Abstract
Background:
Recently, EUS-guided fine-needle biopsy (EUS-FNB) using a Franseen needle was developed for histological tissue acquisition. However, the yield of a 25G Franseen needle when acquiring histological core tissue has been unclear.
Patients and Methods:
We performed a prospective, multicenter, and observational cohort study that included 100 solid lesions scheduled for EUS-FNB using a 25G Franseen needle at eight centers in Hokkaido, Japan. Only EUS-FNB specimens acquired at the first pass were evaluated without a rapid on-site evaluation. The tissue acquisition rate, acquisition rate of an adequate specimen for histological assessment, the quality of tissue sample, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic accuracy, and adverse events were evaluated.
Results:
We analyzed a total of 100 solid lesions in 100 patients. The patients were 57 males and 43 females with a median age of 70 years. The technical success rate was 100%. The tissue acquisition rate was 95.0%. The acquisition rate of an adequate specimen for histological assessment was 82.0%. The sensitivity, specificity, PPV, NPV, and diagnostic accuracy were 87.0%, 100%, 100%, 40.0%, and 88.0%, respectively. The adverse event rate was 1.0%, and it was reported in only one patient who had a moderate pancreatic fistula.
Conclusions:
EUS-FNB using the 25G Franseen needle was feasible, and adequate histological core tissue samples were acquired with this method.
Keywords: EUS, EUS-guided fine-needle biopsy, Franseen needle, tissue acquisition
INTRODUCTION
EUS-guided FNA was initially reported by Vilmann et al. in 1992[1] and it has a high-diagnostic accuracy that ranges from 78% to 95%.[2] In most cases, a cytological assessment is sufficient for a diagnosis. However, all lesions cannot be reliably diagnosed using FNA cytology alone, especially in the presence of chronic pancreatitis.[3] In addition, the adequate tissue architecture and immunohistochemical (IHC) analysis are required for diagnosing some lesions, such as autoimmune pancreatitis, gastrointestinal stromal tumors, and malignant lymphomas.[4,5,6] New-needle types have been developed to acquire not only cytology specimens but also sufficient biopsy specimens (fine-needle biopsy [FNB] sampling). Some EUS-FNB needles (Echotip ProCore®, Cook Japan, Tokyo, Japan; Shark Core®, Covidien, Dublin, Leinster, Ireland, UK) are designed to acquire the core samples.[7,8,9,10]
Recently, a Franseen needle (Acquire™, Boston Scientific Japan, Tokyo, Japan) [Figure 1] was designed for EUS-FNB, and has proven useful in the acquisition of histological core tissue with needle sizes of 22G and 25G.[11,12,13,14,15,16] The specimens obtained with a 22G or 19G EUS-FNA needle are more adequate for histological diagnosis than those obtained with a 25G needle. However, it has been revealed that there is a higher technical success rate with a 25G needle than with a 22G and a 19G needle.[17] We hypothesized that FNB sampling using a 25G needle with Franseen geometry would improve the ability to easily obtain true histological specimens.
Figure 1.

The 25G Franseen needle design with a crown-shaped needle tip with three-symmetrical planes (picture provided by the Boston Scientific Japan)
The aim of our prospective multicenter observational study was to evaluate the ability of the 25G Franseen needle to acquire the histological core tissue samples.
PATIENTS AND METHODS
Study design
A prospective multicenter observational cohort study was conducted at Hokkaido University Hospital, Teine-Keijinkai Hospital, Hokkaido Medical Center, Tonan Hospital, NTT East Sapporo Hospital, Tomakomai City Hospital, Kushiro Rosai Hospital, and Hakodate Municipal Hospital. Consecutive patients with a solid lesion who required EUS-FNB/-FNA for the diagnosis were registered to evaluate the ability of the 25G Franseen needle to acquire the tissue samples in clinical practice. The inclusion criteria were as follows: (1) ≥20 years of age, (2) Use of the 25G Franseen needle for the biopsy, and (3) A written informed consent. The exclusion criteria were as follows: (1) Cystic lesion, (2) Coagulopathy (prothrombin time/international normalized ratio ≥1.5), (3) Thrombocytopenia (platelet count <50,000/mL), (4) Inability to stop anticoagulation therapy, (5) The American society of anesthesiologist's physical status classification >3, (6) An Eastern cooperative oncology group performance status of 4, and (7) Pregnancy. All patients provided written informed consent, and the study was registered at the University Hospital Medical Information Network Clinical Trials Registry (No. 000029612).
