Abstract
Background
Studies evaluating safety of warfarin and direct oral anticoagulants (DOACs) for prevention of stroke in patients with atrial fibrillation (AF) are lacking.
Methods & Results
All patients (n = 196,521) receiving care at veteran's affairs with active cancer and AF from 2010–2015 were included. One-year mortality was significantly higher in unadjusted analysis with warfarin (44.9%) compared to dabigatran (25%, P < 0.001), rivaroxaban (24.4%, P < 0.001) and apixaban (30%, P < 0.001) and after adjusting for age, sex and type of cancer mortality (OR = 2.66, 95% CI: 2.52–2.82, P < 0.001). Risk of ischemic stroke (13.5% vs. 11.1%, 12.0%, 14.0%) was similar, however risk of hemorrhagic stroke was significantly higher among patients receiving warfarin (1.2%) compared to patients receiving dabigatran (0.5%), rivaroxaban (0.7%) and apixaban (0.8%) respectively, P = 0.04.
Conclusions
We demonstrated the superior safety profile of DOACs compared to warfarin among patients with underlying cancer and AF. Warfarin was associated with higher mortality, similar ischemic stroke risk but higher risk of hemorrhagic stroke.
Keywords: Atrial fibrillation, Cancer, Oral anticoagulation
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia affecting 2.66 million Americans in 2010 with number likely rising to 12 million by 2050.[1] The burden of disease treatment and complications is estimated at $ 6.65 billion/year for direct costs and $ 26 billion/year in indirect costs.[2] AF accounts for ≥ 15% of all strokes and 36% of strokes for individuals aged > 80 in the United States.[3] The standard of care for prevention of stroke in patients with AF has traditionally been vitamin K antagonist-warfarin, which has significant side effects and challenges with patient compliance. Since 2010, the Food and Drug Administration has approved four alternative direct oral anticoagulants (DOACs)—dabigatran, rivaroxaban, apixaban, and edoxaban for prevention of stroke in patients with non-valvular AF.[4]–[7]
Although, several clinical trials have demonstrated safety and efficacy of these DOACs in the general population, experience in patients with underlying cancer remains limited. The efficacy and safety of DOACs in patients with venous thromboembolism and cancer has been well described,[8] however similar data regarding use of DOACs in AF patients for prevention of stroke is lacking. Utilizing the Big Data-Scientist Training Enhancement Program (BD-STEP) and our prior research utilizing data from the Department of Veterans Affairs (VA),[9] we developed a study to address the utilization, safety and efficacy of DOACs in AF patients with underlying cancer.
2. Methods
2.1. Study population
The study included all veterans diagnosed with AF with underlying active cancer and have been treated with either warfarin or DOACs from January 2010 to December 2015 for primary or secondary prevention of stroke. All patients had least two risk factors for stroke (age of at least 65 years, symptomatic heart failure within the previous three months, left ventricular ejection fraction of no more than 40%, diabetes mellitus, or hypertension requiring pharmacologic treatment) or documented history of previous stroke, transient ischemic attack, or systemic embolism. Patients in whom survival is expected to be less than one year due to end stage cancer or other comorbid conditions were excluded. Additionally, patients in whom DOACs cannot be used due to moderate or severe mitral stenosis, prosthetic heart valve, severe renal insufficiency, and significant drug interaction with other medications were excluded.
2.2. Patient and procedural characteristics
This is a retrospective observational study performed utilizing the national VA Healthcare data. All VA research was performed on the VA Informatics and Computing Infrastructure (VINCI) Workspace. Data was collected from the following three key resources: (1) Corporate Data Warehouse that contains patient demographics, inpatient, outpatient, laboratory and clinical data; (2) Medical SAS Inpatient and Outpatient Datasets that contain acute care, extended care, observation care and non-VA care inpatient and outpatient data from local VA centers; and (3) Vital Statistics File which is a VA healthcare database of deceased individuals who received benefits from the VA while they were alive.
2.3. Outcomes
Our aim was to determine the utilization of the DOACs (dabigatran, rivaroxaban, apixaban and edoxaban) in patients with AF and underlying cancer among veterans. The primary outcome of the study was unadjusted and adjusted one-year mortality rates among cancer patients with underlying AF. All procedures were approved and monitored by Buffalo VA Healthcare System Institutional Review Board with a waiver of individual informed consent.
2.4. Statistical analysis
Categorical variables are reported as frequency (%) and comparison between the groups were performed by chi-square or Fisher's exact test. Continuous variables were summarized as either mean ± SD or median (interquartile range) and compared across groups using t-test. Univariate regression analysis was performed to identify patient risk factors associated with primary outcomes. These covariates were then entered in a multivariate regression analysis to perform an adjusted analysis comparing oral anticoagulants and mortality. Statistical analysis was performed using STATA v13.0 (StataCorp, College Station, Texas). A P-value of < 0.05 was considered as significant.
3. Results
3.1. Study population
We identified 654,732 patients nationally who received care at the VA from 2010 to 2015 and were diagnosed with active cancer and AF. Among those patients, there were 196,521 (30%) patients who were receiving oral anticoagulation for stroke prevention. The mean age of all patients was 76 ± 10 years and the majority (98.1%) were males. Additional baseline characteristics are outlined in Table 1.
