Abstract
Introduction:
Osteoporosis is a growing major health problem with medical, social, and economic burden. The pathogenesis of osteoporosis involves excessive bone resorption by osteoclasts and decreased new bone formation by osteoblasts. Early diagnosis is the key to minimize the impact of osteoporosis on patients. Dual-energy X-ray absorptiometry at the hip or spine is the main standard test for measuring bone mineral density, and according to the World Health Organization, the T-score value of –2.5 or less determines osteoporosis.
Aim:
In this work, we aimed to study the risk factors associated with osteoporosis among a population sample of osteoporotic and controls in the United Arab Emirates (UAE) and relate them to the T-score value.
Material and Methods:
Two hundred male and female participants were recruited in the study. A questionnaire was used and data were correlated with the T-score value of the participants.
Results:
Results showed a positive correlation between the T-score value of the patients with osteoporosis and their calcium intake, exercise duration, and the age at menopause of female patients, whereas a negative correlation was evident between the T-score value and both caffeine and nicotine consumption. Results also revealed that patients with osteoporosis have significantly low body mass index, low calcium intake, and shorter duration of exposure to the sun than the control group.
Conclusion:
In conclusion, more intervention should be directed toward changing the modifiable risk factors in patients with osteoporosis and more studies should be directed toward osteoporosis in the UAE.
Keywords: Osteoporosis, pathophysiology, risk factors, T-score value, United Arab Emirates (UAE)
Introduction
Osteoporosis is a bone disorder, characterized by loss of bone strength because of an imbalance between the bone resorption and the mechanisms of bone formation, leading to increased fragility and fractures. Pathophysiological mechanisms underlying this disorder include an inadequate formation response during the remodeling process of bone formation, which is an essential factor in osteoporosis pathogenesis. This inadequacy is due to the activation of large numbers of osteoclasts as a response to initiation of hematopoietic precursor cells with failure of normal interaction with the osteoblastic lineage. This will lead to excessive bone resorption that may result in complete loss of trabecular structure and loss of template for new bone formation. The time required for osteoblastic replacement is longer than the resorption phase of bone remodeling by osteoclasts. Therefore, any increase in bone remodeling will lead to damaged architecture and loss of bone mass.[1]
Recently, it has been estimated that more than 200 million people worldwide have osteoporosis. On the basis of a statistics from the International Osteoporosis Foundation in 2017, one in five men and one in three women over the age of 50 years will experience fractures during their lifetime because of osteoporosis. Furthermore, it has been found that an osteoporotic fracture occurs every 3s, with the most common fractures occurring at the hip, spine, and wrist.[2,3]
Osteoporotic fractures may be the first manifestation of the disease, which is why it is called a silent disease. Risk factors of osteoporosis, decreased bone mineral density (BMD), and increased fragility fractures may be modifiable or non-modifiable. Among the modifiable factors are low body mass index (BMI), vitamin D deficiency, low calcium intake, excessive caffeine and alcohol consumption, smoking, sedentary life and low physical activity, endocrine disorders (such as estrogen deficiency and insulin-dependent diabetes mellitus or hyperparathyroidism), some drugs (such as corticosteroids), and previous history of fragility fractures. The non-modifiable factors include female gender, family history, race, and early menopause.[4,5]
According to the World Health Organization (WHO) diagnostic criteria, osteoporosis is diagnosed by BMD at the hip or spine, which is below the young normal mean reference population by 2.5 standard deviations (SDs) or more. This is called the T-score, and osteopenia is diagnosed by BMD less than or equal to 1 SD.[6]
The guidelines for osteoporosis screening vary greatly in various publications. In general, most organizations recommend that all adults older than 50 years of age with a history of fracture must receive BMD screening.[7]
Dual-energy X-ray absorptiometry (DXA) at the hip or spine is the best test for measuring central BMD. An association is present between the T-score values in DXA in patients with osteoporosis or osteopenia and the risk of fractures. A fracture risk assessment tool (FRAX) was designed. It is estimated that for every 1 SD decline in spine BMD, the risk of having a spine fracture increases 2.3 times and the risk of having a hip fracture increases 2.6 times.[8,9]
As the most common bone disease in humans, the prevalence of osteoporosis is steadily increasing due to a growing elderly population, and thus represents a major public health concern with reduced bone strength and a higher risk of fractures.[6,8]
The prevalence of osteoporosis is steadily escalating due to increased life expectancy as a result of developed health services. According to the 2016 WHO report, in the United Arab Emirates (UAE), life expectancy at birth for men was 76 years and for women it was 79 years.[10]
The UAE population projection showed that in 2011, 7% of the population were 50 years of age or over and less than 1% were 70 years of age or over. By 2050, it is estimated that 12% of the population will be 50 years or over and 2% will be 70 years or over.[11]
Materials and Methods
Study design
The study was performed in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines. Ethical approval number UG-H-18-11-7-10 was obtained from the Research Ethics Committee of the college. The study was conducted on a population sample of national and nonnational people in the UAE. Both men and women were recruited in the study. Data were collected from the patients in six governmental and private hospitals.