Procedure technique
Patients were placed in the left lateral decubitus position and were administered sedatives for conscious sedation. A curved linear-array echoendoscope (GF-UCT240-AL5 or GF-UCT260; Olympus medical systems, Tokyo, Japan) was used with the standard station approach. When a lesion was identified, cross-sectional size measurements, including measurement of the greatest dimensions were obtained. The stylet was slightly withdrawn before puncturing. Avoiding intervening vessels, we punctured the lesion through the esophageal, gastric, or duodenal wall using a 25G Franseen needle (Acquire™, Boston Scientific Japan). After the needle was advanced into the target lesion, the stylet was once advanced into the needle and was subsequently withdrawn. A 20-mL syringe with 20-mL negative pressure (NP) was attached to the proximal end of the needle. The needle was then moved back and forth ten times. Rapid on-site cytological evaluation (ROSE) was not performed at the first pass. All procedures were performed at the participating facilities by one of the 14 experienced endosonographers (>50 EUS-FNA/-FNB procedures/year and >100 EUS-FNA/-FNB procedures in total). All EUS-FNB specimens were put into formalin containers for histological examination. The endosonographer (endoscopist) also checked a white-core tissue on the slide before storing a sample in a formalin container at the first pass for evaluating whether or not sufficient specimen was obtained. A white-core tissue was defined as a visible white fragment on the slide with 1–2 mm in length. If a white-core tissue was not identified by the endosonographer, an additional puncture was permitted for clinical practice; however, the results of the additional passes were not included in this study to simplify the protocol and analysis, and also to reduce the burden on the patients as far as possible. If the needle was mounted on a scope and the target lesion could not be punctured, the procedure was interpreted as a technical failure.
Pathological assessment
The specimen obtained at the first pass was fixed in formalin, embedded in paraffin, and sectioned for histopathological analysis. Hematoxylin and eosin (H and E) staining was used for the analysis. All specimens were blindly evaluated by an experienced pathologist (T. M.). Based on the scoring system previously reported by Kudo et al.[18] and Abe et al.,[19] cellularity was scored from 0 to 5 using the following criteria: 0 indicates samples with insufficient material for interpretation; 1, samples allowing for a limited cytological interpretation; 2, samples with sufficient material for an adequate cytological interpretation; 3, samples allowing for a limited histological assessment; 4, samples with sufficient material for an adequate histological interpretation, but a low-quality sample (total material within a ×10 power field in length); and 5, samples sufficient for adequate histological interpretation and a high-quality sample (total tissue sample more than a ×10 power field in length). Samples scoring a 1 or 2 were not sufficient for any histological analysis. Figure 2 shows representative examples with score of 3, 4, and 5.
Figure 2.
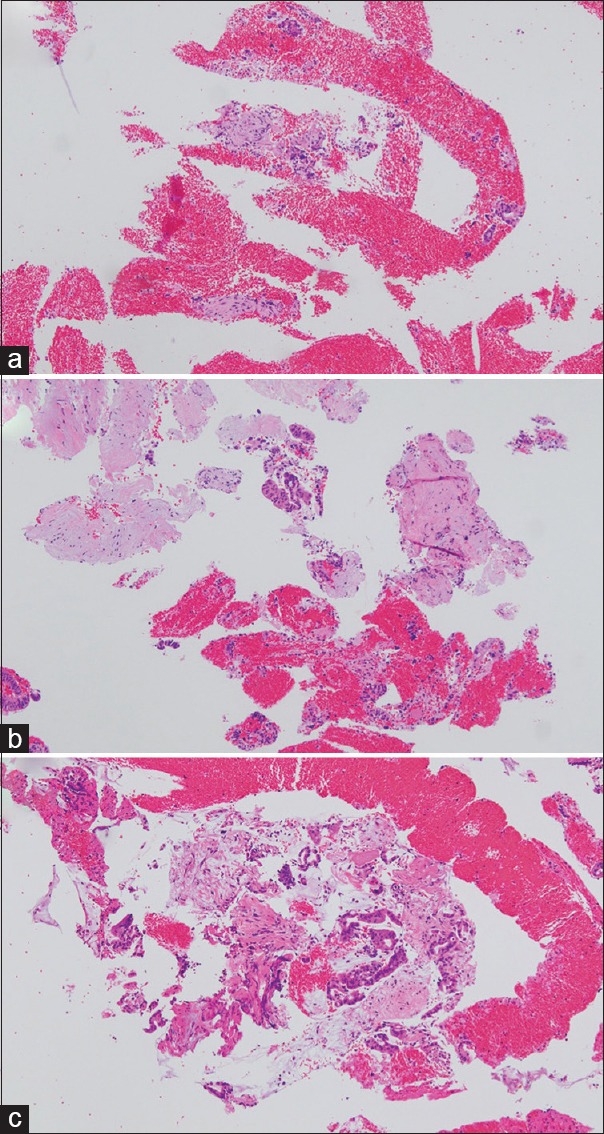
Images of specimens obtained by using the 25G Franseen needle. (a) This specimen (score of 3) is recognizable as a small-tissue cluster. Evaluation of a part of the tissue architecture and limited histological interpretation is possible. (b) In this sample (score of 4), there is sufficient material for adequate histological diagnosis, and the tissue architecture can be evaluated. The area of tissue on the prepared slide is within 10 power field in length. (c) In this sample (score of 5), there is sufficient material for adequate histological diagnosis, and the tissue architecture can be evaluated. The area of tissue on the prepared slide is more than 10 power field in length (H and E, ×100)
