Abstract
Coronary artery aneurysm is a rare disorder, which occurs in 0.3%–4.9% of patients undergoing coronary angiography. Atherosclerosis accounts for >90% of coronary artery aneurysms in adults, whereas Kawasaki disease is responsible for most cases in children. Recently, with the advent of implantation of drug-eluting stents, there are increasing reports suggesting stents causing coronary aneurysms, months or years after the procedure. The pathophysiology of coronary artery aneurysm is not completely understood but is thought to be similar to that for aneurysms of larger vessels, with the destruction of arterial media, thinning of the arterial wall, increased wall stress, and progressive dilatation of the coronary artery segment.
Coronary angiography remains the gold standard tool, providing information about the size, shape, and location and is also useful for planning the strategy of surgical resection. The natural history and prognosis remain unclear.
Despite the important anatomical abnormality of the coronary artery, the treatment options of coronary artery aneuryms are still poorly defined and present a therapeutic challenge.
We describe four cases, which were managed differently followed by a review of the current literature and propose some treatment strategies.
Keywords: Atherosclerosis, coronary angiography, coronary artery disease, drug-eluting stent, Kawasaki disease, percutaneous coronary intervention
INTRODUCTION
Coronary artery aneurysm (CAA) is an uncommon condition and is defined as dilatation of the coronary artery exceeding 50% of the reference vessel diameter.[1] CAAs are termed giant if their diameter transcends the reference vessel diameter by greater than four times or if they are >8 mm in diameter.[2] It occurs in about 0.3%–4.9% of patients undergoing coronary angiography[1] and are reportedly present in 1.4% of postmortem examinations.[1]
CAAs are found in the epicardial coronary arteries, most frequently in the proximal and middle segments of the right coronary artery (RCA) (68%), followed by the proximal left anterior descending (LAD) (60%) and left circumflex (LCx) (50%). CAA of the left main stem (LMS) is exceedingly rare and occurs in only 0.1% of the population.[1,3]
Dilatation may be either focal or diffuse, and aneurysms are classified as either fusiform (longitudinal dimension > transverse dimension) or saccular (transverse dimension > longitudinal dimension) in morphology. It has been noted that about one-third of CAAs are associated with obstructive coronary artery disease and have been associated with myocardial infarction, arrhythmias, or sudden cardiac death.[4]
More recently, with the increasing use of drug-eluting stents (DESs), there are growing reports signifying stents causing coronary aneurysms, months or even years after the intervention.[5] CAAs after coronary intervention are infrequent, with a reported incidence of 0.3%–6.0% and most aneurysms are in actual fact pseudoaneurysms rather than true aneurysms.[6]
We present four cases of CAA from two centers, which were managed differently followed by a review of the literature regarding etiology, pathogenesis, and the management of this rare entity.
CASE 1
A 77-year-old woman with a known history of coronary artery disease, presented with unstable angina. Seven years previously, she underwent coronary angiography, which demonstrated severe, diffuse, three-vessel disease and she was turned down for surgery or percutaneous coronary intervention (PCI). She was managed medically and did well over the last few years until a recent presentation.
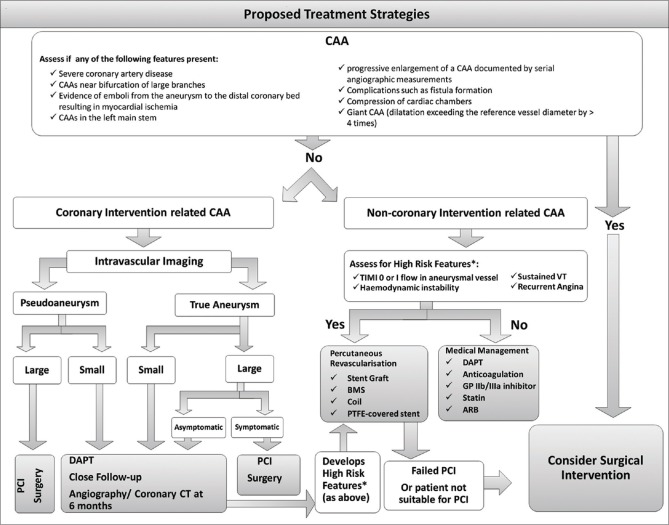
Repeat coronary angiography revealed severe left-sided disease, which was unamenable to PCI, although the occluded LAD artery had severe stenosis into a diagonal and a small LCx which was blocked. The RCA was excessively tortuous with aneurysmal dilatation and severely diseased [Figure 1a]. After informed consent, a decision for revascularization of the RCA with a DES was taken, and subsequently, a 3.5 mm × 30 mm and 3.0 mm × 15 mm Biofreedom stents (Biosensors International Group, Ltd.,) was deployed at 14 atmospheric pressure with good thrombolysis in myocardial infarction-III flow [Figure 1b].
Figure 1.
Coronary angiography: (a) Left anterior oblique view of the right coronary artery demonstrating a tortuous artery with aneurysmal dilatation and severe disease. (b) Right anterior oblique cranial view of the right coronary artery poststenting showing thrombolysis in myocardial infarction-III flow
The case was complicated by perforation distally induced by the guide wire which required a pericardial drain, but she made a good recovery eventually. She was discharged home on the 3rd day on dual anti-platelet therapy (DAPT) along with a statin and a beta blocker. She was followed-up 6 months later and was doing very well and had no cardiac symptoms.