Research tools
A total of 200 male and female participants between the age of 25 and 80 years were recruited in the study. Eighty percent of the sample were women and 20% were men.
After obtaining the required consent, each participant was asked to fill a structured questionnaire consisting of 25 questions, which was used as the primary tool for data collection. The questions were formulated both in Arabic and English, it required 3–4min to be completed. BMD of the participants was assessed in the International Radiology Centre and correlated with their data in the questionnaires. DXA scan was used for the measurement of BMD.
Data collection and data analysis
After ensuring strict confidentiality, data were collected between March 20, 2016 and May 20, 2016. The following information was obtained:
Demographic data (gender, age, race, BMI, and occupation)
Family history of osteoporosis or osteoporotic fractures
Social history and lifestyle, physical activity, and exposure to the sun
Dietary habits such as calcium, caffeine, and soft drinks intake
Medical history of diseases and medicines. For female participants, number of pregnancies, lactation, and age at menopause were included
The following criteria were used to evaluate osteoporosis:
Normal BMD: if T-score between +2.5 and –1
Osteopenia: if T-score between –1 and –2.5
Osteoporosis: if T-score below –2.5
The filled questionnaires were coded and data were analyzed statistically using the Statistical Package for the Social Sciences (SPSS) software (version 24; IBM Corporation, Newyork, USA). Spearman’s correlation test was carried out to assess the association between different variables in the study. A P value of less than 0.05 was considered significant.
Results
Sociodemographic data
The sociodemographic background of the study participants is listed in Table 1. A total of 200 participants were included in the study. The control group and the osteoporotic group consisted of 100 participants each, 20% of them were men, whereas 80% of the respondents were women.
Table 1.
Background demographic data of the respondents
| Control group (%) | Osteoporotic group (%) | Total | |
|---|---|---|---|
| Age group | |||
| <35 years | 50.0 | 6.0 | 56.0 |
| 35–50 years | 40.0 | 18.0 | 58.0 |
| >50 years | 10.0 | 76.0 | 86.0 |
| Gender | |||
| Male | 20.0 | 20.0 | 40.0 |
| Female | 80.0 | 80.0 | 160.0 |
| Occupation | |||
| Doctor | 10.0 | 14.0 | 24.0 |
| Teacher | 8.0 | 12.0 | 20.0 |
| Manager | 4.0 | 8.0 | 12.0 |
| Engineer | 6.0 | 2.0 | 8.0 |
| Housewife | 28.0 | 52.0 | 80.0 |
| Others | 44.0 | 12.0 | 56.0 |
| Total number of respondents | 100 | 100 | 200 |
Body mass index
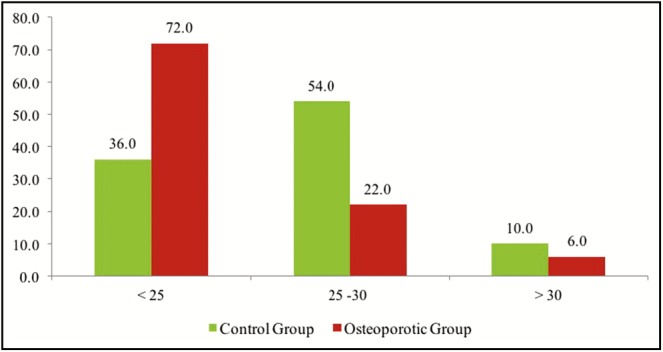
The patients with osteoporosis have significantly lower BMI than the control group (P < 0.05). Results showed that 72% of them have a BMI less than 25 kg/m2 (P < 0.05) [Table 2] [Figure 1].
Table 2.
Body mass index of the respondents
| Control group (%) | Osteoporotic group (%) | Total | |
|---|---|---|---|
| Body mass index (BMI) (kg/m2) | |||
| <25 | 36.0 | 72* | 108 |
| 25–30 | 54.0* | 22 | 76 |
| >30 | 10.0 | 6 | 16 |
| Total number of respondents | 100 | 100 | 200 |
*P < 0.05
Figure 1.