As previously reported,[18,19] contamination and bloodiness were scored from 1 to 3. A contamination score of 1 meant that <25% of the specimen was contaminated with digestive tract mucosa, a score of 2 meant that 25%–50% of the specimen was contaminated, and a score of 3 meant that >50% of the specimen was contaminated. A bloodiness score of 1 meant that the sample had very few red blood cells on the slide, a score of 2 meant that the sample had a medium grade of blood, and a score of 3 meant that the sample on the slide was mostly blood. The pathologist evaluated the degree of red blood cells contamination in the whole under the very-low-magnification.
Clinical diagnostic methodology of benignity and malignancy used for the final diagnosis
All specimens were classified as benign, malignant, or nondiagnostic by histopathological examination. Carcinoma, neuroendocrine tumor, gastrointestinal stromal tumor, and lymphoma were defined as malignant. Slides without malignant cells were defined as benign. When there was either insufficient material to make a diagnosis or indeterminate material on the H and E slide, the sample was defined as nondiagnostic. As tumors such as neuroendocrine tumors and gastrointestinal stromal tumors were nondiagnostic with H and E staining alone, even when an adequate specimen at the first pass was available, in these cases an IHC analysis of the first specimen was performed for an accurate diagnosis.
In this study, malignant diseases were ultimately diagnosed on the basis of histopathological analyses of surgically resected specimens, and radiological or clinical data indicating an evidence of a disease progression. Benign diseases were diagnosed on the basis of a decrease or no change in the mass of the tumor and no change or improvement in its clinical course for >6 months.
Adverse events
The occurrence of immediate adverse events was noted at the time of the procedure, and the patient was followed-up on for 2 weeks after the procedure for the late adverse events. Grading of the adverse events was made according to the severity of the grading system of the American Society for Gastrointestinal Endoscopy Lexicon.[20]
Outcome measures
The primary outcome of the current study was a tissue acquisition rate (cellularity scores ≥3) in the initial pass. The secondary outcomes were an acquisition rate of an adequate specimen for histological assessment (cellularity scores of 4 and 5), quality of the tissue sample, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic accuracy of EUS-FNB, success rate of puncture, and adverse event rate.
Sample size
The sample size was determined on the basis of published data claiming a 72% tissue acquisition rate by using a 25G conventional EUS-FNA needle in two passes with normal NP.[18] We assumed a slightly higher tissue acquisition rate of target samples (cellularity scores ≥3) by using the 25G Franseen needle procedure of 85% in one pass. We set the 95% confidence interval at 15% (77.5%–92.5%), and based on this assumption, we calculated the sample size to be 88. Assuming that some of the enrolled patients would later dropout, we set 100 as the final sample size.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 7.0 (GraphPad Software Inc., San Diego, CA, USA) and the free software, EZR.[21] Results are shown as means (standard deviation) for quantitative variables, medians (range) for nonparametric variables, and percentages for categorical variables. Intragroup comparisons of the histological scores among sites of the target lesions (pancreas versus lymph node; pancreatic head, uncinate process, body, and tail) were done using the Fisher's exact test. Differences were considered as statistically significant at probability P < 0.05.
