INTRODUCTION
Clostridioides difficile (CD) (previously known as Clostridium difficile) is an anaerobic, Gram-positive, spore-forming bacillus that is widespread in the natural environment (found in soils, puddle water, tap water, household surfaces, food, horses, and hay, among other sites), but whose main habitat is the gastrointestinal tract of young mammals.1–3 CD colonizes the gut of up to 70% of neonates and infants and can form part of the commensal intestinal flora of asymptomatic adults.4 However, since 1978, when it was first described as clindamycin colitis or antibiotic-associated pseudomembranous colitis, CD has been recognized to cause a spectrum of human disease, ranging from mild diarrhea to severe illness and fulminant colitis, associated with ileus and bowel dilatation (megacolon), bowel perforation, and septic shock.5–9 Between 2000 and 2005, a hypervirulent strain of CD, known as North American pulsed-field gel electrophoresis type 1, restriction endonuclease analysis group BI, and polymerase chain reaction (PCR) ribotype 027(NAP1/BI/027) spread globally.10–12 The NAP1/027 strain is associated with increased toxin A and B production, produces binary toxin, and is fluoroquinolone-resistant. It harbors a deletion mutation in the TcdC gene (a negative regulator of toxin A and B transcription) that is thought to permit increased toxin production.10,11 Coincident with the global emergence of NAP1/027, increasing reports of CD infection (CDI) in the stem cell transplant (SCT) and hematologic malignancy (HM) populations appeared in the literature.13–22
In the United States, CDI is the leading cause of health care-associated diarrhea, with an estimated incidence rate of 453,000 cases/y and 29,000 deaths, based on 2011 data.23 The length of time spent in the hospital has been shown to have a linear relationship with the risk of CDI.24 More than 90% of cases occur in association with prior or ongoing health care or hospitalization.25 However, in contrast with the early 1980s, most CDI diagnoses are now made in the community or nursing home setting among individuals with recent health care exposure.25
Incidence, Risk Factors, and Outcomes in the Stem Cell Transplant Population
The usual risk factors for CDI in the general population (antecedent antibiotics, particularly fluoroquinolones, clindamycin, carbapenems, higher-generation cephalosporins; health care exposures; age >65 years; proton pump inhibitor use; or achlorhydria) also apply to the transplant population.26,27 However, the incidence among SCT patients has been reported to be up to 9-fold higher among SCT patients and 6.5-fold higher among patients with undifferentiated cancer than for general inpatients. 19,28–30 In the contemporary literature, CDI occurs in adult SCT patients and patients with HM at a frequency of 3% to 33%.31–37 Rates are higher in allogeneic compared with autologous transplant patients by approximately 2-fold.27,29,38 CDI in the SCT population is often of mild to moderate severity32,39,40 and in many studies does not significantly affect survival. CDI is most frequent in the first several weeks after SCT but may also occur in the late posttransplant phase.33
Well-known risk factors for CDI that are amplified in the SCT population include immune suppression, frequent antibiotic use, and health care exposure. Susceptibility to CDI in humans has been associated with lower levels of antibodies to CD toxins A and B.41–43 SCT patients have low levels of protective antibodies because of myeloablative conditioning regimens and unreconstituted immunity following SCT. Several risk factors specific to the SCT and HM populations have been identified in the current literature and are highlighted in Table 1.
Table 1.
Predisposing and protective factors for Clostridioides difficile infection in the stem cell transplant and hematologic malignancy population
| Predisposing Factors | |
|---|---|
| Male gender | Ref.29 |
| Acute leukemia | Refs.29,30 |
| Renal disease | Ref.30 |
| Gastric ulcer | Ref.30 |
| Prior hospitalization | Ref.44 |
| Allogeneic (vs autologous) SCT | Refs.19,21,29,38,45,46 |
| Umbilical cord blood SCT | Ref.20 |
| Total body irradiation | Refs.20,47 |
| Myeloablative conditioning | Refs.32,36,46,48 |
| Chemotherapy | Refs.21,44,49 |
| Neutropenia | Refs.30,50 |
| Receipt of ≥1 high-risk antibiotic or recent antibiotic | Refs.29,39,44,51,52 |
| Mucositis | Ref.39 |
| Reciprocal association reported with GVHD | — |
| GVHD → CDI | Refs.20,21,51,56 |
| CDI → GVHD | Refs.18,21,45,47 |
| CMV reactivation | Ref.39 |
| HSV or VZV reactivation | Ref.39 |
| Precolonization with toxigenic strain | Ref.32,49 |
| VRE colonization | Ref.21,45 |
| Bacterial infection | Ref.30,53 |
| Mechanical ventilation | Ref. 29 |
| Protective Factors | |
| TMP-SMX | Ref.39 |
| Corticosteroids | Ref.46 |
| Proton pump inhibitors | Ref.46 |
| Colonization with nontoxigenic CD strains | Ref.49 |
Arrow (→) indicates direction of temporal relationship.
Abbreviations: CMV, cytomegalovirus; GVHD, graft-versus-host disease; HSV, herpes simplex virus; TMP-SMX, trimethoprim-sulfamethoxazole; VRE, vancomycin-resistant Enterococcus; VZV, varicella zoster virus.
Lavallé e and colleagues39 performed a nested matched case-control study of 65 patients with CDI compared with 123 controls from the same allogeneic SCT cohort between 2002 and 2011. The study was performed at a single center in Quebec that had a high proportion of infection caused by the NAP1/027 strain. CDI was defined as diarrhea plus evidence of toxigenic CD (either Vero cell culture cytotoxicity assay or by an immunoassay to detect CD glutamate dehydrogenase and toxins A and B). The overall incidence of CDI in this cohort was 8.6% (65 out of 760), with approximately half the cases diagnosed in the early posttransplant period (by day 40). In a multivariate analysis, use of trimethoprim-sulfamethoxazole was associated with a marked and significant reduction in the risk of CDI (odds ratio [OR], 0.07). CMV reactivation, HSV or VZV reactivation, and mucositis were all associated with significantly increased risk (OR, 6.2, 3.0, and 5.9, respectively). In a separate analysis of early versus late CDI, CMV reactivation and mucositis were associated with early CDI (OR, 22.1 and 13.2, respectively). Administration of CDI-promoting antibiotics (anti-pseudomonal penicillins, carbapenems, clindamycin, fluoroquinolones, and third-generation and fourth-generation cephalosporins) was associated with late CDI (OR, 4.5). In this study, acute GVHD was not associated with CDI in the multivariate analysis, in contrast with other reports.18,21,45 Clinical manifestations of CDI were modest, with infrequent instances of severe disease (1 patient required colectomy, 2 died). Mortality in patients with CDI was similar to non-CDI controls.39
Alonso and colleagues53 analyzed the incidence, severity, associated outcomes and risk factors for CDI occurring within the first year following umbilical cord blood transplant (UCBT) using a retrospective, case-cohort study design. Patients with CDI were identified from within a total cohort of 226 patients who underwent UCBT between 2003 and 2012. CDI was defined as diarrhea plus a positive stool assay for CD toxin (cell culture cytotoxicity assay, immunoassay for toxin A or toxin A and B combined, or PCR for the toxin gene) without another identified cause. Twenty-two patients (9.7%) in this cohort developed CDI within the first 100 days of transplant (incidence rate, 10.8 out of 10,000 person-days). Thirty patients had CDI within the first year (incidence rate, 5.6 out of 10,000 person-days), with a median onset of 38 days. A risk analysis was performed for total CDI and CDI within 100 days after transplant. In the multivariate analysis, only bacterial infection within 100 days of transplant was significant (hazard ratio [HR], 2.8; P = .03). Corticosteroid use, serum immunoglobulin (Ig) G levels, CD4 count, gut decontamination, transplant conditioning regimen, GVHD, mucositis, gastric acid suppression, and antibiotic exposure had no association with CDI risk. CDI was also not associated with increased mortality, although the investigators noted that markers of severe disease,a such as intensive care unit admission and increased serum creatinine (>1.5 mg/dL more than baseline), were seen in 9% and 32% of patients with CDI.53 Recurrence rates were low (14%). A second, older study of a UCBT cohort from a single center in Japan found a similar rate of CDI (9%) and no association with increased mortality or GVHD.54
Dubberke and colleagues51 prospectively evaluated risk factors for CDI in a cohort of 187 patients who underwent allogenic SCT at a single center (Barnes Jewish Hospital in St Louis, Missouri) between 2007 and 2010. CDI was defined as a positive toxin assay (enzyme immunoassay [EIA] for toxin A/B) and pertinent clinical symptoms occurring within the first year after transplant. Patients were followed for outcomes for a total of 30 months. Sixty-three (34%) patients were diagnosed with CDI, of whom 60% developed illness in the early posttransplant period (preengraftment phase, defined as within 30 days after transplant). Mild disease or moderate disease was seen in 73% of patients, whereas severe disease occurred in 27%. Risk factors for CDI in the preengraftment and postengraftment periods were analyzed separately. Absence of comorbid illness was significantly associated with protection from preengraftment CDI (OR, 0.3; 95% confidence interval [CI], 0.1 to 0.9). In the postengraftment period, relapse of primary disease, exposure to high-risk antibiotics, and GVHD (onset before CDI) were all significant risk factors for CDI (OR, 6.7, 11.8, and 7.8, respectively). No comparative analysis of mortality in patients versus controls was reported. However, death was not increased among those with severe CDI compared with patients with mild CDI.
