Abstract
Background:
Isolated colonic Crohn’s disease (cCD) responds less well to induction therapy with exclusive enteral nutrition (EEN) compared with ileal or ileocolonic disease in adult patients; therefore, we aimed to identify the factors that influence the response to EEN and develop a predictive nomogram model to optimize the use of EEN in cCD patients.
Materials and methods:
Eighty-five cCD patients treated with EEN as first-line therapy at our center between 1 June 2012 and 30 June 2018 were retrospectively analyzed as the primary cohort. The primary endpoint was clinical remission after EEN therapy. Potential predictive factors for the efficacy of EEN were assessed by univariate and multivariate analyses, and a nomogram to predict the response to EEN therapy in cCD patients was designed. Another 19 cCD patients were retrospectively included in the validation cohort to verify the accuracy of the nomogram model.
Results:
The clinical remission rates for the primary cohort and validation cohort were 52.9% and 47.4%, respectively. Pancolitis was the greatest contributor to the risk of failure to respond to EEN [odds ratio (OR) = 4.896; 95% confidence interval (CI) = 1.223–19.607; p = 0.025], lean body mass index (LBMI), colonic lesion features, simple endoscopic scores for Crohn’s disease, C-reactive protein before treatment and ∆prealbumin were also related to the efficacy of EEN in cCD. The nomogram model showed robust discrimination, with an area under the receiving operating characteristic curve of 0.906.
Conclusion:
Several predictive factors for response to EEN therapy in cCD adult patients were identified, and a promising nomogram that can predict the effect of EEN in cCD was developed.
Keywords: exclusive enteral nutrition, isolated colonic Crohn’s disease, nomogram model
Introduction
Isolated colonic Crohn’s disease (cCD) has similar macroscopic features with those of ileal or ileocolonic disease, such as skip lesions, fissuring ulcers, or fibrostenosis, etc.1 but different genetics, epidemiology, intestinal microecology, and disease progress.2 The response to medicine also differs between cCD and ileal or ileocolonic Crohn’s disease (CD). Limited evidence has shown that 5-aminosalicyclic acid and corticosteroids are not as effective for inducing remission in cCD.3–5 Several cohort studies have shown that colonic disease location is a predictor of response to antitumor necrosis factor (anti-TNF) agents;6–8 however, approximately 5.9~54% cCD patients are primary nonresponders to anti-TNF,8,9 and the high cost also limits its use. Exclusive enteral nutrition (EEN) is another effective induction therapy in patients with active Crohn’s disease. Although whether EEN is effective in cCD is controversial; our previous research indicated that EEN still has a considerable success rate of more than 50% for inducing clinical remission among cCD patients,10 which was also supported by other studies.11–13 Therefore, the development of a new predictive model for screening cCD patients with poor EEN response characteristics will be helpful to optimize and personalize the use of EEN in clinical practice.
Nomograms have been applied in many fields of medicine. This type of model can combine multiple sequences and clinical factors to determine the probability of a clinical event with a simple pictorial representation.14 In this study, we reviewed our experience with EEN as the primary treatment for active cCD in adults and aimed to develop and validate a nomogram model to optimize the use of EEN in clinical practice.
Materials and methods
Patients and data collection
This retrospective study was conducted with the approval of the Ethics Committee of Jinling Hospital (Ethics approval number: 2018NZKY-013-03). We obtained informed, written consent from all patients prior to commencement of the study. All clinical data were collected from two independent prospectively maintained inflammatory bowel disease (IBD) databases at Jinling Hospital. One belonged to the IBD center and the other was owned by the gastroenterology department. A total of 91 consecutive cCD patients treated for disease flare-ups at our IBD center from 1 June 2012 to 30 June 2018 were reviewed, and they were identified as the primary cohort for building a nomogram model. From 1 January 2015 to 30 June 2018, another 19 consecutive cCD patients treated with EEN as induction therapy at the Department of Gastroenterology at our hospital were identified as the validation cohort. In order to maximize the predictive efficiency of the nomogram model, the patients in the primary cohort and validation cohort have no overlap or intersection.
The inclusion criteria were as follows: (a) age >18 years; (b) must be in the active disease phase [C-reactive protein (CRP) >10 mg/l and Harvey Bradshaw Index (HBI) > 4]; (c) EEN as the primary induction therapy. The exclusion criteria were as follows: (a) presence of bowel stoma; (b) treatment with EEN as maintenance therapy; (c) EEN treatment interference due to intolerance or noncompliance. All patients underwent colonoscopy before EEN treatment, and abdominal computed tomography (CT) scan or small intestine CT imaging were conducted within 2 weeks before or after admission, and gastroduodenoscopy was performed if upper gastrointestinal disease was suspected. Simple endoscopic scores for Crohn’s disease (SES-CD) were evaluated at the same time. All the patients had been assessed by the nutrition support team to determine their nutritional status, and their body composition was measured using a multifrequency bioelectrical impedance analyzer (Biospace InBody Co., Korea) within 1 week after admission to calculate the lean body mass index (LBMI).