CASE 2
A 52-year-old male, with no significant medical history of note, presented with chest pain and ST elevation in the anterolateral leads on his electrocardiogram (ECG). An emergency coronary angiography demonstrated an acutely occluded LAD, after the origin of the first septal. He subsequently had a 3 mm × 38 mm Promus Element (Boston Scientific Corporation, Marlborough, MA, USA) DES deployed at 16 atmospheric pressure and a 4 mm × 9 mm Azule (Boston Scientific Corporation, Marlborough, MA, USA). A bare metal stent was deployed more proximally with a degree of overlap. The overlap and most part of the first stent was postdilated with a 4-mm noncompliant balloon to moderate pressures. There was an unexpected over-expansion in the distal aspect of the DES.
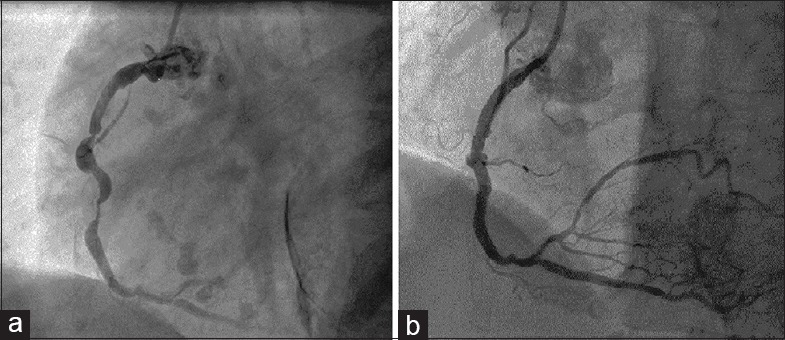
Optimal coherence tomography (OCT) showed a well-apposed stent. There was an ulcerated plaque [Figure 2a, arrow], in the distal aspect of the DES, which resulted in a focal over-expansion of this segment of the stent, because of the lack of resistance in the arterial wall in that segment.
Figure 2.
Coronary angiography: (a) Left anterior oblique cranial view of the left anterior descending artery showing an ulcerated plaque (arrow), beneath the distal portion of the previously placed drug-eluting stent. (b) Posteroanterior caudal view of the left anterior descending artery demonstrating a focal aneurysm in the mid vessel at the site of previous drug-eluting stent insertion. (c) Right anterior oblique caudal view of the left anterior descending artery demonstrating that the aneurysm has nearly sealed-off
The patient was discharged home with aspirin for life and prasugrel for 1 year. A planned surveillance angiogram 9 months later revealed a focal aneurysm formation in the middle of the DES that was deployed in the mid LAD [Figure 2b]. We decided to manage him conservatively with DAPT long term.
A repeat surveillance angiogram 1 year later demonstrated that the aneurysm was much smaller in size and nearly sealed off [Figure 2c]. OCT confirmed the same findings.
CASE 3
A 55-year-old Caucasian male presented with a history of exertional chest pain for few months. The exercise tolerance test demonstrated pathological ST depression in anterior leads, associated with chest pain at 5 min into the standard Bruce protocol.
The patient was obese and diabetic and had a significant family history of premature coronary artery disease. His coronary angiography revealed severe stenoses in the proximal LAD and first diagonal. The LCx had severe disease in the mid vessel with poststenotic aneurysmal dilatation [Figure 3]. The RCA was dominant and occluded in the mid vessel. A mini-multi-disciplinary team (MDT) discussion was held with regard to the revascularization options for him. His Syntax II score was 27, suggesting 4-year mortality of 4.6% with PCI compared to 2.6% with coronary artery bypass grafting (CABG). He was, therefore, referred for CABG.
Figure 3.