Body mass index (kg/m2) of the respondents
Smoking behavior
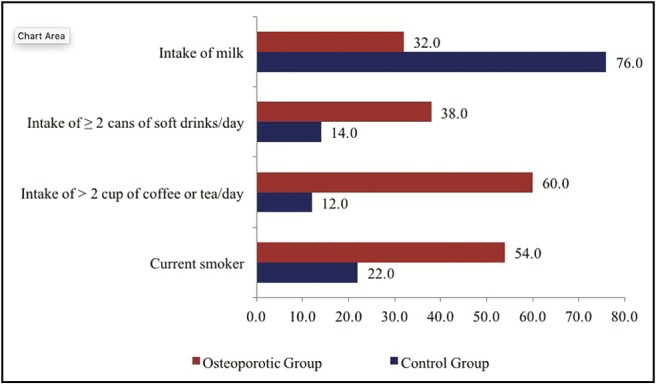
Regarding smoking behavior, a significant value was evident between smoking and osteoporosis (P < 0.01) as 54% of the patients with osteoporosis were current smokers, whereas 72% of the controls had never smoked before [Table 3] [Figure 2].
Table 3.
Smoking behavior and intake of milk, coffee, and soft drinks by the respondents
| Control group (%) | Osteoporotic group (%) | Total | |
|---|---|---|---|
| Smoking behavior | |||
| Current smoker | 22.0 | 54.0** | 76.0 |
| Ex-smoker | 6.0 | 10.0 | 16.0 |
| Never smoke | 72.0** | 36.0 | 108.0 |
| Intake of milk | |||
| 0 glass | 24.0 | 68.0** | 92.0 |
| 1 glass | 60.0** | 24.0 | 84.0 |
| 2 and more glasses | 16.0 | 8.0 | 24.0 |
| Intake of coffee or tea | |||
| 0 cup | 32.0 | 2.0 | 34.0 |
| 1 cup | 38.0** | 14.0 | 52.0 |
| 2 cups | 18.0 | 24.0 | 42.0 |
| >2 cups | 12.0 | 60.0** | 72.0 |
| Intake of soft drinks | |||
| 0 glass | 62.0** | 22.0 | 84.0 |
| 1 glass | 24.0 | 40.0 | 64.0 |
| 2 and more glasses | 14.0 | 38.0** | 52.0 |
| Total number of respondents | 100 | 100 | 200 |
**P < 0.01
Figure 2.
Intake of milk and caffeine and smoking behavior by the respondents
Dietary calcium intake
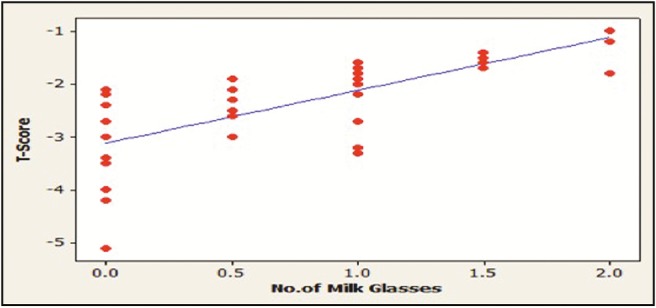
The intake of milk and dairy products by the participants was used to assess the dietary calcium intake. The patients with osteoporosis have a significant low calcium intake (P < 0.01), whereas non-osteoporotic control significantly consume more milk and dairy products (P < 0.01). A positive correlation was found between the intake of milk and dairy products and the T-score value of the participants (P < 0.05) [Table 3] [Figures 2 and 3].
Figure 3.
Correlation between milk intake and T-score in patients with osteoporosis
Caffeine consumption
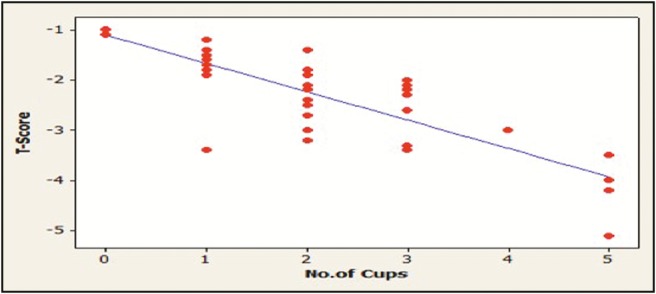
Caffeine intake was evaluated in this study by the amount of coffee, tea, or soft drinks consumed by the participants each day. The results showed that the patients with osteoporosis significantly consume more caffeine (P < 0.01). A negative correlation is present between the amount of caffeine intake and T-score value of the participants (P < 0.05) [Table 3] [Figures 2, 4, and 5].
Figure 4.
Correlation between tea/coffee intake and T-score in patients with osteoporosis
Figure 5.
