Abstract
OBJECTIVE:
In this study, we aimed to investigate the prevalence of thyroid dysfunction in pregnant women in their third trimester and assess its relationship with perinatal outcomes.
METHODS:
A total of 796 women who delivered babies at the Haydarpaşa Numune Training and Research Hospital between January 2014 and January 2015 were evaluated retrospectively. Women with complete data and relevant results from thyroid functions tests were included in the study (n=573). Serum levels of thyroid stimulating hormone (TSH), free triiodothyronine (T3), free thyroxine (T4) were studied for all patients. Patients were classified according to thyroid function test results as having hypothyroidism, subclinical hypothyroidism, hyperthyroidism, or euthyroid state. The perinatal outcomes (Apgar score, birth type and birth weight) were compared.
RESULTS:
A total of 86.7% of pregnant woman (492/573) showed normal thyroid function tests. Out of the remaining participants,0.5% had hypothyroidism, 8.9% had subclinical hypothyroidism, and 2.8% had hyperthyroidism. TSH levels correlated with maternal age. The perinatal outcomes were insignificant between groups.
CONCLUSION:
The prevalence of thyroid dysfunction was 13.2% in our population. Subclinical hypothyroidism and hyperthyroidism had no adverse effects on birth weight, cesarean section rates, and Apgar scores.
Keywords: Hypothyroidsm, perinatal outcome, thyroid
Thyroid hormone is necessary for fetal development and maturation. Until the fetus synthesizes its own thyroid hormones, it is dependent on the T4 hormone that passes through the placenta from the mother [1, 2]. The need for iodine increases during pregnancy because of increased maternal-fetal metabolism and glomerular filtration rate [3]. To meet the increased metabolic needs of the mother and the fetus during pregnancy, physiological changes take place in the thyroid gland. These changes should be considered when evaluating thyroid function tests during pregnancy [4, 5].
Thyroid diseases are the second most common endocrine disorders affecting women in the reproductive period [1]. Women are likely to experience thyroid-related problems during pregnancy. Early diagnosis and treatment of thyroid diseases before and during pregnancy is important for maintaining the health of the mother and the baby [5].
At least 2%–3% of pregnant women are affected by thyroid dysfunction. Hyperthyroidism occurs in 0.2%–0.4% of pregnant women and is most commonly associated with Grave’s disease. The incidence of hypothyroidism in pregnancy is between 0.5%–3.5%. Hashimoto’s thyroiditis is its most common cause, but it is also seen in regions with iodine deficiency [6].
Thyroid dysfunction in pregnancy may be accompanied by both maternal and fetal complications. Hypothyroidism in pregnancy is associated with premature birth, fetal cardiac complications, low birth weight, increased frequency of cesarean delivery, placental complications, preeclampsia and gestational hypertension, perinatal morbidity-mortality, and cognitive dysfunction. In hyperthyroidism during pregnancy, complications such as stillbirth, abortion, premature birth, preeclampsia, heart failure and thyroid storm may develop [6–9].
Subclinical hypothyroidism is associated with increased TSH levels and normal fT4 values and is more common than overt hypothyroidism. Although it has been suggested to be associated with preterm labor and fetal loss, its relationship with pregnancy complications is controversial [8]. As the harmful effects of thyroid diseases for both mother and baby have started to come into prominence, the need for screening thyroid during pregnancy has also been discussed. Although literature shows that screening for subclinical hypothyroidism is cost-effective, the number of studies showing the results and benefits of screening has not yet reached a sufficient level [10–14]. The prevalence of thyroid dysfunction in pregnancy should be known in order to perform community-based screenings in a healthy way. The aim of this study was to investigate the frequency of thyroid dysfunction and its relationship with perinatal outcomes in pregnant women in their 3rd trimester, who applied to our education and research hospital.
MATERIALS AND METHODS
Between January 2014 and January 2015, the thyroid function tests (TFT) of 573 pregnant women who had given birth in our hospital were included in the study. The study was designed retrospectively and approval from the ethical committee was obtained.
Patient Selection: Patients who gave birth at our clinic and who underwent TFT after 24 weeks of gestation were included in the study. These patients had not undergone TFTs during the 1st and 2nd trimesters and had not experienced any thyroid problems previously. Patients with a diagnosis of hyperemesis gravidarum, those who had undergone thyroid surgery previously, had undergone lithium or amiodarone therapy or head and neck radiotherapy, were infertile, and had type 1diabetes were excluded from the study. Out of the 796 screened births, those who did not meet the inclusion criteria were excluded and the study was conducted with 573 patients.