Inflammatory markers [erythrocyte sedimentation rate (ESR) and CRP] and nutritional indicators [serum albumin (ALb) and prealbumin (pre-ALb)] were recorded at the start and once weekly during the exclusive enteral feeding treatment. Basic demographic data [age, sex, course of disease, family history of IBD in first-degree relatives, body mass index (BMI), Montreal classification, maintenance medication, history of surgery, etc.] were recorded at the start of treatment. Blood tests and HBI scores were performed once a week to determine whether the patients achieved clinical remission.
Exclusive enteral nutrition
Exclusive enteral nutrition (EEN) was provided for more than 2 weeks as the exclusive source of energy and nutrition, and all other medical treatments were forbidden. For gustatory improvement, only chewing gum and water were allowed in addition to EEN. In order to evaluate the efficacy of EEN treatment, CRP and HBI scores were recorded weekly. EEN was performed with enteral nutritional suspension (TPF) or with Peptisorb Liquid (SP; both Nutricia, Wuxi, China), in case of intolerance, such as diarrhea, bloating, and belching. The daily caloric intake was calculated depending on individual caloric requirements (approximately 25–30 kcal/kg/d) and was gradually increased from half that amount to the full amount over 2 days. Depending on tolerance, patient choice or doctors’ consideration, the formula was administered via a nasogastric tube (continuous or bolus) or orally in accordance with the patient’s daily routine. Intake of enteral nutrition was recorded by professional nurses in our center daily.
Body composition measurement
The body composition of the patients was estimated using a multifrequency bioelectrical impedance analyzer (Biospace InBody Co., Seoul, Korea) at admission. The patients were not allowed to eat or drink for 6 h before the test and were asked to empty their bladder before testing, if possible. The patients stood barefoot on the detector with both the heel and forefoot resting on the foot electrodes and the hands holding the hand electrodes, with the upper limbs away from the torso. After the test, the fat and skeletal muscle mass, total lean mass, fat ratio, BMI and basal metabolic rate were accurately analyzed. We then calculated the LBMI for every patient as the skeletal muscle mass (kg) divided by height in meters squared (m2).
Definitions
All patients with isolated cCD were diagnosed by endoscopic, histological, or radiological findings according to the European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016.15 Isolated colonic involvement refers to any colonic lesion between the caecum and rectum with no small bowel or upper gastrointestinal involvement. Clinical remission of CD was assessed using the definition of a CRP level < 10 mg/l and an HBI score ⩽ 4. The failure of EEN therapy was determined when one of the following conditions was met: (a) HBI > 4 with or without CRP > 10 mg/l after EEN treatment; (b) the need for further treatment, such as surgery, corticosteroids or anti-TNFα or other medicine, due to no response to EEN treatment. Previous studies have demonstrated that fat mass and lean mass are strongly associated with prognosis in patients with CD.
Statistical analysis
Statistical analyses to identify risk factors were performed using SPSS version 23.0 (SPSS, Inc., an IBM Company, Chicago, IL, US). Potential influential variables were grouped based on clinical findings. Quantitative and qualitative variables were expressed as the mean (SD), frequency and percentage. Frequencies were compared between groups using chi-squared tests or Fisher’s exact test, as appropriate. Continuous variables were compared using the t test. To identify predictors of response to EEN treatment, we used logistic regression analysis for univariate and multivariate analyses.
A nomogram model was formulated based on the results of the multivariate analysis and by using the rms package16 in R version 3.5.1 (http://www.r-project.org/). The discriminative ability of the model was determined by the area under the receiver operating characteristic curve, which ranged from 50% to 100%. Generally, discrimination values below 60% are unacceptable, values of 60–70% may be acceptable, and values from 70% to >90% range from good to excellent. The prediction model was calibrated by a visual calibration plot that compared the predicted and actual probability of clinical remission with EEN therapy.17 In addition, the nomogram was subjected to 1000 bootstrap resamples for internal validation to assess predictive accuracy,18 and external verification was constructed using the validation cohort. The model development and validation code are presented in Appendix 1.
For all the analyses, p < 0.05 was considered statistically significant, and all tests were two tailed unless otherwise indicated.
Results
Patient characteristics
Between 1 June 2012, and 30 June 2018, 91 cCD patients were identified with EEN treatment as inducing therapy in our center. Six cCD patients were excluded from primary cohort due to EEN intolerance. The primary cohort finally comprised 85 enrolled patients in this study. For the validation cohort, we studied 19 consecutive cCD patients from the Department of Gastroenterology. There was no significant difference in terms of the main variables, such as sex (p = 0.35), age (p = 0.86), BMI (p = 0.36), CRP (p = 0.24) or SES-CD (p = 0.07), between the primary and validation cohorts. Among the patients in the primary cohort, aphtha ulcers accounted for approximately 50% of all colonic lesions, and the validation cohort showed a similar composition. The baseline characteristics of the participants are summarized in Table 1.