Subsequently, the investigators augmented this study with a larger, prospective investigation of risk factors and outcomes associated with CDI among 385 SCT recipients.35 The total cohort of SCT patients in this study included the 63 patients previously reported,51 combined with 57 patients from 3 other transplant centers. All CDI episodes occurring within 1 year of SCT were counted, and patients were followed for 2.5 years after transplant. Diagnosis of CDI required a positive assay for CD (toxin immunoassay, PCR, or cellular cytotoxicity) and a clinical diagnosis of gastroenteritis. One-hundred and twenty patients developed CDI, with a median onset of 27 days posttransplant. Rates of CDI ranged from 12% to 38% among the centers. In a risk factor analysis, myeloablative conditioning, cardiovascular disease, GVHD during the past 30 days, and a comorbid condition were more common among patients with CDI. However, when the 63 patients from the previously published cohort were removed from the analysis, only myeloablative conditioning and GVHD in the past month remained significantly associated with CDI. Associations between antimicrobial exposure and CDI were not evaluated because of a lack of data on antibiotic administration.
Evaluated outcomes included subsequent bloodstream infection (BSI), GVHD, and death. Patients with CDI were more likely to experience BSI and GVHD with 30 days following the onset of CDI.35 Mortality was similar between the two groups in the early posttransplant period. However, patients with CDI in the late posttransplant period (31 days after transplant) had increased mortality (HR, 1.8; P = .007) compared with controls.
Several other investigators have found myeloablative conditioning to be associated with increased risk of CDI.32,36,46,48 Similarly, the association of CDI with mortality was found in a large study describing the incidence and associated outcomes of all infections occurring after transplant.36 Schuster and colleagues36 found that CDI was the most frequent bacterial infection caused by a single pathogen in their cohort, occurring in 148 of 444 (33%) enrolled subjects with a median onset at day 27 after SCT. Mortality was higher in patients with CDI (63%) compared with those without CDI (47%) (OR, 1.9; 95% CI, 1.3–2.9). CDI was also significantly more frequent in individuals who had received myeloablative conditioning compared with those who had not (OR, 1.6). Notably, this study relied on the same prospectively enrolled cohorts and transplant centers (University of Michigan, University of Alabama, University of Pennsylvania, and Washington University) as the 385-subject study described earlier35 but spanned a longer period of enrollment (2006–2011).
Other investigators have also reported an association of CDI or recurrent CDI with mortality.29,30,45,47,55,56 Guddati and colleagues29 published an analysis of risk factors and outcomes for hospital-onset CDI in 344,407 SCT patients between 2000 and 2009 using a national administrative database of hospital discharges. A total of 6970 patients (4.7%) developed CDI, of whom 3678 had received autologous SCT (CDI rate of 5.5%) and 3292 had received allogeneic SCT (CDI rate of 8.4%). CDI was not associated with mortality during the so-called engraftment admission. However, during subsequent admissions, CDI was significantly associated with mortality in patients who also had GVHD. Mortality was 19% in patients with simultaneous CDI and GVHD, compared with 8% in patients with CDI without GVHD. Patients with GVHD but not CDI had a mortality of 12.5%.
Although the use of discharge International Classification of Diseases, Ninth Revision, codes to define CDI in this study was a limitation, the results suggest 2 divergent possibilities: (1) CDI is not always a mild illness in the SCT population and may be associated with greater risk of death; or (2) patients with GVHD and gastrointestinal symptoms are more likely to be diagnosed with CDI because of more frequent stool testing. It is unclear in the study by Guddati and colleagues whether the patients with GVHD and CDI died of fulminant CDI or died of severe GVHD with CD colonization. The investigators note that severe CDI was significantly less frequent in SCT compared with non-SCT patients in this database (see further discussion of severity in CDI below).