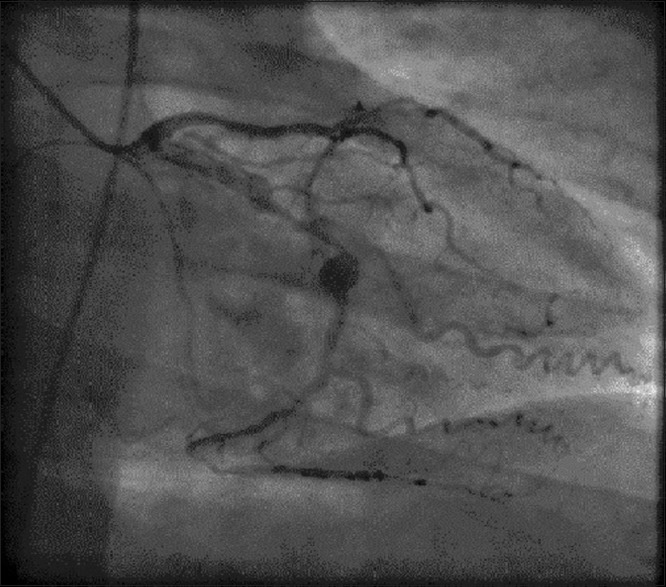
Coronary angiography: Right anterior oblique caudal view of the left circumflex artery showing severe disease in the mid vessel with poststenotic aneurysmal dilatation
CASE 4
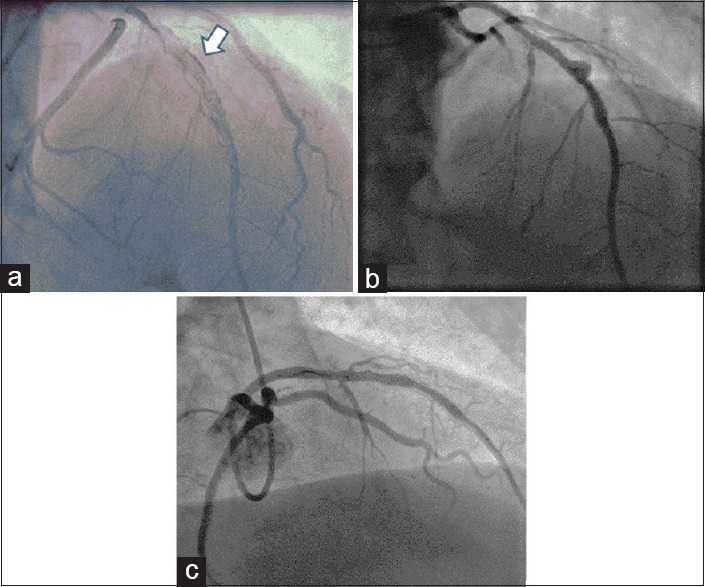
A 67-year-old Caucasian male presented with chest pain and hypotension. He had a history of prior CABG (saphenous vein grafts to LAD, right and intermediate coronary arteries) as well as thoracic and abdominal aneurysm repair. There were no significant dynamic ECG changes and troponin T was elevated. A chest radiograph demonstrated a widened mediastinum. A transthoracic echocardiogram revealed a heterogeneous mediastinal mass and computed tomography confirmed this to be a mediastinal hematoma with active contrast leak from a proximal LAD aneurysm [Figure 4a].
Figure 4.
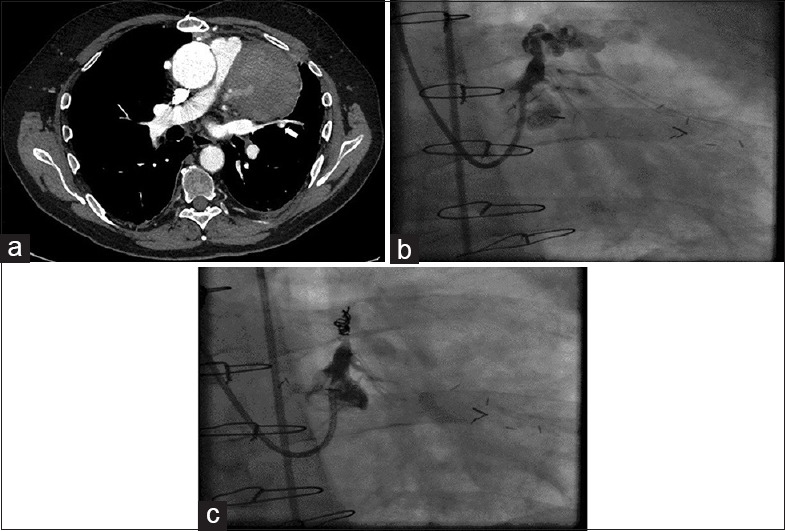
Computed tomography: (a) Computed tomogram showing a mediastinal mass with contrast extravasation (arrow) from the proximal left anterior descending aneurysm. (b) Right anterior oblique caudal view showing aneurysm in the proximal left anterior descending artery with extravasation of the contrast. (c) Right anterior oblique view showing closure of the aneurysm following coils insertion
Following a multi-disciplinary discussion, it was decided to perform percutaneous closure of the fistula between the proximal LAD aneurysm and the mediastinum. Coronary angiography revealed a patent saphenous vein graft to the LAD and a giant proximal LAD aneurysm with evidence of dissection and extravasation of contrast [Figure 4b].
The LMS was cannulated with a 4.0 Extra Back Up Launcher guide catheter (Medtronic, USA). In order to achieve hemodynamic stability, the LAD was temporarily occluded by inflation of a 3.0 mm × 12 mm Trek balloon (Abbott Vascular, USA) inserted on a balanced middle weight coronary guidewire (Abbott Vascular, USA) into the proximal LAD. The LAD was then thrombosed by deployment of 3 0.018” Tornado Embolization coils (Cook Medical, USA) proximal to the aneurysm neck through a MicroFerret microcatheter (Cook Medical, USA) achieving vessel closure [Figure 4c]. The patient made an excellent recovery and was discharged.
He was asymptomatic when reviewed at 6 months, and a gated cardiac computed tomography (CT) demonstrated the mediastinal mass had reduced in size and there was no longer active contrast extravasation.
DISCUSSION
There have been attempts, but with limited success, by the researchers and physicians, in defining abnormal dilatation of the coronary arteries, the dilemma being differentiating between aneurysm and ectasias. At present, CAA is defined as a localized, irreversible dilatation of the blood vessel lumen that exceeds the diameter of the adjacent normal segment by >1.5-fold.[1] On the contrary, ectasia is used to describe a diffuse dilatation of coronary arteries that involves ≥50% of the length of the artery; this classification is based on the appearance and number of vessels involved.[7]
The classification of coronary artery dilatation, as described by Díaz-Zamudio et al.,[8] is shown in Table 1.
