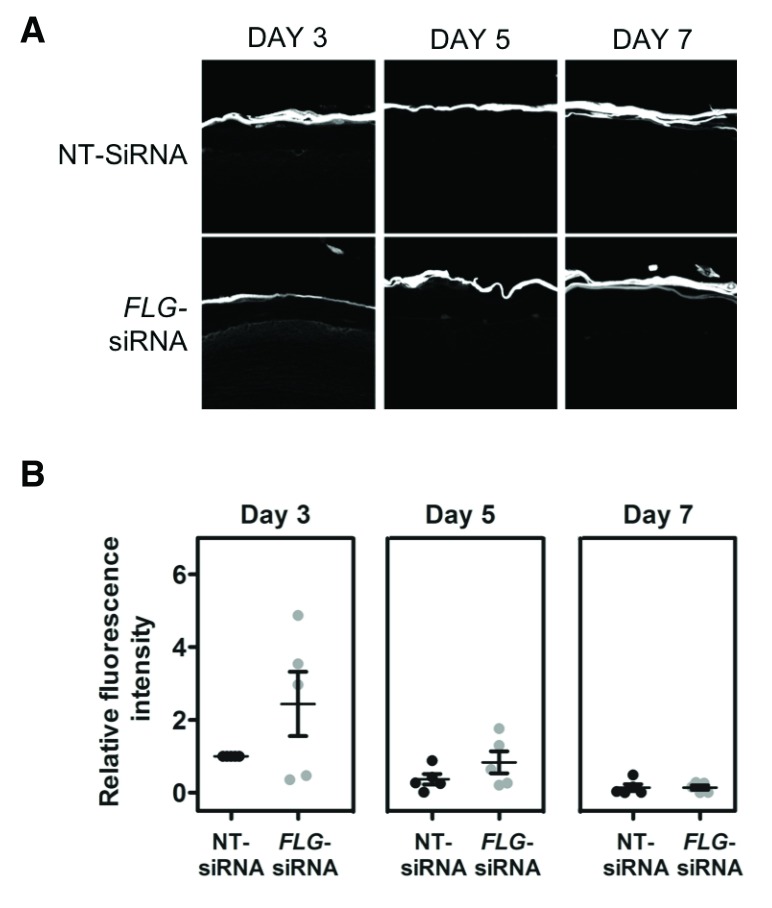
Figure 8. Lucifer yellow dye penetration of organoid samples.
The hydrophilic lucifer yellow dye (Sigma Aldrich) was added to the epidermal surface of the organoid and incubated at 37°C for 4 hours before sectioning for microscopic examination. ( A) Representative image from three of the five biological replicate experiments showing delayed maturation of barrier function. ( B) Dot-plot of densitometry data from all five biological replicates. NT, non-targeting.
Results from five biological replicate experiments showed there was no difference in the mean TEWL ( Figure 6), or mean capacitance measurements ( Figure 7) between the mature organoid models with FLG knockdown and donor-matched non-targeting siRNA-treated controls. Capacitance was increased in the immature FLG-knockdown model (day 5 after air-exposure, Figure 7) and lucifer yellow dye was not fully excluded by the stratum corneum in some of the the FLG-knockdown replicate experiments until day 7 ( Figure 8). However, this delay in maturation of the ‘outside-to-inside’ barrier function was not consistently demonstrated in all biological replicates.
Mass spectrometry proteomic analysis identifies over 8000 protein species per sample
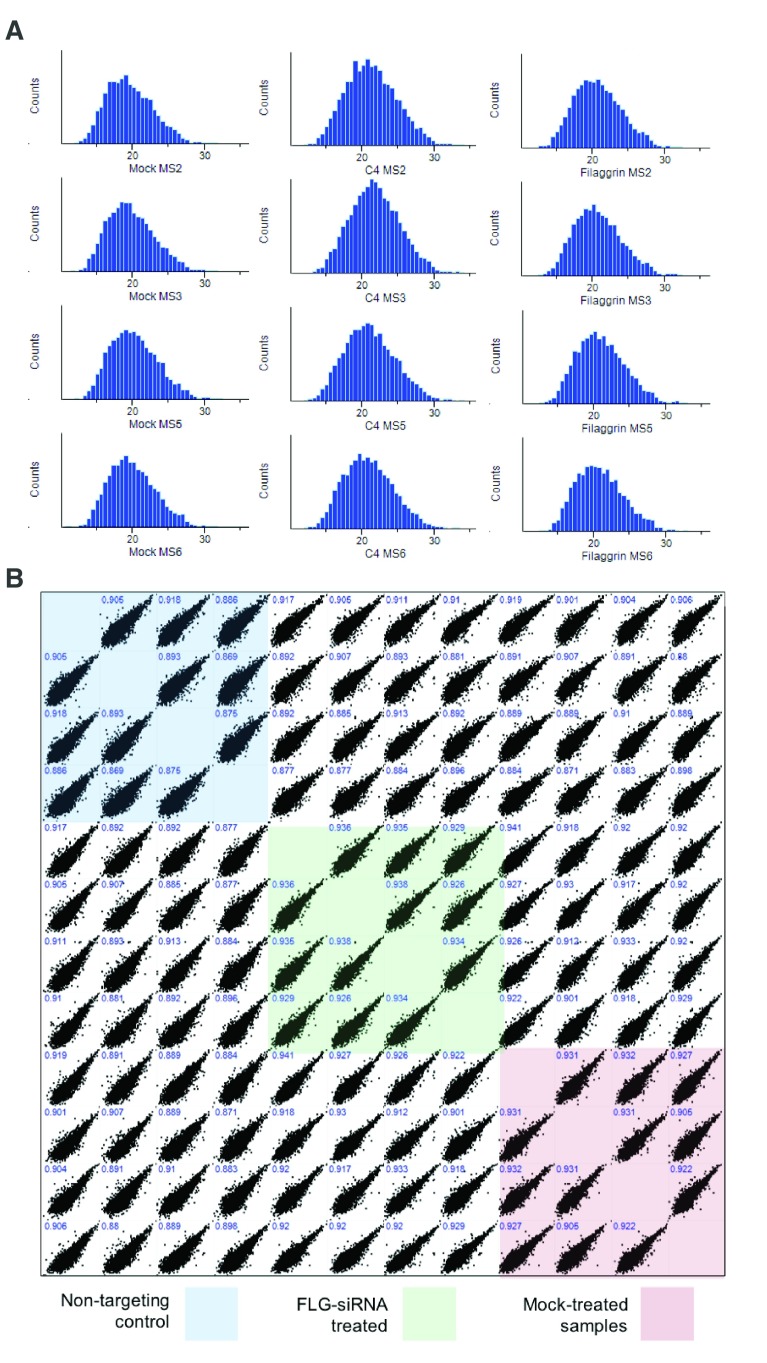
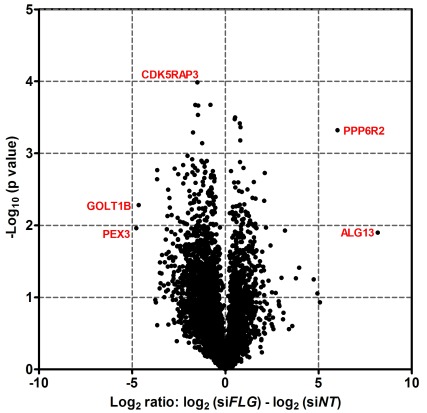
After removal of contaminants and false positive results, >8000 proteins were identified in each epidermal sample. The proteomic data have been deposited to the ProteomeXchange Consortium via the PRIDE 33 partner repository with dataset identifier PXD014875. Quality control analysis confirmed that similar distributions of proteins were identified in each experiment ( Figure 9A); protein extracts were analysed in quadruplicate to test for technical reproducibility and the Pearson correlations were 0.869-0.938 ( Figure 9B). The protein expression changes were visualised using a volcano plot ( Figure 10).
Figure 9. Mass spectrometry proteomic analysis quality control assessments.