Diagnosis and Severity Assessment in the Stem Cell Transplant and Hematologic Malignancy Populations
Current methods for the diagnosis of CDI are imperfect and there is disagreement as to whether the best approach is to use a single, highly sensitive test or a combination of tests. Two older, gold standard reference methods, toxigenic culture (TC) and cell cytotoxicity neutralization assay (CNNA), are rarely used anymore. These reference assays involve cultivating the organism and then demonstrating the presence of toxin. The CNNA method requires 24 to 48 hours and has insufficient sensitivity (65%- 85%).27,57 In contrast, TC is very accurate but can take up to 5 days. It is thus impractical for patient care. The most frequently used laboratory tests have therefore become EIAs or nucleic acid amplification tests (usually PCR for toxin B). The EIAs target glutamate dehydrogenase (GDH), an enzyme present in toxigenic and nontoxigenic CD isolates, or, alternatively, toxins A and B. The EIA for GDH is highly sensitive but not specific. As a result, GDH is often combined with a PCR test or toxin EIA. As standalone tests, toxin EIAs are also not optimal, because of sensitivities that range from 67% to 92% when tested independently of the manufacturer.58
The NAAT (PCR) detects the gene for toxin B (tcdB) rather than its product. In contrast with the toxin EIAs, PCR for tcdB is highly sensitive and moderately specific.58,59 However, this assay can detect strains that possess the toxin B gene but are not elaborating the toxin. Nonetheless, in the correct clinical context (ie, diarrheal stools in a hospitalized patient or person with recent antibiotic exposure), PCR for toxin B is the most sensitive test with the most acceptable specificity.57,60 US guidelines recommend, for health care facilities with established standards for CD stool testing (liquid or unformed stools only), using PCR (NAAT) as a stand-alone test or using a multistep testing algorithm. The multistep algorithm might include, for example, GDH and toxin, PCR plus toxin, or GDH plus toxin, with discrepancies settled by PCR.27 Standards for submitting fecal specimens to the laboratory for testing commonly specify liquid (unformed) stool consistency, new onset of at least 3 diarrheal episodes in 24 hours, and no receipt of laxatives in the preceding 48 hours.27
In the transplant population, the decision to use one or the other CD assay or testing algorithm has added complexity. First, the toxin EIA assays may perform poorly in immune-compromised patients,61 raising the possibility of underdiagnosis of CDI in vulnerable patients. Second, SCT patients and patients with HM receiving chemotherapy are likely to have diarrhea for a variety of reasons other than CDI; for example, as a result of stem cell conditioning, exposure to antineoplastic agents, or supplemental feeding. In these patients, diarrheal symptoms may prompt more frequent testing for CDI, raising the possibility that an unknown subset of patients diagnosed with CDI by PCR may be merely colonized with a toxigenic CD strain. The conflation of CD colonization with CDI could explain the milder disease presentation among SCT patients that has been reported by some investigators, who have relied in whole or in part on PCR testing for CDI in their cohorts.32,40,53,62,63 However, investigators who used EIA for GDH or toxin, followed by confirmation with culture-based methods (TC or CNNA) for CDI diagnosis, have also reported a mild spectrum of illness.21,39,45,54 Some experts have suggested that lower estimates of severe disease in SCT patients and patients with HM stem from inappropriate criteria and have proposed other scoring systems.27,64–67 Leukopenia, for example, is common after SCT and thus leukocytosis, one of the severity criteria in US guidelines, would be an insensitive measure of severe CDI. However, alternative severity scoring systems have not been prospectively validated or consistently applied in studies of CDI in the SCT population.53,68
Reported Association Between Clostridioides difficile Infection and Graft-versus-host Disease
Several studies have reported an association between preceding GVHD and CDI (Table 1).20,21,51,56 in contrast, others have found CDI to be a risk factor for subsequent GVHD.18,21,45,47 A further set of studies uncovered no evidence of association in either direction.22,32,39,52,62 These conflicting findings might be partially explained by differences in rates of GVHD, conditioning regimens, antibiotic prophylaxis, CDI strains, and donor-recipient compatibility across institutions. Other possible explanations include (1) the existence of a true association (either unidirectional or bidirectional) between CDI and GVHD that is inconsistently detected because of lack of power; (2) oversampling patients with GVHD for CDI and mistaking colonization for disease; (3) confounding because of an underlying cause common to both syndromes. The common cause that has been suggested is intestinal dysbiosis, described next.69
The Proposed Role of the Microbiome
Intestinal dysbiosis, or the loss of gastrointestinal microbial diversity and consequent alterations in intestinal metabolism, are features of both CDI and GVHD.70–74 The healthy gut microbiome, which is dominated by anaerobic phyla such as Bacteroidetes and Firmicutes,75 protects mammalian hosts from CD and other invasive bacteria through colonization resistance.76,77 Colonization resistance is the summary of mechanisms by which commensal bacteria interact with host metabolic pathways to exclude pathogenic species.78,79 For example, resistance to toxigenic strains of CD arises from the consumption of available nutrients by commensal species; elaboration of bacteriocins toxic to CD79; and the conversion of primary bile acids to secondary bile acids, which inhibit the germination of CD spores into replicating cells (Fig. 1).71,78 This host-bacteria mutualism is disrupted by antibiotics, which deplete resident microbial communities and result in outgrowth of toxin-producing CD and other pathogenic species (see Fig. 1).
Fig. 1.
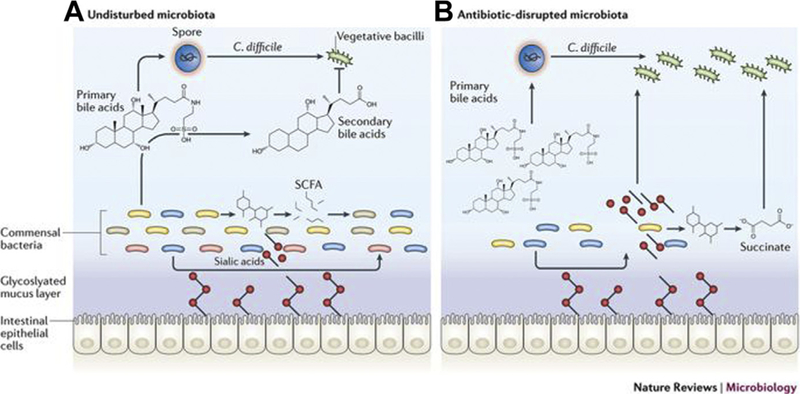
Intestinal microbiota and colonization resistance. (A) In the healthy intestine, commensal bacteria convert primary bile acids into secondary bile acids, which inhibit the growth of CD. Commensal bacteria also convert carbohydrates into short-chain fatty acids (SCFAs), such as succinate, and produce sialidases that cleave sugars attached to gut epithelial cells and release sialic acid into the lumen. These molecules then serve as an energy source for the commensal bacteria. (B) Antibiotics deplete commensal bacterial populations, resulting in a buildup of primary bile acids that facilitate germination of CD spores. CD is then able to take advantage of the abundance of food sources (sialic acid and succinate) in the absence of competition from commensal bacterial communities.
Intestinal dysbiosis may similarly contribute to the immune pathogenesis of GVHD. Investigative work to support this hypothesis has largely been performed at Memorial Sloan Kettering Cancer Center (MSKCC). In one study, Taur and colleagues80 determined that greater bacterial diversity in fecal samples collected before engraftment was associated with lower rates of transplant-related mortality after allogeneic SCT. Individuals with low microbial diversity had a hazard ratio of transplant-related mortality of 5.25 (P = 0.014). In a different study using stool samples prospectively collected 12 days after stem cell infusion, patients with higher fecal biodiversity had less GVHD-related mortality.81 In addition, patients harboring a microbiome enriched with the bacterial genus Blautia experienced less mortality caused by GVHD and greater overall survival. Patients who did not receive anaerobic therapy had higher levels of Blautia. A shorter period on total parenteral nutrition was also associated with greater abundance of Blautia. Conditioning regimen had no impact. Interestingly, Commensal Clostridia are among the species contained within the Blautia genus.
More recently, researchers at MSKCC and an academic cancer center in Regensburg, Germany, investigated the impact of early administration of broad-spectrum antibiotics (used in 92% of Regensburg and 76% of MSKCC patients) on stool biodiversity and outcomes.82 Early administration was defined as receipt of additional antibiotics beyond the prophylactic regimen (to treat neutropenic fever or systemic infection) between day −7 and day 0. Late administration was defined as broad-spectrum antibiotics on day 0 or later. A small group received no additional antibiotics. Gut biodiversity was indirectly assessed through measuring the concentration of 3-indoxyl sulfate (3-IS), a metabolite of intestinal commensal bacteria, in the urine or by detection of commensal Clostridiales in fecal samples using reverse transcription PCR. Both groups received prophylactic antibiotics according to institutional protocols. No differences in intestinal biodiversity were seen between the three groups at baseline before SCT or before antibiotic administration. However, at both sites, the groups that received early antibiotic therapy had lower levels of the urinary marker 3-IS, measured between day 0 and day 28, and also less abundance of Clostridiales compared with the group receiving late or no antibiotic therapy. At both sites, there was an association between early antibiotic treatment and increased transplant-related mortality, including death from acute or chronic GVHD.82 Taken together, the above studies suggest that maintaining the populations of commensal bacteria in the intestine may modulate the deleterious immune responses seen in GVHD and thus reduce transplant mortality.