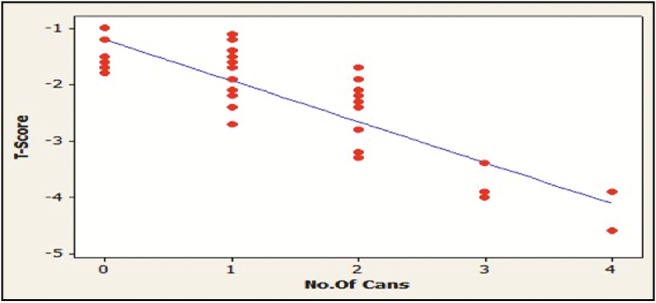
Correlation between soft drinks intake and T-score in patients with osteoporosis
Exercise behavior and exposure to the sun
Results showed a significant positive correlation between the duration of exercise and the T-score value of the participants (P < 0.05) [Table 4] [Figure 6].
Table 4.
Exercise behavior and exposure to sun of the respondents
| Control Group | Osteoporotic Group | Total | |
|---|---|---|---|
| % of respondent do exercise | 62.0** | 26.0 | 88.0 |
| Exposure to the Sun (3-4 times/week) | |||
| 0 – 5 minutes | 8.0 | 74.0** | 82.0 |
| 6 – 15 minutes | 20.0 | 22.0 | 42.0 |
| 16 – 30 minutes | 72.0** | 4.0 | 76.0 |
| Total number of respondents | 100 | 100 | 200 |
**P < 0.01
Figure 6.
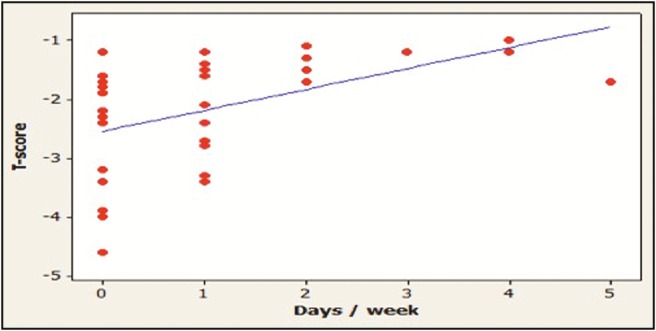
Correlation between exercise duration and T-score in patients with osteoporosis
Regarding the exposure to the sun, the results showed that 96% of the patients with osteoporosis were exposed to the sun for less than 15min, three to four times a week or not exposed at all, whereas most of the normal controls (72%) exposed their bodies to the sun for 16–30min/day, three to four times a week [Table 4] [Figure 7].
Figure 7.
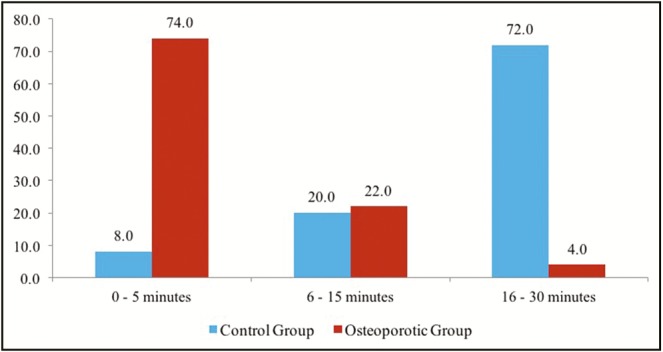
Sun exposure behavior of the respondents
Diseases and medications
Results of the study revealed that 46% of the patients with osteoporosis were diabetic, 42% of them were treated by antidiabetics, 18% had arthritis and 38% had been treated previously by corticosteroids [Table 5].
Table 5.
Distribution of patients with osteoporosis by their diseases and medications
| Osteoporotic group (%) | |
|---|---|
| Type of disease among the patients with osteoporosis | |
| Diabetes | 46.0 |
| Arthritis | 18.0 |
| Chronic liver/kidney disease. | 16.0 |
| Hyperthyroidism | 10.0 |
| Malabsorption | 6.0 |
| Cancer | 4.0 |
| Treatment-seeking behavior | |
| Antidiabetics | 42.0 |
| Corticosteroids | 38.0 |
| Immunosuppressant | 8.0 |
| Chemotherapy | 6.0 |
| No medicine | 6.0 |
| Number of respondents with osteoporosis | 100 |
Number of pregnancies and breastfeeding
Female participants constituted 80% of the study sample, 75% of the females with osteoporosis were menopausal, 75% of them had three or more pregnancies, and 82.5% of them breastfed their children, whereas these values for the control group participants are 30%, 28%, and 33%, respectively. Results showed a significant positive correlation between the age at menopause and the T-score value of females with osteoporosis (P < 0.05) [Table 6] [Figure 8]. The distribution of patients according to the joint affected by Osteoporosis or Osteopenia is shown in [Figure 9] and [Table 7].
Table 6.