( A) Histograms showing the distribution of proteins quantified in each experiment. Bins on the x-axis contain the log2 intensity-based absolute quantification (iBAQ, sum of intensities of tryptic peptides for each protein divided by number of theoretically observable peptides); normalised counts of the proteins in each bin are shown on the y-axis; MS2, MS3, MS5 and MS6 are biological replicate experiments from different human donors; mock, untreated control organotypic; C4, non-targeting control treated organotypic; Filaggrin, FLG si-RNA treated organotypic. ( B) Correlation of protein abundance from replicate analyses. x and y axes show iBAQ intensities of samples mapped against replicate analysis; biological replicates are highly correlated (Pearson correlation coefficient ≥0.86).
Figure 10. Volcano plot to visualise changes in protein expression between control and FLG siRNA-treated organoids.
Adjusted protein intensity data were filtered to include only human proteins detected in three or four replicate experiments. The log2 ratio (log2 FLG knockdown – log2 NT control) for each donor was calculated and averaged; p-values from paired t-test were derived from log10 transformed data and are unadjusted. Selected data-points with more extreme values are labelled using the abbreviated protein name. NT, non-targeting.
648 proteins show a consistent reduction in expression in skin organoids with FLG knockdown
648 human proteins showed a reduction in expression in all four of the replicates and ratio ≤0.5 in three out of four replicates. This list is available as Underlying data, uploaded to Figshare 30.
645 of the proteins are identified in STRING and this group showed significant enrichment for interactions (p<1.0e-16) computed by combining the probabilities from the different evidence channels and corrected for the probability of randomly observing an interaction 21. Gene ontology (GO) analysis in STRING showed evidence for functional enrichment of GO-terms including RNA processing (false discovery rate (FDR) 4.40e-11), catalytic activity (FDR 8.59e-08) and intracellular component (FDR 3.88e-26). A list of the 10 most significant GO analysis results in each of the GO domains is shown in Table 1 (see Underlying data) 34.
