Abstract
Background
The Helicobacter pylori eradication rate has decreased with increasing antibiotic resistance. We conducted a prospective, nationwide, multicenter registry study to monitor the real status of H. pylori eradication therapy and to investigate the association between eradication success and antibiotic use density in Korea.
Materials and Methods
We enrolled 9318 patients undergoing H. pylori eradication therapy from 37 hospitals through “on‐line database registry” from October 2010 to July 2015. Demographic data, detection methods, treatment indications, regimens, durations, compliance, adverse events, and eradication results were collected. The use of all commercially available eradication antibiotics was analyzed through the Korean National Health Insurance data of the Health Insurance Review and Assessment Service. The defined daily dose of antibiotics was used to standardize drug use comparisons.
Results
Finally, 6738 patients were analyzed. The overall eradication rate of first‐line therapy was 71.8%. The eradication success rates were 71.7%, 86.9%, and 74.0% for standard triple therapy for 7 days, quadruple therapy, and concomitant therapy, respectively. The eradication success rate in naive patients was higher than that in those who previously underwent H. pylori eradication. Eradication success was significantly associated with younger age, female sex, and high compliance. Regional differences in eradication rates were observed. The yearly use density of clarithromycin increased statistically in seven regions across the country from 2010 to 2015. The yearly use density of amoxicillin in the Gyeongsang and Chungcheong areas was significantly increased (P < .01), whereas that of other macrolides was significantly lower in the Gyeonggi area than in other areas (P = .01). The overall use of eradication antibiotics has increased while the eradication rate steadily decreased for 5 years. However, there was no significant correlation between antibiotic use density and eradication.
Conclusion
There was no relationship between the eradication rate and antibiotic use density in Korea.
Keywords: antibiotic use, eradication success, first‐line therapy, Helicobacter pylori, online registry
1. INTRODUCTION
Helicobacter pylori infection is associated with chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and non‐Hodgkin's mucosa‐associated lymphoid tissue (MALT) lymphoma of the stomach.1, 2 East Asian countries with a high incidence of gastric cancer, such as Korea and Japan, have been making efforts to eliminate H. pylori to prevent gastric cancer.1, 3 Owing to these efforts, the incidence of H. pylori infection has declined but has remained persistently high in Korea.
Korean guidelines state that a definitive indication for H. pylori eradication is peptic ulcer including scarring, marginal zone B‐cell lymphoma (MALT type), and early gastric cancer after endoscopic resection.4 The selection principle of eradication therapy should be combined with nontolerant antibiotics, synergistic anticipation, high drug compliance, and a second‐line therapy when eradication fails.
Currently, standard triple therapy (STT), comprising a proton‐pump inhibitor (PPI) and the antibiotics amoxicillin and clarithromycin, has been used worldwide including Korea as the first‐line therapy for H. pylori infection.5, 6, 7 However, H. pylori eradication rates with STT have been declining and recently reached <80% in many countries.7, 8, 9 Antibiotic resistance is the main factor that affects the efficacy of current therapeutic regimens against H. pylori. Therefore, many guidelines recommend different treatment options for each region according to antibiotic resistance, particularly against clarithromycin.10, 11 In addition, various therapies have been proposed for a new effective first‐line eradication therapy. Unfortunately, antibiotic resistance tests are not generally recommended before first‐line eradication therapy in the H. pylori treatment guidelines.12
In Korea, it has been reported that the H. pylori eradication rate with first‐line therapy has decreased with increasing antibiotic resistance since 2000.13, 14, 15 Thus, current first‐line therapy does not meet the ideal eradication success rate in Korea.
To develop more appropriate treatments, it is important to monitor the current treatment situation and to identify the compliance of the current guidelines in clinical practice. As the optimal regimen reflects local preference and experience, continuous evaluation of treatment outcomes in clinical practice should be taken into account. Thus, it is of utmost importance to evaluate the efficacy of the currently used first‐line therapy while considering antibiotic resistance.
The aims of the present study were (a) to monitor H. pylori eradication therapy in clinical practice through a prospective systematic registry database on a nationwide scale in Korea within the recent 5 years and (b) to investigate the relationship between the eradication rate and antibiotic use density in a country with a high prevalence of H. pylori infection through national health insurance data.
2. MATERIALS AND METHODS
2.1. Study population
This prospective, multicenter, registry study (cris.nih.go.kr/KCT0001390) was conducted from October 2010 to July 2015 in Korea.
Patients confirmed to be infected by H. pylori and treated for the ensuing infection were enrolled. Patients were not eligible for the study if one of the following criteria was met: known or suspected allergy to PPIs or antibiotics, previous history of gastric surgery, pregnancy or lactation, or therapy with a PPI or antibiotics within 4 weeks of entry.
2.2. Population standardization
In this report, eradication rates were standardized to the mid‐year population of 2010, which was defined as the Korean standard population for this study. The standard population provides the age distributions used in the estimation of age‐standardized rates. The mid‐year population of 2010 was used as the denominator of the eradication rate and was generated using the resident registration population published annually by the Ministry of Government Administration and Home Affairs. In the present study, we divided the country into seven geographic areas according to administrative district.
2.3. Evaluation of H. pylori infection
Helicobacter pylori infection was diagnosed based on histologic evidence of H. pylori according to Giemsa staining, a positive rapid urease test, a positive H. pylori culture test, and a positive 13C‐urea breath test. Patients were diagnosed as infected if any of these tests was positive.
2.4. Eradication therapy
Investigators prescribed the eradication regimen in accordance with the guidelines established by the Korean College of Helicobacter and Upper Gastrointestinal Research group. First‐line STT included standard‐dose PPI (esomeprazole 40 mg, omeprazole 40 mg, lansoprazole 30 mg, pantoprazole 40 mg, or rabeprazole 20 mg) plus amoxicillin (1000 mg), and clarithromycin (500 mg) twice a day for 7‐14 days. Second‐line therapy included quadruple therapy (QT, standard‐dose PPI twice daily, 120 mg bismuth four times a day, 500 mg metronidazole three times a day, and 500 mg tetracycline four times a day for 7‐14 days). Other eradication regimens including concomitant therapy or sequential therapy were also prescribed as first‐line or second‐line therapy.
