Abstract
Thirty years ago, there was an emerging appreciation for the significance of oxidative stress in newborn disease. This prompted a renewed interest in the impact of oxygen therapy for the newborn in the delivery room and beyond, especially in premature infants. Today, the complexity of oxidative stress both in normal regulation and pathology is better understood, especially as it relates to neonatal mitochondrial oxidative stress responses to hyperoxia. Mitochondria are recipients of oxidative damage and have a propensity for oxidative self-injury that has been implicated in the pathogenesis of neonatal lung diseases. Similarly, both intrauterine growth restriction (IUGR) and macrosomia are associated with mitochondrial dysfunction and oxidative stress. Additionally, reoxygenation with 100% O2 in a hypoxic-ischemic newborn lamb model increased the production of pro-inflammatory cytokines in the brain. Moreover, the interplay between inflammation and oxidative stress in the newborn is better understood because of animal studies. Transcriptomic analyses have found a number of genes to be differentially expressed in murine models of bronchopulmonary dysplasia (BPD). Epigenetic changes have also been detected both in animal models of BPD and premature infants exposed to oxygen. Antioxidant therapy to prevent newborn disease has not been very successful; however, new therapeutic principles, like melatonin, are under investigation.
Keywords: Antioxidant therapy, Bronchopulmonary Dysplasia (BPD), Epigenetic changes, Growth, Gen-differentiation, Inflammation Mitochondria, Newborn, Oxidative stress, Persistent Pulmonary Hypertension of the Newborn (PPHN)
In the 1980s, there was an emerging understanding of the significance of oxidative stress in the newborn period, especially in premature infants who were shown to have reduced antioxidant defense systems compared to their term counterparts.[1] Furthermore, free iron, a transitional metal that promotes hydroxyl radical (OH.) production through Fenton chemistry, was detected in preterm as well as in asphyxiated neonates, potentially resulting in increased generation of reactive oxygen species (ROS) in those infants.[2] A decade earlier Babior et al linked inflammation and oxidative stress through discovery of the leucocytes oxidative burst as an important part of antimicrobial defense.[3] The combination of these principles broadened the field’s understanding of oxidative stress in the newborn as a combination of factors beyond just the level of oxygen exposure. In 1988, Saugstad introduced the term “oxygen radical disease in the newborn”. The idea was that, during reoxygenation, oxygen radicals are formed in excess and different organs may be attacked. Thus, we may not be dealing with different diseases in bronchopulmonary dysplasia, necrotizing enterocolitis, or retinopathy of prematurity but different aspects of one disease; an “oxygen radical disease in neonatology,” caused by a common pathogenic mechanism. The clinical manifestation of this disease may differ according to which organ is most severely affected.[4]
At the time, such considerations stimulated a new interest in newborn oxygenation, making it a major research priority. This led to resuscitation studies with different oxygen levels, especially air versus 100% oxygen, and large multicenter studies testing out the optimal oxygenation targets for immature newborn infants. Moreover, these considerations resulted in new therapeutic approaches designed to prevent oxidation injury. Unfortunately, most of these approaches have so far been disappointing. Today, 30 years after the first wave of interest in oxidative stress described above, similar questions are still in focus. ROS clearly are of importance for normal and abnormal development. Although ROS can be harmful, they primarily function as important signaling molecules. The effect of ROS at the mitochondrial, genetic, and epigenetic levels are just emerging. Moreover, new antioxidants are being tested or are in the pipeline as novel therapeutic approaches. Perhaps we still can talk about an “oxygen radical disease in the newborn” and should aim at interventions reducing oxidative stress? In this review, we summarize some of the recent evidence linking oxidative stress to newborn disease (Table 1). We put special emphasis on the relationship between inflammation, growth restriction, oxidative stress, and oxidant injury, and how oxidative stress may induce inflammation. The role of the mitochondria in oxidative stress is also summarized.
Table 1.
Oxidative Stress related factors contributing to neonatal disease
| Newborn Disease | BPD | PPHN | NEC | IVH | HIE | Growth Aberrancy |
|---|---|---|---|---|---|---|
| Mitochondrial OS | + | + | + | + | ||
| Inflammation | + | + | + | + | + | + |
| Genetic/Epigenetic | + | + |
Oxygen and oxygen sensing
Shortly after the discovery of oxygen as an element in the 1790s, its dichotomy as a life-giving as well as a toxic substance was understood. Part of the mechanism behind this was clarified about 100 years later; that oxygen is magnetic. Oxygen receives its magnetic properties due to an inequality in the total electron spin and is therefore reactive. In energy metabolism, oxygen functions as an electron acceptor in the respiratory chain. By accepting 4 electrons, oxygen is reduced to water, but this process requires four steps. Each intermediate step occurs within the mitochondria and generates ROS including superoxide radical (O2−), hydrogen peroxide (H2O2), and OH.. O2− and OH. are free radicals, and therefore highly toxic, causing cell membrane damage by lipid peroxidation, protein and DNA oxidation. On the other hand, H2O2 is not a free radical, in spite of being a ROS, and acts as a signaling molecule essential for cell cross talk (e.g. regulating blood flow within the ductus arteriosus and the pulmonary circulation).[5–8]
As indicated above, there are several reasons that newborn infants, especially those born preterm, are at risk of oxidative stress. First, relative oxidative stress is generally high within the first weeks of birth after transitioning from the low oxygen environment of the uterus to air.[9] Second, sick newborns may need supplemental oxygen if there is respiratory insufficiency. Third, the addition of asphyxia as well as free iron increases the risk of oxidative stress. Most importantly, preterm infants do not have sufficient anti-oxidant defense, missing either de novo or passively acquired from the mother in the 3rd trimester, which increases susceptibility to oxidative stress.
Mitochondrial ROS
Mitochondria are a major source of ROS production, easily surpassing cytosolic ROS sources.[10] Mitochondrial ROS include O2− produced by complexes I and III of the electron transport chain, and H2O2 generated through the dismutation of O2− by mitochondrial superoxide dismutase (MnSOD). Hypoxia leads to elevation of Krebs cycle intermediates, including α-ketoglutarate, succinate, and fumarate. Succinate creates the so-called reverse electron transport from Complex –II to Complex-I, which generates higher ROS compared to the conventional forward electron transport from Complex-I to Complex–II.[11]
Given the importance of mitochondria to ROS signaling, it is perhaps not surprising that exaggerated mitochondrial oxidative stress has been described in a number of animal models that mimic human neonatal disease, including models of persistent pulmonary hypertension of the newborn (PPHN), bronchopulmonary dysplasia (BPD), brain injury, and necrotizing enterocolitis (NEC).
While the etiology of neonatal lung disease is multifactorial, the relevance of mitochondrially-derived ROS to neonatal pulmonary injury has been previously demonstrated in animal models of PPHN and in models of hyperoxic lung injury. Studies utilizing prenatal ligation of the ductus arteriosus in fetal lambs have linked mitochondrial oxidative stress to maladaptive changes in nitric oxide (NO) signaling pathways and mitochondrial dysfunction with the pathogenesis of PPHN.[5, 12] These responses, including activation of phosphodiesterase-5 (PDE5), decreased cGMP-responsiveness to exogenous NO, and decreased expression of endothelial Nitric Oxide Synthase (eNOS), may promote additional injury through increased vasoconstriction, impaired responsiveness to vasodilators, and decreased availability of ATP.[13] Moreover, increased levels of H2O2 in pulmonary arteries have been associated with decreased expression of soluble guanylate cyclase and reductions in cGMP levels, likely contributing to pulmonary vasoconstriction.[14] H2O2 has also been linked to decreased activity of extracellular SOD (ecSOD), an antioxidant that’s a major determinant of NO bioavailability.[6]
Similar to the PPHN model, exaggerated mitochondrial oxidative stress responses have been described in murine hyperoxic lung injury models utilizing chronic neonatal oxygen exposure to mimic BPD.[15, 16] Mitochondrial oxidative injury in the alveolar epithelium is induced by O2−, H2O2, OH., or hypoxanthine/xanthine oxidase.[17, 18] Neonatal chronic hyperoxia increases NADPH oxidase, which induces alveolar epithelial cell apoptosis.[15, 16, 19] Oxygen-induced epithelial cell injury in rodent models are mitigated by MnSOD overproduction alone but not by intracellular SOD (CuZnSOD) or catalase (CAT) in isolation.[18, 19] Additionally, important developmental differences have been identified in murine models, resulting in increased mitochondrial ROS generation in neonatal rodents as compared to adults, suggesting that mitochondrial sources of oxidative stress are particularly important in neonatal responses to hyperoxia.[15] Finally, the possible role of mitochondrial ROS in preterm neonates has been suggested by a study examining genetic polymorphisms of superoxide dismutase (SOD) enzymes linking single nucleotide polymorphisms in MnSOD enzyme to increased risk of BPD development.[20] This retrospective study evaluating the allele frequency and genotype distribution of polymorphisms of SOD enzymes in a population of preterm infants, found that a specific single nucleotide polymorphism on exon 2 of MnSOD gene was associated with increased risk of BPD. Taken together, the available data suggests that ROS originating in the mitochondria are an important contributor to pathophysiology of neonatal lung injury.