Table 1. Baseline characteristics.
| Age, yrs | 76 ± 10 |
| Males | 98.1% (192, 787) |
| Risk factors | |
| Hypertension | 91.0% (178, 742) |
| Diabetes history | 57.0% (111, 988) |
| Congestive heart failure | 38.7% (76, 111) |
| Ischemic stroke history | 13.3% (26, 41) |
| Hemorrhagic stroke history | 2.2% (4, 260) |
| Coronary artery disease/myocardial infarction | 6.8% (13, 447) |
| Tobacco use, % | 18.8% (36, 991) |
Values are mean ± SD, median (interquartile range), % (n).
3.2. Utilization of oral-anticoagulants
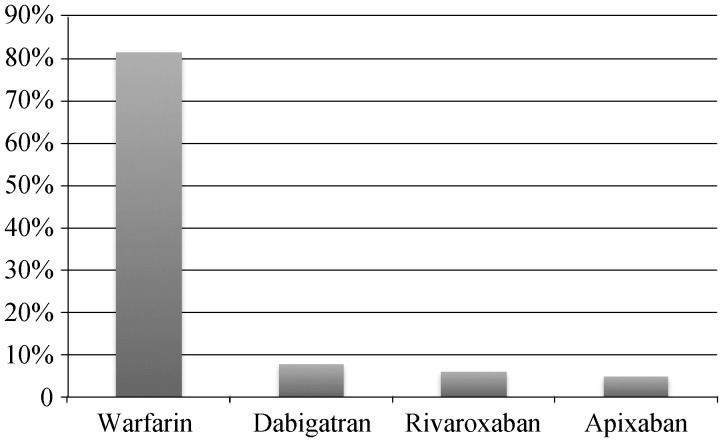
The utilization of oral-anticoagulants is outlined in Figure 1. Among all patients, the most common agent used for oral anticoagulation was Warfarin (160,177/81.5%). The next most common agent used was Dabigatran (14,968/7.6%), followed by Rivaroxaban (11,877/6%) and Apixaban (9495/4.8%) respectively.
Figure 1. Utilization of oral anticoagulants among patients with active cancer and atrial fibrillation.
3.3. Outcomes
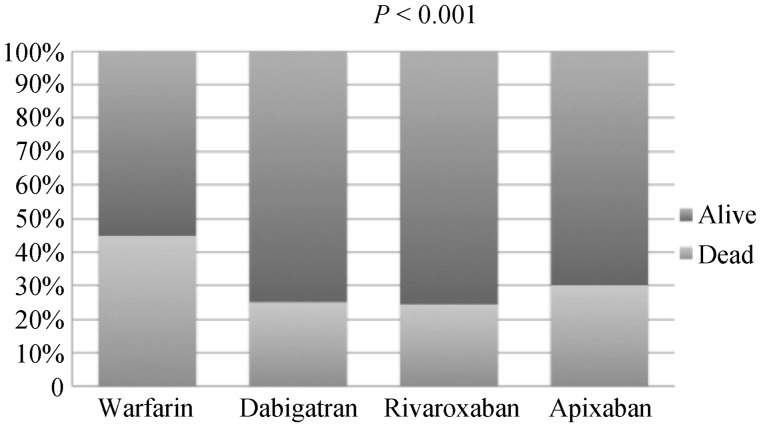
During follow up, all-cause mortality at one year was high at 81,389 (41.4%). All cause mortality was significantly higher among patients receiving Warfarin (44.9% vs. 26.2%, P < 0.001) during follow up (Figure 2). All cause mortality was significantly lower among patients receiving Dabigatran (25% vs. 42.8%, P < 0.001), Rivaroxaban (24.4% vs. 42.5%, P < 0.001) and Apixaban (30% vs. 42%, P < 0.001) respectively, Figure 2. Next, we adjusted for relevant variables identified in a univariate analysis that included age, sex and type of cancer. After adjusting for confounders, the use of warfarin continued to remain significantly associated with increased all cause mortality (OR = 2.66, 95% CI: 2.52–2.82, P < 0.001).
Figure 2. All cause mortality at one year stratified by use of oral anticoagulation.
3.4. Bleeding/Ischemic stroke
Among all patients, there were 26040 (13.3%) events of ischemic stroke during follow up. As shown in Table 2, patients who were on warfarin had similar risk of ischemic stroke compared to those receiving dabigatran, rivaroxaban, and apixaban respectively. Among all patients there were 2130 (1.1%) patients who developed hemorrhagic stroke during follow up. Those patients receiving warfarin had higher hemorrhagic stroke events compared to those receiving dabigatran, rivaroxaban, and apixaban respectively, Table 2.
Table 2. Outcomes among patients based on anticoagulation use.
| Outcome | Warfarin (n = 160177) | Dabigatran (n = 14968) | Rivaroxaban (n = 11877) | Apixaban (n = 9495) |
| Ischemic stroke | 21619 (13.5%) | 1663 (11.1%) | 1427 (12.0%) | 1331 (14.0%) |
| Hemorrhagic stroke | 1875 (1.2%) | 88 (0.6%) | 88 (0.7%) | 79 (0.8%) |
Data are presented as n (%).