Table 1.
Classification of coronary artery dilatation
| Based on the shape or gross structure | |
|---|---|
| Saccular | Transverse diameter is greater than the longitudinal dimension |
| Fusiform | Longitudinal dimension is greater than the transverse diameter |
| Based on the vessel wall composition | |
| True aneurysm | Vessel wall composed of three layers: adventitia, media, and intima |
| Pseudoaneurysm | Vessel wall composed of one or two layers |
| Based on the size | |
| Giant aneurysm (adults) | >20-150 mm in diameter |
| Giant aneurysm (children) | >8 mm in diameter |
| Based on the extent of involvement[16] | |
| Type I | Diffuse dilatation of two or three vessels |
| Type II | Diffuse dilatation in one vessel and localized disease in another |
| Type III | Diffuse dilatation of one vessel only |
| Type IV | Localized or segmental dilatation |
Reproduced with permission from Díaz-Zamudio et al. 2009
Atherosclerosis is accountable for >90% of CAAs in adults, whereas Kawasaki disease is responsible for the majority of cases in children.[8] In recent years, CAA have been described as a sequel of coronary angioplasty. This was first stated by Holmes et al.[9] following a balloon angioplasty. CAA have also been described after placement of DESs.[10]
Table 2 lists the different etiologies which have been postulated for CAAs.
Table 2.
Causes of coronary artery aneurysm
| Causes | Frequency (%) |
|---|---|
| Congenital | 17[5] |
| Acquired | |
| Atherosclerosis | 52[5] |
| Inflammatory disorders | |
| Kawasaki disease | 17[15] |
| Takayasu’s arteritis | |
| Giant cell arteritis | |
| Behcet’s disease | |
| Infectious | |
| Mycotic aneurysm | 11[5] |
| Septic emboli | |
| Bacterial | |
| Syphilis | |
| Connective tissue disorders | |
| Marfan’s syndrome | <10[15] |
| Ehlers-Danlos syndrome | |
| Fibromuscular dysplasia | |
| Drug-related | |
| Cocaine | Rare |
| Amphetamines | |
| Trauma | Rare |
| Iatrogenic (e.g., PTCA, stents, atherectomy, angioplasty, laser angioplasty) | 0.3-0.6[6] |
PTCA: Percutaneous transluminal coronary angioplasty
Pathophysiology
The pathophysiology of CAA still remains unclear but is perceived to be identical to that for aneurysms of larger vessels, with the destruction of arterial media, thinning of the arterial wall, increased wall stress, and progressive dilatation of the coronary artery segment.[11]
The majority of CAAs, about 50%–52%, are considered to be arteriosclerotic in origin. In their study, Berkoff and Rowe[12] hypothesized the presence of a thin, degenerated media, adjacent to an intimal plaque as the major pathological prerequisite leading to plaque rupture and erosion of grumous material by the bloodstream. This excavated plaque then leads to aneurysm formation.
Matrix metalloproteinases (MMPs) have been shown to be involved in the pathogenesis of CAA formation by causing increased proteolysis of extracellular matrix proteins.[13] MMPs (1, 2, 3, 9, and 12) are known to be capable of degrading essentially all components of the arterial wall matrix (elastin, collagen, proteoglycans, laminin, and fibronectin) and are present at elevated concentrations in aneurysms, while decreased levels of tissue inhibitors of MMPs are present. The MMP-3 5A allele is linked with higher promoter activity for transcription of the gene, and this allele is more frequently seen in patients with CAA and atherosclerosis compared to patients with only coronary atherosclerosis.[14]
Connective tissue disorders such as Marfan's syndrome can cause aneurysms without atherosclerosis. Marfan's syndrome is linked to mutations in the gene for fibrillin, and fibrillin is homologous with the family of latent transforming growth factor (TGF)-β binding proteins, which hold TGF-β in an inactive complex.[15,16]
CAA may complicate about 4% of coronary interventions.[17] The suggested pathogenesis of stent-related aneurysm formation is multifactorial. DES consists of immunosuppressants such as Sirolimus, which inhibits inflammation, or chemotherapeutic agents like paclitaxel, which is an anti-inflammatory agent as well as an inhibitor of cell proliferation. It has been proposed that, once the drug is eluted, the polymer in which the drug is embedded may evoke a hypersensitivity reaction and vasculitis which leads to weakening of the vessel wall and subsequent dilatation.[5]
Table 3 describes a classification system for coronary aneurysm that forms after coronary intervention, as proposed by Aoki et al.[10]
Table 3.