Pathway analysis of down-regulated proteins identifies themes including RNA metabolism, adaptive immunity and axon guidance
Reactome detected 39 pathways in this set of proteins (Table 2, Underlying data) 35. Descriptions include: metabolism of RNA (including 67 out of the 652 genes in this pathway, FDR 3.36e-12); class I MHC mediated antigen processing and presentation (30/365 genes in pathway, FDR 0.0031); and axon guidance (34/541 genes, FDR 0.0207).
Down-regulated proteins indicate molecular mechanisms of relevance to atopic eczema
Manual searching of the list of down-regulated proteins identified several of specific interest based on a priori knowledge of eczema pathomechanisms. These highlight several putative therapeutic targets: the aryl hydrocarbon receptor plays a role in signalling pathways for skin barrier repair 22 and has been proposed as a therapeutic target for the treatment of skin inflammation 36; caspase 14 plays a role in filaggrin processing 37 and is required for epidermal protection against water loss and UVB-induced damage 38; dermokine is a soluble regulator of keratinocyte differentiation 39; sequence similarity to a murine protein (UniProtKB: Q8R1R3) indicates that StAR-related lipid transfer protein 7 may play a role in protecting mucosal tissues from exaggerated allergic responses; a reduction in the cytokine TGF-beta 1 may predispose to atopic eczema 40; and loss-of-function mutations in CYP4F22 cause lamellar ichthyosis 41, suggesting that a reduction in CYP4F22 protein expression may contribute to the ichthyotic phenotype observed in ichthyosis vulgaris and atopic skin.
376 proteins show a consistent increase in expression in organoids with FLG knockdown
376 human proteins showed an increase in expression in all four of the replicates and ratio ≥1.2 in three or four out of the four replicates. This list is available as Underlying data, uploaded to Figshare 30.
374 proteins are identified in STRING and the group showed significant enrichment for interactions (p<1.0e-16). GO analysis revealed enrichment of terms including translation (FDR 4.12e-19), RNA binding (FDR 9.77e-12) and cytoplasmic part (FDR 9.64-36). A list of the 10 most significant GO analysis results in each of the GO domains is shown in Table 3 (see Underlying data) 42.
Pathway analysis of up-regulated proteins identifies themes including translation, innate and adaptive immunity and axon guidance
Reactome detected 113 pathways in this set of proteins with FDR p<0.05 (Table 4, Underlying data) 43. Descriptions include: Translation (including 44 out of the 288 genes in this pathway, FDR 1.17e-21); innate immune system (47/1012 genes in pathway, FDR 9.90e-7); cytokine signalling in immune system (32/654 genes in pathway, FDR 5.16e-05); and axon guidance (40/541, FDR 4.69e-11).
Up-regulated proteins indicate molecular mechanisms of relevance to atopic eczema
Manual searching of the list of up-regulated proteins identified several of relevance to atopic eczema, including: STAT1, an intracellular signalling molecule that shows increased expression in atopic eczema, and as part of the JAK-STAT pathway is targeted by several traditional herbal remedies 44 as well as novel small molecule therapies 45; carbonic anhydrase 2, which is increased in skin in a variety of forms of eczema 46; and FK binding protein, which binds to tacrolimus, an established topical treatment for atopic eczema.
The type I keratins 10, 16 and 17 each show increased expression in the FLG-siRNA-treated organoid models with mean ratio 1.7, 1.3 and 1.3, respectively. Keratin 10 is an intermediate filament protein of structural importance in the skin; keratin 16 is an epidermis-specific keratin that plays a role in innate immunity in response to skin barrier breach 47; keratin 17 is expressed in epidermal appendages, including the hair follicle.
Finally, it is noteworthy that GAPDH, which is often considered to be a stable ‘housekeeping’ gene, is in the dataset of consistently up-regulated proteins. However, in the context of this work, filaggrin knockdown is clearly apparent at a greater magnitude than the more subtle change in GAPDH ( Figure 3).
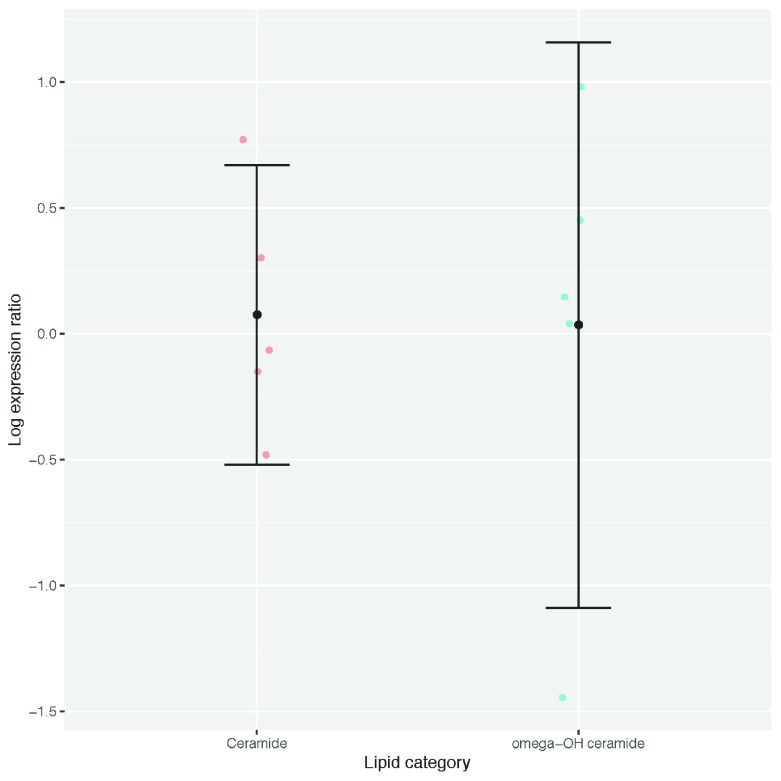
Epidermal ceramide content shows no significant difference in FLG-knockdown organoids compared with controls
Ceramide and omega-hydroxy ceramide species in five biological replicate experiments showed no consistent difference between the FLG knockdown organoids and matched control samples ( Figure 11).