Evaluation of TFTs: The diagnosis of hypothyroidism, hyperthyroidism, and subclinical hypothyroidism was based on the American Thyroid Association (ATA) guidelines [15]. According to these, the normal values are 0.1–2.5 µ/L in the 1st trimester, 0.2–3.0 µ/L in the 2nd trimester, and 0.3–3.0 µ/L in the 3rd trimester. If TSH was >10 µ/L, a diagnosis of overt hypothyroidism was made, and if it was between 3–10 µ/L, a diagnosis of subclinical hypothyroidism was made. Since the participants were in their >24 gestational week, the 3rd trimester values were taken as reference. Perinatal results of the patients were obtained from their computerized records and files.
The levels of TSH (thyroid stimulating hormone), T4 (thyroxine), T3 (triiodothyronine), and anti-TPO (antithyroid peroxidase) antibody were studied as part of the TFTs. The results were recorded by the Abbot Architect 1600 Chemiluminescence method.
Statistical Analysis
Data were analyzed by the two-way method and within a 95% confidence interval. The SPSS 18 program was used for data analysis (SPSS Inc., Chicago, IL, USA). In addition to descriptive statistical analysis (percentage, minimum, maximum, mean, standard deviation), the intergroup comparisons were done according to the distribution of the data. The Student’s t-test, Chi-square test, and Mann Whitney-U test were used. A value of p<0.05 was considered statistically significant.
RESULTS
The patients’ age range was 18-45 years (mean±SD =27.8±5.7). A total of 258 (45%) patients delivered by the vaginal route and 315 patients opted for cesarean sections (55%). Table 1 presents the general characteristics of the patients who participated in the study. Of the 573 pregnant women, 492 (86.7%) had normal TFT results and 76 patients (13.2%) had abnormal results. According to the results of screening tests of thyroid function, the prevalence rates of overt hypothyroidism (n=3, 0.5%), subclinical hypothyroidism (n=51, 8.9%), and hyperthyroidism were as indicated. Figure 1 shows the distribution of thyroid dysfunction during pregnancy.
TABLE 1.
General characteristics of the patients participating in the study (n=573)
| Minimum | Maximum | Mean±SD | |
|---|---|---|---|
| Age | 14 | 45 | 27.8±5.7 |
| Gravida | 1 | 9 | 2.5±1.4 |
| Parity | 0 | 6 | 1.1±1.0 |
| Abortus | 0 | 5 | 0.2±0.6 |
| Survived | 0 | 6 | 1.1±1.0 |
SD: Standard deviation.
FIGURE 1.
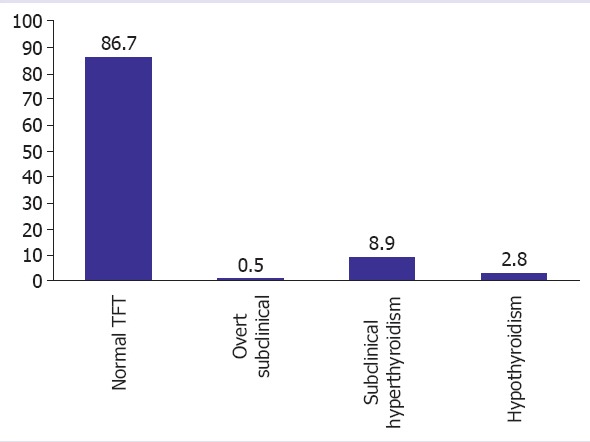
Prevalence rates of hyperthyroidism, hypothyroidism, and subclinical hypothyroidism.
Correlation analysis was performed for TSH elevation using some parameters. Correlation analyses were performed between TSH values and parameters such as, maternal age (r=0.085, p=0.04), anti-TPO (r=0.347, p=0.09), and birth weight (r=-0.07, p=0.873). An increase in TSH correlated positively with maternal age. Although there is a positive correlation with anti-TPO, the number of patients with anti-TPO was limited, because of which statistically significant data could not be found. TSH was found to increase with age. There was no significant correlation between TSH increase and birth weight. Patients with and without anti-TPO antibodies were compared in terms of birth weight and Apgar scores, but no significant difference was found.