At least 4 interventional studies of fecal microbiota transplant (FMT) and its impact on GVHD, colonization with antibiotic-resistant bacteria, and microbiome diversity have now been performed (reviewed in Ref.83). In a recent, open-label, uncontrolled pilot study, oral fecal microbiota capsules were given to 13 patients within 4 weeks of allogeneic stem cell transplant (median of 27 days after SCT).84 Two patients developed GVHD, 1 of whom also had BSI. One patient developed severe abdominal pain, which was considered to be FMT-related, and 1 patient died of acute gastrointestinal GVHD. Overall survival was 85%. In this cohort, fecal microbial diversity increased, as measured by 3-IS concentrations in urine samples following FMT, compared with samples obtained before FMT. Unexpectedly, a significant contraction of microbiota diversity was not observed after SCT compared with pre-SCT baseline, when measured by 16Sribosomal RNAsequencing and no significant increase in microbiota diversity was seen after FMT when assessed by 16S ribosomal RNA sequencing on fecal samples. Thus, it is unclear whether performing 16S ribosomal RNA sequencing on stool specimens is not the optimal method of evaluating the gut microbiome or whether in this cohort the SCT procedure did not contract the intestinal microbiome as expected. The field will benefit from a randomized, prospective clinical trial of FMT to prevent GVHD that uses several different methodologies to assay intestinal biodiversity before and after SCT.
Management of Clostridioides difficile Infection in the Stem Cell Transplant and Hematologic Malignancy Population
Initial episode of Clostridioides difficile infection
Current standard care for the management of moderate to severe CDI in the United States involves withdrawal of the culpable antibiotic, when possible, and oral vancomycin (125 mg 4 times daily) or oral fidaxomicin (200 mg twice daily) given for 10 days.27 Metronidazole (500 mg orally 3 times daily) is a less-effective, second-line agent, most often used for mild cases of CDI. Zar and colleagues67 showed the superiority of vancomycin compared with metronidazole for patients with severe CDI. Among the patients with severe disease in this study, 28% had underlying malignancy. A later, retrospective study of CDI in patients with HM85 showed no difference in outcomes between individuals treated with vancomycin compared with those treated with metronidazole. Thirty-seven percent of patients had severe disease, and treatment efficacy averaged only 50% (vancomycin) to 54% (metronidazole) for the initial episode. This study, apart from being retrospective, was likely underpowered to detect a difference between the two agents.85 Subsequent studies, not focused on the SCT population, have clearly established that vancomycin is superior to metronidazole and reduces mortality in severe CDI.86,87 In a large trial by Johnson and colleagues,86 oral vancomycin had an efficacy of 81%, compared with 73% for metronidazole.
For fulminant CDI, high-dose oral vancomycin (500 mg 4 times daily) and intravenous metronidazole (same dose as oral administration) are recommended.27 Vancomycin by the rectal route (instillation) should be considered for patients with ileus. For patients with fulminant CDI, surgical intervention may be required for cases involving megacolon, refractory septic shock, or colon perforation. Surgical options include total or subtotal colectomy or, alternatively, diverting loop ileostomy with colonic lavage followed by antegrade vancomycin flushes.27,88 Fluid and electrolyte support are other important aspects of management. Gastric acid inhibitors and anti-motility drugs should be avoided.89 Institution-wide infection control measures, including accommodation in private rooms or cohorting, contact precautions, appropriate hand washing (soap and water), and room and equipment disinfection, are key to limiting health care transmission of infection.27
Recurrent Clostridioides difficile infection: vancomycin, fidaxomicin, bezlotoxumab, and fecal microbiota transplant
Recurrent CDI is seen in 15% to 35% of patients after a first episode.90–92 Within the SCT population, recurrence rates are similar.21,53 Risk factors for CDI relapse include older age, proton pump inhibitors, exposure to antibiotics during or after CDI treatment, strain type, inadequate anti-CD antibodies, severity of CDI, immune compromise, underlying disease, and prior episodes of CDI.27 According to current US guidelines, second or higher episodes of CDI may be treated with vancomycin at standard doses (125 mg 4 times a day) for 10 days followed by a tapering dose over several weeks. However, the success rate after a second course of vancomycin for recurrent CDI was only 64% in one large trial.93 Other options for treating recurrent CDI include vancomycin followed by rifaximin, fidaxomicin for 10 days, bezlotoxumab, or FMT.
Fidaxomicin is a macrocyclic antibiotic with potent activity against CD and CD spores but no activity against Gram-negative aerobes or anaerobes.94,95 Consequently, stool microbial diversity is relatively preserved in fidaxomicin-treated patients.96
In 2 unrelated, randomized, double-blind, controlled trials (NCT00468728 and NCT00314951), fidaxomicin was equivalent to vancomycin in the initial response to treatment and was associated with lower levels of recurrent disease compared with vancomycin.90,91 CDI recurrence rates of 13% to 15% and 25% to 27% were seen for fidaxomicin and vancomycin, respectively, in these 2 trials.90,91 A subsequent post hoc analysis compared responses to fidaxomicin and vancomycin in patients with (183) and without cancer (922).97 One-third of those with cancer had a diagnosis of HM. Overall treatment responses were lower among patients with cancer than those without cancer (79% vs 87%), and the duration of diarrhea was nearly twice as long in patients with cancer. In patients without cancer, cure rates for fidaxomicin and vancomycin were not different (89% and 89%, respectively). However, in patients with cancer, treatment with fidaxomicin resulted in a trend toward a higher rate of initial cure (85%) compared with vancomycin (74%) (P = .065). The odds of sustained response, defined as initial cure plus the absence of recurrence at 28 days posttreatment, were 2.6-fold higher in patients with cancer treated with fidaxomicin compared to vancomycin (P = .003).97
The US Food and Drug Administration approved bezlotoxumab, a monoclonal anti-body against CD toxin B, in 2016 for secondary prophylaxis of CDI in patients at high risk of recurrence. In 2 large, double-blind, randomized, placebo-controlled trials, MODIFY I and MODIFY II,98 a single infusion of bezlotoxumab, in addition to standard care for CDI (mostly vancomycin or metronidazole), was associated with a significantly lower CDI recurrence rate compared with placebo (16%−17% vs 26%−28%).98 Approximately 20% of patients in these two trials were immune-compromised. A post hoc analysis in the immunocompromised subgroup showed a 36% recurrence rate in patients who received placebo compared with 19% in patients treated with bezlotoxumab.99 No trials of bezlotoxumab in the SCT population have been performed, to our knowledge. Cost limits the use of bezlotoxumab. The average wholesale cost of a vial is estimated to be $3800 to $4560.100,101 In addition, higher rates of heart failure and increased mortality in patients with a history of heart failure have been observed after bezlotoxumab treatment.102
FMT was first used in 4 cases of pseudomembranous colitis in 1958. Subsequently, it was successful in a case of documented CDI in 1983 (see excellent historical review in Ref.103). Between 1958 and 2008, approximately 100 case reports of FMT used for recurrent CDI were published, with a success rate of 89%.104 Van Nood and colleagues105 compared the efficacy of FMT via duodenal infusion with vancomycin for treatment of recurrent CDI in a small, open-label, randomized controlled trial. Immune-suppressed patients were not included in this trial, nor were patients who were critically ill. Enrolled patients had experienced a median of 3 CDI episodes. Most of the group that received FMT were cured after a single infusion (13 out of 16, or 81%), and 15 out of 16 (94%) were cured with either 1 or 2 infusions. In comparison, only 4 out of 13 patients (31%) treated with vancomycin achieved cure. In a more recent study, Hvas and colleagues106 showed that FMT given after 4 to 10 days of vancomycin in patients with recurrent CDI was superior to 10 days of fidaxomicin or 10 days of vancomycin. Clinical resolution was seen in 92% of patients treated with FMT, compared with 42% of those who received fidaxomicin and 19% of those who were treated with vancomycin.