Figure 11. Ratio of ceramides and omega-hydroxy ceramides in organoid epidermis.
Dots represent values for five individual experiments; bars show 95% confidence interval.
Discussion
The functional and biochemical analyses of a filaggrin-deficient skin model have provided findings that are consistent with our and others’ previous analyses in vitro 19 and in vivo 48. We have included both epidermal and dermal compartments in this model (to allow for dermal-epidermal cross-talk 49, 50) and our more detailed proteomic analysis has revealed additional evidence of molecular mechanisms with relevance to atopic skin. These datasets are shared as a resource for the research community.
Analysis of the immature organoid identified a delay in epidermal maturation following FLG knockdown, but the lack of a replicable functional effect in our model, when mature, reflects previous reports in which epidermal equivalents created from FLG-null keratinocytes do not show impairment in barrier function 20. This is in keeping with the observation that FLG-null mutations are not fully penetrant with respect to ichthyosis vulgaris or atopic eczema 16, 51.
Because of the nature of our data, generated using biological replicate samples, and the focus on modelling a common complex trait with considerable inter-individual variation, we elected to use the criterion of consistency across the biological replicates to define proteins of interest, followed by network and pathway analyses. We recognise that other, more complex data analytical approaches may be applied, and/or more focussed interrogation of specific pathways; we share the proteomics data and welcome such further analysis.
Our analysis has highlighted aspects of keratinocyte-immune signalling. This is an important component of the immunological barrier that may be overlooked in tissue with multiple different cell types. The predicted pathways entitled ‘innate immunity’ and ‘adaptive immunity’ included proteins showing both increased and decreased expression (Table 2 and Table 4, Underlying data) 35, 43. This immune dysregulation is likely to contribute to the predisposition to infection and inflammation observed in filaggrin-deficient skin in vivo: filaggrin-deficient patients show increased viral infection of the skin 52 and increased incidence of irritant 53, 54 and allergic contact dermatitis 55. The regulation of innate and adaptive immune pathways within keratinocytes (in addition to cells of the haematopoietic lineage) may therefore represent a worthwhile target for future therapeutic interventions.
The lack of effect of FLG knockdown on the ceramide content in the model epidermis is also consistent with previously reported findings in vitro 56, 57 and in vivo 58. We did not analyse in detail the other epidermal lipids and therefore we cannot comment on whether there were relevant changes. Differences in lamellar body structure 59 have been reported in FLG-mutant skin biopsies but this was not clearly observed in the organoid model. However, ultrastructural examination of the model stratum corneum did reveal abnormalities in the lamellar bilayer structure and less prominent corneodesmosomes ( Figure 5), as previously reported in vivo 59. It is also noteworthy that pathway analysis in Reactome identified the terms ‘metabolism of lipids’ and ‘phospholipid metabolism’ from the group of consistently down-regulated proteins (Table 4, Underlying data) 43.
The most significant themes identified in the groups of proteins showing increased expression upon FLG knockdown relate to RNA binding and translation; this is in contrast to RNA metabolism and RNA processing, which are demonstrated in the proteins showing decreased expression. The functional significance of this increase in transcriptional and translational activity cannot be determined from the gene ontology and pathway analysis. We may hypothesise that it reflects the increased ‘stress’ response, as we have previously observed in FLG genotype-stratified transcriptome analysis of atopic skin biopsies 18 and/or the activation of innate and adaptive immune mechanisms.
It is tempting to speculate that the increased expression of keratins 10 and 16 may contribute to palmar hyperlinearity and keratin 17 may contribute to keratosis pilaris. These are characteristic features of ichthyosis vulgaris but their pathogenesis has not yet been explained as a direct result of filaggrin haploinsufficiency 16, 60.