In addition to being an important source of ROS, mitochondria are also a prime target of oxidative damage, resulting in defects in oxidative phosphorylation and mitochondrial dysfunction.[21] Mitochondrial dysfunction itself has been implicated in the pathogenesis of BPD and it is possible that the oxidative stress generated by the mitochondria may contribute to defects in mitochondrial function, further worsening lung injury.[22, 23] Mitochondria-derived free radicals have been shown to result in oxidative self-injury and mitochondrial dysfunction in a neonatal brain injury model induced by hypoxia-ischemia (HI).[24] In addition to their role in HI, mitochondrial ROS have also been implicated in brain injury induced by hypoglycemia, and implicated as a major source of intestinal apoptotic signaling in rodent models of NEC.[25, 26] Hypoxia, a common element used in animal models of NEC, is also an important source of oxidative stress and results in increased mitochondrial ROS generation.[27] In that context, it is interesting to note a recent meta-analysis of the five clinical trails investigating the safety and outcomes of different oxygen saturation goals in preterm infants, found that lower oxygen saturation targets were associated with increased rates of NEC, highlighting the importance of hypoxemia in pathophysiology of neonatal diseases associated with increased oxidative stress.[28]
Growth abnormalities and oxidative stress
Fetal growth abnormalities can induce oxidative stress that continues in the newborn period. Both extremes of fetal growth, small or large for gestational age (SGA or LGA), are associated with increased neonatal morbidity and mortality as well as long-term health complications.[29–31] Furthermore, extremely preterm infants are disproportionately affected by intrauterine growth restriction (IUGR) as evidenced by increased mortality, incidence of BPD and BPD-associated PH when using even a liberal definition of growth restriction as birth weight less than the 25th percentile at birth.[29, 32] The concept of “fetal origins of adult disease” was first described by Barker in the early 1990s, as the risk of growth restricted infants to develop cardiovascular disease, diabetes and other metabolic disorders as an adult.[33, 34] In the ensuing three decades, epigenetic change due to oxidative stress has emerged as the mechanism for the life-long disease predilection.[35, 36] Moreover, recent evidence supports that effects of IUGR may be more imminent, especially for preterm infants, in childhood manifesting as global developmental delay as opposed to the previously described endpoints during adulthood.[37] Although fetal IUGR and macrosomia are very disparate growth states, both are associated with mitochondrial dysfunction and oxidative stress.[38]
Fetal growth extremes can be caused by either maternal or fetal factors; however, in high income areas of the world the most common cause is uteroplacental insufficiency (UPI) due to maternal factors, such as preeclampsia, chronic hypertension or obesity.[39] These maternal disease states appear to have overlapping pathogenesis with increased maternal cytokines and inflammatory markers that affect placental function and subsequently cause nutrient deficiencies and hypoxic effects on the fetus.[40, 41] Independent of the cause of the abnormal growth, human and animal data have demonstrated increased markers of oxidative stress along all parts of the maternal-placental-fetal axis.
Early embryonic and placental development occur naturally under hypoxic and oxidant conditions;[42–44] however, when this physiologic condition continues, as in IUGR, the result can be detrimental to the mother or pregnancy outcome.[45–47] Regarding IUGR pathogenesis, it is not known if maternal or placental tissues initiate the oxidative stress cascade. One longitudinal case-control study found elevated maternal urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine at 12 and 28 weeks of gestation in mothers who ultimately had a fetus affected by IUGR; interestingly, this major byproduct of DNA oxidation was present prior to the clinically evident IUGR.[48] Moreover, additional markers of oxidative stress that have been increased with IUGR include plasma protein carbonyls, H2O2, malondialdehyde (MDA), as well as corresponding decreases in antioxidants such as vitamins C and E, SOD and CAT.[49, 50] Many IUGR pregnancies are complicated by preeclampsia but when comparing IUGR with and without preeclampsia, Mert et al found similar measures of increased oxidative stress compared to control.[51] Despite evidence supporting an increased oxidative stress state in women with pregnancies complicated by IUGR, the inciting ROS cause remains unknown.
Although the antecedent for oxidative stress in pregnancies with abnormal growth trajectories is unknown, there is evolving evidence that UPI may result from abnormal placental implantation and development which initiates the pathogenic cascade.[51] The abnormal placental implantation, commonly affecting IUGR pregnancies, produces a state of a relative hypoxia within the intervillous space inducing oxidative stress for both the mother and fetus.[45] Protein carboxylation and lipid peroxidation within the placenta have been shown to impair neutral amino acid and glucose transport.[52, 53] Similarly, placental tissue from UPI affected pregnancies have evidence of insulin receptor and type-1-insulin-like-growth-factor receptor glycosylation; although this did not appear to affect ligand binding, receptor function could be impaired.[53, 54] Moreover, increased presence of CuZnSOD and MnSOD, glutathione peroxidase (GPx) and NADPH oxidase has been found in the vascular endothelium of chorionic and umbilical arteries from IUGR affected pregnancies.[55] In a swine model of natural growth restriction, placental concentrations of H2O2, MDA and 8-hydroxy-2-deoxyguanosine were elevated in growth restriction without differences in NO concentration.[56] The findings in human placental tissues and the swine IUGR model placentas parallel the changes in response to oxidative stress commonly found in the premature neonate with increased free radical moieties, protein oxidation, and decreased antioxidant enzyme production.
Altered biochemical homeostasis is detected in LGA and SGA infants, likely secondary to ongoing oxidative stress and ROS production. In studies simultaneously comparing appropriately grown to both SGA and LGA cohorts, SGA and LGA infants have increased circulating levels of carbonyl proteins and H2O2 as well as decreased vitamin levels.[50] Independently, SGA infants have been found to have increased MDA and xanthine oxidase plasma levels, as well as decreased antioxidant capacity via reduced glutathione (GSH) and CAT levels, with similar signs of oxidative stress present in their mothers as well. The directionality of SOD changes is not consistent between SGA datasets; however, their SOD activity differs from controls by several fold. [40, 57] On the other hand, LGA infants appear to have increased SOD activity, likely a physiologic response to elevated oxidative stress, independent of a change in oxidative stress in their mothers.[50] Animal growth restriction models from several species have supported these finding with similar measures of decreased total antioxidant capacity and increased oxidative stress.[41, 56, 58–60] Similar to the maternal studies, the inciting ROS in LGA and SGA infants is generally unknown. These states likely lead to altered gene expression and epigenetic changes that indefinitely affect the aberrant grown infants through adulthood. With the rising incidence of obesity and common incidence of UPI, effects of abnormal fetal growth states are likely to have public health implications unless the oxidative stress state can be addressed.
Obesity affects more than a third of reproductive age women in the United States with a prevalence that has more than doubled over the last two generations. Although macrosomia is more common in offspring of obese women, affecting 20% of births, the incidence of IUGR in obese women is increased 2–3 fold compared to the general population as well.[61] Obese individuals are in a constant low, metabolic, inflammatory state induced by elevated levels of hypercholesterolemia, non-esterified fatty acid concentrations, hyperglycemia, insulin resistance and adiponectin.[61–63] In addition to this background, pregnant obese women have decreased SOD, GSH, Oxidized to Reduced Glutathione (GSSG/GSH) ratio, and antioxidant vitamin levels, as well as increased MDA, nitrite and carbonyl proteins.[64, 65] Similarly, there are signs of increased oxidative stress and decreased antioxidants in their placentas. Subsequently, the chronic physiologic stress alters the maternal environment, which has physiologic effects on the developing fetus and continues in the neonate following delivery (Table 2).
Table 2.
Summary of the oxidative stress state related to altered fetal and maternal growth status.
| Aberrant Growth State | Antioxidant Reserve | Free Radicals Present | |
|---|---|---|---|
| Enzyme | Vitamin | ||
| Maternal Obesity | ↓SOD, ↓GSH ↓GSSH/GSH | ↓Vitamins B6, C and E | ↑MDA |
| Small for Gestational Age | ↑Xanthine oxidase, ↓GSH, ↓CAT, ⇅ SOD | ↓Vitamins C and E | ↑MDA, ↑H2O2 |
| Large for Gestational Age | ↑SOD | ↓Vitamin A and E | ↑H2O2 |
The antioxidant systems of term neonates from obese mothers are induced during gestation with increased relative activity of CAT and SOD at birth.[64] With the current obesity epidemic in the United States and other industrialized nations, the background oxidative damage caused of obesity needs to be recognized as a cofounder for fetal growth and a risk factor for perinatal disease origins.
Inflammation
For many critically ill neonates, exposure to inflammation begins prenatally. Prenatal inflammation has been associated with a variety of adverse neonatal outcomes, including preterm birth, neonatal sepsis, respiratory distress, NEC, intraventricular hemorrhage, and adverse neurodevelopmental outcomes, underscoring the close relationship between inflammation and oxidative stress in a pathogenesis of a wide variety of neonatal diseases.[66–69] Similarly, postnatal inflammation has long been recognized as an important contributor to a number of neonatal diseases. Studies showing increased inflammatory markers in infants with NEC,[70–72] as well as in neonates who go on to develop BPD,[73, 74] illustrate how ubiquitous inflammation is in neonatal diseases associated with increased oxidative stress. The close link between ROS and inflammation was first understood when the oxidative burst of PMNs were described almost 50 years ago.[3] Activated inflammatory cells release a large amount of oxygen radicals and proteases, resulting in direct tissue injury, while also leading to production of pro-inflammatory cytokines that also act as biomarkers of underlying injury. The inflammatory process can ultimately be a source of excessive ROS production with subsequent damage to cellular components and endothelial dysfunction when inflammatory responses are sustained. In addition, cytokines produced as a result of inflammation have been associated with changes in patterns of nitric oxide synthase (NOS) production, further contributing to oxidative stress through reactions of NO with O2− leading to even greater production of reactive oxidants such as peroxynitrite (ONOO−).