To evaluate the success of the eradication therapy, follow‐up endoscopy with a rapid urease test and histologic examination or a 13C‐urea breath test were performed at least 4 weeks after the completion of the therapy. H. pylori eradication success was defined as a negative rapid urease test and histology or a negative 13C‐urea breath test that was confirmed within 6 months after H. pylori eradication therapy. Eradication failure was defined as a positive result in any of these tests. Compliance was considered to be satisfactory when drug intake exceeded 80%. Adverse events associated with treatment were also investigated.
2.5. Registry data collection
The electronic data management system for this study was developed by the Seoul National University Medical Research Collaborating Center using the Pharmacoepidemiology and Clinical Trial Application X (Phacta X) system. Data entry was completed by selecting appropriate icons. Data from clinical information and laboratory test results were entered into a web‐based electronic case‐reporting form (CRF) following the standard protocol for data entry (http://www.phactax.org). The electronic CRF comprised variables including demographic data (eg, age, sex, residence, smoking status, alcohol consumption, and comorbidity including hypertension, diabetes, ischemic heart disease, liver cirrhosis, chronic renal failure, and malignancy), diagnostic methods for H. pylori infection, treatment indications, regimens, durations, compliance, and treatment‐related adverse events. All patients provided written informed consent to participate before enrollment. The study was approved by the representative institutional review boards of participating hospitals in Korea.
2.6. Measurement of eradication antibiotic use density
All commercially available eradication antibiotics in Korea were evaluated, including clarithromycin, azithromycin, erythromycin, roxithromycin, dirithromycin, telithromycin, and amoxicillin.
We identified the population of the relevant region by year through the National Statistical Office website (http://kostat.go.kr). The data used in the calculation of antibiotic use density included the name, dose, total number of days or number of times of administration, daily dose, and single dose.
We adopted the defined daily dose (DDD) to measure the antibiotic use density. The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. It is used to standardize the comparisons of drug use between different drugs or between different healthcare environments. We calculated the DDD per 1000 inhabitants (DID) of the population scale to compare the density by region. Thus, we assessed the 5 years’ DID of eradication antibiotics using the Health Insurance Review and Assessment (HIRA) database (http://www.hira.or.kr), which was accessible from January 2010 to December 2015 in seven geographic regions in Korea, including the Seoul, Gyeonggi, Gangwon, Chungcheong, Gyeongsang, Jeolla, and Jeju areas, where our eradication data had been collected. The main outcome was any difference in antibiotic (clarithromycin, other macrolide, and amoxicillin) use between any of the seven geographic regions in Korea.
2.7. Statistical analyses
Characteristics of patients and eradication rate of H. pylori were summarized using mean and standard deviation for continuous variables, and frequency or percentage for categorical variables. Comparison of continuous variable was performed using Student's t test. Fisher's exact test was used to compare categorical variables. Age was categorized as <30, 30‐40, 40‐50, 50‐60, 60‐70, or ≥70 years. Eradication success rates for the first‐line therapy were evaluated according to geographic regions. The standardized rates were calculated using patients receiving STT regimens as the standard population within the Korean National Health Insurance database, which covered all patients in 2010. Eradication success rates were calculated by dividing the number of patients with successful eradication by the total number of patients according to the intention‐to‐treat analysis. Logistic regression analysis was used to examine the associations between H. pylori eradication therapy and each of the following factors: age, sex, residence, current smoking, alcohol consumption, medication, previous gastrointestinal disorder, comorbidity, previous H. pylori eradication, duration of treatment, treatment compliance, and treatment‐related complications. A multivariate model was constructed using variables with P < .2 in univariate logistic regression analysis. SAS version 9.4 (SAS Institute Inc) and SAS Enterprise Guide version 6.1 (SAS Institute, Inc) were used for statistical analysis. Statistical significance was considered for P < .05. All P‐values were two‐sided. Annual patterns of antibiotic use density were checked using a regression model with autoregressive error correction.
3. RESULTS
3.1. Baseline characteristics
We prospectively enrolled 9318 patients from 37 tertiary and academic hospitals that were representative of Korean hospitals. The 37 hospitals that participated in the study were major regional hospitals representing different regions of the country. A total of 6738 patients were identified using their eradication results within 6 months after undergoing H. pylori eradication therapy (Figure 1). The baseline characteristics of these patients who received first‐line eradication therapy are summarized in Table 1. Eradication success after first‐line therapy was achieved in 4836 patients (71.8%). Eradication success declined with age (P < .001). However, there was no significant difference (P = .08) in eradication success between sexes. Concerning regional differences, the Gyeongsang area showed the highest eradication rate with first‐line therapy, whereas the Chungcheong area had the lowest (75.2% and 60.9%, respectively, P < .001) eradication rate (Figure 2).
Figure 1.
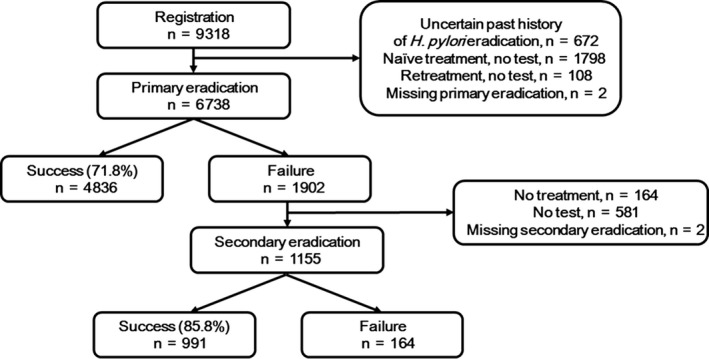
Helicobacter pylori eradication in first‐line and second‐line therapy. A total of 6738 patients participated in the study. Overall eradication rates with first‐line and second‐line therapy were 71.8% and 85.8%, respectively
Table 1.
