Abstract
Background:
Although previous studies have reported negative associations between exposure to air pollution and cognition, studies of the effects of prenatal and postnatal exposures in early childhood have been limited.
Objectives:
We sought to assess the role exposure to fine particulate matter () during different prenatal and postnatal windows may play in children’s cognitive development at school age.
Methods:
Within the Brain Development and Air Pollution Ultrafine Particles in School Children (BREATHE) Project, we estimated residential exposures by land use regression for the prenatal period and first seven postnatal years of 2,221 children from Barcelona, Spain. The participants () completed computerized tests assessing working memory, attentiveness, and conflict network during four visits in 2012–2013. We used linear mixed effects and distributed lag models to assess the period of exposure to in association with cognitive development.
Results:
Inverse associations were identified between exposure during the fifth and sixth postnatal years and working memory, with boys showing much higher vulnerability. Regarding attention functions, exposure to higher levels during the prenatal period and from the fourth postnatal year were associated with a reduction in conflict network performance, though we found no association with attentiveness. The overall estimated cumulative effect of a increase in resulted in a reduction in the working memory score of [95% confidence interval (CI): , ] points and an increase in the conflict attentional network of 11.31 (95% CI: 6.05, 16.57) milliseconds, indicating a poorer performance.
Conclusions:
Early life exposure to was associated with a reduction in fundamental cognitive abilities, including working memory and conflict attentional network. https://doi.org/10.1289/EHP3169
Introduction
Air pollution is the main environmental contributor to the global burden of disease (GBD 2013 Risk Factor Collaborators 2015). Over the last few years, interest in investigating the associations between air pollution and cognitive function has increased (Suades-González et al. 2015), both for children (Calderón-Garcidueñas et al. 2012; Chiu et al. 2013; Sunyer et al. 2015) and adults (Ailshire and Crimmins 2014; Gatto et al. 2014).
Environmental exposures in utero and during early life may permanently modify the body’s structure, physiology and metabolism (Gluckman and Hanson 2004). The structure and function of the brain as well as consequent lifelong developmental potential are established in the early years in a process that is extremely sensitive to external influence (Boucher et al. 2009; Luna et al. 2001). Both positive (e.g., responsive caregiving, early learning) and negative (e.g., nutritional deficiencies, air pollution; Black et al. 2016) environmental factors may determine whether children would be able to reach their full neurodevelopmental potential at adulthood.
Working memory and attention are essential for normal cognitive development. Working memory is a cognitive system that is responsible for temporarily holding information for its manipulation. Its function is crucial for many competencies of cognition, such as learning, reasoning, problem solving, and language comprehension (Vuontela et al. 2003). Most of the development of working memory occurs during childhood, though development of working memory continues until adulthood (Østby et al. 2011; Ullman et al. 2014). Attention involves different processes, such as selectively attending to a particular source of stimulation or voluntarily controlling actions (Anderson 2002). Attention is a basic function required for superior cognitive abilities (e.g., executive functions or memory). The conflict network, also called executive control or executive attention, is one of the three functionally and anatomically differentiated networks that form attention (Posner and Petersen 1990). The conflict network is involved in high-level forms of attention, such as the detection and resolution of conflicts among various options and responses, error detection, response inhibition, as well as in the regulation of thoughts and feelings (Fan et al. 2005). Attention starts to develop early in infancy, and the conflict network presents a longer development period that extends into adolescence (Konrad et al. 2005; Rueda et al. 2015, 2005).
Studies that focused on exposures to air pollution, particularly particulate matter (PM) and , during the prenatal period and the first years of life found associations with reduced psychomotor development (Guxens et al. 2014; Kim et al. 2014), as well as with autism spectrum disorder (Kalkbrenner et al. 2015; Volk et al. 2013) and impairment in cognitive development (Calderón-Garcidueñas et al. 2015; Chiu et al. 2016). However, for similar outcomes, other studies reported no associations (Guxens et al. 2014, 2016; Harris et al. 2015). Children from New York, New York City, showed structural brain alterations related to prenatal air pollution levels, whereas no significant correlation was observed for postnatal exposure at 5 years of age via measures of the cortical thickness or cerebral surface (Peterson et al. 2015). The evidence is still scarce, given the lack of studies focusing on the exposure during the most vulnerable stages of brain development (i.e., prenatal and first one or two postnatal years). Therefore, further research is required to assess how the exposure to traffic-related air pollutants at particular time windows affect brain development.
This study was conducted within the framework of the Brain Development and Air Pollution Ultrafine Particles in School Children (BREATHE) project. In previous publications, we reported a deceleration over a year in the development of working memory and reduced attentiveness among children attending schools with a high concentration of traffic-related air pollution in comparison with children in less-polluted schools (Basagaña et al. 2016; Sunyer et al. 2015). Reduced attentiveness was also associated with short-term exposures to such pollutants (Sunyer et al. 2017). These previous studies evaluated the exposure only during the study period, when the children were 7–10 y old, and omitted the exposure in prenatal and early postnatal periods. Moreover, the conflict network was not assessed before within BREATHE, although the conflict network is of great importance in academic achievement (Checa and Rueda 2011; Posner et al. 2006). Therefore, we aimed to assess whether the exposure to PM with an aerodynamic diameter () at different time windows (prenatal and first years of life) was associated with impaired cognitive development (working memory, attentiveness, and conflict network) in 7- to 10-y-old children and to identify the most vulnerable periods of exposure for the development of these cognitive abilities.
Methods
Participants
Participants were 7- to 10-y-old children who attended 39 schools in Barcelona (Catalonia, Spain). In comparison with the rest of the schools in Barcelona, participating schools had a similar Urban Vulnerability Index [a neighborhood-level index based on data from the Census 2011 on level of education, unemployment, and occupation (Spanish Ministry of Public Works 2012)] (0.46 vs. 0.50, t-test ) and air pollution levels (, 51.5 vs. , t-test ). All children without special needs in second to fourth grades were invited to take part in the study; 2,897 children (59%) participated. All parents or guardians signed the informed consent and the study was approved (No. 2010/41,221/I) by the Clinical Research Ethical Committee of the IMIM-Parc de Salut Mar, Barcelona, Spain.
Outcomes: Working Memory, Attentiveness, and Conflict Network
We used the computerized n-back test for assessing working memory (Nelson et al. 2000b; Vuontela et al. 2003) and the computerized Attentional Network Test (ANT; Rueda et al. 2004) for evaluating attention. Both tests have been validated with brain imaging (Owen et al. 2005; Rueda et al. 2004) and in BREATHE participants (Forns et al. 2014). Participants performed four repeated tests (three months apart) from January, 2012, to March, 2013.