The interplay of inflammation and oxidative stress has been studied in animal models of NEC where, through a variety of insults to the premature intestine, tissue damage and epithelial injury lead to an inflammatory response. Increased levels of pro-inflammatory cytokines have been described in neonates with NEC,[70, 72, 75] and in animal models of the disease.[76] One consequence of increased pro-inflammatory cytokine production is induction of intracellular nitric oxide synthase (iNOS) whose expression is regulated by cytokines.[77, 78] In addition to increased iNOS, NEC is also associated with decreased expression of eNOS and eNOS uncoupling. Collectively, these changes may promote excessive production of NO and result in increased levels of ONOO− when NO combines with superoxide anion, another byproduct of increased oxidative stress, at a rate that is even faster than the dismutation of O2− by superoxide dismutases .[79–81] ONOO− and other reactive nitrogen species can, in turn, increase gut permeability through induction of enterocyte apoptosis and changes in gap junctions that result in decreased intestinal integrity.[82, 83] Finally, mitochondrial ROS production can further exacerbate tissue injury in NEC by increasing production of pro-inflammatory cytokines and promoting apoptotic signaling.[25, 84]
Inflammation has also been implicated in pathogenesis of BPD with increased levels of pro-inflammatory cytokines documented in infants who go on to develop BPD.[74, 85] In addition, exposure to hyperoxia, a risk factor for development of BPD, has been associated with an inflammatory lung response in rodent models of hyperoxic lung injury.[74, 86, 87] Similarly to changes seen in NEC, hyperoxic lung exposure is associated with increased production of pro-inflammatory cytokines and increased expression of iNOS in the lung,[88, 89] resulting in production of ONOO− and other reactive nitrogen species as evidenced by increased plasma 3-nitrotyrosine levels in premature infants who develop BPD.[90]
The complex interplay between inflammation and oxidative stress also appears to be important in pathogenesis of neonatal brain injury. Several studies have demonstrated increased oxidative stress in intraventricular hemorrhage, hypoxic-ischemic encephalopathy (HIE), and epilepsy.[91–93] Preterm neonates with severe perinatal hypoxia-ischemia have been shown to have increased concentrations of IL-6, a pro-inflammatory cytokine, and decreased activity of the antioxidant enzyme glutathione peroxidase in their cerebrospinal fluid, implicating both inflammation and oxidative stress in pathogenesis of HIE.[94] In addition, inflammatory activation of microglial cells in the brain has been associated with increased production of pro-inflammatory cytokines and ROS production.[95] In a newborn lamb model of asphyxia, expressions of IL-1beta, IL-12p40, TLR-2, and TLR-4 were increased in the cortex and subcortex after resuscitation with 100% O2 compared with 21% O2 indicating the pathways by which hyperoxia may induce inflammation in the newborn brain.[96] Finally, similar to the oxidative induction of iNOS in the lung and the intestine, increased iNOS expression has been reported in animal models of hypoxia-ischemia, likely contributing to oxidative stress burden,[97, 98] while selective inhibition of inducible and neuronal NOS has been associated with decreased formation of reactive nitrogen species and decreased apoptotic cell death in a newborn piglet model of HI.[99]
Although much work has been done to elucidate the interplay between inflammation and oxidative stress, the exact mechanisms linking inflammation, oxidative stress, and neonatal pathology remain poorly understood. The end result of excessive inflammation appears to be a vicious cycle whereby oxidative stress induces cytokine release, which then go on to increase ROS production further.[100] Additional studies are needed to investigate the cross talk between inflammatory injury and oxidative stress and their contribution to neonatal injury, and to delineate therapeutic strategies aimed at the inflammatory and oxidative response to injury.
Oxidative stress in neonatal pulmonary disease
While neonatal lung injury is often a result of a multitude of etiologic factors, oxidative stress has been recognized as a critical factor in pathophysiology of several neonatal lung diseases, including PPHN and BPD. Oxidative stress is also a common endpoint for multiple events, including inflammation, hyperoxia, and mechanical ventilation, that contribute to sustained lung injury and result in impaired lung function. The production of oxidative stress originates from a variety of sources and, when combined with immature antioxidant defenses, may ultimately result in oxidative lung injury in an at-risk neonate.
Evidence from animal models of PPHN linking increased oxidative stress to negative effects on NO signaling pathways and aberrant pulmonary vasodilatory responses supports the idea that ROS play an important role in the pathogenesis of PPHN. Lambs with PPHN demonstrate both antenatal and postnatal evidence of increased oxidative stress as compared to control animals, including increased levels of O2− and H2O2 in the pulmonary vasculature of fetal animals,[14, 101, 102] and exaggerated ROS production in response to hyperoxia after birth.[103, 104]
When Dr. Northway first described BPD more than 50 years ago, he outlined the toxic effects of oxygen exposure on the preterm lung as it induced the disease phenotype.[105] Clinicians continue to struggle with the paradigm as the ideal oxygen saturation and exposure for premature infants remain unknown.[106] Recent work has shown that any amount of oxygen exposure in the first few hours of life, or even just in the delivery room, can induce oxidative stress and potentially put patients at higher risk for developing BPD.[107, 108] Murine models have demonstrated that various concentrations and intervals of oxygen exposure during critical developmental windows can induce alveolar simplification.[16, 109] High oxygen exposure is directly toxic to the alveolar epithelium, especially the alveolar type II epithelial cell of which a subpopulation functions as stem cells for growth and injury repair.[19, 110] This is particularly injurious to the lung because oxygen exposure stunts epithelial cell growth potential in culture models even after removal of the oxygen exposure.[18]
A variety of ROS sources have been demonstrated in the lamb model of PPHN as well as in neonatal hyperoxic lung injury models of BPD. In the lamb model of PPHN, mitochondrial ROS have been linked to aberrant changes in the NO signaling pathways while O2− production from uncoupled eNOS and from NADPH oxidase has been associated with impaired vasodilatory responses and decreased angiogenesis.[102, 111–113] Rodent models of hyperoxic lung injury, on the other hand, demonstrate hyperoxic injury to pulmonary endothelial cells via NADPH oxidase resulting in cell death.[114–116] Similarly, hyperoxia exposed human fetal lung endothelial cells demonstrate increases in the NADPH pathway and H2O2 production.
Finally, impaired antioxidant capacity in the premature pulmonary endothelium and epithelium can potentially result in an imbalance between ROS production and antioxidant abilities of the lung, leading to increased oxidative stress. Reductions in superoxide dismutase enzymes, including ecSOD and MnSOD, have been described in PPHN lambs,[111, 117] while treatment with antioxidants results in increased eNOS, decreased PDE5 activation, better vasodilatory responses, and improved oxygenation.[103, 117–119] Preterm human lung tissues are deficient in their ability to increase MnSOD expression when exposed to hyperoxia.[120, 121] Moreover, in premature infants, early high oxygen exposure increases GSSG/GSH ratio that continues for the first several days of life and correlates with later development of BPD.[108]
Due to the crosstalk between the pulmonary endothelium and epithelium during development,[122] the fact that PPHN and BPD have overlapping oxidative stress and antioxidant mechanisms is not surprising. While pulmonary epithelial and endothelial cells have distinct mechanisms of oxidative stress production in hyperoxic lung injury models,[17, 18, 114–116] there are similarities in mechanisms of ROS generation between the PPHN and BPD disease models.[16, 102, 111–113, 123] These cell-type specific responses highlight the difficulty of targeting antioxidant systems for PPHN and BPD therapy as the lung is comprised of more than 40 cell types that may respond differently to oxidative stress stimuli.
Oxidative stress, gene regulation and epigenetic changes
Gene regulation in response to oxidative stress is complex and incompletely understood. As gene expression and regulation are organ specific, and in many cases cell-type specific, research on neonatal organ development and response to injury can be a challenge. Recent work with the LungMAP project has demonstrated that the neonatal period is a dynamic phase of shifting gene expression in order to direct organ maturation. Therefore, neonatal disease modeling is further complicated by this superimposed developmental trajectory.[124, 125] Current knowledge on gene expression or epigenetic changes in human neonates is mostly obtained from cells in blood samples and umbilical cord blood, however this does not allow for systematically revealing temporal pathogenesis at molecular level in lungs, brain, heart etc. Neonatal murine hyperoxic lung injury models are versatile and allow research at the molecular level in tissues. When rodents are born at term, their lungs are developmentally in the saccular stage. However, as opposed to humans, rodents are biologically prepared to meet the extrauterine (21% O2) world in the saccular stage with oxidative defenses genetically programmed to handle this postnatal environment.
In a murine neonatal hyperoxic lung injury model inducing BPD-like alveolar simplification, we determined mRNA gene expression in lung tissue of young mice using microarray technique. 117 genes related to pulmonary vascular disease were differentially regulated, including down-regulation of genes related to contractile elements, as well as up- and down-regulation of factors involved in vascular tone and tissue specific genes. Another group found close to 665 differentially regulated genes in neonatal hyperoxic lung injury; when isolating the genes not affected by sex differences, the remaining ~200 genes were predominantly in the angiogenesis pathways.[126] Moreover, in our hyperoxic lung injury model, 69 genes relevant to the immune system had differential expression including B-cell specific genes. (Revhaug C, Saugstad OD, unpublished data) Additionally, significant enrichment was observed regarding cytokine-cytokine receptor interactions, the P13-AKT, and B cell receptor signaling pathways.[127] Oxidative damage could therefore be partly responsible for the increased susceptibility to respiratory viruses and infections seen in premature infants with BPD through dysregulated genetic pathways involved in immunity and inflammation.
