Abstract
Introduction
Non-invasive vagus nerve stimulation (nVNS; gammaCore®) has the potential to prevent migraine days in patients with migraine on the basis of mechanistic rationale and pilot clinical data.
Methods
This multicentre study included a 4-week run-in period, a 12-week double-blind period of randomised treatment with nVNS or sham, and a 24-week open-label period of nVNS. Patients were to administer two 120-second stimulations bilaterally to the neck three times daily (6–8 hours apart).
Results
Of 477 enrolled patients, 332 comprised the intent-to-treat (ITT) population. Mean reductions in migraine days per month (primary outcome) were 2.26 for nVNS (n = 165; baseline, 7.9 days) and 1.80 for sham (n = 167; baseline, 8.1 days) (p = 0.15). Results were similar across other outcomes. Upon observation of suboptimal adherence rates, post hoc analysis of patients with ≥ 67% adherence per month demonstrated significant differences between nVNS (n = 138) and sham (n = 140) for outcomes including reduction in migraine days (2.27 vs. 1.53; p = 0.043); therapeutic gains were greater in patients with aura than in those without aura. Most nVNS device-related adverse events were mild and transient, with application site discomfort being the most common.
Conclusions
Preventive nVNS treatment in episodic migraine was not superior to sham stimulation in the ITT population. The “sham” device inadvertently provided a level of active vagus nerve stimulation. Post hoc analysis showed significant effects of nVNS in treatment-adherent patients.
Study identification and registration: PREMIUM; NCT02378844; https://clinicaltrials.gov/ct2/show/NCT02378844
Keywords: Neuromodulation, preventive therapy, RCT, migraine prophylaxis, non-pharmacologic treatment, vagal activation
Introduction
Several alternative and emerging therapies provide an opportunity to improve care for patients with migraine by addressing poor tolerability, adherence, and persistence with standard preventive migraine medications (1–3). Non-invasive neuromodulation therapies and injectable monoclonal antibody medications targeting calcitonin gene-related peptide or its receptor are effective and well tolerated for migraine prevention (4–10). The new preventive medications, including the calcitonin gene-related peptide monoclonal antibodies, are not effective for all patients and are currently not recommended for patients under the age of 18 years, pregnant patients, and older adults. There continues to be a need for additional effective, tolerable, and safe treatment options that can be used without concern about potential adverse interactions with existing or emerging treatments (11,12).
Non-invasive vagus nerve stimulation (nVNS; gammaCore®; electroCore, Inc., Basking Ridge, NJ, USA) is a neuromodulation therapy with Class I evidence for the acute treatment of migraine attacks for patients with episodic migraine (13). nVNS has also shown preliminary efficacy for migraine prevention in a small double-blind study of a chronic migraine population (n = 59) and in open-label studies of patients with chronic migraine or treatment-refractory episodic migraine (n = 20) and a menstrually related migraine population (n = 51) (7,14,15). The efficacy of nVNS in these and other primary headache studies combined with its established tolerability and safety profile suggest that nVNS could represent a valuable addition to available migraine prevention treatments (12,16,17). Objectives of the current study were to evaluate the efficacy, tolerability, and safety of nVNS for the preventive treatment of episodic migraine.
Methods
Study design
The PREMIUM trial (NCT02378844) was a phase 3, prospective, multicentre, randomised, double-blind, parallel-group, sham-controlled study conducted from 1 June 2015 through 21 November 2017 at 22 European sites. The study included a 4-week run-in period of no study treatment, a 12-week double-blind period of randomly assigned nVNS or sham stimulation, and a 24-week open-label period of nVNS. All investigators obtained approval from their local independent ethics committee, and all patients provided written informed consent before participating.
Patients
Patients were 18 to 75 years of age with a diagnosis of migraine with or without aura according to International Classification of Headache Disorders, 3rd edition (beta version) criteria (18). The age of migraine onset was <50 years, and patients had experienced 5–12 migraine days per month in the past 4 months, with at least two migraines lasting more than 4 hours. Complete inclusion and exclusion criteria for study participation are provided in Supplemental Table 1.
Those who met any of the following criteria were excluded: Chronic migraine diagnosis; previous diagnosis of medication overuse headache that had reverted to episodic migraine in the past 6 months; medical condition requiring oral/injectable steroids; history of secondary headache, aneurysm, intracranial haemorrhage, brain tumours, significant head trauma, substance abuse, addiction, syncope, or seizure; structural abnormality, pain, or metal cervical spine hardware implantation near the treatment site; another significant pain disorder; cardiovascular/cerebrovascular disease; abnormal electrocardiogram; previous migraine prevention surgery, cervical vagotomy, or electrical or neurostimulator device implantation; uncontrolled hypertension; psychiatric/cognitive disorders; pregnancy; botulinum toxin injections in the past 6 months; head or neck nerve blocks in the past 2 months; failure of at least three classes of migraine prevention drugs; opioid use (more than 2 days per month); marijuana use (more than twice per month); simple analgesic or nonsteroidal anti-inflammatory drug use (more than 15 days per month); or triptan, ergot, or combined analgesic use (more than 10 days per month).
Use of preventive migraine treatments at or within 30 days before baseline was not permitted. Patients were required to refrain from initiating or changing the type, dosage, or frequency of any medications for other indications that were determined to potentially interfere with the study. Those who proceeded to the double-blind period were reassessed for entry criteria.
Randomisation and blinding
After the run-in period, patients were randomly assigned to receive nVNS or a sham control device (allocation, 1:1) under variable block sizes of 4 and 6, where 4 was chosen about 60% of the time and 6 was chosen about 40% of the time. Randomisation was stratified by study site according to independent third party–generated randomisation schedules. At each site, participants' information was entered into the Merge eClinical OS™ interactive web response system, which provided a sequential patient randomisation number and corresponding device serial number. A third-party distributor issued the devices to the sites. A sponsor designee provided a copy of the randomisation schemes to the unblinded trainer at each study centre. The unblinded trainer opened the box and used the study device to provide training. The training was specific to each subject's assigned randomised treatment group and was aimed at instructing the subject to use the device to activate the vagus nerve most efficiently (rather than to achieve a maximal current/stimulation level). After training was complete, the unblinded trainer provided the device to the subject. The subjects, investigators, and study coordinators remained blinded to treatment assignments throughout the double-blind period.