RESULTS
Baseline characteristics
A total of 100 patients (100 lesions) were registered at eight referral centers between September 2017 and March 2018. The patients were 57 males and 43 females with a median age of 70 years (31–87) as shown in Table 1. The sites of target lesions were the pancreas for 81 lesions, lymph node for 13 lesions, duodenum for two lesions, liver for two lesions, gallbladder for one lesion, and the stomach for one lesion. EUS-FNB procedures were performed through the gastric wall in 57 lesions, the duodenal wall in 42 lesions, and esophageal wall in one lesion. The median longest dimension of lesions was 23.5 mm (range 7.0–82.0). Twenty-six lesions could be evaluated using both EUS-FNB specimens and surgically resected specimens, whereas 74 lesions were evaluated using EUS-FNB specimens and by observing the clinical course of the disease. As shown in Table 2, the final diagnoses were pancreatic cancer in 67 lesions, pancreatic neuroendocrine tumor in nine lesions, gastrointestinal stromal tumor in two lesions, a malignant lymphoma in two lesions, ampullary cancer in one lesion, gallbladder cancer in one lesion, lymph node metastasis in eight lesions, liver metastasis in two lesions, chronic pancreatitis in four lesions, autoimmune pancreatitis in two lesions, and benign lymphadenopathy in two lesions.
Table 1.
Baseline characteristics
| Factor | |
|---|---|
| Male/female, n | 57/43 |
| Age (years), median (range) | 70 (31–87) |
| Site of target lesion, n | |
| Pancreas | 81 |
| Lymph node | 13 |
| Duodenum | 2 |
| Liver | 2 |
| Gall bladder | 1 |
| Stomach | 1 |
| Location of a pancreatic lesion, n | |
| Head proper | 33 |
| Uncinate process | 12 |
| Body | 20 |
| Tail | 16 |
| Location of a lymph node, n | |
| #16 | 8 |
| #3 | 2 |
| #15 | 1 |
| #12 | 1 |
| #4 | 1 |
| Puncture route, n | |
| Transgastric | 57 |
| Transduodenal | 42 |
| Transesophageal | 1 |
| Longest dimension of a lesion, mm, median (range) | 23.5 (7.0–82.0) |
| Distribution of longest dimension of a lesion, n | |
| 0–10 | 1 |
| 11–20 | 36 |
| 21–30 | 35 |
| 31–40 | 21 |
| 41–50 | 4 |
| 51–60 | 1 |
| 61–70 | 0 |
| 71–80 | 1 |
| 81–90 | 1 |
Table 2.
Final diagnosis
| Disease | No. |
|---|---|
| Pancreatic cancer | 67 |
| Pancreatic neuroendocrine tumor | 9 |
| Gastrointestinal stromal tumor | 2 |
| Malignant lymphoma | 2 |
| Duodenum papilla cancer | 1 |
| Gallbladder cancer | 1 |
| Lymph node metastasis | 8 |
| Liver metastasis | 2 |
| Chronic pancreatitis | 4 |
| Autoimmune pancreatitis | 2 |
| Benign lymphadenopathy | 2 |
Primary and secondary outcomes
All patients underwent EUS-FNB with the 25G Franseen needle. The tissue acquisition rate at first pass was 95.0% (95/100) in the EUS-FNB samples evaluated by the pathologist as shown in Table 3. The acquisition rate of an adequate specimen for histological assessment at first pass was 82.0% (82/100). The contamination scores were a score of 2 in 4 lesions and 1 in 96 lesions. The bloodiness scores were a score of 3 in 6 lesions, 2 in 67 lesions, and 1 in 27 lesions. The tissue acquisition rate (cellularity scores ≥3) was high, thus the amount of red blood cells did not affect the final diagnosis evaluated by the pathologist. The tissue acquisition rates (cellularity scores ≥3) were not significantly different between the pancreatic lesion (95.1%) and lymph node (91.7%) as shown in Table 4 (P > 0.05). Similarly, the acquisition rates of an adequate specimen for histological assessment (cellularity scores ≥4) were not significantly different between the pancreatic lesion (81.5%) and lymph node (83.3%) (P > 0.05). The similar results were observed among the locations of the pancreatic lesions as shown in Table 5 (P > 0.05). The overall sensitivity, specificity, PPV, NPV, and diagnostic accuracy were 87.0% (80/92), 100% (8/8), 100% (80/80), 40.0% (8/20), and 88.0% (88/100), respectively [Table 6]. There were no early-adverse events and only one late adverse event in one patient (1.0%), who had pancreatic tail cancer (the longest dimension of the lesion was 31.9 mm) and underwent transgastric puncture. The event was a moderate pancreatic fistula successfully treated by the conservative therapy.