Patients with normal thyroid function were compared with cases of subclinical hypothyroidism and hyperthyroidism to evaluate some obstetric and perinatal outcomes (Table 2). Cesarean delivery rates of patients with normal TFT and overt hypo- and hyperthyroidism were similar (p>0.05). The subclinical hypothyroidism group had lower C/S rates (p=0.03). In the overt hypothyroidism group, the 1- and 5-minute Apgar scores were lower, but the variance distribution was not homogeneous, so the statistical significance could not be obtained. The groups were similar in terms of Apgar scores and birth weights (p>0.05).
TABLE 2.
Comparison of some obstetric and perinatal outcomes of patients with normal thyroid functions or subclinical hypothyroidism
| Parameters | Hypothyroidism | Subclinical hypothyroidism | Hyperthyroidism | Normal TFT value | p |
|---|---|---|---|---|---|
| Age | 0.714 | 27.7±5.4 | 28.0±5.2 | 28.3±5.7 | ns |
| Gravida | 3.0±0.0 | 2.1±1.4 | 2.4±1.1 | 2.6±1.3 | ns |
| Parity | 1.5±0.7 | 0.8±0.9 | 1.2±1.1 | 1.2±1.0 | ns |
| Abortus | 0.5±0.7 | 0.2±0.8 | 0.09±0.2 | 0.2 ±0.6 | ns |
| Cesarean delivery rate | %100 | %43* | %54 | % 59 | s |
| Birth weight (g) | 2814±161 | 3322±489 | 3125±410 | 3360±1278 | ns |
| Apgar 1. min | 2.5±3.5 | 7.4±1.5 | 8.1±0.7 | 7.7±1.2 | ns |
| Apgar 5. min | 4.0±5.6 | 8.8±1.5 | 8.8±1.5 | 9.0±0.9 | ns |
p<0.05; s: Significant; ns: Nonsignificant.
DISCUSSION
Normally functioning thyroid glands are able to meet the increasing need for hormones during pregnancy and keep thyroid hormone levels within normal limits. Excessive or deficient maternal thyroid hormone levels are known to have serious effects on the fetal and maternal outcomes at every stage of a pregnancy [9]. However, the performance of screening tests for evaluating thyroid function during routine pregnancies is still controversial.
Maternal hypothyroidism is the most common thyroid dysfunction in pregnancy. In our study, the prevalence of overt hypothyroidism was 0.5% (n=3), which was consistent with the literature. In both new and previously diagnosed cases with overt hypothyroidism, the initiation of a full dose of L-thyroxine is considered necessary to prevent complications related to hypothyroidism [4, 10]. To determine the optimum dose, the trimester-specific intervals for TSH and the total and free thyroid hormone levels should be determined correctly. Reference values may vary in regions where iodine deficiency is common [11].
Iodine deficiency, which is an important public health issue, causes serious problems in different periods of life, starting as early as the intrauterine period. The daily iodine requirement increases 1.5 times in pregnant women and up to 2 times in breastfeeding mothers. Adequate levels of maternal iodine intake should be ensured in regions with iodine deficiency. Its recommended daily average dose is 200 µg [12]. The rate of iodine deficiency in Istanbul has been reported as 46.2% [13]. Severe levels of iodine deficiencies can cause fetal neural developmental disorders, as they can lead to maternal and fetal iodine deficiencies. For normal fetal brain and cognitive development, maternal thyroid functions should remain at normal levels. If this is not treated, hypothyroidism may cause premature births and fetal losses.
In a 3-year follow-up study, Pop et al. detected significantly lower mental and motor scores in babies aged 1–2 years, born to pregnant women who had hypothyroidism in the first trimester [10]. In this study, the Apgar scores were lower in the overt hypothyroid group, but this did not have any statistical significance. Since our cases with hypothyroidism were limited in number, we were not able to comment on its relationship with perinatal outcomes.
The incidence of hyperthyroidism in pregnant women is 0.2%. However, in our study, the incidence of hyperthyroidism was 2.8% (n=31). It has been stated that the most common cause of hyperthyroidism in pregnancy is gestational transient thyrotoxicosis (GTT), which is 10 times more frequent than Graves’ disease. GTT is not an autoimmune condition and may occur during a normal pregnancy as well.