Distribution of female patients with osteoporosis by number of pregnancies and breastfeeding
| Osteoporotic group (%) | ||
|---|---|---|
| Number of pregnancies | ||
| 0 | 6 | 7.5 |
| 1 | 8 | 10.0 |
| 2 | 10 | 12.5 |
| 3 | 14 | 17.5 |
| 4 and more | 42 | 52. 5 |
| % of female that breastfed their children | 66 | 82.5 |
| % of female respondents that reached menopause | 60 | 75.0 |
| Number of female respondents with osteoporosis | 80 | 100 |
Figure 8.
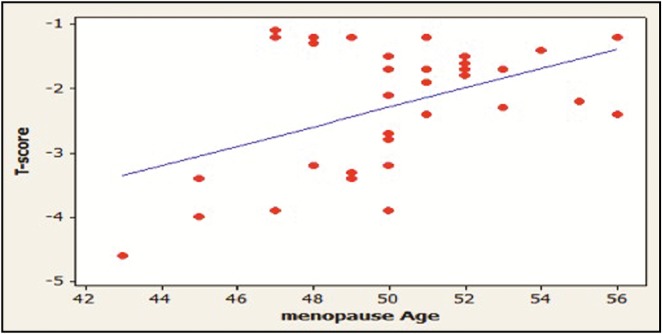
Correlation between age at menopause and T-score in female patients with osteoporosis
Figure 9.
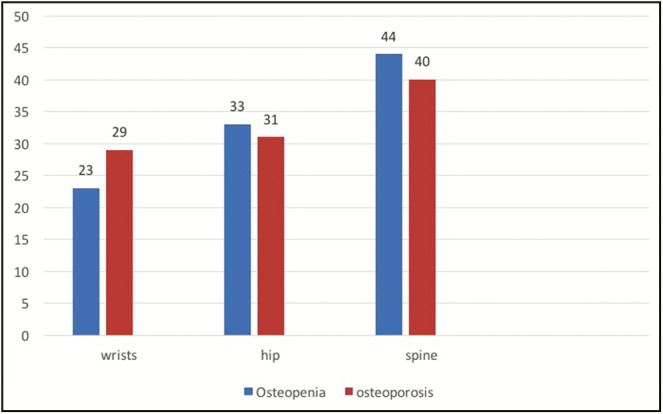
Distribution of patients having osteopenia or osteoporosis in wrists, hips, or spine
Table 7.
Distribution of patients having osteoporosis and/or osteopenia in hips, wrists, and spine
| Osteopenia (%) | Osteoporosis (%) | |
|---|---|---|
| Hips | 23 | 29 |
| Wrists | 33 | 33 |
| Spine | 44 | 33 |
Discussion
To maintain normal bone structure, a remodeling process that consists of osteoclasts, removing old bone, and osteoblasts, synthesizing new bone, occurs. Hematopoietic progenitors produce osteoclasts, whereas mesenchymal stem cells called marrow stromal fibroblasts produce osteoblasts. This process is controlled by certain circulating hormones, growth factors, and locally produced cytokines through their effects on apoptosis of osteoblasts and osteoclasts. Estrogen deficiency or glucocorticoid excess leads to bone loss because of changes in the production of bone cells. This is caused by the prolongation of the life span of osteoclasts and the shortening of the life span of osteoblasts. On the contrary, drugs that aim to treat or prevent osteoporosis prevent apoptosis of osteoblasts and/or stimulate the apoptosis of osteoclasts.[12]
The pathogenesis of osteoporosis is the consequence of various hormonal, genetic, dietary, lifestyle, and physical factors. Genetic factors mainly affect the BMD and bone formation, whereas low levels of estrogen increase parathyroid hormone, and local cytokines are mainly responsible for bone remodeling imbalance.[13]
Age and gender
In this study, 76% of the patients with osteoporosis were women at or above 50 years of age. Osteoporosis is usually considered a disease of the elderly with low BMD. In a recent study, most of the patients with osteoporosis were in the age groups of 45–49 and 50–54 years.[14] Previous studies showed that women were at an increased risk of developing osteoporosis because of their faulty behavior of physical inactivity, sun-avoidance behavior, low intake of dairy products, and poor diets.[15,16,17]
Body mass index
The results of this study showed a significant number of patients of osteoporosis with BMI <25 kg/m2, where P < 0.05. Women that have low BMI are at a higher risk of developing osteoporosis. It is reported that one unit change in BMI has a larger effect on the risk of developing osteoporosis than most other modifiable risk factors. To help reduce the risk of osteoporosis, patients should be advised to maintain a weight within the normal range.[15,16] The risk of osteoporotic fractures increases in adults who have low BMI of less than 20 kg/m2.[18]
On the contrary, patients who are obese (BMI >30 kg/m2) are also at a high risk of developing osteoporosis and fractures because of the risk of repeated falls and the weakness of the skeletal muscles due to loss of its mass as a result of aging.[19]
Exercise and physical activity
The results of this study showed a significant correlation between the exercise time and the T-score value. Previous studies concluded that physical activity and exercises stimulate skeletal growth and bone strength.[4,5]
Estrogen
Estrogen deficiency has a critical effect on the pathogenesis of osteoporosis. The results of this study showed a significant positive correlation between the age at menopause and the T-score value of the female patients. Estrogen has a crucial role in bone formation and physiology. It stimulates the production of T cell cytokines, affects the osteoblastic cell by altering its production of receptor activator of nuclear factor-Kappa B ligand (RANKL) or Osteoprotegerin (OPG), inhibits the differentiation of osteoclasts by direct action, and stimulates the formation of bone performed by osteoblasts and osteocytes, which allows them to enhance their ability to respond to mechanical forces.[20,21]