Table 3.
Histological findings of the specimens
| Cellularity score | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| n | 0 | 0 | 5 | 13 | 56 | 26 |
| Tissue acquisition rate (cellularity scores ≥3) (%) | 95.0 | |||||
| Adequate specimen for histological assessment (cellularity scores ≥4) (%) | 82.0 | |||||
| Contamination score | ||||||
| 1 | 2 | 3 | ||||
| n | 96 | 4 | 0 | |||
| Bloodiness score | ||||||
| 1 | 2 | 3 | ||||
| n | 27 | 67 | 6 | |||
Table 4.
Histological findings of the specimens in the pancreas and lymph node
| Site of a target lesion | Pancreas (n=81) | Lymph node (n=13) | P |
|---|---|---|---|
| Cellularity score, n | |||
| 0 | 0 | 0 | 0.89 |
| 1 | 0 | 0 | |
| 2 | 4 | 1 | |
| 3 | 11 | 1 | |
| 4 | 45 | 8 | |
| 5 | 21 | 3 | |
| Tissue acquisition rate (cellularity scores ≥3) (%) | 95.1 | 92.3 | 0.53 |
| Adequate specimen for histological assessment (cellularity scores ≥4) (%) | 81.5 | 84.6 | 1 |
| Contamination score, n | |||
| 1 | 78 | 12 | 0.45 |
| 2 | 3 | 1 | |
| 3 | 0 | 0 | |
| Bloodiness score, n | |||
| 1 | 22 | 4 | 0.45 |
| 2 | 53 | 9 | |
| 3 | 6 | 0 |
Table 5.
Histological findings of the specimens classified by locations of pancreatic lesions
| Site of target lesion | Head (n=33) | Uncinate process (n=12) | Body (n=20) | Tail (n=16) | P |
|---|---|---|---|---|---|
| Cellularity score, n | |||||
| 0 | 0 | 0 | 0 | 0 | 0.34 |
| 1 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 2 | 2 | |
| 3 | 3 | 3 | 3 | 2 | |
| 4 | 23 | 5 | 10 | 7 | |
| 5 | 7 | 4 | 5 | 5 | |
| Tissue acquisition rate (cellularity scores ≥3), % | 100 | 100 | 90.0 | 87.5 | 0.09 |
| Adequate specimen for histological assessment (cellularity scores ≥4), % | 90.9 | 75.0 | 75.0 | 75.0 | 0.29 |
Table 6.
Yields of EUS-guided fine-needle biopsy
| Final diagnosis of malignancy (n=92) | Surgical histology | Radiological or clinical data | Total |
|---|---|---|---|
| Diagnostic, n | 22 | 58 | 80 |
| Nondiagnostic, n | 4 | 8 | 12 |
| Final diagnosis of benignity (n=8) | Radiological or clinical data | Total | |
| Diagnostic, n | 8 | 8 | |
| Nondiagnostic, n | 0 | 0 | |
| Sensitivity | 87.0% (80/92) | ||
| Specificity | 100% (8/8) | ||
| Positive predictive value | 100% (80/80) | ||
| Negative predictive value | 40.0% (8/20) | ||
| Diagnostic accuracy | 88.0% (88/100) | ||
DISCUSSION
The current study showed the efficacy of EUS-FNB using a 25G Franseen needle for pathological diagnosis. A previous report showed that the tissue acquisition rate using a 25G conventional EUS-FNA needle was 72% after two passes.[18] In the present study, the tissue acquisition rate using a 25G Franseen needle was 95% in one pass alone.