Interventions
The nVNS device (Figure 1) produces a proprietary low-voltage electrical signal comprising a 5-kHz sine wave burst lasting for 1 ms (five sine waves, each lasting 200 μs), with such bursts repeated once every 40 ms (25 Hz), generating a 24-V peak voltage and 60-mA peak output current. The sham device is identical to the active nVNS device in appearance, weight, visual and audible feedback, user application, and control, but produces a low-frequency (0.1 Hz) biphasic direct current signal (amplitude, 0.0–28 V peak to peak ± 10% through 15 kΩ ± 10%) that was intended to be physically perceived without actual stimulation of the vagus nerve or contraction of muscle.
Figure 1.
The nVNS device.
Note: A previous model of the nVNS device was used by patients in the PREMIUM trial. Image provided courtesy of electroCore, Inc. nVNS: non-invasive vagus nerve stimulation.
Preventive treatment protocol
Patients were instructed to administer preventive migraine treatment with their study device using two consecutive bilateral stimulations per treatment. These two stimulations were administered three times per day (TID): Upon waking and at 6–8 hours after the first and second daily treatments. Acute migraine medication was permitted if needed, but preventive migraine medication was not permitted until the open-label period.
Study procedures
Patients gained access to an electronic diary at the start of the run-in period, which was used to record migraine and headache attacks, medication(s) taken for the attacks, adverse events (AEs), and use of the study device throughout the trial. An unblinded trainer instructed the patients on correct study device use at the site before they started home use in the double-blind period; this training was revisited during each study visit.
Study outcomes
The primary efficacy outcome was the mean reduction in number of migraine days from the 4-week run-in period (baseline) to the last 4 weeks of the 12-week double-blind period. Reductions in the number of headache days and acute medication days during the same time period were evaluated as secondary outcomes. For efficacy analysis, ‘migraine day’ was defined as a migraine headache occurring in a 24-hour period, and a ‘headache day’ was defined as any headache occurring in a single calendar day. Other secondary outcomes included ≥50% responder rates for migraine, headache, and acute medication days, with a ‘responder’ defined as a patient who recorded a reduction of at least 50% from baseline to the last 4 weeks of the double-blind period, and migraine and headache day reductions in the open-label period. Blinding effectiveness was evaluated after the first week of the double-blind period and at completion by asking patients to indicate which treatment they thought they had received (active stimulation, sham, or don't know). Device perceptions were determined from patient responses for satisfaction (1, extremely satisfied; 5, not at all satisfied) and ease of use (1, very easy; 4, very difficult). The tolerability and safety of nVNS was assessed using rates of AEs, adverse device effects (ADEs), including those considered serious, and study discontinuations due to AEs.
Statistical methods
A sample size of 320 patients (160 per treatment arm) was determined to provide 90% power to demonstrate statistical significance for the primary outcome, assuming a treatment difference of 1 migraine day, a common standard deviation of 2.5, a type I error of 5%, and an attrition rate of 15% in the double-blind period.
For each 4-week interval, statistics were summarised descriptively by period and by treatment group for both continuous variables (means and 95% confidence intervals [CIs]) and categorical variables (frequency counts, percentages, and 95% CIs). Available data for patients who discontinued from the study were included in the analysis as far as possible. Partially completed data (e.g. 2 weeks of data) for the 4-week run-in period or the last 4 weeks of the 12-week double-blind period were adjusted to reflect an estimated number of days with the outcome of interest per 28-day interval. The adjustment was made using the formula , where x is the number of days with observed data per 28 days and y is the number of observed days with the outcome of interest. If fewer than 21 days of data were observed in the run-in period, the run-in value was not calculated and was considered missing. If fewer than 70 days of data were observed in the double-blind period, the month 3 value was imputed to no change between treatment periods.
The primary efficacy analysis set was the ‘intent-to-treat (ITT) population’, defined as enrolled patients who received ≥1 treatment in the double-blind period. Upon observation of suboptimal rates of adherence to the TID treatment protocol in the ITT population, a ‘modified intent-to-treat (mITT) population’ was defined as those with ≥67% adherence per month for evaluation in a post hoc analysis, which was not adjusted to control for type I error. The electronic database did not measure daily adherence, and the ≥ 67% per month threshold was selected on the basis of reports regarding the nVNS mechanisms of action (19), which suggest benefits lasting up to 6–8 hours, and for practical reasons (i.e. 67% is equivalent to two of the recommended three treatments per day). For continuous and categorical variables, p-values were derived from linear regression (analysis of covariance models) and logistic regression, respectively, adjusted for treatment group, centre, presence/absence of aura, and number of migraine/headache/acute medication days in the run-in period. Two-sided p-values < 0.05 were considered statistically significant.
Responses to blinding effectiveness questions for each treatment group were used to calculate the Bang blinding index (20). This measure assesses the success of blinding in clinical trials using an interval from −1 to 1, with −1 indicating incorrect guessing, 0 indicating perfect blinding, and 1 indicating complete lack of blinding (20). Safety analyses were conducted on the ‘safety population’, defined as all enrolled participants. Data were analysed using SAS® 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
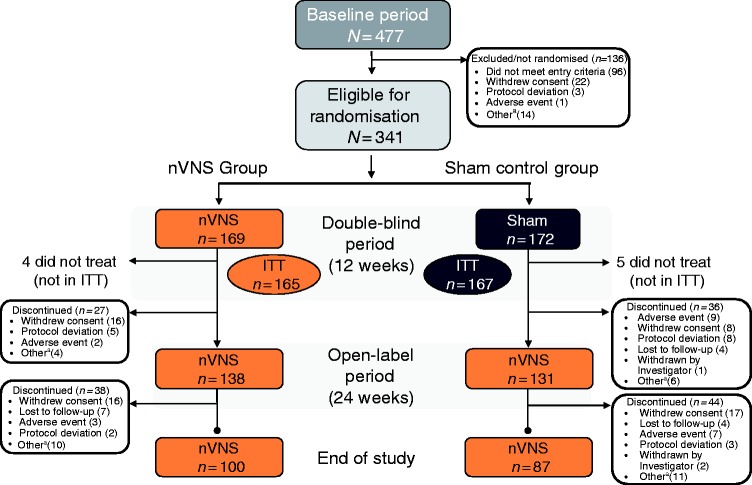
Of 477 patients enrolled in the PREMIUM study, 341 were randomly assigned to receive nVNS (n = 169) or sham (n = 172) in the 12-week double-blind period (Figure 2). A total of 332 patients comprised the ITT population (nVNS, n = 165; sham, n = 167). Two hundred and sixty-nine patients entered the open-label period (nVNS, n = 138; sham, n = 131), with 187 patients completing the study (nVNS, n = 100; sham, n = 87). Demographics and baseline characteristics were well balanced among the nVNS and sham groups (Table 1) and are representative of those from other studies of the prevention of episodic migraine (4,5,8,10).