Table 1.
Demographics and disease characteristics of cCD patients with EEN therapy.
| Demographic or characteristic | Primary cohort (n = 85) |
Validation cohort (n = 19) |
p value | ||
|---|---|---|---|---|---|
| Patients, n | % | Patients, n | % | ||
| Sex | 0.354 | ||||
| Male | 48 | 56.5 | 9 | 47.4 | |
| Female | 37 | 43.5 | 10 | 52.6 | |
| Age, years | 0.867 | ||||
| Mean ± SD | 33.00 ± 13.20 | 32.5 ± 14.34 | |||
| BMI | 0.365 | ||||
| Mean ± SD | 17.99 ± 2.87 | 18.7 ± 2.55 | |||
| Surgery history | 0.682 | ||||
| Yes | 34 | 40 | 8 | 42.1 | |
| No | 51 | 60 | 11 | 57.9 | |
| Smoke | 0.526 | ||||
| Yes | 8 | 9.4 | 1 | 5.3 | |
| No | 77 | 80.6 | 18 | 94.7 | |
| Duration (months) | 0.154 | ||||
| Mean ± SD | 50.8 ± 44.5 | 35.6 ± 33.4 | |||
| B | 0.522 | ||||
| 1 | 22 | 25.9 | 3 | 15.8 | |
| 2 | 30 | 35.3 | 6 | 31.6 | |
| 3 | 33 | 38.8 | 10 | 52.6 | |
| P | 0.980 | ||||
| Yes | 30 | 35.3 | 7 | 36.8 | |
| No | 55 | 64.7 | 12 | 63.2 | |
| Maintenance therapy | 0.534 | ||||
| Mesalazine | 12 | 14.1 | 5 | 26.3 | |
| Azathioprine | 15 | 17.6 | 4 | 21.1 | |
| Thalidomide | 4 | 4.7 | 0 | 0 | |
| Sulfasalazine | 5 | 5.9 | 0 | 0 | |
| Tripterygium | 3 | 3.5 | 5 | 26.3 | |
| None | 46 | 54.1 | 5 | 26.3 | |
| Pancolitis | 0.902 | ||||
| Yes | 19 | 22.4 | 6 | 31.6 | |
| No | 66 | 77.6 | 13 | 68.4 | |
| Feature of colonic lesion | 0.852 | ||||
| Aphtha ulcers | 37 | 43.5 | 9 | 47.3 | |
| Fissuring ulcers | 27 | 27.1 | 2 | 10.5 | |
| Stricturing | 10 | 11.8 | 5 | 26.3 | |
| Stricturing and ulcers | 13 | 15.3 | 3 | 15.6 | |
| HBI | 0.120 | ||||
| Mean ± SD | 7.51 ± 2.17 | 6.75 ± 1.62 | |||
| SES-CD | 0.052 | ||||
| Mean ± SD | 6.52 ± 4.30 | 7.85 ± 5.29 | |||
| CRP (mg/l) | 0.244 | ||||
| Mean ± SD | 50.47 ± 47.0 | 56.3 ± 49.83 | |||
| ESR (mm/h) | 0.322 | ||||
| Mean ± SD | 38.9 ± 26.12 | 43.2 ± 31.48 | |||
| Alb (g/l) | 0.298 | ||||
| Mean ± SD | 36.2 ± 6.3 | 34.6 ± 6.02 | |||
| Pre-Alb (g/l) | 0.839 | ||||
| Mean ± SD | 132.5 ± 70.66 | 128.95 ± 66.72 | |||
| LBMI (kg/m2) | 0.211 | ||||
| Mean ± SD | 8.06 ± 1.38 | 8.56 ± 1.04 | |||
Alb, albumin; BMI, body mass index; B1, nonstricturing, nonpenetrating; B2, stricturing; B3, penetrating; cCD, isolated colonic Crohn’s disease; CRP, C-reactive protein; EEN, exclusive enteral nutrition; ESR, erythrocyte sedimentation rate; HBI, Harvey-Bradshaw Index; LBMI, lean body mass index; P, perianal lesions; Pre-Alb, prealbumin; SD, standard deviation; SES-CD, simple endoscopic score for Crohn’s disease.