A comprehensive description of the tests and the protocol followed in the BREATHE project can be found in Forns et al. (2014). In short, for the n-back task, participants had to click a button when the number stimulus on screen matched the one presented steps before. We evaluated the 3-back load in which the demands on working memory are high (Shelton et al. 2010). In this task, we measured detectability [d prime ()], the normalized proportion of correctly identified targets after subtracting the normalized proportion of false alarms, . A higher indicates more accurate test performance. In the ANT, participants indicated if the central fish in a row of five was pointing to the left or right by pressing the corresponding button. The ANT consisted of 16 training trials and four experimental blocks of 32 trials each (a total of 128 trials). The conflict score (for the evaluation of the conflict network) was obtained by calculating the median reaction time for each flanker condition (central fish pointing toward the same or opposite direction than the other four flanking fish pointed) across four different warning cue conditions and subtracting the reaction times in congruent trials from the incongruent ones. Higher values [in milliseconds (ms)] in the conflict score indicates worse performance. Furthermore, we calculated the hit reaction time standard error (HRT-SE), a measure of response speed consistency throughout the test to measure attentiveness [Continuous Performance Test (CPT); Conners 2000]. Higher HRT-SE indicates less-stable reactions across the test and, hence, lower attentiveness. Therefore, we explored attention through the HRT-SE (for attentiveness) and the conflict score (for the conflict network).
Exposure: Air Pollution Exposures at Early Life
We estimated average concentration of at residence address using the land use regression (LUR) models developed for Barcelona in the European Study of Cohorts for Air Pollution Effects (ESCAPE) and described elsewhere (Eeftens et al. 2012). LUR models are based on simultaneous measurements of air pollutants, such as , taken at many locations throughout an urban area during long periods. Various parameters (e.g., traffic and roadway density, traffic volumes, and population density) derived from geographic information systems (GIS) are used to predict the concentration at specific locations and periods of time (Eeftens et al. 2012).
The history of exposures to from the prenatal period until participants turned 7 y old was created for each participant by estimating the concentration at their residence address using LUR at specific periods of time. To account for the temporal variation of concentrations, we used the time series of daily standardized measurements from the air quality national network (XVPCA) to temporally adjust the LUR models. We estimated the annual average concentrations for every year in the postnatal period (i.e., first year of life, second year of life, etc.), as well as the concentration for the prenatal period. Parents reported the history of residences of their children via questionnaires in which they indicated the periods that the family lived in each residence in case they moved. If they moved, we calculated the time-weighted average of concentration for each residence within the year. In cases of separated parents with shared custody, participants were assigned the time-weighted averaged exposure according to the time that they spent in each home.
From the initial 2,897 participants included in the BREATHE study, we were not able to track back in time exposures of 676 participants (24%, Table 1), either because they were adopted, came from abroad, or we were not able to identify and geocode the address reported by parents for more than 25% of any of the exposure averaging periods (i.e., one year, which is the length of the time window explored). Therefore, in an attempt to minimize exposure misclassification, we did not include participants who did not meet the 75% data availability threshold for each and all of the years.
Table 1.
Description, cognitive outcomes and exposures to of the included and the excluded population (percent or ) from BREATHE participants.
| Characteristic | Included | Excluded | p-Valuea |
|---|---|---|---|
| Number of children () | 2,221 | 676 | — |
| Age | 0.132 | ||
| Gender, (%) | |||
| Girls | 1,088 (49%) | 308 (52%) | 0.205 |
| Boys | 1,133 (51%) | 284 (48%) | |
| Missing observations | 0 | 84 | |
| Adopted, (%) | |||
| Yes | 0 (0%) | 100 (24%) | |
| No | 2,221 (100%) | 318 (76%) | |
| Missing observations | 0 | 258 | |
| Foreign origin, (%) | |||
| Yes | 174 (8%) | 270 (48%) | |
| No | 2,038 (92%) | 291 (52%) | |
| Missing observations | 9 | 115 | |
| Smoking during pregnancy, (%) | |||
| Yes | 214 (10%) | 38 (11%) | 0.621 |
| No | 2,005 (90%) | 319 (89%) | |
| Missing observations | 2 | 319 | |
| Birth weight, (%) | |||
| 210 (10%) | 37 (9%) | 0.623 | |
| 1,996 (90%) | 392 (91%) | ||
| Missing observations | 15 | 247 | |
| Gestational age, (%) | |||
| 166 (8%) | 28 (8%) | 0.895 | |
| 2,034 (92%) | 326 (92%) | ||
| Missing observations | 21 | 322 | |
| Breastfeeding, (%) | |||
| Yes | 1,859 (84%) | 281 (72%) | |
| No | 353 (16%) | 110 (28%) | |
| Missing observations | 9 | 285 | |
| Maternal education, (%) | |||
| Primary or less | 260 (12%) | 73 (17%) | 0.006 |
| Secondary | 627 (28%) | 125 (29%) | |
| University | 1,324 (60%) | 231 (54%) | |
| Missing observations | 10 | 247 | |
| ADHD symptoms, (%) | |||
| Yes, (%) | 212 (10%) | 74 (13%) | 0.022 |
| No, (%) | 1,984 (90%) | 493 (87%) | |
| Missing observations | 25 | 109 | |
| Home SES vulnerability index | 0.010 | ||
| Cognitive outcomes at baseline (visit 1) | |||
| Working memory, | 0.008 | ||
| Attentiveness, HRT-SE (ms) | |||
| Conflict network, conflict score (ms) | 0.178 | ||
| concentrations | |||
| During pregnancy () | — | — | |
| Postnatal exposure (first 7 years) () | — | — | |
Note: Children with more than 6 symptoms from the Criteria of Diagnostic and Statistical Manual of Mental Disorders list (ADHD-DSM-IV). ADHD, Attention Deficit Hyperactivity Disorder; HRT-SE, Hit reaction time standard error from the ANT test; SES, socioeconomic status.
Kruskal-Wallis and chi-square tests.
Contextual and Individual Covariates
We obtained via questionnaire all the information on relevant sociodemographic factors including participants’ age and sex, parental education and occupation, marital status, family origin, and residence history. We used maternal education (primary or less, secondary, university) as an indicator of the individual level of socioeconomic status (SES) and the Urban Vulnerability Index at the home address as the indicator of neighborhood-level SES (Spanish Ministry of Public Works 2012). Parents also completed the Strengths and Difficulties Questionnaire (SDQ) about child behavioral problems (Goodman 2001), which was included as a variable for the inverse probability weighting. Teachers reported each participant’s Attention Deficit Hyperactivity Disorder (ADHD) symptoms (used for stratified sensitivity analysis), using the fourth edition of the ADHD Criteria of Diagnostic and Statistical Manual of Mental Disorders (ADHD-DSM-IV) (American Psychiatric Association 2002). Children were categorized as having ADHD if teachers rated six or more symptoms to be present “Often” or “Very often” out of 18 symptoms on the list. Sex, maternal education, and residential neighborhood SES were identified as potential confounders through directed acyclic graphs (DAG) as detailed in Sunyer et al. (2015).