Modes of delivery of FMT include oral capsules, colonoscopy, nasogastric or naso-duodenal tube, or retention enema.104 There is little difference in the rates of success across these different modes of fecal preparation and delivery.107 The presumed mechanism by which FMT works is by replenishing depleted populations of intestinal commensal bacteria and restoring colonization resistance against CD. Several studies have shown that FMT is associated with an increase in short-chain fatty acids and secondary bile acids, a more diverse intestinal microbiome (enriched for Bacteroides, Lachnospiraceae, and Clostridiales), and a reduction in Proteobacteria.105,108,109
Restoration of metabolic products associated with commensal flora after FMT has been shown in SCT patients.84 However, FMT has not been widely used in the SCT population because of concerns that donor stool may introduce as-yet uncharacterized pathogenic viruses, bacteria, or other organisms into intensely immune- suppressed hosts. Several case reports of successful FMT after SCT or in patients with HM have been published110–112 and at least 3 small, retrospective studies of FMT for recurrent CDI have been performed in allogeneic SCT patients (reviewed in Ref.83). No serious side effects or infections have been reported.
Hefazi and colleagues113 published a case series of FMT used in 23 patients with cancer, including 13 with HM, for recurrent CDI. They reported an 87% success rate and no adverse events or infections. Most patients had completed chemotherapy at least 12 weeks before the FMT. A systemic review of the use of FMT for CDI in immune-compromised patients, which included 44 studies and 303 patients, reported an 87% success rate, a mortality of less than 1%, and a treatment-related infection rate of 1.6%.114 A randomized controlled trial of autologous FMT for prophylaxis of CDI in allogeneic SCT patients (NCT02269150) is ongoing. Enrolled subjects will be followed for 1 year after SCT for CDI events.
Clostridioides difficile Infection Prophylaxis: Vancomycin and Fidaxomicin
Ganetsky and colleagues115 examined the efficacy of vancomycin for primary prophylaxis of CDI in a retrospective study of 105 consecutive SCT patients at a single center. In this study, 0 out of 50 (0%) patients who received vancomycin during their SCT admission developed CDI, compared with 11 out of 50 (20%) patients not on prophylaxis. A second, retrospective study of CDI in allogeneic SCT patients found no difference in the incidence of CDI among patients with a history of CDI who received vancomycin prophylaxis (17% CDI), patients with a history of CDI who received no prophylaxis (14%), and patients without a history of CDI who received no prophylaxis (11%). Details of the timing and duration of vancomycin administration as well as the length of follow-up for CDI detection are not available.116 A further clinical trial is underway to examine the effect of oral vancomycin prophylaxis on CD stool bacterial loads in patients with stool samples that are positive for CD by PCR but negative for CD toxin by EIA (NCT03030248).
Mullane and colleagues117 recently published the results of a randomized controlled trial comparing oral fidaxomicin with placebo for primary prevention of CDI in SCT patients on fluoroquinolone prophylaxis (DEFLECT-1). The primary end point, prophylaxis failure, included all confirmed CDI, any use of CDI antibiotics, as well as missing assessment for CDI as failures. No difference in prophylaxis failure between fidaxomicin and placebo was seen. However, most failures were imputed from missing data. A sensitivity analysis that compared fidaxomicin with placebo, in which prophylaxis failure was more narrowly defined as confirmed CDI or use of anti-CDI agents, found a significantly lower rate of failure for fidaxomicin versus placebo (4% vs 11%; P = .001). Thus, it seems that fidaxomicin may be effective in reducing rates of CDI in SCT patients on fluoroquinolones and other antibiotics often given during engraftment.
Novel Agents for Clostridioides difficile infection
Multiple agents are currently under study for the treatment or prevention of CDI. This armamentarium includes new antimicrobials or repurposed drugs, such as ridinila- zole,118,119 cadazolid,120 and niclosamide121; small molecules, such as bacteriocins directly toxic to CD122; spores from nontoxigenic CD strains123,124; probiotics125; and commensal bacteria bioengineered to overexpress the CD surface adhesin (and thereby competitively outbind CD at the epithelial cell-intestinal lumen inter- face).126 A full discussion of the many anti-CD agents in development is beyond the scope of this article but may be found in 2 excellent reviews.120,127
SUMMARY
CDI is the leading cause of infectious diarrhea in hospitalized patients in the United States. The incidence of CDI is greater in the SCT and HM population, where rates are up to 9-fold higher than for the general population. Although most reports describe mild to moderate disease, CDI in immune-suppressed hosts may be severe, and several studies have found associations with unfavorable outcomes, including mortality, subsequent BSI, and GVHD. Unique risk factors for CDI in SCT patients and patients with HM include receipt of allogeneic (as opposed to autologous) transplant, use of cord blood stem cells, total body irradiation, myeloablative conditioning, chemotherapy, neutropenia, mucositis, herpes virus reactivation, and GVHD. CDI usually occurs within the first month following transplant, although late onset has also been described. The underlying pathophysiology of CDI involves a disruption of commensal microbial communities in the gut, leaving underoccupied niches that are exploited by toxin-producing strains of CD and other pathogens. Intestinal injury, from conditioning regimens, chemotherapy, or infection, is also a precursor to GVHD and may explain the bidirectional association between GVHD and CDI found in the literature.
Diagnostic tests for CDI are imperfect in that PCR testing for toxin B cannot distinguish between colonization and infection, and toxin immunoassays are insufficiently sensitive to rule out disease. Currently recommended antibiotic therapies for primary and recurrent CDI in SCT are identical to those for the general population. Of all the management approaches in use today for recurrent CDI, FMT has shown the greatest efficacy. However, many cancer centers have not adopted routine use of FMT for recurrent CDI in SCT and HM patients because of concerns about safety. Several case reports, small series, and retrospective studies have shown that FMT is effective and safe in SCT patients and patients with HM, and a randomized controlled trial of autologous FMT for prophylaxis of CDI in allogeneic SCT patients (NCT02269150) is underway.
New treatment approaches for CDI are urgently needed, given the frequency of this infection in SCT patients and its association in several studies with nontrivial outcomes, such as GVHD and transplant-related mortality. An abundance of novel therapeutics for CDI is currently in development. In addition, FMT, which is highly effective, may soon become an acceptable option for CDI prophylaxis and treatment and is being explored as a potential preventive measure for GVHD.
KEY POINTS.
Clostridioides difficile infection (CDI) in the stem cell transplant (SCT) population occurs at more than 9-fold the rate in the general population.
Risk factors include allogeneic SCT, use of umbilical cord blood stem cells, neutropenia, myeloablative conditioning, chemotherapy, and possibly graft-versus-host disease (GVHD).
CDI is associated with increased mortality, bacteremia, and GVHD in some studies.
Current first-line therapies for primary or recurrent CDI are vancomycin and fidaxomicin for nonsevere and severe CDI. Intravenous metronidazole is recommended as adjunctive treatment of fulminant CDI, especially if ileus is present.
Fecal microbiota transplant is highly effective for recurrent CDI and primary CDI. Pilot studies in SCT patients have shown safety and efficacy.
Acknowledgments
Nasia Safdar receives grant support from the National Institutes of Health (DP2AI44-244 and U01AI125053) and Agency for Healthcare Research and Quality (R01HS026226 and R01HS025713).