Estrogen produces its effect through a specific cell surface receptor called the estrogen receptor alpha (ERα). This receptor binds and then transports estrogen into the nucleus of the cell where certain genes are then activated by the receptor–hormone complex. ERα receptors and estrogen receptor–related receptor alpha (ERRα) are found on the surface of osteoblasts. ERRα may play a supporting role in the regulation of bone cells.[22] Previous studies also suggest that sex hormone–binding globulin may play a significant role in regulating bone cells as well because it facilitates entry of estrogen into cells.[23]
Because of the natural drop of estrogen level in postmenopausal women, they are at the highest risk of developing osteoporosis. A previous study showed that it is an increase in bone resorption that might be the driving force for bone loss during estrogen deficiency in postmenopausal women not impaired bone formation as was previously thought.[1] However, other studies indicated that both markers of bone resorption and formation also increased, leading to accelerated bone remodeling at menopause.[24,25]
Calcium intake and vitamin D
Calcium and vitamin D are two pivotal contributors of bone formation and mineralization. The results of this study showed that the patients with osteoporosis have a significant low calcium intake, whereas controls significantly consume more milk and dairy products (P < 0.01). This significant result showed the importance of calcium intake. Results also established a positive correlation between calcium intake and the T-score value of the participants.
Vitamin D plays an important role in maintaining calcium homeostasis and bone integrity as it is essential for intestinal calcium absorption.[26]
It is estimated that over one billion people around the world have vitamin D deficiency.[27,28]
Sun exposure is considered as the source of vitamin D, the present results showed a significant value (P < 0.01) as 74% of the patients with osteoporosis exposed their bodies to the sun for 5min or less, three to four times per week, whereas 72% of controls exposed their bodies to the sun for 15–30min, 3 to 4 times a week. A previous study conducted in the UAE showed that 58.2% of the UAE nationals were vitamin D deficient compared to 45% of the patients from other nationalities.[29]
In spite of living in sunny areas, such as UAE, Saudi Arabia, and India, young populations have a high prevalence of vitamin D deficiency because of insufficient knowledge and practice of vitamin D and its health implications.[30,31]
Decreased blood calcium level will lead to secondary hyperparathyroidism. This decrease may result from impaired intestinal calcium absorption due to vitamin D deficiency, aging, or other diseases. Calcitriol, the active form of vitamin D, stimulates the intestinal absorption of calcium and phosphorus. It has an inhibitory effect on the synthesis of parathyroid hormone as well. Therefore, calcitriol deficiency will lead to secondary hyperparathyroidism as well.[32]
There is a clear evidence that the risk of having an osteoporotic fracture increases when vitamin D levels are below 50 nmol.[33,34]
However, a recent study showed that pathological macrophages produced by vitamin D receptor signaling play an important role in the development of myelofibrosis.[35]
Smoking
The results of this study showed that nicotine has a significant effect on the incidence of osteoporosis (P < 0.01). Smoking has been identified as a modifiable risk factor for low BMD and increased osteoporotic fracture risk.[36,37]
Smoking behavior should be evaluated when assessing the fracture risk and FRAX.[16,38]
The role of smoking in decreasing BMD is complicated; smoking is shown to be associated with other risk factors for osteoporosis such as decreased physical activity, low BMI, and poor diet.[38]
Nicotine has both direct and indirect effects on BMD. The direct effect is on bone cell proliferation and is described to be biphasic, small doses have a stimulatory effect, whereas toxic large doses have an antiproliferative effect on the proliferation of osteoblasts.[39]
This effect on osteoblasts is receptor mediated by the nicotinic acetylcholine receptors, where nicotine in low doses upregulates gene expression of alkaline phosphatase, type 1 collagen, and osteocalcin.[40]