Statistical Analysis
Inverse probability weighting.
We used inverse probability weighting (IPW) to account for potential selection bias (Seaman and White 2013) that arises when only participants with available exposure and outcome data are included in comparison with the initial participants recruited. Briefly, we used information from all children at recruitment to predict the probability of participation in the study and used the inverse of those probabilities as weights in the analysis so that results would be representative for the initial population. The variables used to create weights are described in Table S1. We obtained a moderate goodness of fit of the participation model based on the area under the receiver operating characteristic curve (AUROC) ().
Linear mixed effect models and distributed lag models.
We used two approaches to assess the relationship between exposure and cognitive outcomes: linear mixed effects models to examine each exposure window individually (year-by-year analysis) and distributed lag models (DLM), which account for all temporal windows simultaneously (multiple temporal adjustment analysis).
Due to the hierarchical nature of the data (i.e., repeated measurements for each child within schools), we used linear mixed effects models with the cognitive parameters from the four repeated tests as outcomes and random effects for child and school. We estimated the difference in average outcome scores associated with one interquartile range (IQR) increase in concentrations. A different IQR was used for each window of exposure (based on all study participants). We performed separate multivariate models to estimate the associations of each cognitive outcome at 7–10 y old with each exposure time window. Statistical significance was set at .
In addition, we used DLM to incorporate a temporal dimension to the intensity of the exposure, defined as exposure–lag–response (Gasparrini 2014; Gasparrini et al. 2010), that is needed to express the association for past exposures. The DLMs consider that the association at a specific time point varies smoothly as a function of time, while incorporating and controlling for exposure at all other periods. To this end, the data should be structured in equally spaced time periods. In our case, we used yearly averages for the postnatal period (the first 7 y) and the average of the entire pregnancy. The latter corresponds to a shorter period, but pregnancy covers of a year’s length, and the prenatal period is of high vulnerability (because of extensive brain development). DLMs weight past exposures through previously defined functions whose parameters are estimated by the data, creating a cross-basis matrix for the two dimensions of exposures and lags. We modeled the exposure–response function with a linear function. Potential nonlinearity in the exposure–response function was explored. While keeping the same lag-response function, we compared a model with the exposure–response defined by a linear function and another by a natural spline function with two internal knots. The p-value from the analysis of variance (ANOVA) test comparing both models was for all the cognitive outcomes, thus indicating a linear exposure–response relationship. The lag-response was shaped by a quadratic b-spline with 3 degrees of freedom (df), and the knots were placed equally spaced. We then incorporated the matrix, storing the cross-basis variables into a linear mixed effects model. Specifically, the final adjusted model (Equation 1) included additional coefficients for age at the visit, sex, maternal education (less than or primary, secondary, university), and residential neighborhood SES:
| (1) |
where is the cognitive test result for subject i in school s at visit t, ; PM2.5 is the estimated concentration at home address for subject i in the time window j; is random effects at school level, assumed to be normally distributed with mean 0 and variance ; is random effects associated with subject i in school s, assumed to be normally distributed with mean 0 and variance ; and is the model residuals, assumed to be normally distributed with mean 0 and variance . Without the additional structure provided by the cross-basis matrix on the coefficients, multicollinearity among exposures at different time windows results in unstable estimates. With this methodology, the effect estimate of a single exposure event is distributed over a specific period of time, using several parameters to explain the contribution at different lags. The sensitive windows of exposure were identified when the 95% CI did not include zero. We also evaluated the estimated cumulative effect of lifelong exposures with DLM. For the DLM analyses, we reported the associations for an increment of of .
Simulation study.
We conducted a small simulation study to explore possible issues of collinearity in the DLM analyses. We took the observed exposures at pregnancy and at 1 to 7 y as fixed, and we simulated an outcome assuming that the true effect of air pollution was in each of the visits. To simulate the outcome, we used the following values for the residual and random effect variances, which are very similar to the ones obtained in our analysis of working memory: residual standard deviation (SD), 91; SD of the school random effects, 10; SD of the subject random effects, 47. The simulation was conducted 100 times.
Sensitivity analyses.
Stratified analyses were performed by sex, because a study by Chiu et al. (2016) assessing cognitive development and weekly exposures to during the prenatal period suggested that neurotoxic effects of air pollution may be sex-specific effects, and by ADHD symptoms, because previous studies indicate that children with ADHD showed a cortical development delayed by 2–3 y in comparison with their typically developing peers (Shaw et al. 2007). Sensitivity analyses were conducted to assess possible confounding or effect modification by air pollution in the previous day of the test visit and change of residence.
We evaluated the differences of choosing a different function to model the lag–response relationship. In a sensitivity analysis, we selected a b-spline with two internal knots for the lag–response functions. We placed the knots in different positions for working memory and for the attention outcomes (attentiveness and conflict network), based on existing knowledge. The knots for working memory were placed at year 2 and year 4 because the myelination rate of the brain is very high until age 2, when it declines until year 4. From that age, the myelination is almost flat, and children show patterns similar to those of adults (Kinney et al. 1988; Parazzini et al. 2002). For the outcomes on the attention domain (attentiveness and conflict network), the knots were placed at year 4 (when the myelination process is almost complete) and at year 6, because attention may be especially affected by acute exposures (Sunyer et al. 2017).
Statistical environments.
Data management and statistical analyses were carried out with the R statistical software (version 3.2.3; R Development Core Team) and the packages nlme (Pinheiro et al. 2017) and dlnm (Gasparrini 2011).