Footnotes
Disclosure: The authors report no conflicts of interests.
Severe CDI is variably defined in the literature: (1) leukocytosis with white cell count greater than or equal to 15,000 cells/mL and a serum creatinine level greater than 1.5 mg/dL27; (2) a score of 2 points or more in a scoring system including age more than 60 years (1 point), temperature greater than 38. 3°C (1 point), albumin level less than 2.5 mg/dL (1 point), peripheral white blood cell count greater than 15,000 cells/mL (1 point), pseudomembranous colitis at endoscopy (2 points), or treatment in the intensive care (2 points)67; (3) grade 3 or higher diarrhea and/or colitis, with grade 3 diarrhea defined as 1 to 2 L of intestinal output per day65; (4) a score of 2 points or higher in a scoring system based on fever (1 point), ileus (1 point), systolic blood pressure (1 point), white blood cell count (0–2 points), and computed tomography findings (0–2 points).66 Fulminant CDI, or severe CDI with complications, has been defined as CDI accompanied by hypotension (shock), ileus, or megacolon.27
REFERENCES
- 1.Janezic S, Potocnik M, Zidaric V, et al. Highly divergent Clostridium difficile strains isolated from the environment. PLoS One 2016;11(11):e0167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez C, Taminiau B, Van Broeck J, et al. Clostridium difficile in food and animals: a comprehensive review In: Donelli G, editor. Advances in Microbiology, infectious diseases and public health, vol. 4 Cham (Switzerland): Springer International Publishing; 2016. p. 65–92. [DOI] [PubMed] [Google Scholar]
- 3.Allen SD, Emery CL, Lyerly DM. Clostridium. In: Murrray PR, Baron EJ, Jorgensen JH, et al. , editors. Manual of clinical microbiology, vol. 1, 8th edition Washington, DC: American Society for Microbiology; 2003. p. 835–56. [Google Scholar]
- 4.Lees EA, Miyajima F, Pirmohamed M, et al. The role of Clostridium difficile in the paediatric and neonatal gut - a narrative review. Eur J Clin Microbiol Infect Dis 2016;35(7):1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett JG, Moon N, Chang TW, et al. Role of Clostridium difficile in antibiotic- associated pseudomembranous colitis. Gastroenterology 1978;75(5):778–82. [PubMed] [Google Scholar]
- 6.Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev 1988;1(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson S, Gerding DN. Clostridium difficile-associated diarrhea. Clin Infect Dis 1998;26(5):1027–34 [quiz: 1035–6]. [DOI] [PubMed] [Google Scholar]
- 8.Efron PA, Mazuski JE. Clostridium difficile colitis. Surg Clin North Am 2009;89(2): 483–500. [DOI] [PubMed] [Google Scholar]
- 9.Hurley BW, Nguyen CC. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch Intern Med 2002;162(19):2177–84. [DOI] [PubMed] [Google Scholar]
- 10.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353(23):2433–41. [DOI] [PubMed] [Google Scholar]
- 11.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005;366(9491):1079–84. [DOI] [PubMed] [Google Scholar]
- 12.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005;353(23):2442–9. [DOI] [PubMed] [Google Scholar]
- 13.Gorschluter M, Glasmacher A, Hahn C, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis 2001;33(6):786–91. [DOI] [PubMed] [Google Scholar]
- 14.Glasmacher A, Ziske C, Hahn C, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis 2001;33(6):786–91. [DOI] [PubMed] [Google Scholar]
- 15.Arango JI, Restrepo A, Schneider DL, et al. Incidence of Clostridium difficile-associated diarrhea before and after autologous peripheral blood stem cell transplantation for lymphoma and multiple myeloma. Bone Marrow Transplant 2006;37(5):517–21. [DOI] [PubMed] [Google Scholar]
- 16.Fortin É, Dionne M, Hubert B, et al. A portrait of the geographic dissemination of the Clostridium difficile North American Pulsed-Field Type 1 Strain and the Epidemiology of C. difficile-Associated Disease in Quebec. Clin Infect Dis 2007;44(2):238–44. [DOI] [PubMed] [Google Scholar]
- 17.Schalk E, Bohr UR, König B, et al. Clostridium difficile-associated diarrhoea, a frequent complication in patients with acute myeloid leukaemia. Ann Hematol 2009;89(1):9. [DOI] [PubMed] [Google Scholar]
- 18.Dubberke ER, Reske KA, Srivastava A, et al. Clostridium difficile-associated disease in allogeneic hematopoietic stem-cell transplant recipients: risk associations, protective associations, and outcomes. Clin Transplant 2010;24(2):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra T, Chandrasekar P, Salimnia H, et al. Recent epidemiology of Clostridium difficile infection during hematopoietic stem cell transplantation. Clin Transplant 2011;25(1):E82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willems L, Porcher R, Lafaurie M, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 2012;18(8):1295–301. [DOI] [PubMed] [Google Scholar]
- 21.Alonso CD, Treadway SB, Hanna DB, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2012;54(8):1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamboj M, Xiao K, Kaltsas A, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplant: strain diversity and outcomes associated with NAP1/027. Biol Blood Marrow Transplant 2014;20(10):1626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372(9):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerding DN, Johnson S. Clostridium difficile-associated diarrhea. Clin Infect Dis 1998;26(5):1027–34. [DOI] [PubMed] [Google Scholar]
- 25.Gerding DN, Lessa FC. The epidemiology of Clostridium difficile infection inside and outside health care institutions. Infect Dis Clin North Am 2015;29(1):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett JG. Clostridium difficile: progress and challenges. Ann N Y Acad Sci 2010;1213(1):62–9. [DOI] [PubMed] [Google Scholar]
- 27.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66(7):e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamboj M, Son C, Cantu S, et al. Hospital-onset Clostridium difficile infection rates in persons with cancer or hematopoietic stem cell transplant: a C3IC network report. Infect Control Hosp Epidemiol 2012;33(11):1162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guddati AK, Kumar G, Ahmed S, et al. Incidence and outcomes of Clostridium difficile-associated disease in hematopoietic cell transplant recipients. Int J Hematol 2014;99(6):758–65. [DOI] [PubMed] [Google Scholar]
- 30.Selvey LA, Slimings C, Joske DJL, et al. Clostridium difficile infections amongst patients with haematological malignancies: a data linkage study. PLoS One 2016;11(6):e0157839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satlin MJ, Vardhana S, Soave R, et al. Impact of prophylactic levofloxacin on rates of bloodstream infection and fever in neutropenic patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015;21(10):1808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinnebrew MA, Lee YJ, Jenq RR, et al. Early Clostridium difficile infection during allogeneic hematopoietic stem cell transplantation. PLoS One 2014;9(3): e90158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young JH, Logan BR, Wu J, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant 2016;22(2):359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slade M, Goldsmith S, Romee R, et al. Epidemiology of Infections following hap- loldentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis 2017;19(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubberke ER, Reske KA, Olsen MA, et al. Epidemiology and outcomes of Clostridium difficile infection in allogeneic hematopoietic cell and lung transplant recipients. Transpl Infect Dis 2018;20(2):e12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster MG, Cleveland AA, Dubberke ER, et al. Infections in hematopoietic cell transplant recipients: results from the organ transplant infection project, a multicenter, prospective, cohort study. Open Forum Infect Dis 2017;4(2):ofx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Styczynski J, Czyzewski K, Wysocki M, et al. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: a multicentre nationwide study. Clin Microbiol Infect 2016;22(2):179.e1–10. [DOI] [PubMed] [Google Scholar]
- 38.Zacharioudakis IM, Ziakas PD, Mylonakis E. Clostridium difficile infection in the hematopoietic unit: a meta-analysis of published studies. Biol Blood Marrow Transplant 2014;20(10):1650–4. [DOI] [PubMed] [Google Scholar]