Clinical remission after EEN
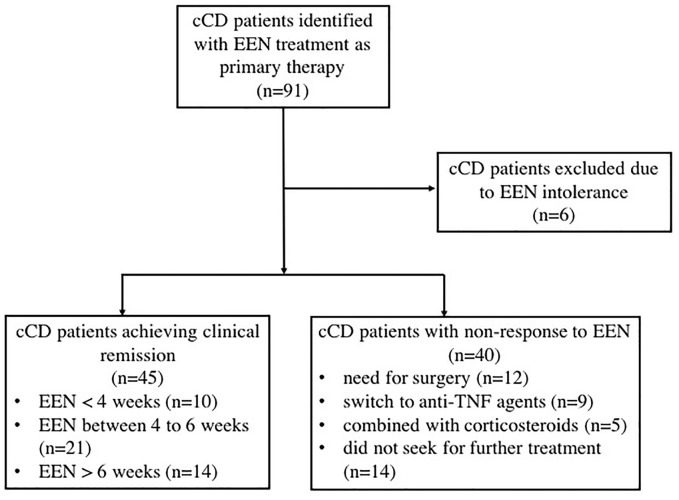
In the primary cohort, 45 patients (52.9%) achieved clinical remission after induction therapy with EEN. The clinical remission rate in the validation cohort was 47.4% (9/19). The mean HBI score and CRP level were 7.51 ± 2.17 and 50.47 ± 47, respectively, before treatment in the primary cohort and 2.43 ± 0.69 and 5.23 ± 1.82, respectively, for those who achieved clinical remission at the end of EEN therapy. Among those who did not respond well to EEN treatment in the primary cohort, 12 patients underwent surgery due to obstruction or fistula; 9 patients switched to anti-TNF-α (6 of them achieved clinical remission); 5 patients used corticosteroids; and the rest of the patients did not seek further treatment for a specific reason (Figure 1).
Figure 1.
Flow chart of cCD patient inclusion in the primary cohort.
Univariate and multivariate analyses of the predictive factors associated with clinical response to EEN in cCD patients
To investigate the predictive factors for the response to EEN therapy in cCD patients, we conducted univariate and multivariate analyses in the primary cohort. From the results presented in Table 2, we found that pancolitis was strongly associated with a poor response to EEN therapy [odds ratio (OR) = 4.896; 95% confidence interval (CI) = 1.223–19.607; p = 0.025]. The characteristics of the colonic lesions were also related to the efficacy of EEN therapy (OR = 2.32; 95% CI = 1.143–4.708; p = 0.020). Using aphtha ulcers as a reference, patients with strictures, or strictures combined with ulcers were much more likely to experience the failure of EEN therapy (p < 0.05, respectively); in contrast, the results for fissuring ulcers did not significantly differ from the results for aphtha ulcers (p = 0.526). In addition, the higher the CRP concentration before treatment and SES-CD scores were, the more likely patients would experience failure of EEN therapy (SES-CD: OR = 1.892; 95% CI = 1.086–4.117; p = 0.014; CRP: OR = 1.014; 95% CI = 1.011–1.030; p = 0.041). Furthermore, we found that the change in pre-Alb (∆pre-Alb) at 1 week after EEN treatment was a predictive factor for the response to EEN (OR = 0.983; 95% CI = 0.972–0.995; p = 0.005). In other words, if the patient has a quick increase in serum pre-Alb levels within a week of starting EEN, a good response to EEN therapy is highly likely. In addition, LBMI was an independent predictive factor for the efficacy of EEN (OR = 0.377; 95% CI = 0.206–0.689; p = 0.002).
Table 2.
Univariate and multivariate analyses of predictive factors associated with clinical response to EEN in the cCD.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Remission (n = 45) | Nonremission (n = 40) | p | Odds ratio (95% CI) | p | |
| Male | 27 | 21 | 0.487 | — | |
| Age | 34.49 ± 12.65 | 31.45 ± 13.82 | 0.291 | — | |
| Duration (months) | 51.02 ± 45.12 | 50.73 ± 44.43 | 0.975 | — | |
| Smoke | 6 | 2 | 0.207 | — | |
| Surgery | 21 | 13 | 0.201 | — | |
| Maintenance therapy | — | ||||
| None | 24 | 22 | 1 | ||
| Mesalazine | 7 | 5 | 0.704 | ||
| Azathioprine | 7 | 8 | 0.711 | ||
| Thalidomide | 2 | 2 | 0.933 | ||
| Sulfasalazine | 4 | 1 | 0.261 | ||
| Tripterygium | 1 | 2 | 0.536 | ||
| BMI | 18.6 ± 2.72 | 17.33 ± 2.9 | 0.032 | 0.905 (0.674–1.214) | 0.506 |
| B | — | ||||
| 1 | 10 | 12 | 1 | ||
| 2 | 17 | 13 | 0.425 | ||
| 3 | 18 | 15 | 0.509 | ||
| P | 16 | 14 | 0.957 | — | |
| Pancolitis | 6 | 13 | <0.001 | 4.896 (1.223–19.607) | 0.025 |
| Feature of lesion | 2.320 (1.143–4.708) | 0.020 | |||
| Aphtha ulcers | 29 | 8 | 1 | ||
| Fissuring ulcers | 7 | 13 | 0.526 | ||
| Stricturing | 7 | 20 | <0.001 | ||
| Stricturing and ulcers | 2 | 9 | 0.001 | ||
| SES-CD | 4.56 ± 3.25 | 8.80 ± 4.28 | <0.001 | 1.892 (1.086–4.117) | 0.014 |
| CRP (mg/l) | 43.0 ± 37.75 | 58.85 ± 54.9 | 0.130 | 1.014 (1.011–1.030) | 0.041 |
| ESR (mm/h) | 41.18 ± 25.80 | 36.38 ± 26.59 | 0.403 | — | |
| Alb (g/l) | 36.89 ± 5.91 | 35.45 ± 6.74 | 0.311 | — | |
| Pre-Alb (g/l) | 136.04 ± 70.86 | 128.4 ± 71.15 | 0.620 | — | |
| ∆Pre-Alb (g/l) | 37.58 ± 73.56 | −4.40 ± 59.68 | 0.008 | 0.983 (0.972–0.995) | 0.005 |
| LBMI (kg/m2) | 8.38 ± 1.22 | 7.7 ± 1.47 | 0.014 | 0.377 (0.206–0.689) | 0.002 |
Data shown as mean ± SD and n (%).