Figure 2.
Patient disposition.
aOther reasons for discontinuation included inability to fulfill visits because of illness, travel, or family commitments, subject decision, and noncompliance with study procedures.
ITT: intent-to-treat; nVNS: non-invasive vagus nerve stimulation.
Table 1.
Demographics and patient characteristics.
| Characteristica | nVNS (n = 165) | Sham (n = 167) |
|---|---|---|
| Age, years | 43.5 ± 11.1 | 41.4 ± 12.3 |
| Age at migraine onset, years | 19.6 ± 9.6 | 19.4 ± 9.8 |
| Female, n (%) | 142 (86.1) | 138 (82.6) |
| Caucasian, n (%) | 160 (97.0) | 154 (92.2) |
| Migraine type, n (%) | ||
| Migraine with aurab | 36 (21.8) | 42 (25.1) |
| Migraine without aurab | 129 (78.2) | 125 (74.9) |
| Migraine days in the last 4 weeks, n | 7.9 ± 2.2 | 8.1 ± 2.0 |
| Headache days in the last 4 weeks, n | 8.9 ± 2.6 | 9.1 ± 2.6 |
| Acute migraine medication use per month, d | 6.8 ± 2.7 | 7.0 ± 2.8 |
Data are mean ± SD unless otherwise indicated and are from the ITT population.
Presence/absence of aura was based on diagnosis provided in subject medical history at enrolment.
ITT: intent-to-treat; nVNS: non-invasive vagus nerve stimulation; SD: standard deviation.
Patients were not fully adherent to the TID treatment protocol, but most (83.6% [138/165]) in the nVNS group and 83.8% ([140/167] in the sham group) demonstrated adherence of at least 67% per month (Supplemental Table 2).
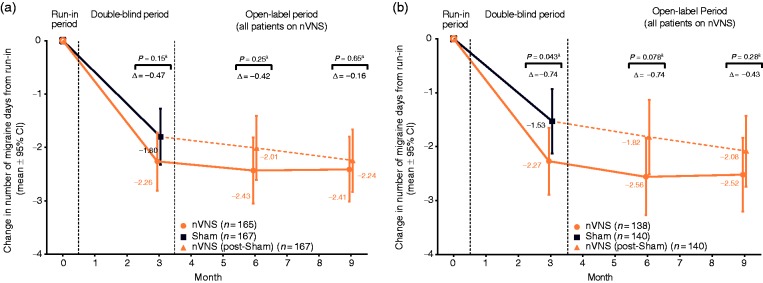
Migraine days
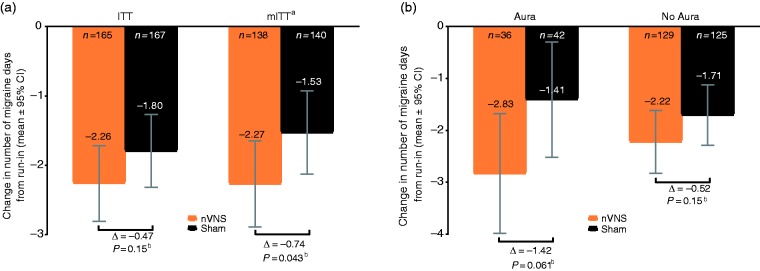
The mean reduction in number of migraine days per month (primary endpoint) was −2.26 days (95% CI: −2.81, −1.72; baseline: 7.9 migraine days) in the nVNS group and −1.80 days (95% CI: −2.32, −1.27; baseline: 8.1 migraine days) in the sham group (p = 0.15) (Figures 3 and 4). The percentage of patients with a ≥50% reduction in number of migraine days was 31.9% (95% CI: 23.4%, 41.8%) in the nVNS group and 25.0% (95% CI: 17.8%, 34.0%) in the sham group (absolute difference, 6.9%; odds ratio [95% CI], 1.40 [0.85, 2.32]; p = 0.19). A prespecified subgroup analysis of patients with and without aura demonstrated no significant differences between treatment groups, but therapeutic gains with nVNS were higher in patients with aura (nVNS, −2.83 days; sham, −1.41 days; p = 0.061) than in those without aura (nVNS, −2.22 days; sham, −1.71 days; p = 0.15) (Figure 3(b)).
Figure 3.
Changes in number of migraine days in the double-blind period in (a) the ITT and mITT populations and (b) subjects with aura and without aura (ITT population).
aPost hoc analysis.
bThe nVNS and sham groups were compared using ANCOVA models; p-values were derived from linear regression adjusted for treatment group, centre, presence/absence of aura (based on diagnosis provided in subject medical history at enrolment), and number of migraine days in the run-in period.
ANCOVA: analysis of covariance; CI: confidence interval; ITT: intent-to-treat; mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation.
Figure 4.
Changes in number of migraine days over time in (a) the ITT population and (b) the mITT population.
aThe nVNS and sham groups were compared using ANCOVA models; p-values were derived from linear regression adjusted for treatment group, centre, presence/absence of aura (based on diagnosis provided in subject medical history at enrolment), and number of migraine days in the run-in period.