This condition is more frequently encountered in twin pregnancies and it is typically associated with hyperemesis gravidarum. Herein, hyperthyroidism is less dominant than Graves’ disease and is more common in the 1st trimester [14]. Antithyroid drugs are used to treat hyperthyroidism during pregnancy [11]. In this study, perinatal outcomes of cases with hyperthyroidism were similar to the other groups. The rate of cesarean sections was largely similar between the groups; it was lower only in the subclinical hypothyroid group. Since our clinic is a reference center, higher rates of cesarean delivery were also detected in the normal group.
As a consensus, screening for thyroid dysfunction in pregnancy is performed during the follow-up of high risk women by assessing symptomatic thyroid dysfunction or recording a history of thyroid disease, autoimmune disease [2], pregnant women carrying high risk for thyroid diseases, and for the diagnosis of subclinical thyroid diseases, [15]. In our study, the TSH elevation was found to be positively correlated with maternal age (p<0.05). This finding is consistent with the literature. According to the Association of Endocrine Metabolism Thyroid Diseases Diagnosis and Treatment Guidelines [15], it is recommended that women older than 35 years be screened for TSH every 5 years.
We have seen that the results of the studies on this subject in our country vary [16–19]. In their studies including the 1st and 2nd trimesters, Temur et al. [16] detected the upper limit of TSH as 5.6 mµ/L and the frequency of hypothyroidism as 3.6%. In these studies, any difference between pregnant women with and without the risk factors for thyroid dysfunction. They also insistently emphasized the need for screening all pregnant women. The prevalence of hypo- and hyperthyroidism according to some studies and regions of our country is given in Table 3 [16–19]. According to these results, in our country, we see that the cut-off values are not standardized in the diagnosis of hypo- and hyperthyroidism.
TABLE 3.
Domestic studies demonstrating the prevalence rates of thyroid dysfunction in Turkey
| Author | City | Year | Hypothyroidism | Hyperthyroidism | TSH reference values |
|---|---|---|---|---|---|
| Temur et al. [16] | Istanbul | 2012 | %3.6 | %1 | 0.34–5.6 MU/L |
| Karakurt et al. [17] | Ankara | 2007 | %2.8 | %0.6 | – |
| Bostanci et al. [18] | Elazig | 2011 | %2.84 | %0.68 | 0.3–4.5 MU/L |
| Guzel et al. [19] | Istanbul | 2015 | %5.38 | %15.8 | 0.1–3 MU/L* |
| Donmez et al. [20] | Istanbul | 2005 | %1.6 | %4 | 0.6–3.42 MU/L |
| Our study | Istanbul | 2016 | %6.8 | %2.8 | 0.3-3 MU/L* |
TSH cut-off values determined by ATA according to the trimesters of pregnancy were taken into consideration.
Similar cut-off values were used in a study by Guzel et al. [19] in Istanbul. Although the prevalence of hypothyroidism among their patients was similar to our study, the prevalence of hyperthyroidism in their study was higher. However, these researchers included all the pregnancy trimesters in the study. In our study, thyroid dysfunction was found in 76 patients (13.2%) and the prevalence of hyperthyroidism was 2.8%. Similar to our study, Donmez et al. performed the study in the 3rd trimester in the metropolitan city of Istanbul, but their reference range of normal thyroid values were different from ours [20].
When we look at the studies performed in our country, we see that different cut-off values were used for TFTs, and they were performed during different gestational weeks. In the light of all these results, we think that countrywide prospective studies where standard cut-off values are used will give more valuable results about the prevalence and perinatal outcomes of thyroid diseases during pregnancy.
The imitations of this study include its retrospective design, the evaluation of TFTs in the last trimester, and the missing anti-TPO data in some pregnant women.
In addition, TFTs were requested from pregnant women in primary care. Since all pregnant women are registered in family medicine, we think that it is more appropriate to include primary care data when conducting prevalence studies.
In conclusion, thyroid diseases that develop during pregnancy can be treated if a diagnosis is made, and the treatment may yield satisfactory results. Otherwise, it is highly probable that this disease may have serious health consequences for both the mother and the newborn.
According to our findings; the prevalence of thyroid dysfunction in the third trimester was 13.2% in the pregnant population. No adverse effects of subclinical hypothyroidism and hyperthyroidism were detected on the perinatal outcomes. Since TSH increase is correlated with age, we can recommend routine screening, especially for pregnant women over 35 years of age. Regarding the participation of those under the age of 35 and those who do not carry risk factors, we believe that a healthy outcome can be achieved after large-scale data is collected.