Results
Study Population and Exposure to
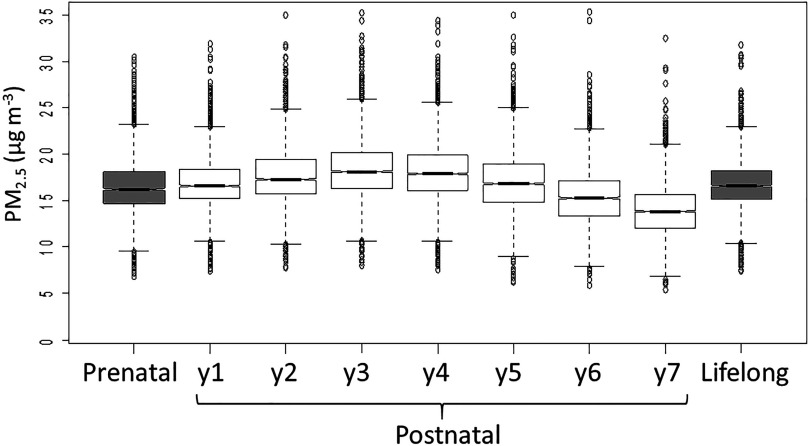
Table 1 shows the characteristics of the included () and excluded () populations, the cognitive outcomes at baseline (first visit), and the average concentrations during pregnancy and the first 7 years of life of included participants. Included participants did not differ by age or sex from the excluded (, ; 49% and 52% female, respectively, ). Included children showed better working memory () and higher attentiveness () than the excluded but similar performance of the conflict network (). Residential areas of the included participants had lower SES vulnerability than the excluded had (0.4 vs. 0.5, respectively, ). A lower proportion of the included participants had a foreign origin (8% of included vs. 48% of excluded, ) and presented ADHD symptoms (10% of included vs. 13% of excluded, ). On the contrary, a higher proportion of the included participants had mothers with a university degree (60% of included vs. 54% of excluded, ). Table S2 shows the population characteristics by sex and by number of residences (1 or residences, as a measure of possible exposure misclassification). The Pearson coefficients between concentrations in the different time windows were moderate to high (between 0.67 and 0.98; see Table S3). The high correlation among periods challenges the interpretation of associations at specific time points separately but allows for the analysis of the patterns of associations. Figure 1 shows the box plots for concentrations at each time window. Average concentrations during the pregnancy period () were slightly lower than the average concentrations for the postnatal period (year 1 to 7; ; ; Table 1).
Figure 1.
Concentrations at different time windows that the participants () are exposed to during prenatal time and childhood. The concentrations were estimated at home address for each year from the temporally adjusted LUR model for Barcelona. Lower and upper bound of the boxes represent 25th and 75th percentile, respectively; central line corresponds to the median; bars outside the box represent the ; and circles are outliers. The notch displays the confidence interval around the median. Note: .
Individual Analysis of Annual Exposures
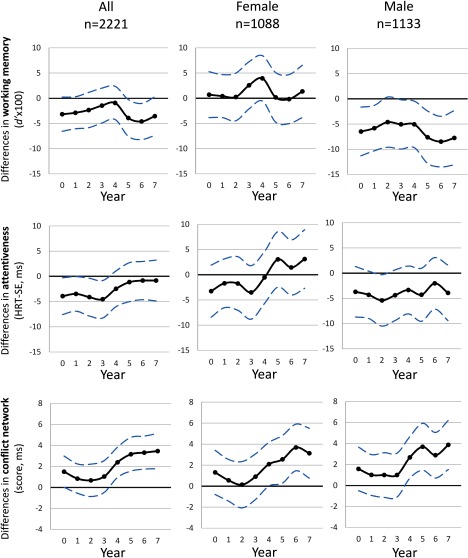
Figure 2 presents the association between an IQR increase in and the three cognitive outcomes for all included participants and is stratified by sex (values reported at Table S4). The results show an almost constant pattern of negative associations between an IQR increase of and working memory throughout the entire period with stronger associations at the most recent years of exposure (year 5 and 6). Considerable differences were observed when stratifying by sex: The overall pattern indicated that higher exposure was associated with lower working memory among boys, but higher exposure was not associated with lower working memory among girls. We further adjusted the models for HRT-SE to evaluate the possible effect of attentiveness on the performance of working memory (Figure S1). The results were very similar to those of the main analysis, with the difference that the associations for some exposure periods became significant after adjusting for HRT-SE: the pregnancy period for the model that included all populations and year 2 for the male population. Second, inattentiveness was not associated with higher concentrations at any time period for boys and girls. Finally, impairment in conflict network was associated with exposure to from age 4 onward. This pattern was similar for both boys and girls. The results for all outcomes were very similar in the complete-cases analysis (without applying IPW; Table S5).
Figure 2.
Association between yearly levels estimated at home address for the pregnancy period (year 0) and the first 7 years of life and different cognitive outcomes at school age (7–10 y old): working memory, attentiveness, and conflict network from the independent linear mixed effect models. Legend: Lower and higher HRT-SE and conflict scores indicate impairment. Models were adjusted for age, sex, maternal education, and residential neighborhood socioeconomic status; school and individual as nested random effects. Solid lines show the difference in the outcomes for an IQR increase in [Interquartile range (IQR) exposure contrasts are reported in Table S4]. Dashed lines indicate 95% CIs. Note: .
Average lifelong exposures to (from pregnancy until the participant turned 7 y old) were not associated with working memory at school age (Table S4; the IQR for lifelong exposure is ). When stratifying by sex, the overall estimated effects for working memory were significant for boys [ (95% CI: , )], with no association for girls [1.28 (95% CI: , 5.60)]. An IQR increase in average lifelong exposures to was associated with an increase in the conflict attentional network score of 2.06 ms (95% CI: 0.67, 3.45), which indicates a worse performance (Table S4). We found an association between lifelong average exposure and the conflict network for boys [2.20 (95% CI: 0.28, 4.12)], although the estimated effect on girls also suggested some impairment [1.84 (95% CI: , 3.83)]. No significant lifelong association was observed for attentiveness.
Multiple Temporal Adjustment
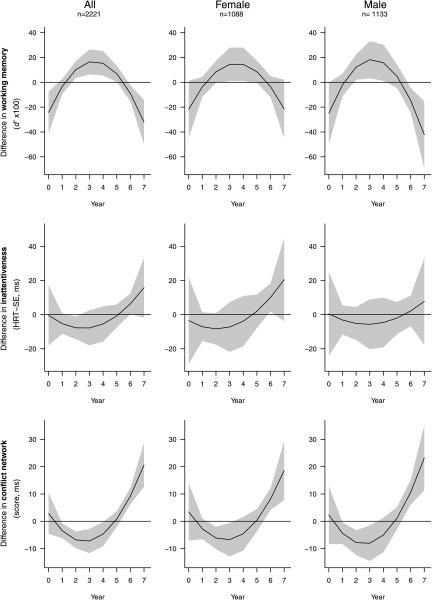
In addition to the independent linear mixed effects models for each cognitive outcome and time window, we also used DLM because this model controls for the exposure at the other periods. Figure 3 presents the association between the exposure to of and the three cognitive outcomes assessed at 7–10 y old for all participants and stratified by sex. With the DLM, the pattern of the model estimates followed a symmetric inverted u-shape with negative associations observed between the exposures during the prenatal period and more recent exposures (years 6 and 7) and working memory at school age (indicating worse performance) and a positive association for the central years (years 2 to 5) of exposure and working memory (indicating better performance). A similar association pattern was observed for boys when stratifying by sex, whereas no inverse association was observed for girls. Regarding attentiveness, the patterns of the coefficients followed a flat and j-shape, with negative estimates (better performance) for the first five years and high positive estimates (worse performance) in years 6 and 7, but overall, exposure to was not associated with impaired attentiveness. Finally, the pattern of the model estimates for the conflict network showed a j-shaped pattern with positive estimates on the most recent years (years 6 and 7) which indicates that exposure to was associated with a reduced performance of the conflict network. On the other hand, and similarly to associations found for working memory, some exposure periods (from years 1 to 4) were associated with better performance of the conflict network. No differences were observed in sex-stratified associations between and conflict network.