Nicotine produces an increased level of tumor necrosis factor α (TNFα) secretion that reduces bone formation by osteoblasts and increases bone resorption by osteoclasts. TNFα has a powerful osteoclastogenic effect through its stimulating effect on RANKL production and by intensifying osteoclasts production.[41]
It is strongly supported that RANK/RANKL/OPG systems have a role in regulating bone resorption and the formation of osteoclasts.[42] The main predisposing factor for chronic obstructive pulmonary disease (COPD) has been found to be smoking. The most important factor in managing COPD continues to be the termination of smoking.[43] It has been shown in numerous studies that smokers with COPD have elevated levels of pro-inflammatory cytokines in the form of interleukin-6. In addition, TNFα levels are much higher in COPD smokers than in asymptomatic smokers. This indicates that COPD affects local and systemic inflammatory responses, which in turn affects bone remodeling.[44,45] Nicotine has an indirect effect on BMD, which is caused by releasing calcitropic hormones and glucocorticoids, which leads to an increase in bone resorption.[43] Furthermore, smoking has been linked to an increase in the level of cortisol[44,46] as well as a decrease in the level of estradiol.[47] Nicotine also harms intestinal calcium absorption, which might lessen the effectiveness of dietary calcium supplements.[48] Also, toxic carcinogenic metabolites of nicotine have also been found to increase osteoclast formation in rats.[49] Moreover, one hip fracture in eight postmenopausal women with osteoporosis is attributable to smoking. This correlation could not be explained by early menopause, low BMI, lower levels of exercise, or by the actions of nicotine on estrogen. This indicates that there is an independent negative effect of nicotine on BMD, which means that smokers lose bone at a faster rate than nonsmokers.[50]
Conclusion
Prevalence of osteoporosis is high in the UAE and is expected to grow due to increased life expectancy and faulty lifestyle of inadequate dietary habits, sun exposure, exercise behavior, and smoking. More intervention should be directed toward changing the modifiable risk factors in the patients with osteoporosis, and more studies should be directed toward osteoporosis in the UAE.
Limitations of the study
To make the study a polycentric research, it would require greater number of participants from all the emirates of the UAE.
Financial support and sponsorship
This work is self-funded by the authors.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lawrence GR. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;11:3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sözen T, Özışık L, Başaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.www.iofbonehealth.org. [homepage on the internet] International Osteoporosis Foundation (IOF) report 2017 [updated 2017; cited 2018 Aug 26] [Last accessed on 2018 Dec 28]. Available from: https://www.iofbonehealth.org/osteoporosis .
- 4.Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, et al. Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthop. 2016;7:171–81. doi: 10.5312/wjo.v7.i3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, et al. Osteoporosis: a review of treatment options. P T. 2018;43:92–104. [PMC free article] [PubMed] [Google Scholar]
- 8.Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Majumdar SR, Lix LM, Johansson H, Oden A, McCloskey E, et al. Manitoba Bone Density Program. High fracture probability with FRAX usually indicates densitometric osteoporosis: implications for clinical practice. Osteoporos Int. 2012;23:391–7. doi: 10.1007/s00198-011-1592-3. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. www.who.int. [homepage on the internet] WHO 2016 report United Arab Emirates. [Last accessed on 2018 Dec 28]. [updated 2018; cited 2018 Aug 27]. Available from: http://www.who.int/countries/are/en/
- 11.www.iofbonehealth.org. [homepage on the internet] UAE report. [Last accessed on 2018 Dec 28]. [cited 2018 Aug 27]. Available from: http://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Middle%20East_Africa/Middle_East_Africa_audit.pdf .
- 12.Jilka RL. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med Pediatr Oncol. 2003;41:182–5. doi: 10.1002/mpo.10334. [DOI] [PubMed] [Google Scholar]
- 13.Cianferotti L, Brandi ML. Pathogenesis of osteoporosis. Osteoporosis. 2013. [[Last accessed on 2018 Dec 30]]. pp. 13–21. Feb 19 [cited 2018 Aug 20]. Available from: https://www.futuremedicine.com/doi/10.2217/ebo.12.159 .