ANCOVA: analysis of covariance; CI: confidence interval; ITT: intent-to-treat; mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation.
Headache days
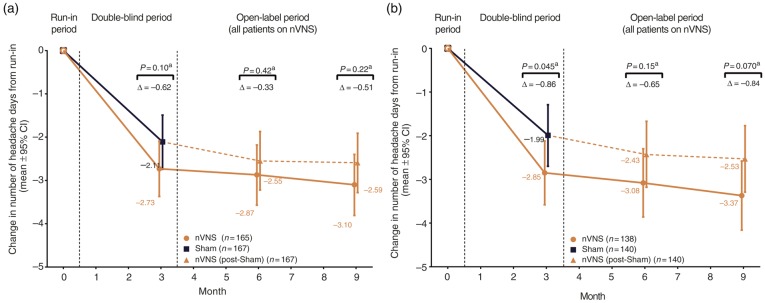
Mean reductions in the number of headache days were −2.73 days (95% CI: −3.37, −2.09; baseline: 8.9 headache days) for the nVNS group and −2.11 days (95% CI: −2.74, −1.49; baseline: 9.1 headache days) for the sham group (p = 0.10) (Figure 5). Headache ≥ 50% responder rates were 28.5% (95% CI: 20.3%, 38.6%) and 25.6% (95% CI: 18.0%, 35.0%) in the nVNS and sham groups, respectively (absolute difference, 2.9%; odds ratio [95% CI], 1.16 [0.69, 1.95]; p = 0.57).
Figure 5.
Changes in number of headache days over time for (a) the ITT population and (b) the mITT population.
aThe nVNS and sham groups were compared using ANCOVA models; p-values were derived from linear regression adjusted for treatment group, centre, presence/absence of aura (based on diagnosis provided in subject medical history at enrolment), and number of migraine days in the run-in period.
ANCOVA: analysis of covariance; CI: confidence interval; ITT: intent-to-treat; nVNS: non-invasive vagus nerve stimulation.
Acute medication days
Reductions in the number of acute medication days were −1.90 days (95% CI: −2.47, −1.32; baseline: 6.8 acute medication days) in the nVNS group and −1.35 days (95% CI: −1.91, −0.79; baseline: 7.0 acute medication days) in the sham group (p = 0.11). The ≥ 50% responder rate for acute medication days was 30.9% (95% CI: 22.2%, 41.3%) with nVNS use and 23.1% (95% CI 15.9%, 32.3%) with sham use (absolute difference, 7.8%; odds ratio [95% CI], 1.49 [0.87, 2.54]; p = 0.14).
Modified intent-to-treat population (mITT)
Upon observation of suboptimal rates of adherence to treatment (Supplemental Table 2), a post hoc analysis of patients who were ≥ 67% adherent was performed. Significant therapeutic gains were observed with nVNS compared with sham for the reduction in migraine days, headache days, and acute medication days (Figure 3(a), Table 2). Therapeutic gains for migraine days were more pronounced in the aura subgroup (nVNS, −2.96 days; sham, −1.38 days; p = 0.055) than in the no aura subgroup (nVNS, −2.29 days; sham, −1.47 days; p = 0.049). Additional data for the mITT population are available in Supplemental Table 3 and Figures 4(b) and 5(b).
Table 2.
Efficacy outcomes (mITT populationa).
| Outcome | nVNS (n = 138) | Sham (n = 140) |
|---|---|---|
| Reduction in migraine daysb | ||
| Mean (95% CI) | −2.27 (−2.89, −1.65) | −1.53 (−2.13, −0.93) |
| Difference (95% CI) | −0.74 (−1.45, −0.02) | |
| p-value | 0.043 | |
| Migraine ≥ 50% responder ratec | ||
| % (95% CI) | 33.6 (23.7, 45.1) | 23.4 (15.7, 33.5) |
| Odds ratio (95% CI) | 1.65 (0.95, 2.87) | |
| p-value | 0.074 | |
| Reduction in headache daysb | ||
| Mean (95% CI) | −2.85 (−3.58, −2.12) | −1.99 (−2.70, −1.29) |
| Difference (95% CI) | −0.86 (−1.70, −0.02) | |
| p-value | 0.045 | |
| Headache ≥ 50% responder ratec | ||
| % (95% CI) | 31.5 (21.9, 43.1) | 25.9 (17.5, 36.5) |
| Odds ratio (95% CI) | 1.32 (0.75, 2.32) | |
| p-value | 0.34 | |
| Reduction in acute medication daysb | ||
| Mean (95% CI) | −1.94 (−2.60, −1.28) | −1.14 (−1.77, −0.50) |
| Difference (95% CI) | −0.80 (−1.56, −0.04) | |
| p-value | 0.039 | |
| Acute medication ≥ 50% responder ratec,d | ||
| % (95% CI) | 34.4 (24.5, 45.9) | 25.1 (16.9, 35.5) |
| Odds ratio (95% CI) | 1.57 (0.88, 2.79) | |
| p-value | 0.13 | |
Post hoc analysis.
Results are from linear regression adjusted for treatment group, centre, presence/absence of aura (based on diagnosis provided in subject medical history at enrolment), and number of migraine days in the run-in period.
Results are from logistic regression adjusted for treatment group, centre, presence/absence of aura (based on diagnosis provided in subject medical history at enrolment), and number of migraine days in the run-in period.
nVNS, n = 134; sham, n = 134.
CI: confidence interval; mITT: modified intent-to-treat; nVNS: non-invasive vagus nerve stimulation.
Blinding
At 1 week into the double-blind period, patients’ guesses for the treatment they thought they had received were higher in the ‘don't know’ category (nVNS, 43.6%; sham, 51.5%) than in the ‘active stimulation’ (nVNS, 33.3%; sham, 29.3%) or ‘sham’ (nVNS, 9.7%; sham, 10.8%) category. Bang blinding index estimates for nVNS (0.27; 95% CI: 0.20, 0.35) and sham (−0.20; 95% CI: −0.28, −0.13) indicated that both groups more commonly thought they were receiving active rather than sham treatment. Blinding was successful at the end of the double-blind period, as demonstrated by well-balanced guesses among categories (active stimulation: nVNS, 33.9%; sham, 26.9%; sham: nVNS, 29.1%; sham, 27.5%; don't know: nVNS, 21.8%; sham, 24.6%) and blinding estimates of 0.06 (95% CI: −0.05, 0.16) for nVNS and 0.01 (95% CI: −0.09, 0.11) for sham, where zero was within the 95% CI.