Any stress or alteration in cellular environment results in some cell or organ response. Gene regulation will change to produce proteins that may protect and repair the cell after damage or exposure to potentially damaging forces. Oxidative stress changes gene expression preferably by up-regulating antioxidant genes and regulating genes responsible for repairing cell damage. ROS also act as messengers to induce expression of genes via signal transduction. ROS from outside the cell (hyperoxia) or intracellular ROS (mostly from the mitochondria but also from cytokines and growth factors) activate redox-sensitive signaling pathways like NF-κB and AP-1, p38 mitogen-activated protein kinase, and JNK and result in expression of antioxidant and proinflammatory genes.[128]
In addition to the direct effects on gene expression, oxidative stress can also result in epigenetic changes. Epigenetic mechanisms regulate the availability of genes for transcription, resulting in altered gene expression without changes to the DNA sequence. Epigenetic research is not new, the term and concept already introduced in the 1940ś when epigenetics were studied in regard to evolution and development. [129] Epigenetic changes are responsible for considerable variability in the phenotypes of complex organisms and it is likely true for pathophysiological and disease phenotypes. The main methods of epigenetic regulation are DNA methylation and histone modifications (chromatin structuring and remodeling) that can lead to short-term and long-term effect on gene regulation. There are some studies that have looked at oxygen treatment and ROS [130] and the effect of oxygen and redox systems in the cells on the enzymes methylating DNA and histones [131]. ROS can have both direct and indirect effects on epigenetic mechanisms. OH. can directly attack DNA bases, leading to the formation of 5-hydroxymethylcytosine (5hmC) from 5-methylcytosine (5mC). 5hmC can then lead to indirect demethylation of CpG sites resulting in changes in gene transcription.[132, 133] An example of lasting epigenetic effect of oxygen in the newborn period was published in 2012, where adult rats exposed to hypoxic episodes in the neonatal period showed increased sleep disordered breathing and hypertension thought to be a result of DNA methylation in redox genes.[134]
Studies on human autopsy lung samples, from preterm infants and stillborn fetuses, have demonstrated a developmental shift in gene methylation affecting gene expression. In patients with established BPD, 32 genes were shown to have differential methylation compared to controls including differential expression of genes in pathways like GSH-mediated detoxification.[135] Moreover, decreased expression of the MnSOD gene was associated with DNA hypermethylation of a single CpG dinucleotide close to the transcription start site. Indirectly, ROS can affect gene regulation via epigenetic changes both locally and globally.[133, 134] Hyperoxic treatment of newborn mice in a BPD model resulted in lasting global hypermethylation of lung tissue homogenates. In this hyperoxic lung injury model, significant global DNA hypermethylation was present in oxygen-exposed animals, as well as enrichment of the TGF-β pathways.[136] Recently, epigenetic data from newborn infants show that the amount of oxygen provided during postnatal stabilization changes the DNA methylome in preterm infants. These authors found 1567 genes to be associated with oxygen load and, of these, 85% were hypomethylated acutely following oxygen exposure. An oxygen load starting at >500 mLO2/kg changed the methylation pattern of pathways involved in cell cycle progression, DNA repair, and oxidative stress. [137]
In the future the role of posttranslational epigenetic changes, like N6-methyladenosine (m6A), may be of interest, as these mechanisms are largely not well understood. A recent study demonstrated that hypoxia induces an increase in methylation of mRNA and that increased m6A stabilizes mRNA, however, the impact of these changes remains to be further elucidated. [138] In conclusion, hypoxia and stress are believed to modify RNAs by complex and poorly understood mechanisms and more work is needed to improve our understanding of these processes. [139]
Antioxidant defense and antioxidant therapy
There is strong evidence that the antioxidant defense system in premature infants is profoundly underdeveloped; however, attempts to therapeutically target these systems have yielded inconsistent results.[121, 140–143] Developmentally, the antioxidant system rapidly matures during the third trimester.[15, 142] This is likely an evolutionary change to adapt from the relatively hypoxic fetal environment, with a partial pressure of oxygen at 3.3 kPa, to the high oxygen tension extrauterine environment after birth.[144] As preterm infants are prematurely exposed to this environmental shift, relevant antioxidants that have been clinically targeted to prevent complications of prematurity include enzymes like CuZnSOD, MnSOD and ecSOD, GSH; vitamins such as A or E; and trace elements which are enzyme cofactors, such as selenium or L-arginine; however, development of these as clinical therapeutics have been limited by biochemical and physiologic factors. Antioxidant therapy developments have been limited by biochemical impediments such as compound half-life, poor cell penetrance, and difficulty targeting intracellular organelles. Moreover, there is a lack of physiologic knowledge regarding ideal antioxidant levels, gestationally appropriate enzyme activity, the relationship between trace element cofactor levels and enzyme activity in the premature neonate, as well as the appropriate disease endpoints to assess therapeutic effect.[120, 145, 146]
The very act of labor may prime the antioxidant system to deal with the transition to extrauterine life. Labor and vaginal delivery change the oxidative state in the mother, as well as in the fetus and the neonate, due to fluctuations in tissue oxygenation tension. Increased neonatal SOD, maternal MDA and placental xanthine oxidase activity have been reported in women who labored versus deliveries accomplished by caesarean without labor.[147, 148] In preterm infants, especially, when this physiologic response occurs it could overload their available antioxidant systems.[148] However, Georgeson et al found that preterm infants were able to produce higher CAT and GPx activity when delivery occurred by spontaneous vaginal delivery as opposed to cesarean section at comparable gestational ages. This supports the theory that the oxidative stress burden produced during labor and delivery induces antioxidant enzyme expression and activity to deal with the transition to extrauterine life.[149]
Animal models have shown beneficial effects of antioxidant therapies regarding prevention and improvement of neonatal disease phenotypes. Mitochondria-targeted antioxidants improve adult mouse survival in hyperoxia, and attenuate alveolar simplification and right ventricular hypertrophy in neonatal hyperoxic lung injury models.[16, 19] Extensive work with MnSOD, CuZnSOD and ecSOD, in hyperoxic lung injury models, have shown improvement in lung epithelial cell function and alveolarization through oxygen scavenging in the pulmonary epithelium.[150–154] In PPHN models, exogenous MnSOD treatment increases eNOS and improves vasodilatory responses in isolated pulmonary arteries.[117] Furthermore, treatment of PPHN lambs with recombinant human-CuZnSOD (rhSOD) improves vasodilation and increases iNO responsiveness,[119] resulting in improvements in oxygenation and decreased oxidative stress,[103] as well as attenuation of hyperoxic PDE5 activation with improvements in cGMP levels.[118] In vitro and in vivo, hydrocortisone treatment is associated with reductions in ROS levels along with decreases in hyperoxic PDE5 activation.[155, 156] Moreover, hypoxic-ischemic brain injury can also be attenuated in neonatal mice by targeting ROS production at the level of mitochondrial complex I, a major source of mitochondria-derived O2−.[157] In a rat NEC model, all-trans-retinoic acid injections were protective against NEC mediated by increased SOD and GPx activity. Supplementation of several different antioxidants, such as all-trans-retinoic acid, N-acetylcysteine, or astragaloside, all decreased the incidence of experimental NEC in the rat model likely through convergent mechanisms of lowering intestinal lipid oxidation as measured by MDA levels.[158] However, some antioxidant supplementation studies have exacerbated the disease of interest,[159, 160] highlighting our limited understanding to the optimal antioxidant balance in the developing neonatal animal.
Due to the direct oxygen toxicity on the lungs that occurs following premature birth, many groups have attempted to prevent BPD with antioxidant therapies ranging from vitamin and cofactor supplementation to replacement of antioxidant enzymes deficient in preterm infants. Supplementation with vitamin E and the enzyme co-factor selenium did not decrease the incidence of BPD in several studies.[161, 162] A non-enzymatic antioxidant intervention to show promise for BPD prevention is intramuscular vitamin A injections for the first month in extremely premature infant. The significant treatment effect was small, but the implementation of this therapy has been variable, likely due to the lack of prolonged clinical effect. Although underpowered for this outcome, Vitamin A therapy showed no difference in neurodevelopmental or pulmonary outcomes at 18–22 months.[163, 164] With regards to enzyme replacement, one of the most promising clinical trials regarding prevention of long-term pulmonary complications was intratracheal administration of rhSOD although this therapy has not become standard of care in the 15 years since the publication. Although the rhSOD intervention did not decrease early death or BPD, at one year, survivors at one year had decreased pulmonary disease burden quantified by less emergency room visits, medications and pulmonary readmissions.[165] On the other hand, attempts to target GSH deficiency in preterm infants with N-acetylcysteine did not affect BPD incidence or lung function in infancy.[166, 167] Similarly, despite promising animal studies, trials using inhaled NO in the early preterm period have not been beneficial in preventing either BPD or brain injury; at this time, routine use is not recommended.[168–172] By contrast, a recent study even indicated increased risk of childhood cancer after NO exposure to newborn infants (Adjusted OR 8.6, 95% CI 4.3– 17.4).[173]
Similar to BPD, animal models and patient studies have implicated oxidative stress and ROS generation in other neonatal disorders such as NEC, IVH and HI; but therapies have not become standard of care as the investigations have been limited by marginal effect or study size.[158, 174] For example, a systematic review regarding the use of L-arginine supplementation was a protective effect against NEC; however, the combined trials within the review only included 235 infants limiting confidence in adapting this therapy as a practice change.[175] However, one emerging therapy to show promise may be the use of melatonin (N-acetyl-5-methoxytryptamine) as an adjunct to hypothermia treatment to prevent HI injury. Melatonin has anti-oxidant, anti-apoptotic and anti-inflammatory properties, and has shown promise in published results from pre – and clinical intervention trials.[176, 177]
Physiologic reasoning supports using antioxidant therapy to prevent or address neonatal diseases where oxidative stress and ROS generation are central to the pathophysiology; despite the promising results in preclinical animal studies, human trials for antioxidant therapies have demonstrated a marginal benefit at best and in most cases no disease effect (Table 3). In addition to the biochemical and physiologic limitations listed above, successful transition of antioxidant therapies from pre-clinical to clinical studies have been limited by poor understanding of the correlations between histologic changes in animal models and physiologic changes in human disease. Many animal models demonstrate a histopathologic change in the target organ and the treatment effect is similarly assessed; however these findings are difficult to translate to the clinical disease spectrum present in humans.[109, 178, 179] Moreover, many of neonatal diseases discussed above are diagnosed on a constellation of clinical parameters, not on the histologic changes that are used to evaluate animal models.[180–182] A current search of clinicaltrials.gov yields a list of 59 studies regarding premature infants and antioxidants, with specifically 13 studies investigating antioxidants and BPD as the outcome.[183] In general, there is a lack of strong evidence in humans to encourage the use of antioxidant therapy as standard of care. Further work is needed to understand the antioxidant system in premature neonates, determine ideal enzyme activity and understand how histopathologic improvements in animals models correlate with physiologic outcomes in human neonates.