Baseline characteristics of 6738 patients who underwent the first‐line Helicobacter pylori eradication therapy in the nationwide H. pylori Registry study in Korea
|
Eradication success (N = 4836) N (%) |
No eradication success (N = 1902) N (%) |
Eradication success rate (N = 4836) %, (95% CI) |
|
|---|---|---|---|
| Age (y) | |||
| <30 | 192 (3.97) | 78 (4.1) | 71.11 (65.70, 76.52) |
| 30‐39 | 392 (8.11) | 117 (6.15) | 77.01 (73.35, 80.67) |
| 40‐49 | 922 (19.07) | 288 (15.14) | 76.20 (73.8, 78.6) |
| 50‐59 | 1577 (32.61) | 612 (32.18) | 72.04 (70.16, 73.92) |
| 60‐69 | 1179 (24.38) | 506 (26.6) | 69.97 (67.78, 72.16) |
| ≥70 | 574 (11.87) | 301 (15.83) | 65.6 (62.45, 68.75) |
| Sex | |||
| Male | 2937 (60.73) | 1111 (58.41) | 72.55 (71.18, 73.92) |
| Female | 1899 (39.27) | 791 (41.59) | 70.59 (68.87, 72.31) |
| Residence | |||
| Seoul | 965 (19.95) | 380 (19.98) | 71.74 (69.33, 74.15) |
| Gyeonggi | 861 (17.8) | 347 (18.24) | 71.27 (68.72, 73.82) |
| Gangwon | 79 (1.63) | 37 (1.95) | 68.10 (59.62, 76.58) |
| Chungcheong | 228 (4.71) | 146 (7.68) | 60.96 (56.02, 65.9) |
| Gyeongsang | 1959 (40.51) | 646 (33.96) | 75.20 (73.54, 76.86) |
| Jeolla | 307 (6.35) | 184 (9.67) | 62.53 (58.25, 66.81) |
| Jeju | 437 (9.04) | 162 (8.52) | 72.95 (69.39, 76.51) |
| Current smoking | |||
| No | 2868 (71.5) | 1123 (70.54) | 71.86 (70.46, 73.26) |
| Yes | 964 (24.03) | 400 (25.13) | 70.67 (68.25, 73.09) |
| NA | 179 (4.46) | 69 (4.33) | 4.46 (1.89, 7.03) |
| Alcohol consumption | |||
| No | 2273 (56.66) | 932 (58.51) | 70.92 (69.35, 72.49) |
| Yes | 1569 (39.11) | 594 (37.29) | 72.54 (70.66, 74.42) |
| NA | 170 (4.24) | 67 (4.21) | 4.24 (1.67, 6.81) |
| Medication | |||
| No | 2393 (59.69) | 862 (54.21) | 73.52 (72, 75.04) |
| Yes | 1495 (37.29) | 686 (43.14) | 68.55 (66.6, 70.5) |
| Aspirin | 274 (18.33) | 127 (18.51) | 68.33 (63.78, 72.88) |
| Nonsteroidal anti‐inflammatory drugs | 70 (4.68) | 45 (6.56) | 60.87 (51.95, 69.79) |
| Antiplatelet/anticoagulation agents, or steroids | 517 (34.58) | 288 (41.98) | 64.22 (60.91, 67.53) |
| NA | 121 (3.02) | 42 (2.64) | 3.02 (0.39, 5.65) |
| Previous GI disorder | |||
| No | 3123 (77.98) | 1185 (74.58) | 72.49 (71.16, 73.82) |
| Yes | 724 (18.08) | 343 (21.59) | 67.85 (65.05, 70.65) |
| Peptic ulcer | 405 (55.94) | 191 (55.69) | 67.95 (64.20, 71.7) |
| Others | 403 (55.66) | 209 (60.93) | 65.85 (62.09, 69.61) |
| NA | 158 (3.95) | 61 (3.84) | 3.95 (1.37, 6.53) |
| Comorbidity | |||
| No | 2366 (59.02) | 845 (53.08) | 73.68 (72.16, 75.2) |
| Yes | 1556 (38.81) | 713 (44.79) | 68.58 (66.67, 70.49) |
| Hypertension | 973 (62.53) | 415 (58.2) | 70.10 (67.69, 72.51) |
| Diabetes | 419 (26.93) | 193 (27.07) | 68.46 (64.78, 72.14) |
| Ischemic heart disease | 82 (5.27) | 45 (6.31) | 64.57 (56.25, 72.89) |
| Liver cirrhosis | 18 (1.16) | 16 (2.24) | 52.94 (36.16, 69.72) |
| Chronic renal failure | 15 (0.96) | 14 (1.96) | 51.72 (33.53, 69.91) |
| Malignancy | 95 (6.11) | 37 (5.19) | 71.97 (64.31, 79.63) |
| Others | 512 (32.9) | 266 (37.31) | 65.81 (62.48, 69.14) |
| NA | 87 (2.17) | 34 (2.14) | 2.17 (0, 4.77) |
Abbreviations: CI, confidence interval; NA, not applicable.
Figure 2.