Alb, albumin; BMI, body mass index; B1, non-stricturing non-penetrating; B2, structuring; B3, penetrating; cCD, isolated colonic Crohn’s disease; CI, confidence interval; CRP, C-reactive protein; EEN, exclusive enteral nutrition; ESR, erythrocyte sedimentation rate; HBI, Harvey-Bradshaw Index; LBMI, lean body mass index; P, perianal lesions; Pre-Alb, prealbumin; SES-CD, simple endoscopic score for Crohn’s disease; SD, standard deviation.
The development of a nomogram model from selected factors
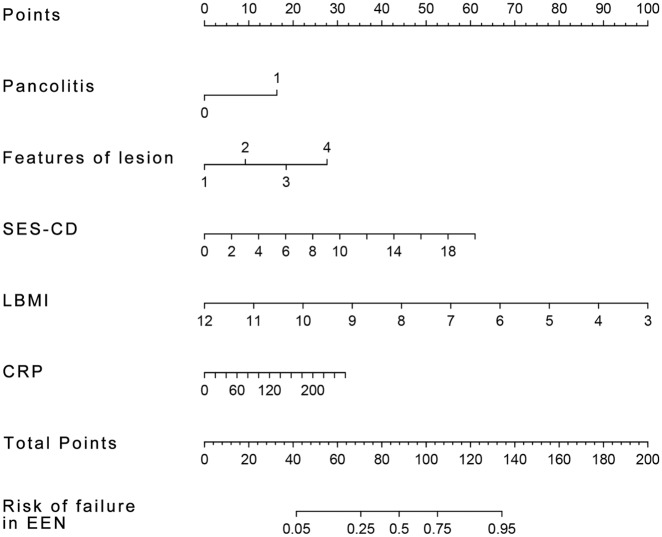
The nomogram was developed by translating multivariate analysis parameters into a visual scoring system, from which the estimated probability of EEN therapy failure could be calculated.13 After the multivariable analyses, the variables pancolitis, colonic lesion features, CRP concentration, SES-CD, and LBMI were selected for the final regression analysis for constructing the nomogram model (Figure 2). In consideration of the rationality and convenience of the nomogram model for predicting the response to EEN, we excluded the ∆pre-Alb from the construction of the model. To optimize the clinical prediction model, we translated the categorical variable (colonic lesion features) into grade variables. Specifically, we replaced the colonic lesion features with numbers (1, 2, 3, and 4 represented aphtha ulcers, fissuring ulcers, strictures, and strictures plus ulcers, respectively). A total score was calculated based on pancolitis, colonic lesion features, CRP, SES-CD, and LBMI. Each of these variables was given a score on the point scale axis. A total score could be easily calculated by adding each single score, and by projecting the total score onto the total point scale, we were able to estimate the probability that EEN therapy would fail to induce clinical remission.
Figure 2.
A nomogram predicting the risk of response to EEN therapy in cCD patients.
Each factor (pancolitis, features of lesion, SES-CD, LBMI, CRP) must be vertically referred to the point line and the sum of points for each variable is then calculated for the single patient. By projecting the total score to the lower total point scale, we were able to estimate the probability of response to EEN.
cCD, isolated colonic Crohn’s disease; CRP, C-reactive protein; EEN, exclusive enteral nutrition; LBMI, lean body mass index; SES-CD, simple endoscopic scores for Crohn’s disease.