Figure 3.
Associations between yearly levels estimated at home address over the pregnancy period (year 0) and the first 7 years of life and different cognitive outcomes at school age (7–10 y old): working memory; attentiveness, and conflict network from the DLM models. Legend: Lower and higher HRT-SE and conflict scores indicate impairment. The associations are presented for all the population and stratified by sex. Models were adjusted for age, sex (only in the model including all population), maternal education, and residential neighborhood socioeconomic status; school and individual included as nested random effects. Solid lines show the predicted difference in the outcomes associated with an increase of of . Grey areas indicate 95% CIs. Note: .
The shape of the patterns obtained from the estimates of the individual models is similar to the shapes from the DLM, although the value of the estimates differs considerably. The main results (i.e., the negative associations between working memory and exposures during school age—years 6 and 7—in boys and in the conflict network in both females and males) were found with both models. However, the results from DLM analyses estimated positive effects of the exposure to in working memory and conflict network that were not present in the independent linear mixed effects models. Given that the latter results were seen only in the DLM, they could be a product of collinearity. Figure S2 shows the results from the simulation study that was conducted to assess collinearity. The gray curves in Figure S2 correspond to each simulation run, which can be thought of as the curve obtained in a single study. Although the average of all the curves (blue line) approximates the truth (red line), showing that the procedure is unbiased (i.e., it gets to the right answer when averaging over multiple studies), the individual curves show patterns with both positive and negative associations. This result is a consequence of collinearity between predictors. The simulation study also indicated that the estimated cumulative effect is less sensitive to collinearity. The true cumulative effect over all lags in this case is . If we compute the cumulative estimate in each simulation run, we obtain the distribution shown in Figure S3. In this case, the cumulative effect fluctuates around the true value, and all estimates are negative, in part because in this particular case, the cumulative effect is much stronger.
The overall estimated cumulative effect for an exposure to of during prenatal and first 7 years of life was points (95% CI: , ) for working memory and 11.31 ms (95% CI: 6.05, 16.57) for the conflict network, which in both cases indicate a worse performance (Table 2). When stratifying by sex, the estimated cumulative effects for working memory were stronger for boys [ (95% CI: , )], whereas we found no association for girls [ (95% CI: , 11.91)]. On the other hand, the estimated cumulative effect on conflict was similar for both strata [11.31 (95% CI: 6.05, 16.57) for all]. The cumulative effect estimate was not significant for attentiveness in any of the models (all participants and by sex).
Table 2.
Estimated cumulative effect of the exposure to of from the prenatal period until the seventh year of life.
| Outcome | 95% CI | |
|---|---|---|
| All () | ||
| Working memory, | (, ) | |
| Attentiveness, HRT-SE (ms) | (, 7.49) | |
| Conflict network, conflict score (ms) | 11.31 | (6.05, 16.57) |
| Female () | ||
| Working memory, | (, 11.91) | |
| Attentiveness, HRT-SE (ms) | 2.55 | (, 20.75) |
| Conflict network, conflict score (ms) | 10.39 | (3.00, 17.78) |
| Male () | ||
| Working memory, | (, ) | |
| Attentiveness, HRT-SE (ms) | (, 7.17) | |
| Conflict network, conflict score (ms) | 12.03 | (4.67, 19.41) |
Note: Estimates from the DLM. Models were adjusted for age, sex (only in the model including all population), maternal education, and residential neighborhood socioeconomic status; school and individual included as nested random effects. CI, confidence interval; HRT-SE: hit reaction time standard error from the ANT test.
Sensitivity Analysis
We performed several sensitivity analyses. Figure S4 shows the results for the DLM analyses with the exposure–response modeled by a b-spline with two specifically located internal knots (see sensitivity analysis in the “Methods” section). Generally, the results are in agreement with the patterns observed in the main DLM results: Exposure at prenatal/first year of age and school age (from year 5 or 6) resulted in significant effect estimates for working memory and the conflict network (negative estimates for working memory and positive for conflict network, indicating worse performance in both cases). However, the associations between exposure at the prenatal period and working memory and the associations between exposure at year 7 and both working memory and conflict network no longer showed an association.
For the individual linear mixed effect models, stratified analysis for teacher-rated ADHD symptoms (Figure S5) showed that the associations remained the same as the main analysis across all the time windows for participants without ADHD symptoms. The CI for the models with only participants with ADHD (with a much smaller sample size) were much wider. For children with ADHD symptoms, no associations were found for working memory and attentiveness, whereas a pattern similar to that of the non-ADHD population was observed for conflict, although with much higher estimates for children with ADHD symptoms (for exposures from year 4 onward, Figure S5).
When performing the analyses including only those participants with no residential mobility (Figure S6), we observed no differences in the association between and the three cognitive parameters in comparison with the test including all participants. We also performed the models adjusting by air pollution concentrations on the previous day to control for the short-term effect of air pollution (Figure S6), with no change to the main analysis.
Discussion
This study is one of the largest conducted to date on the impact of air pollution on cognitive development in children. We, for the first time, evaluated the associations between the exposure to during different prenatal and early postnatal 1-y time windows and various cognitive outcomes (i.e., working memory, attentiveness, and the conflict network) at school age (7–10 y old). We used two computerized tests repeated four times to characterize different aspects of cognitive function and applied a validated modeling approach to estimate residential exposure to at different time windows. The cumulative exposure during the prenatal period and the first 7 years of life was associated with worse performance of the conflict network in both boys and girls and with working memory in boys.
Different patterns of exposure and magnitudes of the effect estimates were observed for the different cognitive outcomes assessed. We observed negative associations between exposure to during years 5 and 6 and working memory at 7–10 y old. Working memory is fundamental for learning and is associated with intelligence (Gathercole et al. 2003). This cognitive function depends on the maturation of the frontal lobes, which are developing from the prenatal period to adolescence (Bell 2001; Nelson 2000a). The prenatal period and the first 7 years of life include important intervals of the frontal lobes’ development, such as the fetal formation of brain structures and their connections (synapse formation and myelination; Rice and Barone 2000) and the preceding year of a rapid connectivity growth between the ages of 7 and 9 y (Anderson 2002; Pelegrina et al. 2015; Vuontela et al. 2003). The finding of negative association between cumulative exposure during the prenatal period and the first 7 years of life with working memory is in accordance with our previous studies in which we demonstrated that the 12-month exposure to traffic-related air pollutants (elemental carbon, ultrafine particles, and ) at schools was negatively associated with the progress of working memory capacity during that same period (Basagaña et al. 2016; Sunyer et al. 2015).