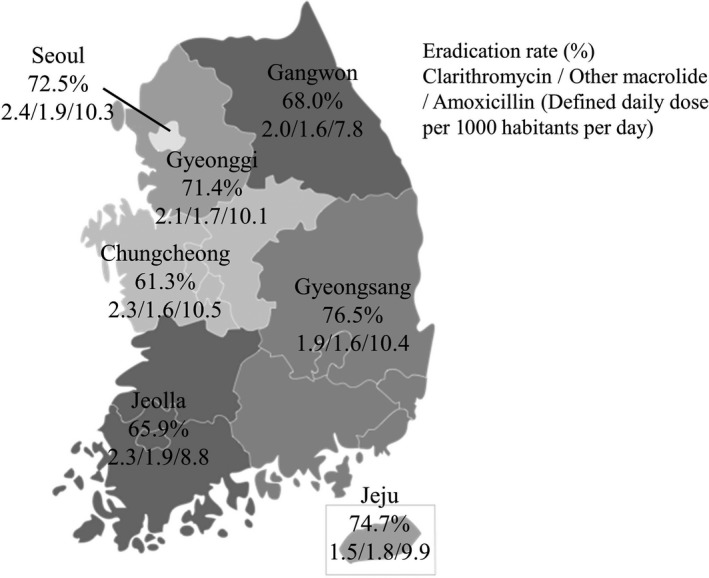
Overall Helicobacter pylori eradication rate and antibiotic use according to seven regions in Korea. Eradication rate is represented as a percentage; antibiotic use (clarithromycin/other macrolide/ amoxicillin) is represented as defined daily dose per 1000 habitants per day. The overall eradication rate in seven regions in Korea was 71.8%. Gyeongsang province showed the highest eradication rate with first‐line therapy, whereas Chungcheong province had the lowest eradication rate. Overall use of clarithromycin, other macrolide, and amoxicillin varied among regions. In particular, Seoul showed the highest use of clarithromycin among the seven regions
There was no significant difference between patients with a current smoking status or currently consuming alcohol and those not currently smoking or drinking (P = .68 and P = .43, respectively). The eradication rate in patients who had taken other medications, especially nonsteroidal anti‐inflammatory drugs, was lower than that in those who had not (P < .001). Patients with previous gastrointestinal disorders or comorbidities had significantly lower eradication rates (P = .011 and P < .001, respectively). The eradication rate in patients with liver cirrhosis or chronic renal failure was significantly lower than that in patients who were free of these diseases (52.9% and 51.7%, respectively).
3.2. First‐line H. pylori eradication
The results of H. pylori eradication with first‐line therapy are summarized in Table 2. The overall eradication rate in Korea was 71.8%. The eradication rates were significantly different according to treatment indication. The eradication rates in patients with benign gastric and duodenal ulcers were 67.1% and 74.8%, respectively (P < .001 and P = .005, respectively). The eradication success rate in treatment‐naive patients was higher than that in patients who had previously undergone H. pylori eradication treatment (72.8% vs 60.2%, P < .001). There were significant differences in the eradication rates according to treatment regimen: 71.4% for STT, 86.9% for QT, and 74.0% for concomitant therapy (CT, P < .001). The most common regimen and treatment duration were STT for 7 days (5383/6738, 79.8%), which achieved a 71.7% eradication success rate (Table 3). When extended to a 2‐week duration, the eradication rates were slightly increased. Other treatment regimens except STT, QT, and CT were also applied to eradicate H. pylori and were proved to be inferior to STT.
Table 2.
Eradication success rates and related characteristics of 6738 patients who underwent the first‐line Helicobacter pylori eradication therapy in the Nationwide H. pylori Registry study in 2010‐2015 in Korea
|
Eradication success (N = 4836) N (%) |
No eradication success (N = 1902) N (%) |
Eradication success rate (N = 4836) %, (95% CI) |
|
|---|---|---|---|
| Indication for eradication | |||
| BGU | 1075 (22.23) | 525 (27.6) | 67.19 (64.89, 69.49) |
| BDU | 1021 (21.11) | 343 (18.03) | 74.85 (72.55, 77.16) |
| BGU + BDU | 2287 (47.29) | 938 (49.32) | 70.91 (69.35, 72.48) |
| Bleeding peptic ulcer | 20 (0.41) | 4 (0.21) | 83.33 (68.42, 98.24) |
| After endoscopic resection for early gastric cancer | 663 (13.71) | 229 (12.04) | 74.33 (71.46, 77.19) |
| Gastric MALT lymphoma | 61 (1.26) | 15 (0.79) | 80.26 (71.31, 89.21) |
| Others | 1996 (41.27) | 786 (41.32) | 71.75 (70.07, 73.42) |
| Previous H. pylori eradication | |||
| No | 4564 (94.38) | 1699 (89.33) | 72.87 (71.77, 73.97) |
| Yes (within 1 y) | 63 (1.3) | 65 (3.42) | 49.22 (40.56, 57.88) |
| Yes (before 1 y) | 209 (4.32) | 138 (7.26) | 60.23 (55.08, 65.38) |
| STT | 154 (3.18) | 122 (6.41) | 55.8 (49.94, 61.66) |
| PBMT | 28 (0.58) | 9 (0.47) | 75.68 (61.85, 89.5) |
| CT | 13 (0.27) | 1 (0.05) | 92.86 (79.37, 106.35) |
| Others | 14 (0.29) | 6 (0.32) | 70 (49.92, 90.08) |
| First‐line therapy regimens | |||
| STT | 4422 (91.44) | 1766 (92.85) | 71.46 (70.34, 72.59) |
| PBMT | 127 (2.63) | 19 (1) | 86.99 (81.53, 92.44) |
| CT | 151 (3.12) | 53 (2.79) | 74.02 (68, 80.04) |
| Others | 136 (2.81) | 64 (3.36) | 68 (61.53, 74.47) |
| Duration | |||
| 7 d | 4064 (84.04) | 1593 (83.75) | 71.84 (70.67, 73.01) |
| 8‐13 d | 244 (5.05) | 118 (6.2) | 67.4 (62.57, 72.23) |
| 14 d | 527 (10.9) | 189 (9.94) | 73.6 (70.37, 76.83) |
| Others | 1 (0.02) | 2 (0.11) | 33.33 (0, 86.68) |
| Compliance | |||
| ≥80% | 4764 (98.51) | 1811 (95.22) | 72.46 (71.38, 73.54) |
| <80% | 40 (0.83) | 40 (2.1) | 50 (39.04, 60.96) |
| NA | 32 (0.66) | 51 (2.68) | 0.66 (0, 49.03) |
| Complication | |||
| No | 3807 (78.72) | 1490 (78.34) | 71.87 (70.66, 73.08) |
| Yes | 925 (19.13) | 344 (18.09) | 72.89 (70.45, 75.34) |
| Abdominal pain | 74 (1.53) | 44 (2.31) | 62.71 (53.99, 71.44) |
| Nausea, vomiting | 149 (3.08) | 89 (4.68) | 62.61 (56.46, 68.75) |
| Diarrhea | 373 (7.71) | 113 (5.94) | 76.75 (72.99, 80.5) |
| Others | 536 (11.08) | 198 (10.41) | 73.02 (69.81, 76.24) |
| NA | 104 (2.15) | 68 (3.58) | 2.15 (0, 67.77) |
| Second‐line therapy regimens | |||
| PBMT | 819 (16.94) | 115 (6.05) | 87.69 (85.58, 89.79) |
| PAC | 13 (0.27) | 7 (0.37) | 65 (44.1, 85.9) |
| PTM | 42 (0.87) | 7 (0.37) | 85.71 (75.92, 95.51) |
| PAM | 2 (0.04) | 0 (0) | 100.0 (34.2, 100.0) |
| Others | 115 (2.38) | 35 (1.84) | 76.67 (69.9, 83.44) |
STT: Proton‐pump inhibitor + amoxicillin + clarithromycin.