Table 3.
Summary of trials evaluating antioxidant therapy for prevention of neonatal disorders.
| Therapy | Mechanism/ROS Target | Trial Outcome | Reference |
|---|---|---|---|
| BPD | |||
| Inhaled NO | NO | No disease effect | Hasan et al.[171]; Askie et al. [172]; Askie et al. [28] |
| N-acetylcystine | GSH | No disease effect; no long term effect on lung function | Ahola et al.[167]; Sandberg et al.[166] |
| rhSOD | CuZnSOD | No decrease in death or BPD; survivors with less pulmonary disease burden in infancy | Davis et al.[165] |
| Selenium | Enzyme co-factor supplementation | No disease effect | Darlow et al.[162] |
| Vitamin A | Supplementation | Decreased incidence of BPD No pulmonary effect at 18–22 months | Ambalavanan et al. [163]; Tyson et al. [164] |
| Vitamin E | Supplementation | No disease effect | Watts et al. [161] |
| NEC | |||
| L-arginine | Enzyme co-factor Supplementation | Mitchell et al.[175] | |
| Neurodevelopment | |||
| Inhaled NO | NO | No effect for preventing IVH | Askie et al.[172] |
| Melatonin | GSH, MnSOD, ecSOD | Aly et al. [176] | |
| Vitamin A | Supplementation | No developmental effects at 18–22 months | Tyson et al. [164] |
Discussion
Oxidative stress is involved in a wide spectrum of newborn disease. In this review, we have summarized evidence for oxidative stress involvement not only in pulmonary diseases, such as BPD and PPHN, but also in HIE, NEC and fetal growth, as well as vascular changes. Although not addressed in as much detail here, previous reviews have detailed the evidence regarding retinopathy of prematurity as an oxygen dependent condition.[184, 185] Moreover, we summarized the epigenetic changes and subsequent effects on the transcriptome caused by oxidative stress and the subsequent long-term health implications of these neonatal changes.
Since those early articles linking neonatal disease to oxidative stress more than 30 years ago, the complexity and interplay between different ROS mechanisms and cell types have progressed significantly. The specific role and significance of mitochondrial derived oxidative stress has enhanced our understanding how oxidative stress may contribute to disease and injury especially in the newborn. Taken together with a better understanding of risk factors for oxidative stress, such as growth restriction and neonatal oxygen exposure, there is a hope that development of a new generation of antioxidants, in combination with other modulators, may contribute to prevention of oxidative stress induced injury of the newborn.
Genetic and epigenetic regulation occurs through several mechanisms; moreover, evidence supports that oxygen exposure induces epigenetic changes. Whether these are transitory or more permanent remains to be proven. However, such genetic and epigenetic investigations are also of importance for augmentation of our understanding how to prevent newborn disease; perhaps most importantly, how neonatal diseases and interventions have lasting effects into adult life, and thus subsequently contribute to adult diseases.
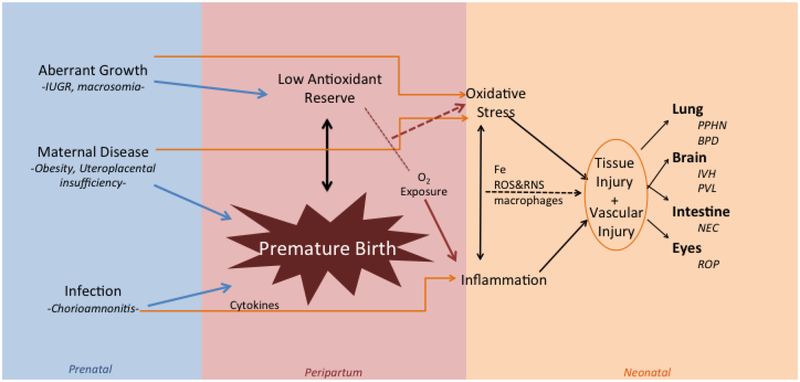
In figure 1 are summarized some of the concepts discussed in this review. Aberrant growth, maternal disease, such as obesity and UPI, infection, like chorioamnionitis, may all have more profound effects on the premature than the term infant. Oxidative stress with or without inflammation may be translated to injury of several organs such as the lung, brain, intestine and eye.
Figure 1.
Mechanisms contributing to Oxygen Radical Disease in the Newborn
References
- [1].Frank L, Groseclose EE, Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung, Pediatr Res 18(3) (1984) 240–4. 10.1203/00006450-198403000-00004. [DOI] [PubMed] [Google Scholar]
- [2].Buonocore G, Zani S, Sargentini I, Gioia D, Signorini C, Bracci R, Hypoxia-induced free iron release in the red cells of newborn infants, Acta Paediatr 87(1) (1998) 77–81. [DOI] [PubMed] [Google Scholar]
- [3].Babior BM, Kipnes RS, Curnutte JT, Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent, J Clin Invest 52(3) (1973) 741–4. 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Saugstad OD, Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production, Pediatr Res 23(2) (1988) 143–50. 10.1203/00006450-198802000-00001. [DOI] [PubMed] [Google Scholar]
- [5].Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, Schumacker PT, Steinhorn RH, Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn, Respir Physiol Neurobiol 174(3) (2010) 272–81. 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH, Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension, Antioxid Redox Signal 15(6) (2011) 1497–506. 10.1089/ars.2010.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sanderud J, Norstein J, Saugstad OD, Reactive oxygen metabolites produce pulmonary vasoconstriction in young pigs, Pediatr Res 29(6) (1991) 543–7. 10.1203/00006450-199106010-00005. [DOI] [PubMed] [Google Scholar]
- [8].Clyman RI, Saugstad OD, Mauray F, Reactive oxygen metabolites relax the lamb ductus arteriosus by stimulating prostaglandin production, Circ Res 64(1) (1989) 1–8. [DOI] [PubMed] [Google Scholar]
- [9].Friel JK, Friesen RW, Harding SV, Roberts LJ, Evidence of oxidative stress in full-term healthy infants, Pediatr Res 56(6) (2004) 878–82. 10.1203/01.PDR.0000146032.98120.43. [DOI] [PubMed] [Google Scholar]
- [10].Balaban RS, Nemoto S, Finkel T, Mitochondria, oxidants, and aging, Cell 120(4) (2005) 483–95. 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [11].Sahni PV, Zhang J, Sosunov S, Galkin A, Niatsetskaya Z, Starkov A, Brookes PS, Ten VS, Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxic-ischemic brain injury in mice, Pediatr Res 83(2) (2018) 491–497. 10.1038/pr.2017.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Afolayan AJ, Eis A, Alexander M, Michalkiewicz T, Teng RJ, Lakshminrusimha S, Konduri GG, Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 310(1) (2016) L40–9. 10.1152/ajplung.00392.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT, Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells, Antioxid Redox Signal 17(3) (2012) 460–70. 10.1089/ars.2011.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM, Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn, Am J Physiol Lung Cell Mol Physiol 289(4) (2005) L660–6. 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berkelhamer SK, Kim GA, Radder JE, Wedgwood S, Czech L, Steinhorn RH, Schumacker PT, Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung, Free Radic Biol Med 61 (2013) 51–60. 10.1016/j.freeradbiomed.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Datta A, Kim GA, Taylor JM, Gugino SF, Farrow KN, Schumacker PT, Berkelhamer SK, Mouse lung development and NOX1 induction during hyperoxia are developmentally regulated and mitochondrial ROS dependent, Am J Physiol Lung Cell Mol Physiol 309(4) (2015) L369–77. 10.1152/ajplung.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tiedge M, Lortz S, Munday R, Lenzen S, Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species, Diabetes 47(10) (1998) 1578–85. [DOI] [PubMed] [Google Scholar]
- [18].Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, Pollack S, Horowitz S, Davis JM, Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury, Am J Respir Cell Mol Biol 24(4) (2001) 436–41. 10.1165/ajrcmb.24.4.4240. [DOI] [PubMed] [Google Scholar]
- [19].Budinger GR, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, Hawkins K, Chiarella SE, Radigan KA, Eisenbart J, Agrawal H, Berkelhamer S, Hekimi S, Zhang J, Perlman H, Schumacker PT, Jain M, Chandel NS, Epithelial cell death is an important contributor to oxidant-mediated acute lung injury, Am J Respir Crit Care Med 183(8) (2011) 1043–54. 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Giusti B, Vestrini A, Poggi C, Magi A, Pasquini E, Abbate R, Dani C, Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants, Free Radic Res 46(9) (2012) 1130–9. 10.3109/10715762.2012.692787. [DOI] [PubMed] [Google Scholar]