Open-label period
Treatment responses to nVNS during the double-blind period were maintained with further nVNS treatment during the open-label period for migraine and headache days (Figures 3(b) and 4).
Device perceptions
The percentages of patients who were at least a little satisfied with their treatment at the end of the double-blind period were similar between the nVNS group (77.5%) and the sham group (73.5%). At the end of the open-label period, patient satisfaction remained consistent for those who continued receiving nVNS (78.2%) and increased for double-blind sham subjects after they switched to open-label nVNS (85.4%). Most patients in each treatment group (nVNS, 95%; sham, 93%) reported that their device was somewhat or very easy to use.
Tolerability and safety
Preventive nVNS therapy was well tolerated and safe (Table 3). Across all study periods, the most common ADEs were rash, pain, erythema, discomfort at the application site, and dizziness. No serious ADEs were reported during the study.
Table 3.
AE summary.
| AEs and ADEsa | Double-blind period |
Open-label period (n = 269) | |
|---|---|---|---|
| nVNS (n = 169) | Sham (n = 172) | ||
| Subjects with ≥1 AE | 74 (43.8) | 91 (52.9) | 118 (43.9) |
| Subjects with ≥1 SAE | 2 (1.2) | 1 (0.6) | 2 (0.7) |
| Subjects with ≥1 ADE | 31 (18.3) | 57 (33.1) | 29 (10.8) |
| Subjects with ≥1 AE leading to discontinuation | 2 (1.2) | 9 (5.2) | 10 (3.7) |
| All study periods |
|||
| Most common AEs and ADEsa |
nVNS (n = 169) |
Sham (n = 172) |
|
| AEs | |||
| Nasopharyngitis | 29 (17.2) | 17 (9.9) | |
| Influenza | 16 (9.5) | 12 (7.0) | |
| Application site pain | 6 (3.6) | 10 (5.8) | |
| Oropharyngeal pain | 9 (5.3) | 7 (4.1) | |
| Dizziness | 8 (4.7) | 4 (2.3) | |
| ADEs | |||
| Application site rash | 1 (0.6) | 12 (7.0) | |
| Application site pain | 5 (3.0) | 10 (5.8) | |
| Application site erythema | 3 (1.8) | 8 (4.7) | |
| Application site discomfort | 7 (4.1) | 5 (2.9) | |
| Dizziness | 5 (3.0) | 3 (1.7) | |
Data are n (%) of patients with the event and are from the safety population.
ADE: adverse device effect; AE: adverse event; nVNS: non-invasive vagus nerve stimulation; SAE: serious adverse event.
Discussion
In this large, randomised, double-blind, sham-controlled trial, nVNS was not superior to sham stimulation in the ITT population, with migraine reductions of −2.26 days with nVNS and −1.80 days with sham (p = 0.15). To better understand the role of adherence in the findings of this neutral study, we conducted a post hoc analysis of patients who were ≥ 67% adherent. In the mITT population, nVNS demonstrated statistically significant benefits versus sham in the prevention of migraine, headache, and acute medication days. Adverse events were mostly mild and transient.
Findings from previous clinical and mechanistic studies provide the rationale for evaluating nVNS for migraine prevention. Migraine/headache day reductions with nVNS were previously reported in a smaller randomised, double-blind, sham-controlled study of chronic migraine and an open-label study of menstrual and menstrually related migraine (7,14). The mechanism of action of nVNS in migraine is probably multifactorial. In the current study, therapeutic gains with nVNS were greater for patients with aura than for those without aura, which is consistent with the reported effects of nVNS on cortical spreading depression (CSD), a common mechanistic target of preventive migraine treatments and the underlying triggering event of migraine with aura (21,22). In adult rats, nVNS has been found to suppress CSD (22), which suggests that nVNS benefits in patients who have migraine with aura might be related to the suppression of CSD. In the PREMIUM study, the presence/absence of migraine with aura was based on the diagnosis provided in each subject's medical history at the time of enrolment. Considering that patients typically do not experience aura with every attack, the occurrence of aura might be underrepresented in subjects' existing diagnoses. The consideration and use of alternative definitions to categorize subjects as experiencing migraine with aura (e.g. the documentation of ≥1 attack with symptoms consistent with aura during a study) might allow for a more robust understanding of the effect of nVNS on subjects with aura as well as the effects of nVNS specifically on attacks with aura.
The effect of nVNS on CSD may be complemented by other acute mechanisms, including the inhibition of dural-intracranial evoked trigeminocervical neuronal responses (23,24). Taken together, the preventive and acute mechanistic effects may provide a greater synergistic effect. More recently, nVNS was shown to exert a significant bilateral inhibitory effect on parasympathetic functions within the trigeminal autonomic reflex (25). This effect has been shown to relieve symptoms of migraine and the trigeminal autonomic cephalalgias and could complement the previous mechanistic findings (25–27).
Study limitations
This study had three notable limitations: a) the vagal activity of the sham device, b) the use of bilateral stimulations, and c) suboptimal subject adherence to the TID treatment regimen. Subsequent to the PREMIUM trial, another study revealed parasympathetic activity caused by the sham device used in this study, indicating vagal activation and representing a key limitation of the current study (28). The vagal activity of the sham device likely decreased the therapeutic gain seen in this study. This is also consistent with the finding that the group that received sham treatment during the double-blind phase had little additional improvement during active nVNS therapy during the open-label phase (see Figure 4(a) and Figure 5).