PBMT: Proton‐pump inhibitor + denol + metronidazole + tetracycline.
CT (Concomitant therapy): PPI + AMX + CLA + MTZ.
PAC: Proton‐pump inhibitor + amoxicillin + clarithromycin.
PTM: Proton‐pump inhibitor + tetracycline + metronidazole.
PLM: Proton‐pump inhibitor + levofloxacinvmetronidazole.
Abbreviations: CI, confidence interval; NA, not applicable; BGU, benign gastric ulcer including scar; BDU, benign duodenal ulcer including scar; BGU + BDU, both benign gastric and duodenal ulcers.
Table 3.
First‐line Helicobacter pylori eradication rate according to the regimen and treatment duration
| STT N (%, 95% CI) | PBMT N (%, 95% CI) | CT N (%, 95% CI) | Others N (%, 95% CI) | Total | |
|---|---|---|---|---|---|
| 7 d | 3859/5383 (71.7%, 70.5‐72.9) | 66/80 (82.5%, 74.2‐90.8) | 20/24 (83.3%, 68.4‐98.2) | 119/170 (70.0%, 63.1‐76.9) | 5657 |
| 8‐13 d | 74/136 (54.4%, 46.0‐62.8) | 55/59 (93.2%, 86.8‐99.6) | 112/159 (70.4%, 63.4‐77.5) | 3/8 (37.5%, 4.0‐71.1) | 362 |
| 14 d | 489/667 (73.3%, 70.0‐76.7) | 6/7 (85.7%, 59.8‐100.0) | 19/21 (90.5%, 77.9‐100.0) | 13/21 (61.9%, 41.1‐82.7) | 716 |
| Others | 0/2 (0.0%, 0.0‐84.2) | 0/0 (‐) | 0/0 (‐) | 1/1 (100.0%, 2.5‐100.0) | 3 |
| Total | 6188 | 146 | 204 | 200 | 6738 |
Abbreviation: CI, confidence interval.
STT: Proton‐pump inhibitor + amoxicillin + clarithromycin.
PBMT: Proton‐pump inhibitor + denol+metronidazole + tetracycline.
CT (concomitant therapy): PPI + AMX+CLA + MTZ.
Patients with good compliance with therapy had higher eradication rates than those with poor compliance (72.4% vs 50.0%, P < .001). Of the 1902 patients whose first‐line therapy failed, 1155 (60.7%) tried the second‐line therapy. The overall eradication rate of second‐line therapy was 85.8% (Figure 1). There was a statistically significant difference (P = .001) in the eradication rate among various treatment regimens. In particular, bismuth‐containing quadruple therapy achieved an eradication success rate of 87.7% (Table 2).
Although the eradication success rates varied among regions, the overall eradication success rate has been gradually decreasing for 5 years (Table 4).
Table 4.
Annual Helicobacter pylori eradication rate according to seven regions in Korea
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total | |
|---|---|---|---|---|---|---|---|
| Seoul | 70.5 | 77.1 | 70.3 | 73.5 | 70.4 | 71.6 | 72.5 |
| Gyeonggi | 66.7 | 76.0 | 69.0 | 71.7 | 71.2 | 67.7 | 71.4 |
| Gangwon | 58.2 | 65.6 | 82.4 | ‐ | ‐ | ‐ | 68.0 |
| Chungcheong | 67.0 | 58.9 | 57.9 | 54.4 | 87.1 | 74.8 | 61.3 |
| Gyeongsang | 74.7 | 75.3 | 74.5 | 75.6 | 75.9 | 85.2 | 76.5 |
| Jeolla | ‐ | 75.9 | 73.6 | 54.1 | 30.2 | 15.5 | 65.9 |
| Jeju | ‐ | 79.0 | 79.3 | 63.6 | 76.0 | 72.0 | 74.7 |
Age‐standardized eradication rate (%).
3.3. Factors related to first‐line H. pylori eradication success
In univariate analysis, age, residential area, medication, previous gastrointestinal disorder, comorbidity, previous H. pylori eradication, and compliance with treatment were related to the success of eradication. In multivariate analysis, age, sex, residential area, comorbidity, and compliance with treatment were related to the success of eradication. The following were the significant risk factors on performing multivariate analysis after adjustment for age: residential area, previous gastrointestinal disorder, comorbidity, previous H. pylori eradication, and compliance with treatment (P < .01).
3.4. Density of antibiotic use according to geographic region
The total amount of antibiotics including clarithromycin, azithromycin, erythromycin, roxithromycin, dirithromycin, telithromycin, and amoxicillin was estimated as the DDD per 1000 inhabitants per day. Table 5 shows the annual density of antibiotic use for clarithromycin, other macrolides, and amoxicillin (daily dose per 1000 population) in seven geographic regions of Korea from October 2010 to July 2015. The annual use density of clarithromycin (DID) increased statistically in seven regions across the country from 2010 to 2015. In particular, the annual use density of amoxicillin in the Gyeongsang and Chungcheong areas increased significantly (P < .01), whereas that of other macrolides was significantly lower in the Gyeonggi area than in other areas (P = .01).