Several of the proteins showing reduced expression relate to aspects of differentiation, including filaggrin itself, and caspase 14 which plays a role in the terminal degradation of filaggrin 37. The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that responds to multiple different environmental and metabolic stimuli to control transcriptional pathways of relevance to atopic eczema, including immunity and differentiation 61. Activation of AhR reduces skin inflammation 62 and enhances barrier repair 63; therefore, a reduction in AhR expression in the FLG knockdown organoid would be anticipated to increase the eczema diathesis by opposing these effects. AKT1 activity is known to be reduced in atopic skin, leading to an alteration in protease expression and reduced filaggrin expression and processing 64. This mechanism may further exacerbate the filaggrin deficiency in our FLG knockdown organoid. Dermokine acts as a soluble regulator of keratinocyte differentiation and mice deficient in the epidermal dermokines (beta and gamma isoforms) show defective cornification 65. Transforming growth factor (TGF) beta-1 is a multifunctional protein: it regulates the growth and differentiation of multiple cell types, including keratinocytes and it can promote either Th17 or T-regulatory cell lineage differentiation in a concentration-dependent manner; it appears to have an immunosuppressive effect in atopic disease since a genetically-determined lower production of TGF-beta-1 is associated with increased risk of atopic dermatitis 40.
The Reactome term ‘axon guidance’ was detected in proteins showing increased and decreased expression. It has long been recognised that there is an increase in the density of sensory neurons in eczematous skin 66 and nerve growth factor expression is upregulated in the keratinocytes of patients with atopic eczema 67. Therefore, dysregulation of axon guidance may be of relevance to the pruritus that is so characteristic of atopic eczema and contributes substantially to the morbidity of this condition 3. Antagonists of transient receptor potential vanilloid subfamily member 1 (TRPV1), expressed on sensory nerves and keratinocytes, are now being investigated for the control of pruritus 68, 69.
Taken together, our data demonstrate that filaggrin haploinsufficiency leads to abnormalities in both structural and immune features within the keratinocyte compartment of the epidermis. Keratinocytes display immune activity, producing multiple cytokines and chemokines in addition to their roles in innate immunity 70, 71. Together with the evidence of dysregulation in axon guidance, these findings indicate that patients with filaggrin deficiency – either haploinsufficiency or a reduction in filaggrin expression that occurs secondary to atopic inflammation 72 – may derive clinical benefit from future therapies targeting keratinocyte-immune crosstalk mechanisms and neurogenic pruritus.
The skin organoid model described here represents a valuable opportunity to study genetic effects on keratinocytes in relative isolation. However, the lack of other cell types (notably T cells, B cells, dermal dendritic cells and innate lymphoid cells), the absence of complex dermal structures (including neurons and blood vessels) and skin appendages (for example hair follicles, sweat and sebaceous glands) is also a limitation in that it precludes assessment of the complex interactions that are likely to occur between different tissue compartments in skin.
In conclusion, we share these experimental results in detailed form, because of the additional insight provided by this in vitro analysis of filaggrin deficiency in a skin organoid model; our findings compliment and extend previous work in vitro and in vivo. Our analyses have re-emphasised the role of keratinocyte-specific mechanisms in contributing to immune and neurological as well as structural aspects of skin barrier function and offer new insight into molecular mechanisms for the ichthyosis vulgaris phenotype.
Data availability
Underlying data
Mass spectrometry proteomics data deposited in the ProteomeXchange Consortium via the PRIDE partner repository 33, Accession number PXD014875: https://identifiers.org/pride.project:PXD014875
Figshare: Proteomic analysis of FLG knockdown in skin organoid. https://doi.org/10.6084/m9.figshare.c.4606052.v3 30.
This project contains the following underlying data:
-
-
TEM images (zip file containing raw, unedited TEM images in .tif format)
-
-
TEWL and corneometer readings (raw readings in .xlsx format)
-
-
Western blots (zip file containing unedited western blot scans, plus an annotated composite image with labels in .tif format)
-
-
Lucifer yellow dye penetration quantification.xlsx
-
-
Lucifer yellow dye penetration (zip file containing raw, unedited microscopy images for five replicates in .czi format)
-
-
Histology quantification (raw histology quantification data in .xlsx format)
-
-
H&E images (raw, unedited histology images for replicates 1-11 in .bmp and .tif format)
-
-
Filaggrin densitometry (densitometry measurements for seven replicate experiments in .xlsx format)
-
-
FLG qPCR data (raw qPCR data in .xlsx format)
-
-
FLG genotyping results (raw genotyping results in .xlsx format)
-
-
Consistently down-regulated proteins in FLG kd organoids.xlsx
-
-
Consistently up-regulated proteins in FLG kd organoids.xlsx
Figshare: Table 1. Gene Ontology (GO) analysis of proteins showing a consistent reduction in expression with FLG knockdown. https://doi.org/10.6084/m9.figshare.9710585.v1 34.
Figshare: Table 2. Reactome pathway analysis of down-regulated proteins. https://doi.org/10.6084/m9.figshare.9710666.v1 35.
Figshare: Table 3. Gene Ontology (GO) analysis of proteins showing a consistent increase in expression with FLG knockdown. https://doi.org/10.6084/m9.figshare.9710738.v1 42.