Previous studies have reported the acquisition rate of an appropriate and sufficient specimen for histological assessment using a 22G Franseen needle. A previous animal experiment showed that the mean amount of core tissue-acquired using a 22G Franseen needle was significantly higher than that of a conventional 22G needle.[12] In addition, a previous randomized trial showed that the procurement of histological core tissue indicated by the total tissue and tumor area acquired was significantly higher for a 22G Franseen needle than that of a 22G conventional needle.[16] Furthermore, a retrospective pilot study and randomized, controlled trial showed that the mean number of needle passes was 1.5 times (±1.2) and 1.04 times (±0.20) using a 22G Franseen needle, respectively.[11,14] These results and our data suggest that EUS-FNB using a Franseen needle is effective for histological tissue procurement using a lesser number of passes, and thus can help in an efficient histological diagnosis.
Meanwhile, a previous study showed that a higher technical success rate of EUS-FNA can be achieved with a thin-25G conventional needle than with a thick-22G or 19G conventional needle.[17] Some previous studies using a thick-22G Franseen needle revealed a high-technical success rate (96.7%–100.0%).[11,14] However, these clinical trials were conducted at tertiary referral centers, and therefore, the results might not be applicable to all centers. Because a thick-needle can skid on the surface of the gastrointestinal mucosa, it is more difficult to puncture the target lesions with a 22G Franseen needle than with a 25G Franseen needle. The current study revealed that there were no instances of technical failure while using a 25G Franseen needle, despite the involvement of multiple centers and multiple endoscopists. These data indicate that EUS-FNB approaches using a 25G Franseen needle are feasible regardless of the skill and experience of the endosonographer.
There is no consensus regarding EUS-FNB approaches with NP suction (NPS) using Franseen needles. A previous animal experiment showed that there was no significant difference in the mean amount of core tissue score and blood score in EUS-FNB with suction and without suction.[12] Whereas, a previous randomized and controlled trial using a conventional 25G needle showed that EUS-FNA approaches with NPS were superior to those without NPS,[22,23] and based on this trial, we chose an EUS-FNB approach with NPS for the current study. Further studies are needed to determine the necessity of NPS in EUS-FNB approaches.
In the current study using a 25G Franseen needle, NPV was low, whereas the overall sensitivity, specificity, PPV, and diagnostic accuracy were high. There are several explanations for this. One, the rate of benign tumor was only 8.0%. Another is that only specimens obtained at the first pass were evaluated without ROSE. The role of ROSE remains unclear while using a Franseen needle. However, a previous report showed that ROSE had a positive effect on the diagnostic accuracy of EUS-FNA.[24] Thus, we speculate that ROSE using a 25G Franseen needle can further improve the diagnostic yields.
In the current study, the rate of adverse events was low. A recent meta-analysis of the results of 31 prospective studies showed a cumulative FNA-related adverse event rate of 1.72%.[25] Regarding FNB-related adverse events using Franseen needles, some recent studies have showed a range of adverse event rates from 0% to 3.3%.[13,14,15] These studies, along with our data, indicate that adverse event rates using a Franseen needle are low and similar to the rates observed using a conventional needle.
There are several limitations to the current study. First, we evaluated specimens obtained at the first pass alone without ROSE. Second, as a variety of lesions were targeted, it is unclear whether the results would change according to the type of target lesion.
CONCLUSIONS
The 25G Franseen needle was useful in the acquisition of histological core tissue samples and can be a safe first-line diagnostic technique for an accurate diagnosis using a histological analysis.
Financial support and sponsorship
This study was supported by a research grant for young researchers from Japan Gastroenterological Endoscopy Society Hokkaido Chapter.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We express our deepest appreciation to Dr. Kazumichi Kawakubo, Shin Kato, Koji Hirata (Department of Gastroenterology and Hepatology, Hokkaido University Faculty of Medicine and Graduate School of Medicine); Dr. Hiroyuki Maguchi, Kuniyuki Takahashi, Tsuyoshi Hayashi, Toshifumi Kin, Masato Endo, Naohiro Komatsu, Kazumasa Nagai, Kazunari Tanaka, Yousuke Kobayashi, Yukiko Takigawa, Ran Utsunomiya (Center for Gastroenterology, Teine-Keijinkai Hospital); Dr. Toshio Kimura, Shuichi Muto, Yuki Tsukamoto, Urara Baba, Seiji Tsunematsu, Mio Matsumoto, Rui Sasaki, Kensuke Sakurai (Department of Gastroenterology, Hokkaido Medical Center); Dr. Hitoshi Kondo, Michiaki Hirayama, Tetsuya Sumiyoshi, Kotaro Morita, Yutaka Okagawa, Takeyoshi Minagawa, Shota Yoshida, Kaho Tokuchi, Shutaro Ohiwa (Department of Gastroenterology, Tonan Hospital); Dr. Shinji Yoshii, Masayoshi Dazai, Daisuke Miyamoto, Marina Kubo, Hiroki Egami (Department of Gastroenterology, NTT East Sapporo Hospital); Dr. Fumiyasu Yamamoto, Tomoe Kobayashi, Tatsuya Nishizawa, Ken Ito, Ren Yamada (Department of Gastroenterology, Tomakomai City Hospital); Takuto Miyagishima, Hisashi Oda, Yoshimitsu Kobayashi, Kazuhiro Takahashi, Katsumi Terashita, Kazuaki Harada, Hajime Hirata, Koichiro Sarashina, Soichiro Matsuda, Chihiro Nishida (Department of Internal Medicine, Kushiro Rosai Hospital); Dr. Hirohito Naruse, Yoshiya Yamamoto, Kazuteru Hatanaka, Masayoshi Ono, Jun Ito, Yoshihiko Shimoda, Takashi Kitagataya (Department of Gastroenterology and Hepatology, Hakodate Municipal Hospital). We also thank Boston Scientific Japan (Tokyo, Japan) for providing a picture of the Franseen needle.