Determining optimal dosing for neuromodulatory devices is challenging. For nVNS, several stimulation paradigms – including right-side, bilateral, and ipsilateral-to-pain stimulations and varying numbers of stimulations – have been evaluated in multiple studies of primary headache (7,13,29–31). The PREMIUM study called for bilateral stimulations (once per side). In subsequent research by de Morais et al. (32) presented at the 12th European Headache Federation Congress, the effect of two unilateral stimulations on CSD suppression was greater than the effect of several other stimulation paradigms, including the treatment paradigm used in this study. This suggests that the use of bilateral stimulations could have also mitigated the overall efficacy reported in this study. The inability of some patients to use nVNS consistently was likely due to its TID treatment protocol.
Adherence to the TID treatment regimen was directly examined in a post hoc analysis of the mITT population and appears to be a factor in the success of nVNS for migraine prevention. nVNS may therefore be particularly attractive for use by patients who are highly motivated to consistently treat on a TID basis because of overall headache burden and/or more convenient times of stimulation during the day (i.e. morning, noon, and before bed) (2,7). An additional randomised controlled trial of nVNS for migraine prevention that is currently under way in the United States (NCT03716505) addresses the limitations identified in this study through the use of an inactive sham device and unilateral stimulations (ipsilateral to the pain) in patients with a greater headache burden.
Application of findings
Results of the PREMIUM study demonstrate a favourable risk-benefit ratio for nVNS in the prevention of migraine, as the apparent benefits of the therapy outweigh the minimal risks. Efficacy findings from this study are comparable to those reported in other studies of non-invasive neuromodulation therapies (6,7,9). The International Headache Society–recommended primary outcome for the reduction in migraine days from run-in to 3 months was −2.26 days with nVNS and −2.06 days with supraorbital transcutaneous stimulation (6,33). The clinical benefit of nVNS in this study, which may have been understated because of the partially active effects of the sham device (28), complements the good tolerability and safety profile of nVNS.
The proven benefits of nVNS in the acute treatment of migraine attacks (13) could be complemented by preventive nVNS use. In a previous open-label pilot study of 20 patients with migraine who received preventive and acute nVNS therapy for 3 months, the reduction in headache days per month was −5.8 days (15), which compares favourably with the headache day reduction in this study (−2.7 days) in which patients received preventive nVNS only. These clinical data are consistent with the mechanistic evidence supporting the potential synergy of acute and preventive nVNS treatment in migraine (15,22–24,34). The ability of nVNS to provide acute and preventive benefits would represent a novel clinical option, as pharmacologic migraine therapies are indicated only as either preventive or acute treatment.
Conclusions
Preventive effects of nVNS in episodic migraine were not superior to sham stimulation (ITT). The “sham” device used in this study, however, produced a level of active vagus nerve stimulation (28) that likely affected the therapeutic gain observed in the study. A post hoc analysis demonstrated some significant effects of nVNS for patients with ≥67% adherence to treatment. nVNS may have clinical utility in migraine prevention, which will be defined further in a second study that addresses the key limitations of this study by using an inactive sham device, unilateral stimulation, and patients with a higher headache burden.
Supplemental Material
Supplemental Material for Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: The multicentre, double-blind, randomised, sham-controlled PREMIUM trial by Hans-Christoph Diener, Peter J Goadsby, Messoud Ashina, Mohammad Al-Mahdi Al-Karagholi, Alexandra Sinclair, Dimos Mitsikostas, Delphine Magis, Patricia Pozo-Rosich, Pablo Irimia Sieira, Miguel JA Làinez, Charly Gaul, Nicholas Silver, Jan Hoffmann, Juana Marin, Eric Liebler, Michel D Ferrari and on behalf of the PREMIUM Study Group in Cephalalgia
Acknowledgements
The authors acknowledge all patients, coinvestigators, research nurses, and sites for their participation in the study. Annelie Andersson and Emma de Launay of electroCore, Inc., were responsible for coordination and execution of the study. Statistical analyses for the study were conducted by Candace McClure, PhD, and Lisa Thackeray, MS, of North American Science Associates Inc. (Minneapolis, MN). Editorial support was provided by Stefanie Dorlas, BMath, BEd, of MedLogix Communications, LLC, in cooperation with the authors.
The PREMIUM Study Group
Investigators are listed by study site at the time of the trial. 1. Klinik für Neurologie, Universitätsklinikum Essen, Essen, Germany – Professor Hans-Christoph Diener (principal investigator), Dr. Kasja Solbach (co-investigator), and Michael Kleinschmidt (study coordinator); 2. Klinik für Neurologie, Ludwig-Maximilians-University Munich, Klinikum Grosshadern, Munich, Germany – Professor Andreas Straube (principal investigator), Dr. Katarina Kamm (co-investigator), and Dr. Ruth Ruscheweyh (co-investigator); 3. Migräne- und Kopfschmerzklinik Königstein, Königstein im Taunus, Germany – Privatdozent. Dr. Charly Gaul (principal investigator); 4. Neurologische Klinik und Poliklinik, Charité Campus Mitte, Berlin, Germany – Dr. Uwe Reuter (principal investigator) and Dr. Heike Israel-Willner (co-investigator); 5. Institut für Systemische Neurowissenschaften, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany – Professor Arne May (principal investigator) and Priv.-Doz. Dr. Jan Hoffmann (sub-investigator); 6. King’s College London, Neurology Department, Wellcome Foundation Building, Denmark Hill Campus, London, United Kingdom – Professor Peter J Goadsby (principal investigator); 7. Hull Royal Infirmary, Neurology Department, Hull, United Kingdom – Dr. Fayyaz Ahmed (principal investigator); 8. The Southern Hospital, Neurology Department, Glasgow, United Kingdom – Dr. Alok Tyagi (principal investigator); 9. The Walton Centre, Neurology Department, Liverpool, United Kingdom – Dr. Nicholas Silver (principal investigator); 10. School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, Institute of Biomedical Research (West), University of Birmingham, Birmingham, United Kingdom – Dr. Alexandra Sinclair (principal investigator) and Elizabeth Dwenger (study coordinator); 11. Danish Headache Centre, Glostrup, Denmark – Dr. Messoud Ashina (principal investigator) and Dr. Mohammad Al-Mahdi Al-Karagholi (sub-investigator); 12. Sandvika Nevrosenter AS, Sandvika, Norway – Dr. Anne Christine Buckley Poole (principal investigator); 13. DKD HELIOS Klinik Wiesbaden, Wiesbaden, Germany – Professor Till Sprenger (principal investigator); 14. Catholic University of Valencia, Department of Neurology, University Clinic Hospital, Valencia, Spain – Dr. Miguel JA Làinez (principal investigator); 15. Leiden University Medical Centre, Department of Neurology, Leiden, The Netherlands – Professor Michel D Ferrari (principal investigator) and Dr. Ilse de Coo (co-investigator); 16. University of Liège, CHR de la Citadelle, Neurology Department, Liège, Belgium – Dr. Delphine Magis (principal investigator) and Romain Nonis (study coordinator); 17. Neurology Clinic, Aeginition Hospital, Athens, Greece – Dr. Dimos Mitsikostas (principal investigator); 18. Servicio de Neurología, Pamplona, Spain – Dr. Pablo Irimia Sieira (principal investigator); 19. Basildon University Hospital, Nethermayne, Basildon, Essex, United Kingdom – Dr. Farooq Maniyar (principal investigator); 20. Zentrum für Neurologie und Epileptologie, Hertie-Institut für Klinische Hirnforschung, Universitätsklinikum Tübingen, Tübingen, Germany – Dr. Tobias Freilinger (principal investigator); 21. Servicio de Neurología, Hospital Ruber Internacional, Madrid, Spain – Dr. Margarita Sánchez del Rio (principal investigator); 22. Vall d’Hebron University Hospital, Headache Unit, Barcelona, Spain – Dr. Patricia Pozo-Rosich (principal investigator), Marta Torres-Ferrus and Juan B Gomez (subinvestigators), and Victor J Gallardo (study coordinator).