- [21].Galam L, Failla A, Soundararajan R, Lockey RF, Kolliputi N, 4-hydroxynonenal regulates mitochondrial function in human small airway epithelial cells, Oncotarget 6(39) (2015) 41508–21. 10.18632/oncotarget.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kandasamy J, Olave N, Ballinger SW, Ambalavanan N, Vascular Endothelial Mitochondrial Function Predicts Death or Pulmonary Outcomes in Preterm Infants, Am J Respir Crit Care Med 196(8) (2017) 1040–1049. 10.1164/rccm.201702-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ratner V, Starkov A, Matsiukevich D, Polin RA, Ten VS, Mitochondrial dysfunction contributes to alveolar developmental arrest in hyperoxia-exposed mice, Am J Respir Cell Mol Biol 40(5) (2009) 511–8. 10.1165/rcmb.2008-0341RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, Ten VS, The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice, J Neurosci 32(9) (2012) 3235–44. 10.1523/JNEUROSCI.6303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baregamian N, Song J, Papaconstantinou J, Hawkins HK, Evers BM, Chung DH, Intestinal mitochondrial apoptotic signaling is activated during oxidative stress, Pediatr Surg Int 27(8) (2011) 871–7. 10.1007/s00383-011-2880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McGowan JE, Chen L, Gao D, Trush M, Wei C, Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia, Neurosci Lett 399(1–2) (2006) 111–4. 10.1016/j.neulet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- [27].Smith KA, Waypa GB, Schumacker PT, Redox signaling during hypoxia in mammalian cells, Redox Biol 13 (2017) 228–234. 10.1016/j.redox.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, Whyte R, Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants, Cochrane Database Syst Rev 4 (2017) CD011190 10.1002/14651858.CD011190.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK, Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia, J Perinatol 33(7) (2013) 553–7. 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gluckman PD, Hanson MA, Living with the past: evolution, development, and patterns of disease, Science 305(5691) (2004) 1733–6. 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- [31].Ozkan H, Cetinkaya M, Koksal N, Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia, J Matern Fetal Neonatal Med 25(12) (2012) 2681–5. 10.3109/14767058.2012.708371. [DOI] [PubMed] [Google Scholar]
- [32].Zeitlin J, El Ayoubi M, Jarreau PH, Draper ES, Blondel B, Kunzel W, Cuttini M, Kaminski M, Gortner L, Van Reempts P, Kollee L, Papiernik E, Group MR, Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort, J Pediatr 157(5) (2010) 733–9 e1. 10.1016/j.jpeds.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [33].Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM, Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth, Diabetologia 36(1) (1993) 62–7. [DOI] [PubMed] [Google Scholar]
- [34].Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME, Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease, BMJ 298(6673) (1989) 564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].MacLennan NK, James SJ, Melnyk S, Piroozi A, Jernigan S, Hsu JL, Janke SM, Pham TD, Lane RH, Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats, Physiol Genomics 18(1) (2004) 43–50. 10.1152/physiolgenomics.00042.2004. [DOI] [PubMed] [Google Scholar]
- [36].Sebert S, Sharkey D, Budge H, Symonds ME, The early programming of metabolic health: is epigenetic setting the missing link?, Am J Clin Nutr 94(6 Suppl) (2011) 1953S–1958S. 10.3945/ajcn.110.001040. [DOI] [PubMed] [Google Scholar]
- [37].El Ayoubi M, Patkai J, Bordarier C, Desfrere L, Moriette G, Jarreau PH, Zeitlin J, Impact of fetal growth restriction on neurodevelopmental outcome at 2 years for extremely preterm infants: a single institution study, Dev Med Child Neurol 58(12) (2016) 1249–1256. 10.1111/dmcn.13218. [DOI] [PubMed] [Google Scholar]
- [38].Rashid CS, Bansal A, Simmons RA, Oxidative Stress, Intrauterine Growth Restriction, and Developmental Programming of Type 2 Diabetes, Physiology (Bethesda) 33(5) (2018) 348–359. 10.1152/physiol.00023.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tanbe AF, Khalil RA, Circulating and Vascular Bioactive Factors during Hypertension in Pregnancy, Curr Bioact Compd 6(1) (2010) 60–75. 10.2174/157340710790711737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gupta P, Narang M, Banerjee BD, Basu S, Oxidative stress in term small for gestational age neonates born to undernourished mothers: a case control study, BMC Pediatr 4 (2004) 14 10.1186/1471-2431-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen F, Wang T, Feng C, Lin G, Zhu Y, Wu G, Johnson G, Wang J, Proteome Differences in Placenta and Endometrium between Normal and Intrauterine Growth Restricted Pig Fetuses, PLoS One 10(11) (2015) e0142396 10.1371/journal.pone.0142396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].De Marco CS, Caniggia I, Mechanisms of oxygen sensing in human trophoblast cells, Placenta 23 Suppl A (2002) S58–68. 10.1053/plac.2002.0809. [DOI] [PubMed] [Google Scholar]
- [43].Dunwoodie SL, The role of hypoxia in development of the Mammalian embryo, Dev Cell 17(6) (2009) 755–73. 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- [44].Sharp AN, Heazell AE, Crocker IP, Mor G, Placental apoptosis in health and disease, Am J Reprod Immunol 64(3) (2010) 159–69. 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Myatt L, Cui X, Oxidative stress in the placenta, Histochem Cell Biol 122(4) (2004) 369–82. 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- [46].Vest AR, Cho LS, Hypertension in pregnancy, Curr Atheroscler Rep 16(3) (2014) 395 10.1007/s11883-013-0395-8. [DOI] [PubMed] [Google Scholar]
- [47].Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, Harper A, Hulbert D, Lucas S, McClure J, Millward-Sadler H, Neilson J, Nelson-Piercy C, Norman J, O’Herlihy C, Oates M, Shakespeare J, de Swiet M, Williamson C, Beale V, Knight M, Lennox C, Miller A, Parmar D, Rogers J, Springett A, Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom, BJOG 118 Suppl 1 (2011) 1–203. 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- [48].Potdar N, Singh R, Mistry V, Evans MD, Farmer PB, Konje JC, Cooke MS, First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus, BJOG 116(5) (2009) 637–42. 10.1111/j.1471-0528.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- [49].Karowicz-Bilinska A, Suzin J, Sieroszewski P, Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation, Med Sci Monit 8(3) (2002) CR211–6. [PubMed] [Google Scholar]
- [50].Saker M, Soulimane Mokhtari N, Merzouk SA, Merzouk H, Belarbi B, Narce M, Oxidant and antioxidant status in mothers and their newborns according to birthweight, Eur J Obstet Gynecol Reprod Biol 141(2) (2008) 95–9. 10.1016/j.ejogrb.2008.07.013. [DOI] [PubMed] [Google Scholar]
- [51].Mert I, Oruc AS, Yuksel S, Cakar ES, Buyukkagnici U, Karaer A, Danisman N, Role of oxidative stress in preeclampsia and intrauterine growth restriction, J Obstet Gynaecol Res 38(4) (2012) 658–64. 10.1111/j.1447-0756.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- [52].Araujo JR, Correia-Branco A, Pereira AC, Pinho MJ, Keating E, Martel F, Oxidative stress decreases uptake of neutral amino acids in a human placental cell line (BeWo cells), Reprod Toxicol 40 (2013) 76–81. 10.1016/j.reprotox.2013.06.073. [DOI] [PubMed] [Google Scholar]
- [53].Robajac D, Masnikosa R, Mikovic Z, Mandic V, Nedic O, Oxidation of placental insulin and insulin-like growth factor receptors in mothers with diabetes mellitus or preeclampsia complicated with intrauterine growth restriction, Free Radic Res 49(8) (2015) 984–9. 10.3109/10715762.2015.1020798. [DOI] [PubMed] [Google Scholar]
- [54].Robajac D, Vanhooren V, Masnikosa R, Mikovic Z, Mandic V, Libert C, Nedic O, Preeclampsia transforms membrane N-glycome in human placenta, Exp Mol Pathol 100(1) (2016) 26–30. 10.1016/j.yexmp.2015.11.029. [DOI] [PubMed] [Google Scholar]
- [55].Schneider D, Hernandez C, Farias M, Uauy R, Krause BJ, Casanello P, Oxidative stress as common trait of endothelial dysfunction in chorionic arteries from fetuses with IUGR and LGA, Placenta 36(5) (2015) 552–8. 10.1016/j.placenta.2015.02.003. [DOI] [PubMed] [Google Scholar]
- [56].Luo Z, Luo W, Li S, Zhao S, Sho T, Xu X, Zhang J, Xu W, Xu J, Reactive oxygen species mediated placental oxidative stress, mitochondrial content, and cell cycle progression through mitogen-activated protein kinases in intrauterine growth restricted pigs, Reprod Biol (2018). 10.1016/j.repbio.2018.09.002. [DOI] [PubMed] [Google Scholar]
- [57].Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I, Role of oxidative stress in intrauterine growth restriction, Gynecol Obstet Invest 64(4) (2007) 187–92. 10.1159/000106488. [DOI] [PubMed] [Google Scholar]
- [58].Plata Mdel M, Williams L, Seki Y, Hartil K, Kaur H, Lin CL, Fiallo A, Glenn AS, Katz EB, Fuloria M, Charron MJ, Vuguin PM, Critical periods of increased fetal vulnerability to a maternal high fat diet, Reprod Biol Endocrinol 12 (2014) 80 10.1186/1477-7827-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tarry-Adkins JL, Fernandez-Twinn DS, Hargreaves IP, Neergheen V, Aiken CE, Martin-Gronert MS, McConnell JM, Ozanne SE, Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth, Am J Clin Nutr 103(2) (2016) 579–88. 10.3945/ajcn.115.119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gonzalez-Anover P, Torres-Rovira L, Garcia-Contreras C, Vazquez-Gomez M, Pesantez JL, Sanz-Fernandez MV, Astiz S, Gonzalez-Bulnes A, Antioxidant homeostasis is disturbed in fetuses with leptin-resistant genotypes: A cross-sectional study, Int J Reprod Biomed (Yazd) 16(8) (2018) 497–500. [PMC free article] [PubMed] [Google Scholar]
- [61].Chandrasekaran S, Neal-Perry G, Long-term consequences of obesity on female fertility and the health of the offspring, Curr Opin Obstet Gynecol 29(3) (2017) 180–187. 10.1097/GCO.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Suganami T, Nishida J, Ogawa Y, A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha, Arterioscler Thromb Vasc Biol 25(10) (2005) 2062–8. 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- [63].Chabrolle C, Tosca L, Rame C, Lecomte P, Royere D, Dupont J, Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells, Fertil Steril 92(6) (2009) 1988–96. 10.1016/j.fertnstert.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [64].Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, Narce M, Oxidative stress and maternal obesity: feto-placental unit interaction, Placenta 35(6) (2014) 411–6. 10.1016/j.placenta.2014.03.010. [DOI] [PubMed] [Google Scholar]