REFERENCES
- 1.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 2.Itoi T, Sofuni A, Itokawa F, et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23(Suppl 1):17–21. doi: 10.1111/j.1443-1661.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 3.Weynand B, Deprez P. Endoscopic ultrasound guided fine needle aspiration in biliary and pancreatic diseases: Pitfalls and performances. Acta Gastroenterol Belg. 2004;67:294–300. [PubMed] [Google Scholar]
- 4.Deshpande V, Mino-Kenudson M, Brugge WR, et al. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: Diagnostic criteria and pitfalls. Am J Surg Pathol. 2005;29:1464–71. doi: 10.1097/01.pas.0000173656.49557.48. [DOI] [PubMed] [Google Scholar]
- 5.Na HK, Lee JH, Park YS, et al. Yields and utility of endoscopic ultrasonography-guided 19-gauge trucut biopsy versus 22-gauge fine needle aspiration for diagnosing gastric subepithelial tumors. Clin Endosc. 2015;48:152–7. doi: 10.5946/ce.2015.48.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 7.Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: A prospective comparison study. Endoscopy. 2013;45:792–8. doi: 10.1055/s-0033-1344217. [DOI] [PubMed] [Google Scholar]
- 8.Adler DG, Witt B, Chadwick B, et al. Pathologic evaluation of a new endoscopic ultrasound needle designed to obtain core tissue samples: A pilot study. Endosc Ultrasound. 2016;5:178–83. doi: 10.4103/2303-9027.183976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues-Pinto E, Jalaj S, Grimm IS, et al. Impact of EUS-guided fine-needle biopsy sampling with a new core needle on the need for onsite cytopathologic assessment: A preliminary study. Gastrointest Endosc. 2016;84:1040–6. doi: 10.1016/j.gie.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest Endosc. 2016;84:1034–9. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 11.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 12.Mukai S, Itoi T, Katanuma A, et al. An animal experimental study to assess the core tissue acquisition ability of endoscopic ultrasound-guided histology needles. Endosc Ultrasound. 2018;7:263–9. doi: 10.4103/eus.eus_16_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitri RD, Rimbaş M, Attili F, et al. Performance of a new needle for endoscopic ultrasound-guided fine-needle biopsy in patients with pancreatic solid lesions: A retrospective multicenter study. Endosc Ultrasound. 2018;7:329–34. doi: 10.4103/eus.eus_33_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the franseen and fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–8. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Abdelfatah MM, Grimm IS, Gangarosa LM, et al. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest Endosc. 2018;87:495–500. doi: 10.1016/j.gie.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Bang JY, Hebert-Magee S, Navaneethan U, et al. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081–4. doi: 10.1136/gutjnl-2017-315154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–90. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 18.Kudo T, Kawakami H, Hayashi T, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–70. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Abe Y, Kawakami H, Oba K, et al. Effect of a stylet on a histological specimen in EUS-guided fine-needle tissue acquisition by using 22-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2015;82:837–440. doi: 10.1016/j.gie.2015.03.1898. [DOI] [PubMed] [Google Scholar]
- 20.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri R, Vilmann P, Săftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 25.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73:283–90. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]