Clinical implications
nVNS was not superior to sham stimulation in patients with episodic migraine.
Therapeutic difference was greater in patients with aura than in those without aura.
Significant benefits were observed for migraine, headache, and acute medication reductions for those with ≥67% adherence with treatment (post hoc, mITT).
The sham treatment activated the vagus nerve, demonstrated by unexpected inhibitory effects on the trigeminal autonomic reflex (28). This likely decreased the therapeutic gain observed in this study.
nVNS was further established as a well tolerated and safe therapy for primary headache.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HCD has received honoraria for participation in clinical trials and for contributions to advisory boards and oral presentations sponsored by 3M Medica; Addex Pharma; Adler; Allergan; Almirall; Amgen; AstraZeneca; Autonomic Technologies; Bayer; Berlin-Chemie; Boehringer Ingelheim; Bristol-Myers Squibb; Chordate Medical; Coherex Medical; CoLucid Pharmaceuticals; electroCore, Inc.; Eli Lilly and Company; GlaxoSmithKline; Grünenthal; Janssen-Cilag; Johnson & Johnson; Labrys Biologics; La Roche; Medtronic; Menarini; Minster Pharmaceuticals; MSD; NeuroScore; Novartis; Pfizer; Pierre Fabre; Sanofi; Schaper and Brümmer; St. Jude Medical; Vital; and Weber & Weber. HCD has also received research funding from Allergan; Almirall; AstraZeneca; Bayer; electroCore, Inc.; GlaxoSmithKline; Janssen-Cilag; MSD; and Pfizer. He has received additional research support from the European Union; the German Ministry of Education and Research; and the German Research Council. HCD has no ownership interests and does not own any pharmaceutical company stocks.
PJG has received grants and personal fees from Allergan; Amgen; and Eli Lilly and Company. He has also received personal fees from Akita Biomedical; Alder Biopharmaceuticals; Autonomic Technologies; Avanir Pharmaceuticals; Cipla Ltd; CoLucid Pharmaceuticals, Inc.; Dr. Reddy’s Laboratories; electroCore, Inc.; eNeura; Journal Watch; Medico-Legal Journal; Novartis; Oxford University Press; Pfizer Inc; Promius Pharma; Quest Diagnostics; Scion; Teva Pharmaceuticals; Trigemina, Inc.; and Up-to-Date. In addition, PJG has a patent for magnetic stimulation for headache pending assigned to eNeura.
MA has received honoraria for contributions to advisory boards and oral presentations sponsored by Allergan; Alder; Amgen; Eli Lilly and Company; Novartis; and Teva Pharmaceuticals. MA has been the primary investigator for Alder, Amgen, electroCore, Novartis, and Teva trials. MA has no ownership interests and does not own any pharmaceutical company stocks.
MAA has received travel grants from electroCore, Inc.
AS is funded by an NIHR Clinician Scientist Fellowship (NIHR-CS-011-028) and by the Medical Research Council, UK (MR/K015184/1).
D. Mitsikostas has received advisory fees, honoraria, research grants, or travel grants from Allergan; Amgen; Biogen; Cefaly; electroCore, Inc.; Eli Lilly and Company; Merz Pharma; Novartis; Roche; Sanofi Genzyme; and Teva Pharmaceuticals.
D. Magis has received travel grants from electroCore, Inc.; is a consultant for Novartis Belgium and Eli Lilly Benelux; and is an associate editor for Cephalalgia.
PP-R has received honoraria as a consultant and speaker for Allergan; Almirall; Chiesi; Eli Lilly and Company; Novartis; and Teva Pharmaceuticals. PP-R does not own stocks from any pharmaceutical company.
PIS has received honoraria from Allergan; Novartis; and Teva Pharmaceuticals. PIS has no ownership interests and does not own any pharmaceutical company stocks.
MJAL has received advisory fees, speaker fees, research grants, and/or research support from ATI Pharma; Allergan; Amgen; Boehringer Ingelheim; electroCore, Inc.; Eli Lilly and Company; Lupin Pharmaceuticals; Medtronic; Novartis; Otsuka; Roche; and Teva Pharmaceuticals.
CG has received honoraria from Allergan; Bayer; Boehringer Ingelheim; Desitin Arzneimittel; electroCore, Inc.; Eli Lilly and Company; Grünenthal; Hormosan Pharma; Novartis; Ratiopharm; Reckitt Benckiser Group; and Teva Pharmaceuticals. CG has no ownership interests and does not own any pharmaceutical company stocks.