Table 5.
Annual antibiotic use density according to seven regions in Korea
| Region | Classification | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | P‐value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Seoul | Clarithromycin | 2.1 | 0.5 | 2.3 | 0.4 | 2.3 | 0.4 | 2.4 | 0.4 | 2.7 | 0.6 | 2.7 | 0.7 | <.01 |
| Other macrolides | 1.9 | 0.4 | 1.9 | 0.3 | 1.8 | 0.4 | 1.8 | 0.3 | 1.9 | 0.3 | 1.8 | 0.4 | .50 | |
| Amoxicillin | 10.1 | 1.7 | 10.0 | 1.2 | 10.2 | 1.7 | 10.5 | 1.4 | 10.9 | 1.7 | 10.2 | 1.8 | .56 | |
| Gyeonggi | Clarithromycin | 1.8 | 0.5 | 2.0 | 0.5 | 2.0 | 0.4 | 2.0 | 0.4 | 2.3 | 0.5 | 2.4 | 0.6 | <.01 |
| Other macrolide | 1.8 | 0.4 | 1.7 | 0.3 | 1.6 | 0.3 | 1.6 | 0.3 | 1.7 | 0.3 | 1.6 | 0.3 | .01 | |
| Amoxicillin | 10.1 | 2.0 | 9.8 | 1.4 | 10.0 | 1.8 | 10.1 | 1.5 | 10.5 | 1.9 | 9.8 | 1.8 | .39 | |
| Gangwon | Clarithromycin | 1.5 | 0.4 | 1.8 | 0.4 | 1.9 | 0.4 | 2.0 | 0.4 | 2.3 | 0.5 | 2.5 | 0.6 | <.01 |
| Other macrolide | 1.7 | 0.4 | 1.7 | 0.2 | 1.7 | 0.3 | 1.5 | 0.3 | 1.5 | 0.3 | 1.6 | 0.3 | .43 | |
| Amoxicillin | 8.1 | 1.4 | 7.6 | 1.0 | 7.7 | 1.2 | 7.6 | 1.0 | 7.8 | 1.2 | 7.8 | 1.3 | .39 | |
| Chungcheong | Clarithromycin | 1.9 | 0.5 | 2.3 | 0.6 | 2.2 | 0.5 | 2.2 | 0.5 | 2.5 | 0.6 | 2.7 | 0.8 | .01 |
| Other macrolide | 1.7 | 0.4 | 1.7 | 0.3 | 1.5 | 0.4 | 1.5 | 0.3 | 1.6 | 0.3 | 1.6 | 0.4 | .55 | |
| Amoxicillin | 10.3 | 1.8 | 10.1 | 1.5 | 10.3 | 1.7 | 10.7 | 1.5 | 10.9 | 1.8 | 10.6 | 1.8 | <.01 | |
| Jeolla | Clarithromycin | 1.7 | 0.4 | 1.8 | 0.3 | 1.9 | 0.4 | 1.8 | 0.4 | 2.0 | 0.4 | 2.1 | 0.5 | .00 |
| Other macrolide | 1.7 | 0.4 | 1.6 | 0.3 | 1.5 | 0.3 | 1.5 | 0.3 | 1.7 | 0.3 | 1.8 | 0.4 | .55 | |
| Amoxicillin | 10.6 | 1.9 | 9.9 | 1.4 | 10.3 | 1.7 | 10.5 | 1.7 | 10.8 | 1.8 | 10.6 | 1.9 | .90 | |
| Gyeongsang | Clarithromycin | 1.9 | 0.5 | 2.2 | 0.5 | 2.2 | 0.5 | 2.2 | 0.4 | 2.5 | 0.6 | 2.7 | 0.6 | <.01 |
| Other macrolide | 1.9 | 0.4 | 1.9 | 0.3 | 1.9 | 0.4 | 1.8 | 0.4 | 1.8 | 0.4 | 2.0 | 0.4 | .58 | |
| Amoxicillin | 8.7 | 1.6 | 8.4 | 1.2 | 8.8 | 1.6 | 8.6 | 1.4 | 9.1 | 1.7 | 9.0 | 1.5 | <.01 | |
| Jeju | Clarithromycin | 1.3 | 0.2 | 1.3 | 0.3 | 1.3 | 0.3 | 1.3 | 0.3 | 1.9 | 0.5 | 2.2 | 0.5 | <.01 |
| Other macrolide | 2.2 | 0.4 | 1.9 | 0.5 | 1.7 | 0.4 | 1.5 | 0.4 | 1.8 | 0.3 | 1.7 | 0.5 | .12 | |
| Amoxicillin | 10.5 | 1.6 | 9.4 | 1.6 | 9.6 | 1.7 | 9.4 | 1.4 | 10.0 | 1.5 | 10.3 | 1.7 | 1.00 | |
P‐value was obtained using regression model with autoregressive error correction.
3.5. Relationship between antibiotic use density and eradication success
In the present study, the density of antibiotic use has continued to increase over the recent 5 years, while the success rate of H. pylori eradication has been gradually decreasing. However, there was no significant correlation between antibiotic use density and eradication rate according to regional differences (Figure 3). Furthermore, there was no significant correlation between the antibiotic use density and eradication rate over time (Figure 4).
Figure 3.