Figshare: Table 4. Reactome pathway analysis of up-regulated proteins. https://doi.org/10.6084/m9.figshare.9710783.v1 43.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
We are very grateful to the anonymous skin donors who gave consent for their samples to be accessed for research and the governance of the NHS Research Scotland Biorepository Tayside in collecting these samples. We thank Dr Andrew South, Dr Celine Pourreyron, Dr Michael Mildner and Prof Erwin Tschachler for expert advice on skin organoid culture methods; Dr Paul Appleton for technical expertise and advice on microscopy; and Mr Seshu Tammireddy for his technical work in lipid analysis.
Funding Statement
This work was supported by the Wellcome Trust [106865], a Senior Research Fellowship in Clinical Science awarded to SJB; the Brown laboratory has also received financial support from the Manknell Charitable Trust, the Tayside Dermatology Research Charity and the British Skin Foundation. The mass spectrometry proteomic analysis was supported by grants to AIL from the Wellcome Trust [105024]. Dundee Imaging Facility is supported by a Wellcome Trust Technology Platform award [097945]. UHI Lipidomics Research Facility acknowledges the support of European Regional Development Fund, Scottish Funding Council and Highlands and Islands Enterprise.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 3 approved]
References
- 1. Johansson SG, Bieber T, Dahl R, et al. : Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–6. 10.1016/j.jaci.2003.12.591 [DOI] [PubMed] [Google Scholar]
- 2. Silverberg JI, Thyssen JP, Paller AS, et al. : What's in a name? Atopic dermatitis or atopic eczema, but not eczema alone. Allergy. 2017;72(12):2026–2030. 10.1111/all.13225 [DOI] [PubMed] [Google Scholar]
- 3. Weidinger S, Novak N: Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. 10.1016/S0140-6736(15)00149-X [DOI] [PubMed] [Google Scholar]
- 4. Paternoster L, Savenije OEM, Heron J, et al. : Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol. 2018;141(3):964–971. 10.1016/j.jaci.2017.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SJ: Molecular mechanisms in atopic eczema: insights gained from genetic studies. J Pathol. 2017;241(2):140–145. 10.1002/path.4810 [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez E, Baurecht H, Herberich E, et al. : Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123(6):1361–70.e7. 10.1016/j.jaci.2009.03.036 [DOI] [PubMed] [Google Scholar]
- 7. Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. : Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–6. 10.1038/ng1767 [DOI] [PubMed] [Google Scholar]
- 8. Ferreira MA, Vonk JM, Baurecht H, et al. : Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49(12):1752–1757. 10.1038/ng.3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu Z, Lee PH, Chaffin MD, et al. : A genome-wide cross-trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat Genet. 2018;50(6):857–864. 10.1038/s41588-018-0121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown SJ, Asai Y, Cordell HJ, et al. : Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127(3):661–7. 10.1016/j.jaci.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kretz M, Siprashvili Z, Chu C, et al. : Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493(7431):231–5. 10.1038/nature11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elias MS, Wright SC, Remenyi J, et al. : EMSY expression affects multiple components of the skin barrier with relevance to atopic dermatitis. J Allergy Clin Immunol. 2019;144(2):470–481. 10.1016/j.jaci.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finan C, Gaulton A, Kruger FA, et al. : The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9(383): pii: eaag1166. 10.1126/scitranslmed.aag1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandilands A, Sutherland C, Irvine AD, et al. : Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122(Pt 9):1285–94. 10.1242/jcs.033969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown SJ, McLean WH: One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132(3 Pt 2):751–62. 10.1038/jid.2011.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown SJ, Relton CL, Liao H, et al. : Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol. 2009;161(4):884–9. 10.1111/j.1365-2133.2009.09339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flohr C, England K, Radulovic S, et al. : Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. 2010;163(6):1333–6. 10.1111/j.1365-2133.2010.10068.x [DOI] [PubMed] [Google Scholar]
- 18. Cole C, Kroboth K, Schurch NJ, et al. : Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;134(1):82–91. 10.1016/j.jaci.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elias MS, Long HA, Newman CF, et al. : Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J Allergy Clin Immunol. 2017;140(5):1299–1309. 10.1016/j.jaci.2017.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niehues H, Schalkwijk J, van Vlijmen-Willems IMJJ, et al. : Epidermal equivalents of filaggrin null keratinocytes do not show impaired skin barrier function. J Allergy Clin Immunol. 2017;139(6):1979–1981.e13. 10.1016/j.jaci.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 21. Purdie KJ, Pourreyron C, South AP: Isolation and culture of squamous cell carcinoma lines. Methods Mol Biol. 2011;731:151–159. 10.1007/978-1-61779-080-5_14 [DOI] [PubMed] [Google Scholar]