Classification of percutaneous intervention-associated coronary artery aneurysm
| Types | Description |
|---|---|
| Type I | Aneurysm that demonstrates rapid growth with pseudoaneurysm formation detected within 4 weeks[46] |
| Type II | Detected incidentally during angiography for recurrent symptoms or as part of protocol mandated follow-up ≥6 months after the procedure[47] |
| Type III | Mycotic or infectious in etiology[48] |
Clinical features
No clinical features are distinctive of CAAs. Chest pain, suggestive of stable angina, is the most frequent presentation in patients with coronary aneurysm.[18] Patients may present with ST-elevation myocardial infarction,[19] non-ST elevation myocardial infarction,[20] sudden cardiac death or complications such as thrombus formation, embolization, fistula formation, rupture, hemopericardium, tamponade, compression of surrounding structures, or congestive cardiac failure.[5,21,22]
Clinical presentation of the giant coronary aneurysm may mimic aneurysms of ascending aorta or the pulmonary trunk, or other conditions such as cardiac tumors, pericardial tumors or thymomas.[5] Giant coronary aneurysms are linked to advanced age, the tendency for complications, including rupture and may present as mediastinal, intra-cardiac mass or superior vena cava syndrome in addition to symptoms suggestive of ischemia.[21]
Diagnosis
CAA can be diagnosed by noninvasive and invasive techniques, such as echocardiography, CT, magnetic resonance imaging (MRI), and coronary angiography.
Coronary angiography remains the gold standard tool as it provides information about the shape, size, location, and co-existing anomalies such as coronary artery disease, and is also useful for setting up the strategy of surgical resection.[23] However, it is invasive with associated risks, expensive, and the true size of the coronary aneurysms may be underestimated if they contain a substantial amount of thrombus.[24]
Among noninvasive modalities, coronary CT is an alternative to invasive coronary angiography that can be suggested as a technique of choice for the follow-up of patients with CAAs because of improvements in terms of radiation dose with the current protocols.[25] However, this modality may have limitations in demonstrating clots or thrombus inside the vessel, in delineating the distal part of the coronary arteries, and in simulating a large coronary aneurysm as an inhomogenous mass because of the blood turbulence within it.[26]
Coronary magnetic resonance angiography (MRA) is an another noninvasive technique for the diagnosis and assessment of CAAs, avoiding the large radiation dose associated with coronary CT.[21] However, coronary MRA has its limitations; it is not available in all medical centers, has inferior spatial resolution compared to coronary CTA, and does not show the characteristic linear peripheral calcifications of the CAA, which are essential for making the correct diagnosis.[27]
Intravascular ultrasound (IVUS) has become the “gold standard” technique that produces transluminal images of the coronary arteries, including information on the composition of the lumen and the arterial wall structure.[28] IVUS is very helpful in differentiating true from false aneurysms caused by plaque rupture.[28]
Management
All patients with angiographic evidence of coronary aneurysms should have their cardiac risk factors aggressively dealt with, whether or not they have obstructive coronary artery disease. The options for managing coronary aneurysms are still ill-defined and present a therapeutic challenge to interventional cardiologists.[29] There are no randomized trials to evaluate different management strategies and their outcomes. Most of the information, we have at present, is based on the anecdotal case reports and expert consensus.
The treatment of CAA consists of medical management, surgical resection, and stent placement; however, the appropriate treatment for CAAs depends on the precise clinical situation. The clinical decision-making process is further complicated by the fact that the complete resolution of these aneurysms has been noticed in some cases without any treatment, contrary to the possibility of rupture if the aneurysm is left untreated. In their study, Aoki et al.[10] proposed that the treatment of CAA be “individualized” using a combination of the size of the aneurysm, expansion history, pathophysiology, and symptoms to decide when and if to apply therapy alternatives.
We discussed four cases with CAA, which were managed differently - conventional stenting, conservative management, coil occlusion of the coronary artery, and CABG. All management strategies were undertaken according to individual anatomical and clinical information, with excellent outcomes achieved in all cases.
Medical management
The evidence suggests that asymptomatic small CAAs may not require any treatment, but the dilatation may get progressively worse,[30] increasing the risk of complications.
Medical therapy generally consists of attempts to prevent thrombo-embolic complications in patients with aneurysmal arteries who are at increased thrombotic risk through the administration of antiplatelets and anticoagulant medications.[31] Aspirin is the preferred choice; and a second antiplatelet agent (such as clopidogrel, prasugrel, or ticagrelor) considered unless there is clinical contraindication.[32]
Lima et al. reported two cases of left main CAA that were managed conservatively with warfarin and aspirin who remained well at 6 months follow-up.[33] Other studies have demonstrated angiographic thrombus resolution and outstanding clinical outcomes with the use of intravenous eptifibatide, heparin, and aspirin, and long-term DAPT on discharge.[34]
There is evidence to suggest that excessive TGF-β[15] and metalloproteinase[5] may have some role in the development and progression of CAAs. Angiotensin II type-1 receptor antagonists, such as Losartan, can inhibit TGF-β.[35] Similarly, statins have been shown to inhibit the secretion of metalloproteinase-1, -2, -3, and -9 from macrophages and vascular smooth muscle cells.[36] Therefore, these drugs may be helpful in the treatment and prevention of progression of coronary aneurysms, though there have been no long-term randomized data available.