Footnotes
Ethics Committee Approval: Haydarpasa Numune Training and Research Hospital Clinical Research Ethics Committee HNEAH-KAEK 2016/79 (HNEAH KAEK 2016/KK/79) date: 27.09.2016, decision number: 2479.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – HD, FV; Design – FV; Supervision – FV; Materials – HD; Data collection and/or processing – HD, SZ; Analysis and/or interpretation – FV; Writing – HD; Critical review – NA.
REFERENCES
- 1.Negro R, Mestman JH. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:927–43. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetrics and Gynecology. ACOG practice bulletin. Thyroid disease in pregnancy. Number 37, August 2002. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 2002;79:171–80. doi: 10.1016/s0020-7292(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 3.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation:trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddow JE, McClain MR, Lambert-Messerlian G, Palomaki GE, Canick JA, Cleary-Goldman J, et al. First and Second Trimester Evaluation of Risk for Fetal Aneuploidy Research Consortium. Variability in thyroid-stimulating hormone suppression by human chorionic [corrected] gonadotropin during early pregnancy. J Clin Endocrinol Metab. 2008;93:3341–7. doi: 10.1210/jc.2008-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 7.Susan J, Mandel SC, David SC. The use of antithyroid drugs in pregnancy and lactation. J Clin Endocrinol Metab. 2001;86(6):2354–9. doi: 10.1210/jcem.86.6.7573. [DOI] [PubMed] [Google Scholar]
- 8.Tekin Bayoğlu Y, Güven Güvendağ E. Thyroid Disease in Pregnancy and Neonatal Outcome. Jinekoloji-Obstetrik ve Neonatoloji Tıp Dergisi. 2014;4:150–3. [Google Scholar]
- 9.Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283–92. doi: 10.1097/01.AOG.0000244103.91597.c5. [DOI] [PubMed] [Google Scholar]
- 10.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development:a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 11.Soldin OP. Thyroid function testing in pregnancy and thyroid disease:trimester-specific referenceintervals. Ther Drug Monit. 2006;28:8–11. doi: 10.1097/01.ftd.0000194498.32398.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lao TT. Thyroid disorders in pregnancy. Curr Opin Obstet Gynecol. 2005;17:123–7. doi: 10.1097/01.gco.0000162179.15360.08. [DOI] [PubMed] [Google Scholar]
- 13.Gür E, Ercan O, Can G, Akkuş S, Güzelöz S, Ciftcili S, et al. Prevalence and risk factors of iodine deficiency among schoolchildren. J Trop Pediatr. 2003;49:168–71. doi: 10.1093/tropej/49.3.168. [DOI] [PubMed] [Google Scholar]
- 14.Glinoer D. Management of hypo- and hyperthyroidism during pregnancy. Growth Horm IGF Res. 2003;13(Suppl A):S45–54. doi: 10.1016/s1096-6374(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 15.American Thyroid Association. Consensus Statement #2:American Thyroid Association statement on early maternal thyroidal insufficiency:recognition, clinical management and research directions. Thyroid. 2005;15:77–9. doi: 10.1089/thy.2005.15.77. [DOI] [PubMed] [Google Scholar]
- 16.Temur M, Cengiz H, Arıcı B, Yaşar L, Özdemir İA. Detection of Thyroid Dysfunction in Early Pregnancy. Gazi Med J. 2012;23:6–9. [Google Scholar]
- 17.Karakurt F, Gümüşİnegöl İ, Kargılı A, Uz B, Keskin E, Köroğlu M. Gebe Hastalarımızda Tiroid Fonksiyon Testleri. Yeni Tıp Dergisi. 2007;24:54. [Google Scholar]
- 18.Bostancı MS, Taşkesen F. Thyroid dysfunction during pregnancy and evaluation of its results. J Clin Exp Invest. 2011;2:196–201. [Google Scholar]
- 19.Güzel E, Sivri Aydın D, Çilesiz Göksedef B, Boran Birtan A. The incidence of thyroid dysfunction in pregnant women. Perinatal Journal. 2015;23:96–100. [Google Scholar]
- 20.Dönmez Kesim M, Aydın Y, Atış A, Şişli T. Thyroid function disturbances in third trimester pregnancy. Turkiye Klinikleri J Gynecol Obst. 2005;15:132–6. [Google Scholar]