We explored the association of past exposures to and attention at 7–10 y old through the HRT-SE (for attentiveness) and the conflict network score. Attentiveness develops earlier than working memory develops (Anderson 2002), which we also observed in the BREATHE population (López-Vicente et al. 2016; Suades-González et al. 2017). Moreover, we observed larger associations with the exposure to air pollution at baseline than in the growth trajectory during the 1-y follow-up for attentiveness (Sunyer et al. 2015). The negative-effect estimates observed in attentiveness (indicating better performance) for the exposure during the prenatal and first 3 years of life could not be explained. However, we did not observe an association between overall lifelong or cumulative exposure and attentiveness. This lack of association might be explained by attentiveness varying on a daily basis by acute exposures to ambient air pollution, as previously observed by Sunyer et al. (2017) in the BREATHE population when evaluating the exposure on the previous day of the test to and elemental carbon (EC) and inattentiveness. Furthermore, the broad measure of attentiveness is the result of the development and coordination of several elements linked to attention (Mirsky et al. 1991), such as the three independent attentional networks (alerting, orienting, and conflict network) that develop at different periods during childhood (Rueda et al. 2004). Thus, attentiveness would show a continuous development from childhood to adulthood (Rueda et al. 2004), with no specific time of vulnerability. Early life exposures to were associated with reduced performance of the conflict network at school age, with the highest estimates being observed for exposures from year 4 onward, but also for exposures during the prenatal period. As for working memory, the development of the conflict network is not linear during childhood but may be characterized by periods of rapid growth. For instance, a considerable degree of development of the conflict network during the period from 4 to 7 years of age was observed by Rueda et al. (2004), with a manifest progression from 6 to 7 years of age which stabilizes after that. Contrary to findings for attentiveness, we observed an association between cumulative exposure during the prenatal period and the first 7 years of life and the assessment of the conflict network at school age.
The mechanisms underlying the impairment of cognitive development associated with exposure to air pollution are yet to be established. Endocrine disruption, DNA damage (genotoxicity), systemic inflammation, and oxidative stress are the most plausible pathways proposed in the literature (Block and Calderón-Garcidueñas 2009; Calderón-Garcidueñas et al. 2011; Perera et al. 2006). Specifically, Calderón-Garcidueñas et al. (2008) suggested that a prolonged brain inflammation could interfere with subcortical pathways that connect the prefrontal cortex with decisive brain regions for cognitive functions.
Even though a spate of publications on the associations between air pollution and cognitive development has begun to appear, the literature on this topic is still scarce (Suades-González et al. 2015). Few of these studies focused on working memory or attention functions as outcomes, and the air pollutants assessed may differ from . Therefore, comparisons between studies is difficult. For instance, in a cohort of 4-y-old children in Granada, Spain (), exposure to high levels of () was associated with a decrease of 7.4 points of working memory (McCarthy Scales of Children’s Abilities), among other cognitive impairments (Freire et al. 2010). Suglia et al. (2008) observed a negative association between black carbon (BC) and various cognitive parameters, including verbal and nonverbal intelligence and memory in 202 10-y-old children in Boston, Massachusetts (USA). In the same population, Chiu et al. (2013) observed an association between higher lifelong exposure to BC and higher commission of errors and slower reaction time (indicating lower attentiveness in the Conners’ Continuous Performance Test). Porta et al. (2016) found an association between a prenatal exposure increase of and a reduction in points in the verbal intelligence quotient (IQ) and verbal comprehension score (Wechsler Intelligence Scale for Children-III) in a cohort of 719 children in Rome. They found the same association when using the lifelong averaged exposure to , but the association was weaker. Harris et al. (2015) explored the associations of exposure to BC and proximity to major roadways with cognitive outcomes at different time windows. They observed an association between lower nonverbal and verbal intelligence in 8-y-old children and residential proximity to major roads (), with these association found to be stronger for road proximity at birth than at mid-childhood. Chiu et al. (2016) used DLM to identify sensitive weeks of exposure during pregnancy. They found higher levels at 31–38 wk to be associated with lower IQ at age 6.5 y and levels at 22–40 wk to be associated with increased HRT-SE in the Conners’ Continuous Performance Test-II (CPT-II) at age 6.5 y old. The findings of our study, in which lifetime exposure to is associated with some outcomes, are in line with the results of the studies described above.
Sex-differential susceptibility plays a major role in the associations of air pollution with working memory. In our study, although cumulative exposure was inversely associated with working memory in boys, such an association was null for girls. This observation was in line with those of other studies (Chiu et al. 2016, 2013). Different mechanisms may explain this difference, such as the sex-specific alteration of the dopamine function as observed in animals (Curtis et al. 2010) or the influence of sex hormones. For instance, estrogens show an anti-inflammatory effect by regulating cytokine expression (Shivers et al. 2015), which could help to counteract the inflammatory response to exposure in girls. Genotoxic effects have also been associated with exposure to air pollution (Knudsen et al. 1999). Existing literature suggests an over-representation of genes linked to intelligence in the X chromosome (Johnson et al. 2009; Lehrke 1972; Turner 1996). Laumonnier et al. (2007) reported that of the 800 genes coding for proteins in the X chromosome, 500 are expressed in the brain. Any genotoxicity in genes on the X chromosome is more likely to affect males, because they count on only one X chromosome.
Our study faced some limitations. We assessed exposure at home, omitting the contribution of exposure in other microenvironments. We expected that our participants spent most of their time at home during the first years of life (Leech et al. 2002). However, with increasing age, time spent at home decreases, and exposures that occur in other microenvironments, such as school or friends’ homes, could increase the risk of exposure misclassification. Moreover, the participants who changed residence during the course of the study were subject to recall errors for the timing of the move or the address(es) of previous residences, which could have been another source of exposure misclassification. However, limiting our analyses to participants who never moved did not result in a significant change in the interpretation of our findings. Furthermore, the high correlation between exposure concentrations across different time periods challenges the identification of sensitive periods of exposure regardless of the modeling approach. In addition, we cannot rule out the likelihood of residual SES confounding, but we adjusted for indicators of individual and neighborhood SES, which should have minimized such likelihood. Furthermore, no data were available for some potentially relevant confounders such as maternal age at delivery (Tearne 2015), parental mental health status, or childrearing environment (Fergusson and Woodward 1999), nor for potential effect modifiers such as mother’s and child’s diets (Daniels et al. 2004).
Conclusions
This study demonstrates that exposure to during early life (i.e., prenatal period and first years of life) was negatively associated with fundamental cognitive abilities at school age (7–10 y old), such as working memory and the attentional conflict network. We found associations between exposure to during early life and reduced working memory. For attention functions, was also associated with reduced performance of the conflict network, particularly from year 4 onward, but not for attentiveness. Exposure to air pollutants during childhood jeopardizes the achievement of full neurodevelopmental potential and diminishes mental capital at the population level. Future studies should include refined exposure assessment by carrying out precise personal monitoring of air pollutants at different time windows (including prenatal) as well as parallel evaluations of working memory and attention in the same time windows.