Percutaneous coronary intervention
Percutaneous intervention is a fairly new option with a markedly smaller data set. The described techniques include conventional stent implantation, coil embolization, autologous saphenous vein-covered stent grafting, and one case has been reported when DES implantation superimposed on a polytetrafluoroethylene (PTFE)-covered stent graft.[37]
PTFE-covered stents have emerged as a new tool for the treatment of CAAs.[38] However, some multicenter randomized trials in comparing expanded PTFE-stent graft with bare metal stents have demonstrated that these stents do not improve clinical outcomes and may be linked to a higher incidence of restenosis and early thrombosis.[39]
Szalat et al.[38] reported one of the largest retrospective studies comparing outcomes of patients treated with surgery (n = 18) or with PTFE-covered stents (n = 24). It was demonstrated that patients treated with stents were older (60.5 vs. 47.7 years old) and had smaller aneurysms (9.8 vs. 35.1 mm). No deaths were reported in either group. Only 5 of 24 patients who received stents were found to have restenosis on follow-up angiography and these patients tended to have aneurysms >10 mm in diameter.
Other authors have described successful treatment of CAA using coil embolization. Saccà et al.[40] reported a case of successful coil embolization and occlusion of CAA in the terminal LMS in a patient with prior coronary artery bypass graft surgery, including left internal mammary artery to LAD artery. The authors successfully deployed four Guglielmi detachable coils (Boston Scientific) into the aneurysm resulting in complete resolution of the aneurysm and a patent native left main at final angiography.
Surgery
Surgical management is appropriate in symptomatic patients who have obstructive coronary artery disease or evidence of embolization leading to myocardial ischemia and in patients with coronary aneurysm with a risk of rupture.[41] Various surgical strategies have been described including resection, aneurysm ligation, marsupialization with interposition graft, and coronary artery bypass surgery. The major bulk of experience regarding these strategies stem from atherosclerosis – induced CAAs.[41]
In symptomatic patients unsuitable for PCI, surgical excision or ligation of CAA combined with bypass grafting of the affected coronary arteries is the preferred option.[42]
Surgical approach is considered to be safer and more reliable for repair of a CAA/pseudoaneurysm. The indications for the surgical treatment of CAA in general are:
Severe coronary artery disease
CAAs near the bifurcation of large branches
Evidence of emboli from the aneurysm to the distal coronary bed resulting in myocardial ischemia
Progressive enlargement of a CAA documented by serial angiographic measurements; and
CAAs in the LMS
Complications such as fistula formation
Compression of cardiac chambers
Giant CAA (dilatation exceeding the reference vessel diameter by > four times).[43]
Based on the current literature, our proposed management strategies are highlighted in Figure 5. These are our own proposed strategies and are not endorsed by any of the American or European Cardiovascular Societies.
Figure 5.
Management algorithm: Our proposed management algorithm for managing coronary artery aneurysm
Prognosis
The prognosis of CAA depends on the size of the aneurysm. In general, small aneurysms have a favorable prognosis with a low risk of myocardial ischemic events and/or mortality.[44,45] On the contrary, giant CAAs (i.e., those with an internal diameter >8 mm) have a high risk of morbidity and mortality.[19] About one-half of such aneurysms become obstructed, and are associated with myocardial infarction, arrhythmias, or sudden death.
CONCLUSIONS
CAA is an uncommon entity and is frequently found incidentally during coronary angiography. The majority of the coronary aneurysms are atherosclerotic in origin, but they can also be congenital or secondary to inflammatory or connective tissue disorders with a well-known association with Kawasaki disease. The precise pathogenesis leading to CAA formation remains unclear. Treatment may consist of surgical, percutaneous or medical interventions, but the optimal treatment for CAA still remains debatable. With the increase in coronary angiography and more widespread use of imaging modalities, like high resolution CT scans and MRI, the diagnosis of coronary aneurysms is likely to become more frequent, and we need to have evidence-based management strategies to deal with this uncommon but complex condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors appreciate the support of the Catheterization Laboratory Staff of University Hospital of Wales, Cardiff and Morriston Hospital, Swansea for providing the images for this article.