Performance of the nomogram
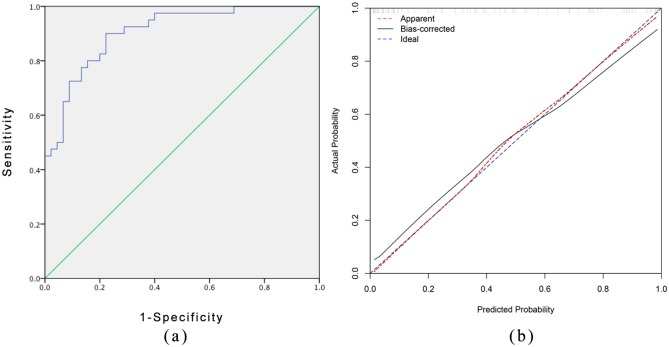
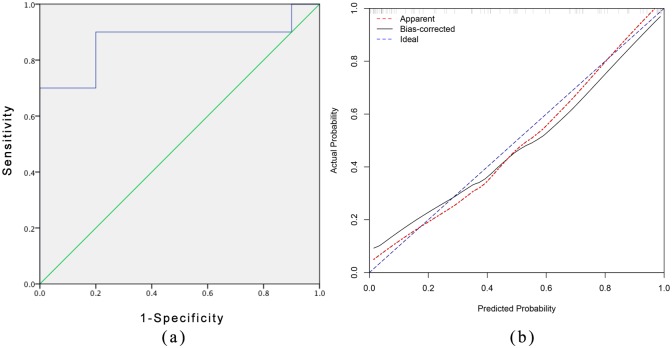
The nomogram model showed robust discrimination based on the receiver operating characteristic analysis in Figure 3(a), with a C-index of 0.906 (95% CI 0.844–0.967). We then conducted 1000 bootstrap resamples for internal calibration validation using the package of rms in R. The result of the calibration curve of the nomogram is presented in Figure 3(b), which shows that the ideal probabilities of EEN therapy predicted by the nomogram agreed well with the actual probabilities. We then assessed the accuracy of the nomogram model using the validation cohort as an external verification. The C-index of the nomogram for predicting the effect of EEN therapy was 0.867 (95% CI 0.691–1.0), and the calibration curve of the nomogram also indicated good agreement between the predicted probability and the actual occurrence in the validation cohort [Figure 4(a) and (b)]. For clinical use of the model, we generalized the sensitivity, specificity, positive predictive value, and negative predictive value for estimating the risk cutoff points in Table 3.
Figure 3.
Measures of accuracy of the nomogram for the prediction in primary cohort.
(a) Discrimination based on ROC with C-index = 0.906 (95% confidence interval 0.844–0.967) in primary cohort. (b) The calibration curves for the nomogram. The x axis represents the nomogram-predicted probability and y axis represents the actual probability of response of EEN therapy. Perfect prediction would correspond to the 45 blue dashed line. The red dotted line represents the primary cohort (n = 85), and the black solid line is bias corrected by bootstrapping (B = 1000 repetitions), indicating observed nomogram performance.
EEN, exclusive enteral nutrition; ROC, receiver operating characteristic curve.
Figure 4.
Measures of accuracy of the nomogram for the prediction in validation cohort.
(a) External validation for nomogram based on ROC with C-index = 0.867 (95% confidence interval 0.691–1) by validation cohort. (b) The calibration curves for the nomogram with validation cohort. The x axis represents the nomogram-predicted probability and y axis represents the actual probability of response of EEN therapy. Perfect prediction would correspond to the 45 blue dashed line. The red dotted line represents the validation cohort (n = 19), and the black solid line is bias corrected.
EEN, exclusive enteral nutrition; ROC, receiver operating characteristic curve.
Table 3.
Classification accuracy for prediction of response to EEN therapy in cCD patients at different risk cutoff points for the model.
| Risk score threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| >5% | 97.5 | 33.3 | 69.1 | 98.2 |
| >25% | 92.5 | 64.4 | 53.2 | 95.4 |
| >50% | 80.2 | 84.4 | 87.5 | 75.3 |
| >75% | 65.3 | 93.3 | 98.0 | 49.4 |
| >90% | 45.6 | 97.8 | 98.9 | 5.2 |
cCD, isolated colonic Crohn’s disease; EEN, exclusive enteral nutrition; NPV, negative predictive value; PPV, positive predictive value.
Discussion
EEN has been widely accepted as a first-line therapy to treat active CD in children.19–21 However, other than in the Japanese population, this intervention is not routinely utilized in adults due to heterogeneous response rate. Given that cCD patients have intact small bowel and relatively uninjured absorption function, EEN may be effective as an inducing therapy.
Our study showed that 52.9% of patients with cCD achieved clinical remission after EEN therapy, which is consistent with the results we reported previously.10 Given that more than half of the patients with isolated CD patients benefited from EEN therapy, we identified six factors that were predictive for the response to EEN, namely, pancolitis, colonic lesion features, SES-CD, CRP before treatment, LBMI and ∆pre-Alb, and we developed a simple, intuitive graph of a statistically predictive nomogram model that quantified the risk of nonresponse to EEN based on the five factors.