- 14.Boschitsch EP, Durchschlag E, Dimai HP. Age-related prevalence of osteoporosis and fragility fractures: real-world data from an Austrian menopause and osteoporosis clinic. Climacteric. 2017;20:157–63. doi: 10.1080/13697137.2017.1282452. [DOI] [PubMed] [Google Scholar]
- 15.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt) 2006;15:1028–34. doi: 10.1089/jwh.2006.15.1028. [DOI] [PubMed] [Google Scholar]
- 16.Snelling AM, Crespo CJ, Schaeffer M, Smith S, Walbourn L. Modifiable and non-modifiable factors associated with osteoporosis in postmenopausal women: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Women’s Health Gender-Based Med. 2001;10:57–65. doi: 10.1089/152460901750067124. [DOI] [PubMed] [Google Scholar]
- 17.Zareef TA, Jackson RT, Alkahtani AA. Vitamin D intake among premenopausal women living in Jeddah: food sources and relationship to demographic factors and bone health. J Nutr Metab. 2018;2018:8570986. doi: 10.1155/2018/8570986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 19.Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175–80. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Krassas GE, Papadopoulou P. Oestrogen action on bone cells. J Musculoskelet Neuronal Interact. 2001;2:143–51. [PubMed] [Google Scholar]
- 21.Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23:576–81. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnelye E, Aubin JE. Estrogen receptor-related receptor alpha: a mediator of estrogen response in bone. J Clin Endocrinol Metab. 2005;90:3115–21. doi: 10.1210/jc.2004-2168. [DOI] [PubMed] [Google Scholar]
- 23.Goderie-Plomp HW, van der Klift M, de Ronde W, Hofman A, de Jong FH, Pols HA. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J Clin Endocrinol Metab. 2004;89:3261–9. doi: 10.1210/jc.2002-022041. [DOI] [PubMed] [Google Scholar]
- 24.Parfitt AM, Villanueva AR, Foldes J, Rao DS. Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res. 1995;10:466–73. doi: 10.1002/jbmr.5650100319. [DOI] [PubMed] [Google Scholar]
- 25.Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81:3366–71. doi: 10.1210/jcem.81.9.8784098. [DOI] [PubMed] [Google Scholar]
- 26.Parfitt AM, Gallagher JC, Heaney RP, Johnston CC, Neer R, Whedon GD. Vitamin D and bone health in the elderly. Am J Clin Nutr. 1982;36:1014–31. doi: 10.1093/ajcn/36.5.1014. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080–6. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 28.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–45. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haql A, Sofi NY, Atef S. Vitamin D deficiency status, measurement and its treatment in the United Arab Emirates. EJPMR. 2016;3:131–7. [Google Scholar]
- 30.Yammine K, Al Adham H. The status of serum vitamin D in the population of the United Arab Emirates. East Mediterr Health J. 2016;22:682–6. doi: 10.26719/2016.22.9.682. [DOI] [PubMed] [Google Scholar]
- 31.Muhairi SJ, Mehairi AE, Khouri AA, Naqbi MM, Maskari FA, Al Kaabi J, et al. Vitamin D deficiency among healthy adolescents in Al Ain, United Arab Emirates. BMC Public Health. 2013;13:33. doi: 10.1186/1471-2458-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 33.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA study of elderly women. Osteoporos Int. 2005;16:1425–31. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 34.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. IOM dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. p. 231. [PubMed] [Google Scholar]
- 35.Kobe University. Vitamin D and immune cells stimulate bone marrow disease. ScienceDaily. 2019. [Last accessed on 2019 April 9]. 8 February www.sciencedaily.com/releases/2019/02/190208094330.htm .
- 36.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 37.Vestergaard P, Mosekilde L. Fracture risk associated with smoking: a meta-analysis. J Intern Med. 2003;254:572–83. doi: 10.1111/j.1365-2796.2003.01232.x. [DOI] [PubMed] [Google Scholar]
- 38.Wong PK, Christie JJ, Wark JD. The effects of smoking on bone health. Clin Sci (Lond) 2007;113:233–41. doi: 10.1042/CS20060173. [DOI] [PubMed] [Google Scholar]
- 39.Kallala R, Barrow J, Graham SM, Kanakaris N, Giannoudis PV. The in vitro and in vivo effects of nicotine on bone, bone cells, and fracture repair. Expert Opin Drug Saf. 2013;12:209–33. doi: 10.1517/14740338.2013.770471. [DOI] [PubMed] [Google Scholar]
- 40.Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab. 2009;27:555–61. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- 41.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–75. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 44.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int. 2012;23:2081–92. doi: 10.1007/s00198-012-1940-y. [DOI] [PubMed] [Google Scholar]
- 46.Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol. 2006;59:228–35. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause. 2004;11: 104–9. doi: 10.1097/01.GME.0000079740.18541.DB. [DOI] [PubMed] [Google Scholar]
- 48.Need AG, Kemp A, Giles N, Morris HA, Horowitz M, Nordin BE. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13:83–8. doi: 10.1007/s198-002-8342-9. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal J, Sun L, Cao J, Yuen T, Lu P, Bab I, et al. Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci U S A. 2013;110:11115–20. doi: 10.1073/pnas.1220919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841–6. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]