REFERENCES
- 1.Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, et al. Aneurysmal coronary artery disease. Circulation. 1983;67:134–8. doi: 10.1161/01.cir.67.1.134. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–85. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Elahi MM, Dhannapuneni RV, Keal R. Giant left main coronary artery aneurysm with mitral regurgitation. Heart. 2004;90:1430. doi: 10.1136/hrt.2004.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Indolfi C, Achille F, Tagliamonte G, Spaccarotella C, Mongiardo A, Ferraro A, et al. Polytetrafluoroethylene stent deployment for a left anterior descending coronary aneurysm complicated by late acute anterior myocardial infarction. Circulation. 2005;112:e70–1. doi: 10.1161/CIRCULATIONAHA.104.497891. [DOI] [PubMed] [Google Scholar]
- 5.Nichols L, Lagana S, Parwani A. Coronary artery aneurysm: A review and hypothesis regarding etiology. Arch Pathol Lab Med. 2008;132:823–8. doi: 10.5858/2008-132-823-CAAARA. [DOI] [PubMed] [Google Scholar]
- 6.Bell MR, Garratt KN, Bresnahan JF, Edwards WD, Holmes DR., Jr Relation of deep arterial resection and coronary artery aneurysms after directional coronary atherectomy. J Am Coll Cardiol. 1992;20:1474–81. doi: 10.1016/0735-1097(92)90439-t. [DOI] [PubMed] [Google Scholar]
- 7.Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R, et al. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976;37:217–22. doi: 10.1016/0002-9149(76)90315-5. [DOI] [PubMed] [Google Scholar]
- 8.Díaz-Zamudio M, Bacilio-Pérez U, Herrera-Zarza MC, Meave-González A, Alexanderson-Rosas E, Zambrana-Balta GF, et al. Coronary artery aneurysms and ectasia: Role of coronary CT angiography. Radiographics. 2009;29:1939–54. doi: 10.1148/rg.297095048. [DOI] [PubMed] [Google Scholar]
- 9.Holmes DR, Jr, Vlietstra RE, Mock MB, Reeder GS, Smith HC, Bove AA, et al. Angiographic changes produced by percutaneous transcutaneous coronary angioplasty. Am J Cardiol. 1983;51:676–83. doi: 10.1016/s0002-9149(83)80114-3. [DOI] [PubMed] [Google Scholar]
- 10.Aoki J, Kirtane A, Leon MB, Dangas G. Coronary artery aneurysms after drug-eluting stent implantation. JACC Cardiovasc Interv. 2008;1:14–21. doi: 10.1016/j.jcin.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch GM, Casey PJ, Raza-Ahmad A, Miller RM, Hirsch KJ. Thrombosed giant coronary artery aneurysm presenting as an intracardiac mass. Ann Thorac Surg. 2000;69:611–3. doi: 10.1016/s0003-4975(99)01369-7. [DOI] [PubMed] [Google Scholar]
- 12.Berkoff HA, Rowe GG. Atherosclerotic ulcerative disease and associated aneurysms of the coronary arteries. Am Heart J. 1975;90:153–8. doi: 10.1016/0002-8703(75)90114-3. [DOI] [PubMed] [Google Scholar]
- 13.Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb. 1994;14:1315–20. doi: 10.1161/01.atv.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 14.Lamblin N, Bauters C, Hermant X, Lablanche JM, Helbecque N, Amouyel P, et al. Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. J Am Coll Cardiol. 2002;40:43–8. doi: 10.1016/s0735-1097(02)01909-5. [DOI] [PubMed] [Google Scholar]
- 15.Gelb BD. Marfan's syndrome and related disorders – More tightly connected than we thought. N Engl J Med. 2006;355:841–4. doi: 10.1056/NEJMe068122. [DOI] [PubMed] [Google Scholar]
- 16.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 17.Berkalp B, Kervancioglu C, Oral D. Coronary artery aneurysm formation after balloon angioplasty and stent implantation. Int J Cardiol. 1999;69:65–70. doi: 10.1016/s0167-5273(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 18.Aboeata AS, Sontineni SP, Alla VM, Esterbrooks DJ. Coronary artery ectasia: Current concepts and interventions. Front Biosci (Elite Ed) 2012;4:300–10. doi: 10.2741/377. [DOI] [PubMed] [Google Scholar]
- 19.Mrdović I, Jozić T, Asanin M, Perunicić J, Ostojić M. Myocardial reinfarction in a patient with coronary ectasia. Cardiology. 2004;102:32–4. doi: 10.1159/000077000. [DOI] [PubMed] [Google Scholar]
- 20.Kühl M, Varma C. A case of acute coronary thrombosis in diffuse coronary artery ectasia. J Invasive Cardiol. 2008;20:E23–5. [PubMed] [Google Scholar]
- 21.Channon KM, Wadsworth S, Bashir Y. Giant coronary artery aneurysm presenting as a mediastinal mass. Am J Cardiol. 1998;82:1307–8. doi: 10.1016/s0002-9149(98)00626-2. A11. [DOI] [PubMed] [Google Scholar]
- 22.Augustin N, Wessely R, Pörner M, Schömig A, Lange R. Giant coronary aneurysm obstructing the right heart. Lancet. 2006;368:386. doi: 10.1016/S0140-6736(06)69112-5. [DOI] [PubMed] [Google Scholar]
- 23.Pahlavan PS, Niroomand F. Coronary artery aneurysm: A review. Clin Cardiol. 2006;29:439–43. doi: 10.1002/clc.4960291005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy PA, Mohammed TL, Read K, Gilkeson RC, White CS. MDCT of coronary artery aneurysms. AJR Am J Roentgenol. 2005;184:S19–20. doi: 10.2214/ajr.184.3_supplement.01840s19. [DOI] [PubMed] [Google Scholar]
- 25.Devabhaktuni S, Mercedes A, Diep J, Ahsan C. Coronary artery ectasia-A review of current literature. Curr Cardiol Rev. 2016;12:318–23. doi: 10.2174/1573403X12666160504100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konen E, Feinberg MS, Morag B, Guetta V, Shinfeld A, Smolinsky A, et al. Giant right coronary aneurysm: CT angiographic and echocardiographic findings. AJR Am J Roentgenol. 2001;177:689–91. doi: 10.2214/ajr.177.3.1770689. [DOI] [PubMed] [Google Scholar]