NS has received honoraria from Allergan; electroCore, Inc.; Eli Lilly and Company; Novartis; and Teva Pharmaceuticals; and investigator fees paid to the Walton Centre.
JH has consulted for and/or served on advisory boards for Allergan; Autonomic Technologies Inc. (ATI); Chordate Medical AB; Eli Lilly and Company; Hormosan Pharma; Novartis; and Teva Pharmaceuticals. He received honoraria for speaking from Allergan; Chordate Medical AB; Novartis; and Teva Pharmaceuticals. JH is an associate editor for Cephalalgia and the Journal of Oral & Facial Pain and Headache. JH received personal fees and/or honoraria from Oxford University Press, Quintessence Publishing, and Sage Publishing.
JM has received honoraria and travel grants from electroCore, Inc.
EL is an employee of electroCore, Inc., and receives stock ownership.
MDF has received consultancy fees from Medtronic and research support from the Netherlands Organisation for Scientific Research (NWO); the European Community; ZonMw; and the Dutch Heart Foundation. MDF is a member of the editorial board for Cephalalgia.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by electroCore, Inc. Employees of the sponsor were involved in study design, data collection, analysis, and interpretation. All authors, including an employee of the study sponsor, drafted and/or revised the manuscript and approved the final version for submission. Statistical analysis for the study conducted by North American Science Associates Inc. (Minneapolis, MN, USA) and editorial support from MedLogix Communications, LLC (Itasca, IL, USA) were funded by electroCore, Inc.
References
- 1.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: Results from the second international burden of migraine study (IBMS-II). Headache 2013; 53: 644–655. [DOI] [PubMed] [Google Scholar]
- 2.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia 2017; 37: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolley JM, Bonafede MM, Maiese BA, et al. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache 2017; 57: 1399–1408. [DOI] [PubMed] [Google Scholar]
- 4.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA 2018; 319: 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017; 377: 2123–2132. [DOI] [PubMed] [Google Scholar]
- 6.Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology 2013; 80: 697–704. [DOI] [PubMed] [Google Scholar]
- 7.Silberstein SD, Calhoun AH, Lipton RB, et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 2016; 87: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia 2018; 38: 1442–1454. [DOI] [PubMed] [Google Scholar]
- 9.Starling AJ, Tepper SJ, Marmura MJ, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia 2018; 38: 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurol 2018; 75: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients – a review of pros and cons. J Headache Pain 2017; 18: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsikostas DD, Belesioti I, Arvaniti C, et al. Patients' preferences for headache acute and preventive treatment. J Headache Pain 2017; 18: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassorelli C, Grazzi L, de Tommaso M, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study. Neurology 2018; 91: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grazzi L, Egeo G, Calhoun AH, et al. Non-invasive vagus nerve stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: An open-label study. J Headache Pain 2016; 17: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinfe TM, Pintea B, Muhammad S, et al. Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: A prospective observational cohort study. J Headache Pain 2015; 16: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puledda F, Shields K. Non-pharmacological approaches for migraine. Neurotherapeutics 2018; 15: 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao H, Wang T, Dong X, et al. Effectiveness of transcutaneous electrical nerve stimulation for the treatment of migraine: A meta-analysis of randomized controlled trials. J Headache Pain 2018; 19: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 19.Simon B, Blake J. Mechanism of action of non-invasive cervical vagus nerve stimulation for the treatment of primary headaches. Am J Manag Care 2017; 23: S312–S316. [PubMed] [Google Scholar]
- 20.Bang H, Flaherty SP, Kolahi J, et al. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin Res Regul Aff 2010; 27: 42–51. [Google Scholar]
- 21.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 2006; 59: 652–661. [DOI] [PubMed] [Google Scholar]
- 22.Chen SP, Ay I, de Morais AL, et al. Vagus nerve stimulation inhibits cortical spreading depression. Pain 2016; 157: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis 2017; 102: 96–104. [DOI] [PubMed] [Google Scholar]
- 24.Oshinsky ML, Murphy AL, Hekierski H, Jr, et al. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 2014; 155: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Möller M, Schroeder CF, May A. Vagus nerve stimulation modulates the cranial trigeminal autonomic reflex. Ann Neurol 2018; 84: 886–892. [DOI] [PubMed] [Google Scholar]
- 26.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 27.Avnon Y, Nitzan M, Sprecher E, et al. Autonomic asymmetry in migraine: Augmented parasympathetic activation in left unilateral migraineurs. Brain 2004; 127: 2099–2108. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder CF, Möller M, May A. nVNS sham significantly affects the trigeminal-autonomic reflex: A randomized controlled study. Neurology 2019; 93: e518–e521. [DOI] [PubMed] [Google Scholar]
- 29.Gaul C, Diener HC, Silver N, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): A randomised controlled study. Cephalalgia 2016; 36: 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goadsby PJ, de Coo IF, Silver N, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double-blind, sham-controlled ACT2 study. Cephalalgia 2018; 38: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: Findings from the randomized, double-blind, sham-controlled ACT1 study. Headache 2016; 56: 1317–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Morais AL, Takizawa T, Mulder I, et al. Optimization of vagus nerve stimulation efficacy on cortical spreading depression (abstract). J Headache Pain 2018; 19: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia 2012; 32: 6–38. [DOI] [PubMed] [Google Scholar]
- 34.Marin J, Giffin N, Consiglio E, McClure C, Liebler E and Davies B. Non-invasive vagus nerve stimulation for treatment of cluster headache: Early UK clinical experience. J Headache Pain 2018; 19: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: The multicentre, double-blind, randomised, sham-controlled PREMIUM trial by Hans-Christoph Diener, Peter J Goadsby, Messoud Ashina, Mohammad Al-Mahdi Al-Karagholi, Alexandra Sinclair, Dimos Mitsikostas, Delphine Magis, Patricia Pozo-Rosich, Pablo Irimia Sieira, Miguel JA Làinez, Charly Gaul, Nicholas Silver, Jan Hoffmann, Juana Marin, Eric Liebler, Michel D Ferrari and on behalf of the PREMIUM Study Group in Cephalalgia