Supplementary Material
Acknowledgments
The research leading to these results has received funding from the European Research Council (ERC-Advanced Grant) under grant agreement number 268479—the BREATHE project. We are grateful to A. Dalmau-Bueno for his help in estimating exposure to air pollution. We thank all the families participating in the study and particularly to the schools. P.D. is funded by a Juan de la Cierva fellowship (JCI-2011-09937) awarded by the Spanish Ministry of Science and Innovation.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP3169).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Ailshire JA, Crimmins EM. 2014. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 180(4):359–366, PMID: 24966214, 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 2002. Manual Diagnóstico y Estadístico de los Trastornos Mentales. [in Spanish]. 4th ed Barcelona, Spain:Masson. [Google Scholar]
- Anderson P. 2002. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8(2):71–82, PMID: 12638061, 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Basagaña X, Esnaola M, Rivas I, Amato F, Alvarez-Pedrerol M, Forns J, et al. . 2016. Neurodevelopmental deceleration by urban fine particles from different emission sources: a longitudinal observational study. Environ. Health Perspect 124(10):1630–1636, PMID: 27128166, 10.1289/EHP209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA. 2001. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B task. Infancy 2(3):311–330, 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, et al. . 2016. Early childhood development coming of age: science through the life course. Lancet 1–14, PMID: 27717614, 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: 19716187, 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH. 2009. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ. Health Perspect 117(1):7–16, PMID: 19165381, 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Engle R, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H, et al. . 2011. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn 77(3):345–355, PMID: 22032805, 10.1016/j.bandc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Franco-Lira M, Zhu H, Lu Z, Solorio E, et al. . 2015. Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein ε4 children exposed to air pollution. J Alzheimers Dis 45(3):757–770, PMID: 25633678, 10.3233/JAD-142685. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Styner M, Zhu H, Torres-Jardón R, Carlos E, et al. . 2012. White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J Alzheimers Dis 31(1):183–191, PMID: 22531421, 10.3233/JAD-2012-120610. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, et al. . 2008. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36(2):289–310, PMID: 18349428, 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Checa P, Rueda MR. 2011. Behavioral and brain measures of executive attention and school competence in late childhood. Dev Neuropsychol 36(8):1018–1032, PMID: 22004022, 10.1080/87565641.2011.591857. [DOI] [PubMed] [Google Scholar]
- Chiu YHM, Bellinger DC, Coull BA, Anderson S, Barber R, Wright RO, et al. . 2013. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environ. Health Perspect 121(7):859–864, PMID: 23665743, 10.1289/ehp.1205940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y-H, Hsu H-H, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. . 2016. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 87:56–65, PMID: 26641520, 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. 2000. Conners' Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual . MHS Staff, eds. North Tonawanda, NY:Multi-HealthSystems. [Google Scholar]
- Curtis JT, Hood AN, Chen Y, Cobb GP, Wallace DR. 2010. Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: an animal model of autism. Behav. Brain Res 213(1):42–49, PMID: 20433873, 10.1016/j.bbr.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JL, Longnecker MP, Rowland AS, Golding J. 2004. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology 15(4):394–402, PMID: 15232398, 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, et al. . 2012. Development of Land Use Regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–11205, PMID: 22963366, 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. 2005. The activation of attentional networks. Neuroimage 26(2):471–479, PMID: 15907304, 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ. 1999. Maternal age and educational and psychosocial outcomes in early adulthood. J Child Psychol Psychiatry 40(3):479–489, PMID: 10190348, 10.1111/1469-7610.00464. [DOI] [PubMed] [Google Scholar]
- Forns J, Esnaola M, López-Vicente M, Suades-González E, Alvarez-Pedrerol M, Julvez J, et al. . 2014. The n-back test and the attentional network task as measures of child neuropsychological development in epidemiological studies. Neuropsychology 28(4):519–529, PMID: 24819069, 10.1037/neu0000085. [DOI] [PubMed] [Google Scholar]
- Freire C, Ramos R, Puertas R, Lopez-Espinosa M-J, Julvez J, Aguilera I, et al. . 2010. Association of traffic-related air pollution with cognitive development in children. J Epidemiol Community Health 64(3):223–228, PMID: 19679705, 10.1136/jech.2008.084574. [DOI] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: The Package dlnm. J Stat Softw 43(8):1–20, PMID: 22003319. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: 20812303, 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2014. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med 33(5):881–899, PMID: 24027094, 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Brown L, Pickering SJ. 2003. Working memory assessments at school entry as longitudinal predictors of National Curriculum attainment levels. Educational and Child Psychology 20(3):109–122.https://shop.bps.org.uk/publications/publication-by-series/educational-and-child-psychology/educational-child-psychology-vol-20-no-3-2003-memory-and-education.html. [Google Scholar]
- Gatto NM, Henderson VW, Hodis HN, St. John JA, Lurmann F, Chen J-C, et al. . 2014. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 40:1–7, PMID: 24148924, 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Risk Factor Collaborators. 2015. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:2287–2323, PMID: 26364544, 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P, Hanson M. 2004. Living with the past: evolution, development, and patterns of disease. Science 305(5691):1733–1736, PMID: 15375258, 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Goodman R. 2001. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry 40(11):1337–1345, PMID: 11699809, 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, et al. . 2014. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology 25(5):636–647, PMID: 25036432, 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Guxens M, Ghassabian A, Gong T, Garcia-Esteban R, Porta D, Giorgis-Allemand L, et al. . 2016. Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: The ESCAPE project. Environ. Health Perspect 124(1):133–140, PMID: 26068947, 10.1289/ehp.1408483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. . 2015. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the Project Viva cohort (Massachusetts, USA). Environ. Health Perspect 123(10):1072–1078, PMID: 25839914, 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Carothers A, Deary IJ. 2009. A role for the X chromosome in sex differences in variability in general intelligence? Perspect Psychol Sci 4(6):598–611, PMID: 26161735, 10.1111/j.1745-6924.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, et al. . 2015. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 26(1):30–42, PMID: 25286049, 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Kim E, Park H, Hong YC, Ha M, Kim Y, Kim BN, et al. . 2014. Prenatal exposure to PM10 and NO2 and children’s neurodevelopment from birth to 24 months of age: Mothers and Children’s Environmental Health (MOCEH) study. Sci Total Environ 481:439–445, PMID: 24631606, 10.1016/j.scitotenv.2014.01.107. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles F. 1988. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 47(3):217–234, PMID: 3367155. [DOI] [PubMed] [Google Scholar]