In our nomogram model, pancolitis is the greatest contributor to the risk of nonresponse to EEN, followed by LBMI and colonic lesion features. CRP before treatment showed the smallest effect on the probability of response to EEN. According to our findings, the risk of EEN therapy failure is approximately five times higher in isolated CD patients with pancolitis than in those without pancolitis. Pancolitis, characterized by high levels of inflammatory cytokines, will destroy the immunonutrition and anti-inflammatory effects of enteral nutrition.
We also observed that features of colonic lesions are related to the efficacy of EEN therapy. In particular, strictures or strictures combined with ulcers have a greater negative impact on the effectiveness of EEN therapy than fissuring ulcers. A likely explanation is that fibrostenosis and stricture are well-recognized endpoints in Crohn’s disease and chronic inflammation sequelae.22 Strictures, the result of the wound-healing process, are accumulations of collagenous scarring that thicken the muscle layers and contract the lumina. These changes play a role in producing critical architectural changes in the intestinal wall that impede the normal movement of intestinal contents.23 EEN may be less effective in such luminal situations. Intestinal inflammation is not noticeable in some cases, but it does consistently coexist with strictures.24 Therefore, some newly diagnosed CD patients who are undergoing EEN for the first time still cannot achieve a satisfactory response to EEN therapy.
SES-CD can quantify colonic lesions, including ulcers and stricture.25 The more ulcers and strictures in the colon, the higher the scores will be, so it is understandable that SES-CD scores are a risk factor for response to EEN.
Our analyses indicated that LBMI was a protective factor for the efficacy of EEN. Some research has shown a significantly lower lean body mass in patients with high disease activity than in those with mild-to-moderate disease activity,26 and preoperative lean body mass is a protective factor for postoperative overall and major complications in CD patients with severe malnutrition.27,28 Lean mass was determined by the balance between muscle protein synthesis and breakdown, but loss of lean mass driven by inflammation and a suboptimal muscle protein synthetic response to enteral nutrition, termed anabolic resistance, may destroy this mechanism and finally lead to reduced lean mass.29 Recently, Davies and colleagues30 confirmed the theory in adolescent CD patients that inability to respond to nutrition could be implicated in low muscle mass in CD. Those CD patients with anabolic resistance cannot maintain a positive protein balance, even in remission. We believe that LBMI, as a reflection of both nutritional status and the inflammatory condition, plays a role in predicting the response to EEN treatment.
To our knowledge, this study is the first to construct a quantitative nomogram to predict the probability of failure to respond to EEN therapy in patients with cCD. However, this study has several limitations. First, the analysis of patients from a single center and the retrospective design may have caused some bias. Second, the accuracy of the nomogram model was based on large-sample learning. Thus, the relatively small number of patients enrolled in this study may have affected the accuracy of our model. Third, although internal and external validation of the model yielded optimal discrimination and calibration, the generalizability of this nomogram requires additional validation using other cohorts.
In conclusion, we identified several factors that predict the efficacy of EEN induction therapy and developed a novel nomogram for predicting the effect of EEN in adult patients with cCD. Our nomogram is easy to operate and has good accuracy and calibration. This nomogram might help clinicians make individualized predictions of each patient’s likelihood of response to EEN and improve individualized recommendations for clinical treatment.
Appendix 1
Library(rms)
#build a data set
#Set the parameters
ddist <- datadist(pancolitis, LBMI,. . .)
options(datadist=‘ddist’)
#For Logistic Regression Model
f <- lrm(Remission ~ pancolitis+LBMI. . ., x=T, y=T)
#For Nomogram
nom <- nomogram(f, fun=plogis, fun.at=c(.05,.25,.75,.99), lp=F, funlabel= “Risk of failure in response to EEN”)
plot (nom)
#For Resampling Validation of Nomogram#
validate (f, method=“boot”, B=1000, dxy=T)
#For Calibration Curve
cal<-calibrate (f, method=“boot”, B=1000)
plot (cal)
Footnotes
Author contributions: Yihan Xu and Zhen Guo contributed equally to this work. Weiming Zhu supervised the whole project. Liangyu Huang collected data, and Jianfeng Gong and Lili Gu analyzed the dataset. Yihan Xu and Zhen Guo conducted data analyses and drafted the manuscript. All authors critically reviewed the article and approved the final manuscript.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Nanjing Science and Technology Project (201715008).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Zhen Guo  https://orcid.org/0000-0002-1541-421X
https://orcid.org/0000-0002-1541-421X
Weiming Zhu  https://orcid.org/0000-0002-9138-4614
https://orcid.org/0000-0002-9138-4614
Contributor Information
Yihan Xu, Nanjing Medical University, Nanjing, China Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Zhen Guo, Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Liangyu Huang, Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Jianfeng Gong, Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Yi Li, Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Lili Gu, Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Weisong Shen, Research Institute of General Surgery, Jinling Hospital, Nanjing, China.