- 39.Lavallée C, Labbé AC, Talbot JD, et al. Risk factors for the development of Clostridium difficile infection in adult allogeneic hematopoietic stem cell transplant recipients: a single-center study in Quebec, Canada. Transpl Infect Dis 2017; 19(1):e12648. [DOI] [PubMed] [Google Scholar]
- 40.Salamonowicz M, Ociepa T, Fraczkiewicz J, et al. Incidence, course, and outcome of Clostridium difficile infection in children with hematological malignancies or undergoing hematopoietic stem cell transplantation. Eur J Clin Microbiol Infect Dis 2018;37(9):1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson S, Gerding DN, Janoff EN. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J Infect Dis 1992;166(6): 1287–94. [DOI] [PubMed] [Google Scholar]
- 42.Kyne L, Warny M, Qamar A, et al. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000;342(6): 390–7. [DOI] [PubMed] [Google Scholar]
- 43.Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection. Vaccine 2010;28(4):965–9. [DOI] [PubMed] [Google Scholar]
- 44.Aldrete S, Kraft CS, Magee MJ, et al. Risk factors and epidemiology of Clostridium difficile infection in hematopoietic stem cell transplant recipients during the peri-transplant period. Open Forum Infect Dis 2015;2(Suppl 1) [abstract: S235]. [DOI] [PubMed] [Google Scholar]
- 45.Trifilio SM, Pi J, Mehta J. Changing epidemiology of Clostridium difficile-associated disease during stem cell transplantation. Biol Blood Marrow Transplant 2013;19(3):405–9. [DOI] [PubMed] [Google Scholar]
- 46.Scardina TL, Martinez EK, Balasubramanian N, et al. Evaluation of risk factors for Clostridium difficile infection in hematopoietic stem cell transplant recipients. Pharmacotherapy 2017;37(4):420–8. [DOI] [PubMed] [Google Scholar]
- 47.Chakrabarti S, Lees A, Jones SG, et al. Clostridium difficile infection in allogeneic stem cell transplant recipients is associated with severe graft-versus-host disease and non-relapse mortality. Bone Marrow Transplant 2000;26(8):871–6. [DOI] [PubMed] [Google Scholar]
- 48.Boyle NM, Magaret A, Stednick Z, et al. Evaluating risk factors for Clostridium difficile infection in adult and pediatric hematopoietic cell transplant recipients. Antimicrob Resist Infect Control 2015;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cannon CM, Musuuza JS, Barker AK, et al. Risk of Clostridium difficile infection in hematology-oncology patients colonized with toxigenic C. difficile. Infect Control Hosp Epidemiol 2017;38(6):718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon YK, Kim MJ, Sohn JW, et al. Predictors of mortality attributable to Clostridium difficile infection in patients with underlying malignancy. Support Care Cancer 2014;22(8):2039–48. [DOI] [PubMed] [Google Scholar]
- 51.Dubberke ER, Reske KA, Olsen MA, et al. Risk for Clostridium difficile infection after allogeneic hematopoietic cell transplant remains elevated in the postengraftment period. Transplant Direct 2017;3(4):e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vehreschild MJ, Weitershagen D, Biehl LM, et al. Clostridium difficile infection in patients with acute myelogenous leukemia and in patients undergoing allogeneic stem cell transplantation: epidemiology and risk factor analysis. Biol Blood Marrow Transplant 2014;20(6):823–8. [DOI] [PubMed] [Google Scholar]
- 53.Alonso CD, Braun DA, Patel I, et al. A multicenter, retrospective, case-cohort study of the epidemiology and risk factors for Clostridium difficile infection among cord blood transplant recipients. Transpl Infect Dis 2017;19(4):e12728. [DOI] [PubMed] [Google Scholar]
- 54.Hosokawa K, Takami A, Tsuji M, et al. Relative incidences and outcomes of Clostridium difficile infection following transplantation of unrelated cord blood, unrelated bone marrow, and related peripheral blood in adult patients: a single institute study. Transpl Infect Dis 2014;16(3):412–20. [DOI] [PubMed] [Google Scholar]
- 55.Mani S, Rybicki L, Jagadeesh D, et al. Risk factors for recurrent Clostridium difficile infection in allogeneic hematopoietic cell transplant recipients. Bone Marrow Transplant 2016;51:713. [DOI] [PubMed] [Google Scholar]
- 56.Chang K, Kreuziger LMB, Angell K, et al. Recurrence of Clostridium difficile infection after total colectomy in an allogeneic stem cell transplant patient. Bone Marrow Transplant 2012;47:610–1. [DOI] [PubMed] [Google Scholar]
- 57.Wilcox M, Planche T, Fang F, et al. Point-counterpoint: what is the current role of algorithmic approaches for diagnosis of Clostridium difficile infection? J Clin Microbiol 2010;48(12):4347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcox MH. Evaluation report: Clostridium difficile toxin detection assays (CEP08054). London: Centre for Evidence-Based Purchasing, National Health Service (UK); 2009. [Google Scholar]
- 59.Tenover FC, Novak-Weekley S, Woods CW, et al. Impact of strain type on detection of toxigenic Clostridium difficile: comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol 2010;48(10):3719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tenover FC, Baron EJ, Peterson LR, et al. Laboratory diagnosis of Clostridium difficile infection can molecular amplification methods move us out of uncertainty? J Mol Diagn 2011;13(6):573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erb S, Frei R, Strandén AM, et al. Low sensitivity of fecal toxin A/B enzyme immunoassay for diagnosis of Clostridium difficile infection in immunocompromised patients. Clin Microbiol Infect 2015;21(11):998.e9–15. [DOI] [PubMed] [Google Scholar]
- 62.Bruminhent J, Wang Z-X, Hu C, et al. Clostridium difficile colonization and disease in patients undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20(9):1329–34. [DOI] [PubMed] [Google Scholar]
- 63.Crobach MJT, Vernon JJ, Loo VG, et al. Understanding Clostridium difficile colonization. Clin Microbiol Rev 2018;31(2). e00021–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang MS, Evans CT, Rodriguez T, et al. Clostridium difficile infection and limitations of markers for severity in patients with hematologic malignancy. Infect Control Hosp Epidemiol 2013;34(2):127–32. [DOI] [PubMed] [Google Scholar]
- 65.Dubberke E, Sadhu J, Gatti R, et al. Severity of Clostridium difficile-associated disease (CDAD) in allogeneic stem cell transplant recipients: evaluation of a CDAD severity grading system. Infect Control Hosp Epidemiol 2007;28(2): 208–11. [DOI] [PubMed] [Google Scholar]
- 66.Belmares J, Gerding DN, Parada JP, et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect 2007; 55(6):495–501. [DOI] [PubMed] [Google Scholar]
- 67.Zar FA, Bakkanagari SR, Moorthi KMLST, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45(3):302–7. [DOI] [PubMed] [Google Scholar]
- 68.Alonso CD, Marr KA. Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr Opin Infect Dis 2013;26(4): 326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shallis RM, Terry CM, Lim SH. Changes in intestinal microbiota and their effects on allogeneic stem cell transplantation. Am J Hematol 2018;93(1):122–8. [DOI] [PubMed] [Google Scholar]
- 70.Schaffler H, Breitruck A. Clostridium difficile - from colonization to infection. Front Microbiol 2018;9:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 2016;14(10):609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khoruts A, Hippen KL, Lemire AM, et al. Toward revision of antimicrobial therapies in hematopoietic stem cell transplantation: target the pathogens, but protect the indigenous microbiota. Transl Res 2017;179:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peled JU, Hanash AM, Jenq RR. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood 2016;2016(1):119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 2017; 129(8):927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2008;457:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballen K, Woo Ahn K, Chen M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22(9):1636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng KM, Ferreyra JA, Higginbottom SK, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013; 502(7469):96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorbara MT, Pamer EG. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol 2019; 12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rea MC, Dobson A, O’Sullivan O, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A 2011;108(Supplement 1):4639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taur Y, Jenq RR, Perales M-A, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014;124(7):1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 2015;21(8): 1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weber D, Jenq RR, Peled JU, et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2017;23(5): 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeFilipp Z, Hohmann E, Jenq RR, et al. Fecal microbiota transplantation: restoring the injured microbiome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019;25(1):e17–22. [DOI] [PubMed] [Google Scholar]