- Knudsen LE, Norppa H, Gamborg MO, S-transferase M, Nielsen PS, Okkels H, et al. . 1999. Chromosomal aberrations in humans induced by urban air pollution: influence of DNA repair and polymorphisms of glutathione S-transferase M1 and N-acetyltransferase 2. Cancer Epidemiol Biomarkers Prev 8(4 pt 1):303–310, PMID: 10207633. [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, et al. . 2005. Development of attentional networks: an fMRI study with children and adults. Neuroimage 28(2):429–439, PMID: 16122945, 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Cuthbert PC, Grant S. 2007. The role of neuronal complexes in human X-linked brain diseases. Am J Hum Genet 80(2):205–220, PMID: 17236127, 10.1086/511441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. 2002. It’s about time: a comparison of Canadian and American time-activity patterns. J Expo Anal Environ Epidemiol 12(6):427–432, PMID: 12415491, 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- Lehrke R. 1972. A theory of X-linkage of major intellectual traits. Am J Ment Defic 76(6):611–619, PMID: 5031080. [PubMed] [Google Scholar]
- López-Vicente M, Forns J, Suades-González E, Esnaola M, García-Esteban R, Álvarez-Pedrerol M, et al. . 2016. Developmental trajectories in primary schoolchildren using n-back task. Front Psychol 7:1–11, PMID: 27242625, 10.3389/fpsyg.2016.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. . 2001. Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13(5):786–793, PMID: 11304075, 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. 1991. Analysis of the elements of attention: a neuropsychological approach. Neuropsychol Rev 2(2):109–145, PMID: 1844706, 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Nelson CA. 2000a. Neural plasticity and human development: the role of early experience in sculpting memory systems. Dev Sci 3(2):115–136, 10.1111/1467-7687.00104. [DOI] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. 2000b. Functional neuroanatomy of spatial working memory in children. Dev Psychol 36(1):109–116, PMID: 10645748, 10.1037/0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Walhovd KB. 2011. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia 49(14):3854–3862, PMID: 22001853, 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25(1):46–59, PMID: 15846822, 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini C, Baldoli C, Scotti G, Triulzi F. 2002. Terminal zones of myelination: MR evaluation of children aged 20-40 months. Am J Neuroradiol 23(10):1669–1673, PMID: 12427621. [PMC free article] [PubMed] [Google Scholar]
- Pelegrina S, Lechuga MT, García-Madruga JA, Elosúa MR, Macizo P, Carreiras M, et al. . 2015. Normative data on the n-back task for children and young adolescents. Front Psychol 6:1–11, PMID: 26500594, 10.3389/fpsyg.2015.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. . 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114(8):1287–1292, PMID: 16882541, 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, et al. . 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 72(6):531, PMID: 25807066, 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2017. nlme: Linear and Nonlinear Mixed Effects Models. R package version 31–131. [Google Scholar]
- Porta D, Narduzzi S, Badaloni C, Bucci S, Cesaroni G, Colelli V, et al. . 2016. Air pollution and cognitive development at age 7 in a prospective Italian birth cohort. Epidemiology 27(2):228–236, PMID: 26426942, 10.1097/EDE.0000000000000405. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. 1990. The attention system of the human brain. Annu Rev Neurosci 13:25–42, PMID: 2183676, 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Sheese BE, Odludaş Y, Tang Y. 2006. Analyzing and shaping human attentional networks. Neural Networks 19(9):1422–1429, PMID: 17059879, 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. 2000. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect 108:511–533, PMID: 10852851, 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. . 2004. Development of attentional networks in childhood. Neuropsychologia 42(8):1029–1040, PMID: 15093142, 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Pozuelos JP, Cómbita LM. 2015. Cognitive neuroscience of attention from brain mechanisms to individual differences in efficiency. AIMS Neurosci 2:183–202, 10.3934/Neuroscience.2015.3.183. [DOI] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. 2005. Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci U S A 102(41):14931–14936, PMID: 16192352, 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman SR, White IR. 2013. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 22(3):278–295, PMID: 21220355, 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. . 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A 104(49):19649–19654, PMID: 18024590, 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JT, Elliott EM, Matthews RA, Hill BD, Gouvier WD. 2010. The relationships of working memory, secondary memory, and general fluid intelligence: working memory is special. J Exp Psychol Learn Mem Cogn 36(3):813–820, PMID: 20438278, 10.1037/a0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V. 2015. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 72(2):121–129, PMID: 25647266, 10.1016/j.cyto.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanish Ministry of Public Works. 2012. Atlas of Urban Vulnerability in Spain. Methodology and Contents. http://www.fomento.gob.es/NR/rdonlyres/40668D5E-26B6-4720-867F-286BD55E1C6B/135960/20160201METODOLOGIAATLASVULNERABILIDAD2001Y2011.pdf [accessed 21 January 2013].
- Suades-González E, Forns J, García-Esteban R, López-Vicente M, Esnaola M, Álvarez-Pedrerol M, et al. . 2017. A longitudinal study on attention development in primary school children with and without teacher-reported symptoms of ADHD. Front Psychol 8:655, PMID: 28559855, 10.3389/fpsyg.2017.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suades-González E, Gascon M, Guxens M, Sunyer J. 2015. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology 156(10):3473–3482, PMID: 26241071, 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. 2008. Association of black carbon with cognition among children in a prospective birth cohort study. Am. J. Epidemiol 167(3):280–286, PMID: 18006900, 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, López-Vicente M, et al. . 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med 12(3):e1001792, PMID: 25734425, 10.1371/journal.pmed.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, Suades-González E, García-Esteban R, Rivas I, Pujol J, Alvarez-Pedrerol M, et al. . 2017. Traffic-related air pollution and attention in primary school children. Epidemiology 28(2):181–189, PMID: 27922536, 10.1097/EDE.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tearne JE. 2015. Older maternal age and child behavioral and cognitive outcomes: a review of the literature. Fertil Steril 103(6):1381–1391, PMID: 26041693, 10.1016/j.fertnstert.2015.04.027. [DOI] [PubMed] [Google Scholar]
- Turner G. 1996. Intelligence and the X chromosome. Lancet 347(9018):1814–1815, PMID: 8667929, 10.1016/S0140-6736(96)91623-2. [DOI] [PubMed] [Google Scholar]
- Ullman H, Almeida R, Klingberg T. 2014. Structural maturation and brain activity predict future working memory capacity during childhood development. J Neurosci 34(5):1592–1598, PMID: 24478343, 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. 2013. Traffic-related air pollution, particulate matter, and autism. JAMA psychiatry 70(1):71, PMID: 23404082, 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuontela V, Steenari M-R, Carlson S, Koivisto J, Fjällberg M, Aronen ET. 2003. Audiospatial and visuospatial working memory in 6 – 13 year old school children. Learn Mem 10(1):74–81, PMID: 12551966, 10.1101/lm.53503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.