- 22. Rheinwald JG, Green H: Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–343. 10.1016/s0092-8674(75)80001-8 [DOI] [PubMed] [Google Scholar]
- 23. Sandilands A, Terron-Kwiatkowski A, Hull PR, et al. : Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39(5):650–4. 10.1038/ng2020 [DOI] [PubMed] [Google Scholar]
- 24. He C, Holme J, Anthony J: SNP genotyping: the KASP assay. Methods Mol Biol. 2014;1145:75–86. 10.1007/978-1-4939-0446-4_7 [DOI] [PubMed] [Google Scholar]
- 25. Alexander H, Brown S, Danby S, et al. : Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J Invest Dermatol. 2018;138(11):2295–2300.e1. 10.1016/j.jid.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 26. Folch J, Lees M, Sloane Stanley GH: A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 27. McIlroy GD, Tammireddy SR, Maskrey BH, et al. : Fenretinide mediated retinoic acid receptor signalling and inhibition of ceramide biosynthesis regulates adipogenesis, lipid accumulation, mitochondrial function and nutrient stress signalling in adipocytes and adipose tissue. Biochem Pharmacol. 2016;100:86–97. 10.1016/j.bcp.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szklarczyk D, Franceschini A, Wyder S, et al. : STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–52. 10.1093/nar/gku1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fabregat A, Jupe S, Matthews L, et al. : The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46(D1):D649–D655. 10.1093/nar/gkx1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown S: Proteomic analysis of FLG knockdown in skin organoid.2019. 10.6084/m9.figshare.c.4606052.v3 [DOI]
- 31. Nirunsuksiri W, Zhang SH, Fleckman P: Reduced stability and bi-allelic, coequal expression of profilaggrin mRNA in keratinocytes cultured from subjects with ichthyosis vulgaris. J Invest Dermatol. 1998;110(6):854–61. 10.1046/j.1523-1747.1998.00208.x [DOI] [PubMed] [Google Scholar]
- 32. Markova NG, Marekov LN, Chipev CC, et al. : Profilaggrin is a major epidermal calcium-binding protein. Mol Cell Biol. 1993;13(1):613–25. 10.1128/mcb.13.1.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perez-Riverol Y, Csordas A, Bai J, et al. : The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47(D1):D442–D450. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown S: Table 1. Gene Ontology (GO) analysis of proteins showing a consistent reduction in expression with FLG knockdown.2019. 10.6084/m9.figshare.9710585.v1 [DOI]
- 35. Brown S: Table 2. Reactome pathway analysis of down-regulated proteins.2019. 10.6084/m9.figshare.9710666.v1 [DOI] [Google Scholar]
- 36. Smith SH, Jayawickreme C, Rickard DJ, et al. : Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J Invest Dermatol. 2017;137(10):2110–2119. 10.1016/j.jid.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 37. Hoste E, Kemperman P, Devos M, et al. : Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131(11):2233–41. 10.1038/jid.2011.153 [DOI] [PubMed] [Google Scholar]
- 38. Denecker G, Hoste E, Gilbert B, et al. : Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9(6):666–74. 10.1038/ncb1597 [DOI] [PubMed] [Google Scholar]
- 39. Matsui T, Hayashi-Kisumi F, Kinoshita Y, et al. : Identification of novel keratinocyte-secreted peptides dermokine-alpha/-beta and a new stratified epithelium-secreted protein gene complex on human chromosome 19q13.1. Genomics. 2004;84(2):384–97. 10.1016/j.ygeno.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 40. Arkwright PD, Chase JM, Babbage S, et al. : Atopic dermatitis is associated with a low-producer transforming growth factor beta(1) cytokine genotype. J Allergy Clin Immunol. 2001;108(2):281–4. 10.1067/mai.2001.117259 [DOI] [PubMed] [Google Scholar]
- 41. Lefèvre C, Bouadjar B, Ferrand V, et al. : Mutations in a new cytochrome P450 gene in lamellar ichthyosis type 3. Hum Mol Genet. 2006;15(5):767–76. 10.1093/hmg/ddi491 [DOI] [PubMed] [Google Scholar]
- 42. Brown S: Table 3. Gene Ontology (GO) analysis of proteins showing a consistent increase in expression with FLG knockdown.2019. 10.6084/m9.figshare.9710738.v1 [DOI]
- 43. Brown S: Table 4. Reactome pathway analysis of up-regulated proteins.2019. 10.6084/m9.figshare.9710783.v1 [DOI]
- 44. Gil TY, Kang YM, Eom YJ, et al. : Anti-Atopic Dermatitis Effect of Seaweed Fulvescens Extract via Inhibiting the STAT1 Pathway. Mediators Inflamm. 2019;2019: 3760934. 10.1155/2019/3760934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Damsky W, King BA: JAK inhibitors in dermatology: The promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–744. 10.1016/j.jaad.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kamsteeg M, Jansen PA, van Vlijmen-Willems IM, et al. : Molecular diagnostics of psoriasis, atopic dermatitis, allergic contact dermatitis and irritant contact dermatitis. Br J Dermatol. 2010;162(3):568–78. 10.1111/j.1365-2133.2009.09547.x [DOI] [PubMed] [Google Scholar]
- 47. Elango T, Sun J, Zhu C, et al. : Mutational analysis of epidermal and hyperproliferative type I keratins in mild and moderate psoriasis vulgaris patients: a possible role in the pathogenesis of psoriasis along with disease severity. Hum Genomics. 2018;12(1): 27. 10.1186/s40246-018-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cole C, et al. : Transcriptome analysis of atopic skin characterises filaggrin-dependent differences in the extracellular space and response to viral infection. Manuscript in preparation. 2013. [Google Scholar]