- 84.DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv 2018;2(7): 745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parmar SR, Bhatt V, Yang J, et al. A retrospective review of metronidazole and vancomycin in the management of Clostridium difficile infection in patients with hematologic malignancies. J Oncol Pharm Pract 2013;20(3):172–82. [DOI] [PubMed] [Google Scholar]
- 86.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014;59(3):345–54. [DOI] [PubMed] [Google Scholar]
- 87.Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med 2017; 177(4):546–53. [DOI] [PubMed] [Google Scholar]
- 88.Neal MD, Alverdy JC, Hall DE, et al. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg 2011;254(3):423–7 [discussion: 427–9]. [DOI] [PubMed] [Google Scholar]
- 89.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014;20:1–26. [DOI] [PubMed] [Google Scholar]
- 90.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a doubleblind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12(4): 281–9. [DOI] [PubMed] [Google Scholar]
- 91.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364(5):422–31. [DOI] [PubMed] [Google Scholar]
- 92.Huebner ES, Surawicz CM. Treatment of recurrent Clostridium difficile diarrhea. Gastroenterol Hepatol 2006;2(3):203–8. [PMC free article] [PubMed] [Google Scholar]
- 93.Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Babakhani F, Bouillaut L, Gomez A, et al. Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis 2012;55(Suppl 2):S162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldstein EJC, Babakhani F, Citron DM. Antimicrobial activities of fidaxomicin. Clin Infect Dis 2012;55(suppl_2):S143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (0PT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 2010;156(11):3354–9. [DOI] [PubMed] [Google Scholar]
- 97.Cornely OA, Miller MA, Fantin B, et al. Resolution of clostridium difficile-associated diarrhea in patients with cancer treated with fidaxomicin or vancomycin. J Clin Oncol 2013;31(19):2493–9. [DOI] [PubMed] [Google Scholar]
- 98.Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent clostridium difficile infection. N Engl J Med 2017;376(4):305–17. [DOI] [PubMed] [Google Scholar]
- 99.Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018;67(5):649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee Y, Lim W, Bloom C, et al. Bezlotoxumab (Zinplava) for Clostridium difficile infection. P T 2017;42:735–8. [PMC free article] [PubMed] [Google Scholar]
- 101.Bezlotoxumab (Zinplava) for prevention of recurrent Clostridium difficile infection. Med Lett Drugs Ther 2017;59(1517):49–50. [PubMed] [Google Scholar]
- 102.Gerding DN, Johnson S. Bezlotoxumab. Clin Infect Dis 2018;68(4):699–704. [DOI] [PubMed] [Google Scholar]
- 103.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011;9(12):1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009;15(6):285–9. [DOI] [PubMed] [Google Scholar]
- 105.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368(5):407–15. [DOI] [PubMed] [Google Scholar]
- 106.Hvas CL, Jorgensen SMD, Jorgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 107.Khan MY, Dirweesh A, Khurshid T, et al. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2018;30(11): 1309–17. [DOI] [PubMed] [Google Scholar]
- 108.Seekatz AM, Theriot CM, Rao K, et al. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe 2018;53:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jalanka J, Mattila E, Jouhten H, et al. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med 2016;14(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Castro CG, Ganc AJ, Ganc RL, et al. Fecal microbiota transplant after hematopoietic SCT: report of a successful case. Bone Marrow Transplant 2015; 50(1):145. [DOI] [PubMed] [Google Scholar]
- 111.Trubiano JA, George A, Barnett J, et al. A different kind of ‘allogeneic transplant’: successful fecal microbiota transplant for recurrent and refractory Clostridium difficile infection in a patient with relapsed aggressive B-cell lymphoma. Leuk Lymphoma 2015;56(2):512–4. [DOI] [PubMed] [Google Scholar]
- 112.Mittal C, Miller N, Meighani A, et al. Fecal microbiota transplant for recurrent Clostridium difficile infection after peripheral autologous stem cell transplant for diffuse large B-cell lymphoma. Bone Marrow Transplant 2015;50(7):1010. [DOI] [PubMed] [Google Scholar]
- 113.Hefazi M, Patnaik MM, Hogan WJ, et al. Safety and efficacy of fecal microbiota transplant for recurrent Clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin Proc 2017;92(11):1617–24. [DOI] [PubMed] [Google Scholar]
- 114.Shogbesan O, Poudel DR, Victor S, et al. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol 2018;2018:1394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ganetsky A, Han JH, Hughes ME, et al. Oral vancomycin is highly effective in preventing Clostridium difficile infection in allogeneic hematopoietic stem cell transplant recipients. Blood 2016;128(22):2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pereiras MA, Urnoski E, Wynd M, et al. Does oral vancomycin prophylaxis for clostridium difficile infection improve allogeneic hematopoietic stem cell transplant outcomes? Biol Blood Marrow Transplant 2017;23(3):S395. [Google Scholar]
- 117.Mullane KM, Winston DJ, Nooka A, et al. A randomized, placebo-controlled trial of fidaxomicin for prophylaxis of Clostridium difficile-associated diarrhea in adults undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2019;68(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goldstein EJC, Citron DM, Tyrrell KL, et al. Comparative in vitro activities of SMT19969, a new antimicrobial agent, against Clostridium difficile and 350 gram-positive and gram-negative aerobic and anaerobic intestinal flora isolates. Antimicrob Agents Chemother 2013;57(10):4872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vickers RJ, Tillotson GS, Nathan R, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study Lancet Infect Dis 2017;17(7):735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petrosillo N, Granata G, Cataldo MA. Novel antimicrobials for the treatment of Clostridium difficile infection. Front Med 2018;5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tam J, Hamza T, Ma B, et al. Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota. Nat Commun 2018;9(1):5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gebhart D, Lok S, Clare S, et al. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. MBio 2015;6(2) [pii:e02368–14]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C difficile infection: a randomized clinical trial. JAMA 2015;313(17):1719–27. [DOI] [PubMed] [Google Scholar]
- 124.Gerding DN, Sambol SP, Johnson S. Non-toxigenic Clostridioides (formerly Clostridium) difficile for Prevention of C. difficile infection: from bench to bedside back to bench and back to bedside. Front Microbiol 2018;9:1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barker AK, Duster M, Valentine S, et al. A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). J Antimicrob Chemother 2017;72(11):3177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vedantam G, Kochanowsky J, Lindsey J, et al. An engineered synthetic biologic protects against Clostridium difficile infection. Front Microbiol 2018;9:2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Darkoh C, Deaton M, DuPont HL. Nonantimicrobial drug targets for Clostridium difficile infections. Future Microbiol 2017;12(11):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