4. Discussion
We present the first Veterans study evaluating the utilization and safety of DOACs and comparing it to warfarin among cancer patients with atrial fibrillation. Using a robust national Veterans Affairs database, the study identified the following key findings: (1) in spite of wide availability of DOACs within the VA healthcare system, warfarin continues to be extensively utilized amongst cancer patients with AF; (2) Warfarin was associated with worse mortality outcomes when compared to DOACs; (3) Warfarin was associated with increased risk of bleeding and thromboembolic events compared to DOACs; and (4) The increased association of warfarin with adverse outcomes remains even after adjusting for the type of cancer and other risk factors.
Although DOACs were available, warfarin was extensively utilized amongst cancer patients with atrial fibrillation (81.5%). A study conducted by Zhu, et al.[10] found that warfarin was routinely prescribed to elderly patients above age 75, patients with extensive comorbidities, and patients with low socioeconomic status. The study also found that warfarin utilization in the male population was higher compared to females. Our study is unable to make a correlation for gender usage because the study consisted mainly of men (98.1%). In addition, the popularity of DOACs has been growing since its initiation and more patients are switching from warfarin in the last few years to DOACs. Norway experienced a 20% patient initiation rate from warfarin to DOACs in 2015, which had consistently grown since the beginning of the study in 2010.[11]
Although warfarin was the most prescribed anticoagulant in the study, warfarin was also associated with higher mortality rates after one year when compared to other DOACs. Even with the data adjusted for relevant variables, there was a higher incidence of mortality in patients taking warfarin versus DOACs (OR = 2.66). Likewise, in a pool meta-analysis, Chai-Adisaksopha, et al.[12] found higher mortality amongst patients receiving warfarin compared to DOACs. In addition, the study also found that there was a lower incidence of adverse bleeding in patients taking DOACs compared to warfarin.[12]
Our findings supported an associated risk of bleeding but similar risk of thromboembolic events when taking warfarin compared to DOACs. Chai-Adisaksopha, et al.[13] conducted a meta-analysis comparing the risks of bleeding in patients taking warfarin and DOACs. The results showed that patients taking warfarin had a significant increase in major, non-major, fatal, and intracranial bleeding when compared to DOACs. However, the incidence of gastrointestinal bleeding was comparable. Wu, et al.[14] compared DOACs and warfarin outcomes for Chinese patients. The results showed a comparable, but decreased rate of ischemic stroke and bleeding for patients taking DOACs when compared to warfarin.
4.1. Limitations
Since the study population is limited to veterans, females are underrepresented in this study. The study population was selected from a registry and hence may not be representative of the general population. Specific type of mortality data could not be obtained since that data could not be reliably queried from the VA healthcare database. Due to the retrospective nature of the study there is potential for selection bias inherent to the study design.
4.2. Conclusions
In a large national VA cohort we demonstrated for the first time the superior safety profile of DOACs compared to Warfarin among patients with underlying cancer and AF. This remained true even after adjusting for the other covariates including the type of cancer. Warfarin was associated with similar risk of ischemic stroke, but significantly higher risk of hemorrhagic stroke compared to DOACs. Future prospective studies confirming our findings for preferential use of DOACs in patients with cancer and AF are warranted.
Acknowledgments
The study was funded by the Thomas F. Frawley, MD, Research Fellowship Fund awarded to Dr. Sawant. All other co-authors do not have any relevant financial.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127:e15–e16. doi: 10.1016/j.amjmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus Warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus Warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 7.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 8.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer. Chest. 2015;147:475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 9.Sawant AC, Josey K, Plomondon ME, et al. Temporal trends, complications, and predictors of outcomes among nonagenarians undergoing percutaneous coronary intervention: insights from the veterans affairs clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv. 2017;10:1295–1303. doi: 10.1016/j.jcin.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Alexander GC, Nazarian S, et al. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy. 2018;38:907–920. doi: 10.1002/phar.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjerpeseth LJ, Ellekjær H, Selmer R, et al. Trends in use of warfarin and direct oral anticoagulants in atrial fibrillation in Norway, 2010 to 2015. Eur J Clin Pharmacol. 2017;73:1417–1425. doi: 10.1007/s00228-017-2296-1. [DOI] [PubMed] [Google Scholar]
- 12.Chai-Adisaksopha C, Hillis C, Isayama T, et al. Mortality Outcomes in Patients Receiving Direct Oral Anticoagulants: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Thromb Haemost. 2015;13:2012–2020. doi: 10.1111/jth.13139. [DOI] [PubMed] [Google Scholar]
- 13.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. the impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-Analysis. Blood. 2014;124:2450–2458. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Xie S, Xu Y, et al. Persistence and outcomes of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with non-valvular atrial fibrillation. J Clin Nurs. 2019;28:1839–1846. doi: 10.1111/jocn.14797. [DOI] [PubMed] [Google Scholar]