- 49. Mateu R, Živicová V, Krejčí ED, et al. : Functional differences between neonatal and adult fibroblasts and keratinocytes: Donor age affects epithelial-mesenchymal crosstalk in vitro. Int J Mol Med. 2016;38(4):1063–74. 10.3892/ijmm.2016.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hausmann C, Zoschke C, Wolff C, et al. : Fibroblast origin shapes tissue homeostasis, epidermal differentiation, and drug uptake. Sci Rep. 2019;9(1): 2913. 10.1038/s41598-019-39770-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith FJ, Irvine AD, Terron-Kwiatkowski A, et al. : Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38(3):337–42. 10.1038/ng1743 [DOI] [PubMed] [Google Scholar]
- 52. Gao PS, Rafaels NM, Hand T, et al. : Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124(3):507–13, 513.e1–7. 10.1016/j.jaci.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Timmerman JG, Heederik D, Spee T, et al. : Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations. Br J Dermatol. 2016;174(2):348–55. 10.1111/bjd.14215 [DOI] [PubMed] [Google Scholar]
- 54. Visser MJ, Landeck L, Campbell LE, et al. : Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168(2):326–32. 10.1111/bjd.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Novak N, Baurecht H, Schäfer T, et al. : Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol. 2008;128(6):1430–5. 10.1038/sj.jid.5701190 [DOI] [PubMed] [Google Scholar]
- 56. van Drongelen V, Alloul-Ramdhani M, Danso MO, et al. : Knock-down of filaggrin does not affect lipid organization and composition in stratum corneum of reconstructed human skin equivalents. Exp Dermatol. 2013;22(12):807–12. 10.1111/exd.12271 [DOI] [PubMed] [Google Scholar]
- 57. Vávrová K, Henkes D, Strüver K, et al. : Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J Invest Dermatol. 2014;134(3):746–753. 10.1038/jid.2013.402 [DOI] [PubMed] [Google Scholar]
- 58. Baurecht H, Rühlemann MC, Rodríguez E, et al. : Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J Allergy Clin Immunol. 2018;141(5):1668–1676.e16. 10.1016/j.jaci.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 59. Gruber R, Elias PM, Crumrine D, et al. : Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178(5):2252–63. 10.1016/j.ajpath.2011.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gruber R, Sugarman JL, Crumrine D, et al. : Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185(4):1012–21. 10.1016/j.ajpath.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rothhammer V, Quintana FJ: The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19(3):184–197. 10.1038/s41577-019-0125-8 [DOI] [PubMed] [Google Scholar]
- 62. Di Meglio P, Duarte JH, Ahlfors H, et al. : Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40(6):989–1001. 10.1016/j.immuni.2014.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. : Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123(2):917–27. 10.1172/JCI65642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Naeem AS, Tommasi C, Cole C, et al. : A mechanistic target of rapamycin complex 1/2 (mTORC1)/V-Akt murine thymoma viral oncogene homolog 1 (AKT1)/cathepsin H axis controls filaggrin expression and processing in skin, a novel mechanism for skin barrier disruption in patients with atopic dermatitis. J Allergy Clin Immunol. 2017;139(4):1228–1241. 10.1016/j.jaci.2016.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leclerc EA, Huchenq A, Kezic S, et al. : Mice deficient for the epidermal dermokine β and γ isoforms display transient cornification defects. J Cell Sci. 2014;127(Pt 13):2862–72. 10.1242/jcs.144808 [DOI] [PubMed] [Google Scholar]
- 66. Tobin D, Nabarro G, Baart de la Faille H, et al. : Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90(4 Pt 1):613–22. 10.1016/0091-6749(92)90134-n [DOI] [PubMed] [Google Scholar]
- 67. Dou YC, Hagströmer L, Emtestam L, et al. : Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006;298(1):31–7. 10.1007/s00403-006-0657-1 [DOI] [PubMed] [Google Scholar]
- 68. Xie Z, Hu H: TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals (Basel). 2018;11(4): pii: E100. 10.3390/ph11040100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee YW, Won CH, Jung K, et al. : Efficacy and safety of PAC-14028 cream - a novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: a phase IIb randomized trial. Br J Dermatol. 2019;180(5):1030–1038. 10.1111/bjd.17455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bergboer JGM, Zeeuwen PLJM, Schalkwijk J: Genetics of psoriasis: evidence for epistatic interaction between skin barrier abnormalities and immune deviation. J Invest Dermatol. 2012;132(10):2320–2331. 10.1038/jid.2012.167 [DOI] [PubMed] [Google Scholar]
- 71. Han H, Roan F, Ziegler SF: The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116–130. 10.1111/imr.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim BE, Howell MD, Guttman-Yassky E, et al. : TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011;131(6):1272–9. 10.1038/jid.2011.24 [DOI] [PMC free article] [PubMed] [Google Scholar]