Weiming Zhu, Research Institute of General Surgery, Nanjing Jinling Hospital, 305 Zhongshan East Road, Nanjing 210002, China; Nanjing Medical University, 305 Zhongshan East Road, Nanjing, Jiangsu 210029, China.
References
- 1. Warren BF. Classic pathology of ulcerative and Crohn’s colitis. J Clin Gastroenterol 2004; 38: S33–S35. [DOI] [PubMed] [Google Scholar]
- 2. Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn’s disease: the third IBD? Gut 2017; 66: 362–381. [DOI] [PubMed] [Google Scholar]
- 3. Ford AC, Kane SV, Khan KJ, et al. Efficacy of 5-aminosalicylates in Crohn’s disease: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 617–629. [DOI] [PubMed] [Google Scholar]
- 4. Moja L, Danese S, Fiorino G, et al. Systematic review with network meta-analysis: comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn’s disease. Aliment Pharmacol Ther 2015; 41: 1055–1065. [DOI] [PubMed] [Google Scholar]
- 5. Malchow H, Ewe K, Brandes JW, et al. European cooperative Crohn’s disease study (ECCDS): results of drug treatment. Gastroenterology 1984; 86: 249–266. [PubMed] [Google Scholar]
- 6. Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn’s disease. Aliment Pharmacol Ther 2003; 17: 1451–1457. [DOI] [PubMed] [Google Scholar]
- 7. Laharie D, Salzmann M, Boubekeur H, et al. Predictors of response to infliximab in luminal Crohn’s disease. Gastroenterol Clin Biol 2005; 29: 145–149. [DOI] [PubMed] [Google Scholar]
- 8. Dupont-Lucas C, Sternszus R, Ezri J, et al. Identifying patients at high risk of loss of response to infliximab maintenance therapy in paediatric Crohn’s disease. J Crohns Colitis 2016; 10: 795–804. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Guo Z, Cao L, et al. Isolated colonic Crohn’s disease is associated with a reduced response to exclusive enteral nutrition compared to ileal or ileocolonic disease. Clin Nutr 2019; 38: 1629–1635. [DOI] [PubMed] [Google Scholar]
- 11. Afzal NA, Davies S, Paintin M, et al. Colonic Crohn’s disease in children does not respond well to treatment with enteral nutrition if the ileum is not involved. Dig Dis Sci 2005; 50: 1471–1475. [DOI] [PubMed] [Google Scholar]
- 12. De Bie C, Kindermann A, Escher J. Use of exclusive enteral nutrition in paediatric Crohn’s disease in the Netherlands. J Crohn’s Colitis 2013; 7: 263–270. [DOI] [PubMed] [Google Scholar]
- 13. Rubio A, Pigneur B, Garnier-Lengliné H, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther 2011; 33: 1332–1339. [DOI] [PubMed] [Google Scholar]
- 14. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015; 16: e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohn’s Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 16. Frank E, Harrell Jr. rms: regression modeling strategies. Package rms version 4.3–0. http://www.r-project.org (accessed 20 February 2016).
- 17. Miller ME, Langefeld CD, Tierney WM, et al. Validation of probabilistic predictions. Med Decis Making 1993; 13: 49–58. [DOI] [PubMed] [Google Scholar]
- 18. Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 19. Connors J, Basseri S, Grant A, et al. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-term avoidance of corticosteroids: results of a propensity-score matched cohort analysis. J Crohns Colitis 2017; 11: 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swaminath A, Feathers A, Ananthakrishnan AN, et al. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment Pharmacol Ther 2017; 46: 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Day AS. Exclusive enteral nutrition in children with Crohn’s disease. World J Gastroenterol 2015; 21: 6809–6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masterson JC, Capocelli KE, Hosford L, et al. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn’s ileitis. Inflamm Bowel Dis 2015; 21: 2429–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salvo-Romero E, Alonso-Cotoner C, Pardo-Camacho C, et al. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig 2015; 107: 686–696. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto T. Diagnosis and monitoring of postoperative recurrence in Crohn’s disease. Expert Rev Gastroenterol Hepatol 2015; 9: 55–66. [DOI] [PubMed] [Google Scholar]
- 25. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 26. Yan T, Li L, Wu Q, et al. Analysis of body composition in patients with Crohn’s disease. Zhonghua Wei Chang Wai Ke Za Zhi 2014; 17: 981–984. [PubMed] [Google Scholar]
- 27. Zhang T, Cao L, Cao T, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection. J Parenter Enteral Nutr 2017; 41: 592–600. [DOI] [PubMed] [Google Scholar]
- 28. Bamba S, Sasaki M, Takaoka A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS One 2017; 12(6): e0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 2013; 98: 2604e12. [DOI] [PubMed] [Google Scholar]
- 30. Davies A, Nixon A, Muhammed R, et al. Reduced skeletal muscle protein balance in paediatric Crohn’s disease. Clin Nutr 2019; pii: S0261-5614(19)30228-6. [DOI] [PubMed] [Google Scholar]