- 27.Halapas A, Lausberg H, Gehrig T, Friedrich I, Hauptmann KE. Giant right coronary artery aneurysm in an adult male patient with non-ST myocardial infarction. Hellenic J Cardiol. 2013;54:69–76. [PubMed] [Google Scholar]
- 28.Sanidas EA, Vavuranakis M, Papaioannou TG, Kakadiaris IA, Carlier S, Syros G, et al. Study of atheromatous plaque using intravascular ultrasound. Hellenic J Cardiol. 2008;49:415–21. [PubMed] [Google Scholar]
- 29.Bajaj S, Parikh R, Hamdan A, Bikkina M. Covered-stent treatment of coronary aneurysm after drug-eluting stent placement: Case report and literature review. Tex Heart Inst J. 2010;37:449–54. [PMC free article] [PubMed] [Google Scholar]
- 30.Bhindi R, Testa L, Ormerod OJ, Banning AP. Rapidly evolving giant coronary aneurysm. J Am Coll Cardiol. 2009;53:372. doi: 10.1016/j.jacc.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 31.Demopoulos VP, Olympios CD, Fakiolas CN, Pissimissis EG, Economides NM, Adamopoulou E, et al. The natural history of aneurysmal coronary artery disease. Heart. 1997;78:136–41. doi: 10.1136/hrt.78.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer N, Gupta R, Schevchuck A, Hindnavis V, Maliske S, Sheldon M, et al. Coronary artery aneurysms in acute coronary syndrome: Case series, review, and proposed management strategy. J Invasive Cardiol. 2014;26:283–90. [PubMed] [Google Scholar]
- 33.Lima B, Varma SK, Lowe JE. Nonsurgical management of left main coronary artery aneurysms: Report of 2 cases and review of the literature. Tex Heart Inst J. 2006;33:376–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Vik-Mo H, Wiseth R, Hegbom K. Coronary aneurysm after implantation of a paclitaxel-eluting stent. Scand Cardiovasc J. 2004;38:349–52. doi: 10.1080/14017430410016413. [DOI] [PubMed] [Google Scholar]
- 35.Guo ZX, Qiu MC. Losartan downregulates the expression of transforming growth factor beta type I and type II receptors in kidney of diabetic rat. Zhonghua Nei Ke Za Zhi. 2003;42:403–8. [PubMed] [Google Scholar]
- 36.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23:769–75. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 37.Ghanta RK, Paul S, Couper GS. Successful revascularization of multiple coronary artery aneurysms using a combination of surgical strategies. Ann Thorac Surg. 2007;84:e10–1. doi: 10.1016/j.athoracsur.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Szalat A, Durst R, Cohen A, Lotan C. Use of polytetrafluoroethylene-covered stent for treatment of coronary artery aneurysm. Catheter Cardiovasc Interv. 2005;66:203–8. doi: 10.1002/ccd.20448. [DOI] [PubMed] [Google Scholar]
- 39.Schächinger V, Hamm CW, Münzel T, Haude M, Baldus S, Grube E, et al. A randomized trial of polytetrafluoroethylene- membrane-covered stents compared with conventional stents in aortocoronary saphenous vein grafts. J Am Coll Cardiol. 2003;42:1360–9. doi: 10.1016/s0735-1097(03)01038-6. [DOI] [PubMed] [Google Scholar]
- 40.Saccà S, Pacchioni A, Nikas D. Coil embolization for distal left main aneurysm: A new approach to coronary artery aneurysm treatment. Catheter Cardiovasc Interv. 2012;79:1000–3. doi: 10.1002/ccd.23195. [DOI] [PubMed] [Google Scholar]
- 41.LaMotte LC, Mathur VS. Atherosclerotic coronary artery aneurysms: 8-year angiographic follow-up. Tex Heart Inst J. 2000;27:72–3. [PMC free article] [PubMed] [Google Scholar]
- 42.Badmanaban B, Mallon P, Campbell N, Sarsam MA. Repair of left coronary artery aneurysm, recurrent ascending aortic aneurysm, and mitral valve prolapse 19 years after Bentall's procedure in a patient with Marfan syndrome. J Card Surg. 2004;19:59–61. doi: 10.1111/j.0886-0440.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- 43.Bradbury AW, Milne AA, Murie JA. Surgical aspects of Behçet's disease. Br J Surg. 1994;81:1712–21. doi: 10.1002/bjs.1800811205. [DOI] [PubMed] [Google Scholar]
- 44.Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364:533–44. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 45.Roberts WC. Natural history, clinical consequences, and morphologic features of coronary arterial aneurysms in adults. Am J Cardiol. 2011;108:814–21. doi: 10.1016/j.amjcard.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F, Qian JY, Ge JB. Rapid development of late stent malappositon and coronary aneurysm following implantation of a paclitaxel-eluting coronary stent. Chin Med J (Engl) 2007;120:614–6. [PubMed] [Google Scholar]
- 47.Nilsen DW, Melberg T, Larsen AI, Barvik S, Bonarjee V. Late complications following the deployment of drug eluting stents. Int J Cardiol. 2006;109:398–401. doi: 10.1016/j.ijcard.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 48.Singh H, Singh C, Aggarwal N, Dugal JS, Kumar A, Luthra M, et al. Mycotic aneurysm of left anterior descending artery after sirolimus-eluting stent implantation: A case report. Catheter Cardiovasc Interv. 2005;65:282–5. doi: 10.1002/ccd.20338. [DOI] [PubMed] [Google Scholar]