- [65].Sen S, Iyer C, Meydani SN, Obesity during pregnancy alters maternal oxidant balance and micronutrient status, J Perinatol 34(2) (2014) 105–11. 10.1038/jp.2013.153. [DOI] [PubMed] [Google Scholar]
- [66].Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR, Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years, Am J Obstet Gynecol 182(3) (2000) 675–81. [DOI] [PubMed] [Google Scholar]
- [67].Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG, Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis, J Pediatr 162(2) (2013) 236–42 e2. 10.1016/j.jpeds.2012.07.012. [DOI] [PubMed] [Google Scholar]
- [68].Liu Z, Tang Z, Li J, Yang Y, Effects of placental inflammation on neonatal outcome in preterm infants, Pediatr Neonatol 55(1) (2014) 35–40. 10.1016/j.pedneo.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [69].Bastek JA, Weber AL, McShea MA, Ryan ME, Elovitz MA, Prenatal inflammation is associated with adverse neonatal outcomes, Am J Obstet Gynecol 210(5) (2014) 450 e1–10. 10.1016/j.ajog.2013.12.024. [DOI] [PubMed] [Google Scholar]
- [70].Edelson MB, Bagwell CE, Rozycki HJ, Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis, Pediatrics 103(4 Pt 1) (1999) 766–71. [DOI] [PubMed] [Google Scholar]
- [71].Caplan MS, Sun XM, Hseuh W, Hageman JR, Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis, J Pediatr 116(6) (1990) 960–4. [DOI] [PubMed] [Google Scholar]
- [72].Viscardi RM, Lyon NH, Sun CC, Hebel JR, Hasday JD, Inflammatory cytokine mRNAs in surgical specimens of necrotizing enterocolitis and normal newborn intestine, Pediatr Pathol Lab Med 17(4) (1997) 547–59. [PubMed] [Google Scholar]
- [73].Joung KE, Kim HS, Lee J, Shim GH, Choi CW, Kim EK, Kim BI, Choi JH, Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia, Free Radic Res 45(9) (2011) 1024–32. 10.3109/10715762.2011.588229. [DOI] [PubMed] [Google Scholar]
- [74].An H, Nishimaki S, Ohyama M, Haruki A, Naruto T, Kobayashi N, Sugai T, Kobayashi Y, Mori M, Seki K, Yokota S, Interleukin-6, interleukin-8, and soluble tumor necrosis factor receptor-I in the cord blood as predictors of chronic lung disease in premature infants, Am J Obstet Gynecol 191(5) (2004) 1649–54. 10.1016/j.ajog.2004.04.014. [DOI] [PubMed] [Google Scholar]
- [75].Ford H, Watkins S, Reblock K, Rowe M, The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis, J Pediatr Surg 32(2) (1997) 275–82. [DOI] [PubMed] [Google Scholar]
- [76].Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B, Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis, Pediatr Res 51(6) (2002) 733–9. 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- [77].Soufli I, Toumi R, Rafa H, Touil-Boukoffa C, Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases, World J Gastrointest Pharmacol Ther 7(3) (2016) 353–60. 10.4292/wjgpt.v7.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chu SC, Marks-Konczalik J, Wu HP, Banks TC, Moss J, Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters, Biochem Biophys Res Commun 248(3) (1998) 871–8. 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- [79].Drucker NA, Jensen AR, Te Winkel JP, Ferkowicz MJ, Markel TA, Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis, J Pediatr Surg 53(6) (2018) 1208–1214. 10.1016/j.jpedsurg.2018.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Whitehouse JS, Xu H, Shi Y, Noll L, Kaul S, Jones DW, Pritchard KA Jr., Oldham KT, Gourlay DM, Mesenteric nitric oxide and superoxide production in experimental necrotizing enterocolitis, J Surg Res 161(1) (2010) 1–8. 10.1016/j.jss.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ferretti E, Tremblay E, Thibault MP, Grynspan D, Burghardt KM, Bettolli M, Babakissa C, Levy E, Beaulieu JF, The nitric oxide synthase 2 pathway is targeted by both pro- and anti-inflammatory treatments in the immature human intestine, Nitric Oxide 66 (2017) 53–61. 10.1016/j.niox.2017.03.003. [DOI] [PubMed] [Google Scholar]
- [82].Kandasamy J, Huda S, Ambalavanan N, Jilling T, Inflammatory signals that regulate intestinal epithelial renewal, differentiation, migration and cell death: Implications for necrotizing enterocolitis, Pathophysiology 21(1) (2014) 67–80. 10.1016/j.pathophys.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Grishin A, Bowling J, Bell B, Wang J, Ford HR, Roles of nitric oxide and intestinal microbiota in the pathogenesis of necrotizing enterocolitis, J Pediatr Surg 51(1) (2016) 13–7. 10.1016/j.jpedsurg.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM, Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS), J Exp Med 208(3) (2011) 519–33. 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schultz C, Tautz J, Reiss I, Moller JC, Prolonged mechanical ventilation induces pulmonary inflammation in preterm infants, Biol Neonate 84(1) (2003) 64–6. 10.1159/000071446. [DOI] [PubMed] [Google Scholar]
- [86].Warner BB, Stuart LA, Papes RA, Wispe JR, Functional and pathological effects of prolonged hyperoxia in neonatal mice, Am J Physiol 275(1 Pt 1) (1998) L110–7. [DOI] [PubMed] [Google Scholar]
- [87].Buczynski BW, Maduekwe ET, O’Reilly MA, The role of hyperoxia in the pathogenesis of experimental BPD, Semin Perinatol 37(2) (2013) 69–78. 10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Potter CF, Kuo NT, Farver CF, McMahon JT, Chang CH, Agani FH, Haxhiu MA, Martin RJ, Effects of hyperoxia on nitric oxide synthase expression, nitric oxide activity, and lung injury in rat pups, Pediatr Res 45(1) (1999) 8–13. 10.1203/00006450-199901000-00003. [DOI] [PubMed] [Google Scholar]
- [89].Cucchiaro G, Tatum AH, Brown MC, Camporesi EM, Daucher JW, Hakim TS, Inducible nitric oxide synthase in the lung and exhaled nitric oxide after hyperoxia, Am J Physiol 277(3) (1999) L636–44. 10.1152/ajplung.1999.277.3.L636. [DOI] [PubMed] [Google Scholar]
- [90].Banks BA, Ischiropoulos H, McClelland M, Ballard PL, Ballard RA, Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia, Pediatrics 101(5) (1998) 870–4. [DOI] [PubMed] [Google Scholar]
- [91].Waldbaum S, Patel M, Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy?, J Bioenerg Biomembr 42(6) (2010) 449–55. 10.1007/s10863-010-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ten VS, Starkov A, Hypoxic-ischemic injury in the developing brain: the role of reactive oxygen species originating in mitochondria, Neurol Res Int 2012 (2012) 542976 10.1155/2012/542976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Esiaba I, Angeles DM, Holden MS, Tan JB, Asmerom Y, Gollin G, Boskovic DS, Urinary Allantoin Is Elevated in Severe Intraventricular Hemorrhage in the Preterm Newborn, Transl Stroke Res 7(2) (2016) 97–102. 10.1007/s12975-015-0405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Vasiljevic B, Maglajlic-Djukic S, Gojnic M, Stankovic S, Ignjatovic S, Lutovac D, New insights into the pathogenesis of perinatal hypoxic-ischemic brain injury, Pediatr Int 53(4) (2011) 454–62. 10.1111/j.1442-200X.2010.03290.x. [DOI] [PubMed] [Google Scholar]
- [95].Kaur C, Rathnasamy G, Ling EA, Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina, J Neuroimmune Pharmacol 8(1) (2013) 66–78. 10.1007/s11481-012-9347-2. [DOI] [PubMed] [Google Scholar]
- [96].Markus T, Hansson S, Amer-Wahlin I, Hellstrom-Westas L, Saugstad OD, Ley D, Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep, Pediatr Res 62(1) (2007) 71–7. 10.1203/PDR.0b013e31811ead6e. [DOI] [PubMed] [Google Scholar]
- [97].Chang CC, Wang YH, Chern CM, Liou KT, Hou YC, Peng YT, Shen YC, Prodigiosin inhibits gp91(phox) and iNOS expression to protect mice against the oxidative/nitrosative brain injury induced by hypoxia-ischemia, Toxicol Appl Pharmacol 257(1) (2011) 137–47. 10.1016/j.taap.2011.08.027. [DOI] [PubMed] [Google Scholar]
- [98].Cai Z, Hutchins JB, Rhodes PG, Intrauterine hypoxia-ischemia alters nitric oxide synthase expression and activity in fetal and neonatal rat brains, Brain Res Dev Brain Res 109(2) (1998) 265–9. [DOI] [PubMed] [Google Scholar]
- [99].Peeters-Scholte C, Koster J, Veldhuis W, van den Tweel E, Zhu C, Kops N, Blomgren K, Bar D, van Buul-Offers S, Hagberg H, Nicolay K, van Bel F, Groenendaal F, Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia, Stroke 33(9) (2002) 2304–10. [DOI] [PubMed] [Google Scholar]
- [100].Crapo JD, Oxidative stress as an initiator of cytokine release and cell damage, Eur Respir J Suppl 44 (2003) 4s–6s. [DOI] [PubMed] [Google Scholar]