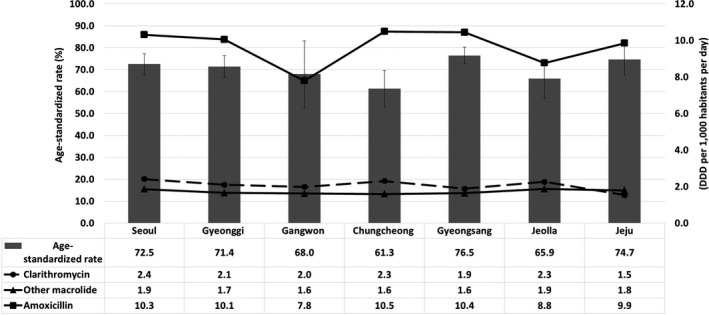
Antibiotic use density and eradication rate according to seven geographic areas in Korea. There was no significant correlation between antibiotic use density and eradication rate by region
Figure 4.
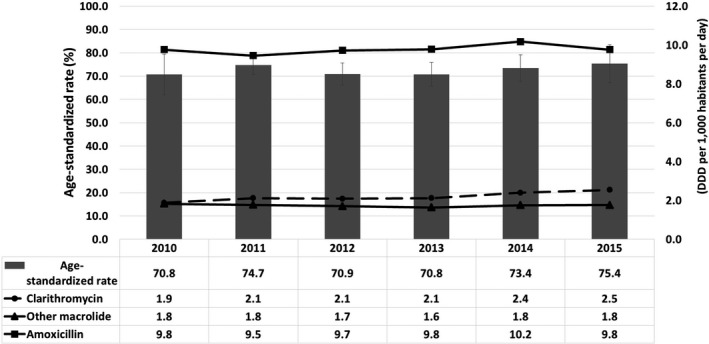
Yearly antibiotic use density and eradication rate from 2010 to 2015. There was no significant correlation between antibiotic use density and eradication rate over time
4. DISCUSSION
The present study reports on the final results of a 5‐year nationwide online registry in Korea aimed at tracking the current H. pylori eradication rate. In addition, this study verified the relationship between the H. pylori eradication rate and antibiotic use density by investigating the total amount of antibiotics used in H. pylori eradication treatment in Korea. In particular, we compared the antibiotic use density in each region of the country and evaluated the therapeutic efficacy of the currently used first‐line therapy.
This study demonstrated the efficacy of first‐line therapy in H. pylori eradication over the recent 5 years in Korea. The overall 71.8% eradication success rate found in this study indicates the decreasing tendency of current eradication success rates when compared with the success rates of 73.1% in our interim report,11 84.9%‐87.5% from 2001 to 2007, 80.0%‐81.4% from 2008 to 2010, with a decreasing trend in our previous study before 2010 in Korea.16 In the present study, the eradication rates were standardized to the population of 2010 as the Korean standard population in order to keep track of the eradication rate over 15 years from 2000 to 2015. Furthermore, we revealed the influence of antibiotic resistance on eradication success by estimating the total amount of antibiotics nationwide from HIRA, which guarantees complete data collection. This allows us to infer the association between antibiotic resistance and eradication success.
Since the Korean guidelines were established in 1998, STT has been recommended as the first‐line therapy for H. pylori eradication in Korea. However, since 2000, the eradication rate has been declining, falling below 80% with increasing antibiotic resistance since 2000.13, 14, 15
Resistance to commonly used antimicrobials has resulted in increasingly poor cure rates, leading to clinical trials that compare treatment regimens, often without regard to the key role of antimicrobial resistance on outcomes.17, 18 Based on this, assessing the current therapeutic efficacy of first‐line therapy is essential for the development of new treatment regimens. In the present study, the overall eradication rate was only 71.8% for first‐line therapy, suggesting that current H. pylori eradication therapy has become suboptimal in Korea. Considering that the majority (91.8%) of cases underwent STT for 7 days, a decline in the eradication rate could imply a serious problem in the management of H. pylori infection in Korea. Fortunately, treatment‐naive patients had a significantly higher eradication rate than those treated previously for H. pylori infection. These results strongly suggest that more effective first‐line therapy is necessary to treat H. pylori infection as early as possible. Indeed, QT showed a higher eradication rate than STT even in first‐line eradication therapy. However, taking into consideration the higher resistance of H. pylori to clarithromycin than to metronidazole, prior macrolide exposure may be a possible cause of the reduced eradication rate later.
Antibiotic resistance is the main factor that affects the efficacy of current therapeutic regimens against H. pylori.19 In Korea, 5.9% of those treated were resistant to clarithromycin before 2000.15 However, clarithromycin resistance rate remained high since 2000.14 The resistance rate was 17.2% in 2003‐2005 and had a sharp increase from 2007 to 2009, reaching 38.5%.13, 20 A recent study reported an increase in resistance to clarithromycin and an overall increase in multidrug resistance.18 Antibiotic resistance rates against clarithromycin, amoxicillin, tetracycline, metronidazole, and levofloxacin for the 2011‐2012 isolates were 16.0% (15/94), 2.1% (2/94), 0% (0/94), 56.3% (53/94), and 22.3% (21/94), respectively. Furthermore, multidrug resistance for two or more antibiotics increased to 23.4% (22/94) in the 2011‐2012 isolates.
In the present study, there were regional differences in eradication rates: the Gyeongsang area showed the highest eradication rate for the first‐line therapy, whereas the Chungcheong area had the lowest eradication rate (75.2% vs 60.9%, P < .001). One Korean study reported that the rate of resistance to clarithromycin, prepared using the agar dilution method, was 42.1% in Busan city, the Gyeongsang area, whereas that in the Gangwon area was 12.5%.21 This result indicates that different guidelines should be applied for H. pylori eradication based on the antibiotic resistance even within the same country or ethnic group.16
Regional differences in eradication rates are possibly associated with the amount of antibiotics used and population density, which are closely related to antibiotic resistance. We postulated that population density might be an important factor for the development of antibiotic resistance through interpersonal transmission. Therefore, we calculated the DID for the entire nation of Korea. Furthermore, we included all kinds of macrolides for calculating DID because it is well known that macrolides have high cross‐resistance with each other.
As a result, the eradication rate has continued to decrease along with an increasing density of antibiotic use. However, the association between the density of antibiotic use and eradication rate according to geographic or chronological differences was unclear. We speculate that the link between eradication rate and antibiotic use density has an overall significant trend, but it does not seem to be meaningful in terms of geography or chronology.
There is no doubt that previous exposure to clarithromycin is one of the major causes of resistance development according to some studies reporting that H. pylori resistance to second‐line clarithromycin therapy was significantly higher than that to first‐line therapy.13, 22 Furthermore, we cannot exclude the possibility of multidrug resistance or potential mutations.
To date, this is the most comprehensive, systematic, and easily accessible online registration database tracking H. pylori eradication in Korea. This registration database based on the real status will serve as a valuable resource for future establishment of new guidelines for a better treatment strategy on H. pylori infection. It also helps to set up a global network for H. pylori control. In particular, the complete enumeration of a nationwide survey on the DDD of clarithromycin, other macrolides, and amoxicillin from the HIRA data minimized bias and strengthened the quality of this study.
There are some limitations in our study. First, as it was an observational study using a registry, not all the patients (6186/6738, 91.8%) observed the current Korean guidelines in real clinical practice. Moreover, 27% of the patients did not confirm eradication results. Therefore, new guidelines accommodating the real status should be implemented in the future. Second, no antimicrobial susceptibility tests were performed before or after eradication therapy. Unfortunately, the data obtained from HIRA could not provide real antibiotic resistance in Korea.
In summary, the current guideline is not completely implemented in real world. The rate of choosing first‐line therapy in accordance with the guideline was 91.8%. The eradication rate of first‐line therapy has decreased to 71.8% over the recent 5 years, indicating that the currently used first‐line therapy including STT has not been satisfying therapeutic expectations in Korea. At the same time, the amount of antibiotics used has been increasing, although the relationship between antibiotic use density and the eradication rate was not found to be significant.
In conclusion, our data present the real status of H. pylori eradication in Korea and support the evidence that the eradication rate of first‐line therapy for H. pylori infection has been decreasing in the recent 5 years, irrespective of an increase in antibiotic use in Korea. Our findings provide strong support to the need for alternate regimens to improve the efficacy of first‐line therapy.
Kim BJ, Yang C‐H, Song HJ, et al.; Korean College of Helicobacter, Upper Gastrointestinal Research . Online registry for nationwide database of Helicobacter pylori eradication in Korea: Correlation of antibiotic use density with eradication success. Helicobacter. 2019;24:e12646 10.1111/hel.12646
Beom Jin Kim and Chang‐Hun Yang contributed equally to this work.
Funding information
This work was supported by a Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant.
REFERENCES
- 1. Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther. 2016;14:577‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harb AH, Chalhoub JM. Abou Mrad R, Sharara AI. Systematic review and meta‐analysis: full‐ vs. half‐dose anti‐microbials in clarithromycin‐based regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2015;42:131‐141. [DOI] [PubMed] [Google Scholar]
- 3. Hsu P‐I, Wu D‐C, Chen W‐C, et al. Randomized controlled trial comparing 7‐day triple, 10‐day sequential, and 7‐day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother. 2014;58:5936‐5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014;29:1371‐1386. [DOI] [PubMed] [Google Scholar]
- 5. Fock KM, Talley N, Moayyedi P, et al. Asia‐Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351‐365. [DOI] [PubMed] [Google Scholar]
- 6. Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heo J, Jeon SW. Optimal treatment strategy for Helicobacter pylori: era of antibiotic resistance. World J Gastroenterol. 2014;20:5654‐5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeo YH, Shiu SI, Ho HJ, et al. First‐line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: a systematic review and network meta‐analysis. Gut. 2018;67:20‐27. [DOI] [PubMed] [Google Scholar]
- 9. Kuo Y‐T, Liou J‐M, El‐Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia‐Pacific region: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2017;2:707‐715. [DOI] [PubMed] [Google Scholar]
- 10. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence Consensus Report. Gut. 2017;66:6‐30. [DOI] [PubMed] [Google Scholar]
- 11. Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci. 2016;31:1246‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savoldi A, Carrara E, Graham Prof DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta‐analysis in World Health Organization regions. Gastroenterology. 2018;155:1372‐1382.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010;44:536‐543. [DOI] [PubMed] [Google Scholar]
- 14. Jung YS, Park CH, Park JH, Nam E, Lee HL. Efficacy of Helicobacter pylori eradication therapies in Korea: a systematic review and network meta‐analysis. Helicobacter. 2017;22. [DOI] [PubMed] [Google Scholar]
- 15. Kim JJ, Reddy R, Lee M, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47:459‐461. [DOI] [PubMed] [Google Scholar]
- 16. Shin WG, Lee SW, Baik GH, et al. Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016;21:266‐278. [DOI] [PubMed] [Google Scholar]
- 17. Zhang M. High antibiotic resistance rate: A difficult issue for Helicobacter pylori eradication treatment. World J Gastroenterol. 2015;21:13432‐13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. An B, Moon BS, Kim H, et al. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med. 2013;33:415‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ang TL, Fock KM, Song M, et al. Ten‐day triple therapy versus sequential therapy versus concomitant therapy as first‐line treatment for Helicobacter pylori infection. J Gastroenterol Hepatol. 2015;30:1134‐1139. [DOI] [PubMed] [Google Scholar]
- 20. Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first‐line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006;48:156‐161. [PubMed] [Google Scholar]
- 21. Kwon YH, Kim N, Lee JY, et al. Comparison of the efficacy of culture‐based tailored therapy for Helicobacter pylori eradication with that of the traditional second‐line rescue therapy in Korean patients: a prospective single tertiary center study. Scand J Gastroenterol. 2016;51:270‐276. [DOI] [PubMed] [Google Scholar]
- 22. Chang WL, Sheu BS, Cheng HC, Yang YJ, Yang HB, Wu JJ. Resistance to metronidazole, clarithromycin and levofloxacin of Helicobacter pylori before and after clarithromycin‐based therapy in Taiwan. J Gastroenterol Hepatol. 2009;24:1230‐1235. [DOI] [PubMed] [Google Scholar]