- [101].Konduri GG, Bakhutashvili I, Eis A, Pritchard K Jr., Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension, Am J Physiol Heart Circ Physiol 292(4) (2007) H1812–20. 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- [102].Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM, Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase, Circ Res 92(6) (2003) 683–91. 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- [103].Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH, Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension, Am J Respir Crit Care Med 174(12) (2006) 1370–7. 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH, Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells, Circ Res 102(2) (2008) 226–33. 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Northway WH Jr., Rosan RC, Porter DY, Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia, N Engl J Med 276(7) (1967) 357–68. 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- [106].Saugstad OD, Is oxygen more toxic than currently believed?, Pediatrics 108(5) (2001) 1203–5. [DOI] [PubMed] [Google Scholar]
- [107].Bonikos DS, Bensch KG, Ludwin SK, Northway WH Jr., Oxygen toxicity in the newborn. The effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice, Lab Invest 32(5) (1975) 619–35. [PubMed] [Google Scholar]
- [108].Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ 2nd, Arduini A, Escobar JJ, Sastre J, Asensi MA, Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease, Pediatrics 124(3) (2009) e439–49. 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- [109].Nardiello C, Mizikova I, Silva DM, Ruiz-Camp J, Mayer K, Vadasz I, Herold S, Seeger W, Morty RE, Standardisation of oxygen exposure in the development of mouse models for bronchopulmonary dysplasia, Dis Model Mech 10(2) (2017) 185–196. 10.1242/dmm.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ, Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells, Science 359(6380) (2018) 1118–1123. 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH, Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn, Antioxid Redox Signal 18(14) (2013) 1765–76. 10.1089/ars.2012.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG, Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 297(1) (2009) L184–95. 10.1152/ajplung.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Konduri GG, Ou J, Shi Y, Pritchard KA Jr., Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension, Am J Physiol Heart Circ Physiol 285(1) (2003) H204–11. 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- [114].Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, Barazzone Argiroffo C, NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice, Am J Respir Crit Care Med 180(10) (2009) 972–81. 10.1164/rccm.200902-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM, Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation, J Biol Chem 282(3) (2007) 1718–26. 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- [116].Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JG, Natarajan V, Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive oxygen species production in caveolin-enriched microdomains of the endothelium, J Biol Chem 284(50) (2009) 34964–75. 10.1074/jbc.M109.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG, Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn, Am J Physiol Lung Cell Mol Physiol 303(10) (2012) L870–9. 10.1152/ajplung.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH, Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 295(6) (2008) L979–87. 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM, Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension, Am J Respir Crit Care Med 164(5) (2001) 834–9. 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- [120].Asikainen TM, White CW, Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy, Antioxid Redox Signal 6(1) (2004) 155–67. 10.1089/152308604771978462. [DOI] [PubMed] [Google Scholar]
- [121].Berkelhamer SK, Farrow KN, Developmental regulation of antioxidant enzymes and their impact on neonatal lung disease, Antioxid Redox Signal 21(13) (2014) 1837–48. 10.1089/ars.2013.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Thebaud B, Abman SH, Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease, Am J Respir Crit Care Med 175(10) (2007) 978–85. 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Mamo LB, Suliman HB, Giles BL, Auten RL, Piantadosi CA, Nozik-Grayck E, Discordant extracellular superoxide dismutase expression and activity in neonatal hyperoxic lung, Am J Respir Crit Care Med 170(3) (2004) 313–8. 10.1164/rccm.200309-1282OC. [DOI] [PubMed] [Google Scholar]
- [124].Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M, Potter SS, Dexheimer P, Aronow B, Jobe AH, Whitsett JA, Xu Y, Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development, Thorax 72(5) (2017) 481–484. 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ardini-Poleske ME, Clark RF, Ansong C, Carson JP, Corley RA, Deutsch GH, Hagood JS, Kaminski N, Mariani TJ, Potter SS, Pryhuber GS, Warburton D, Whitsett JA, Palmer SM, Ambalavanan N, Lung MAPC, LungMAP: The Molecular Atlas of Lung Development Program, Am J Physiol Lung Cell Mol Physiol 313(5) (2017) L733–L740. 10.1152/ajplung.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Coarfa C, Zhang Y, Maity S, Perera DN, Jiang W, Wang L, Couroucli X, Moorthy B, Lingappan K, Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway, Am J Physiol Lung Cell Mol Physiol 313(6) (2017) L991–L1005. 10.1152/ajplung.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Revhaug C, Zasada M, Rognlien AGW, Gunther CC, Grabowska A, Ksiazek T, Madetko-Talowska A, Katarzyna, Szewczyk, Bik-Multanowski M, Kwinta P, Pietrzyk JJ, Baumbusch LO, Saugstad OD, pulmonary vascular disease is evident in gene regulation of experimental bronchopulmonary dysplasia, J Matern Fetal Neonatal Med (2018) 1–241. 10.1080/14767058.2018.1541081. [DOI] [PubMed] [Google Scholar]
- [128].Morgan MJ, Liu ZG, Crosstalk of reactive oxygen species and NF-kappaB signaling, Cell Res 21(1) (2011) 103–15. 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Waddington CH, The epigenotype. 1942, International journal of epidemiology 41(1) (2012) 10–3. 10.1093/ije/dyr184. [DOI] [PubMed] [Google Scholar]
- [130].Chen Z, Li S, Subramaniam S, Shyy JY-J, Chien S, Epigenetic Regulation: A New Frontier for Biomedical Engineers, Annual Review of Biomedical Engineering 19(1) (2017) 195–219. 10.1146/annurev-bioeng-071516-044720. [DOI] [PubMed] [Google Scholar]
- [131].Lamadema N, Burr S, Brewer AC, Dynamic regulation of epigenetic demethylation by oxygen availability and cellular redox, Free Radical Biology and Medicine 131 (2019) 282–298. [DOI] [PubMed] [Google Scholar]
- [132].Tirone F, Vigano A, Groppetti A, Parenti M, Effects of the desensitization by morphine of the opiate-dependent adenylate cyclase system in the rat striatum on the activity of the inhibitory regulatory G protein, Biochem Pharmacol 37(6) (1988) 1039–44. [DOI] [PubMed] [Google Scholar]
- [133].Kietzmann T, Petry A, Shvetsova A, Gerhold JM, Gorlach A, The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system, Br J Pharmacol 174(12) (2017) 1533–1554. 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR, Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis, Proc Natl Acad Sci U S A 109(7) (2012) 2515–20. 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Cuna A, Halloran B, Faye-Petersen O, Kelly D, Crossman DK, Cui X, Pandit K, Kaminski N, Bhattacharya S, Ahmad A, Mariani TJ, Ambalavanan N, Alterations in gene expression and DNA methylation during murine and human lung alveolar septation, Am J Respir Cell Mol Biol 53(1) (2015) 60–73. 10.1165/rcmb.2014-0160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Bik-Multanowski M, Revhaug C, Grabowska A, Dobosz A, Madetko-Talowska A, Zasada M, Saugstad OD, Hyperoxia induces epigenetic changes in newborn mice lungs, Free Radic Biol Med 121 (2018) 51–56. 10.1016/j.freeradbiomed.2018.04.566. [DOI] [PubMed] [Google Scholar]
- [137].Lorente-Pozo S, Parra-Llorca A, Nunez-Ramiro A, Cernada M, Hervas D, Boronat N, Sandoval J, Vento M, The Oxygen Load Supplied during Delivery Room Stabilization of Preterm Infants Modifies the DNA Methylation Profile, J Pediatr 202 (2018) 70–76 e2. 10.1016/j.jpeds.2018.07.009. [DOI] [PubMed] [Google Scholar]
- [138].Fry NJ, Law BA, Ilkayeva OR, Holley CL, Mansfield KD, N(6)-methyladenosine is required for the hypoxic stabilization of specific mRNAs, RNA (New York, N.Y.) 23(9) (2017) 1444–1455. 10.1261/rna.061044.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Licht K, Jantsch MF, Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications, The Journal of cell biology 213(1) (2016) 15–22. 10.1083/jcb.201511041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lindeman JH, Lentjes EG, Berger HM, Diminished protection against copper-induced lipid peroxidation by cord blood plasma of preterm and term infants, JPEN J Parenter Enteral Nutr 19(5) (1995) 373–5. 10.1177/0148607195019005373. [DOI] [PubMed] [Google Scholar]
- [141].Lindeman JH, Lentjes EG, van Zoeren-Grobben D, Berger HM, Postnatal changes in plasma ceruloplasmin and transferrin antioxidant activities in preterm babies, Biol Neonate 78(2) (2000) 73–6. 10.1159/000014252. [DOI] [PubMed] [Google Scholar]
- [142].Lavoie JC, Chessex P, Development of glutathione synthesis and gamma-glutamyltranspeptidase activities in tissues from newborn infants, Free Radic Biol Med 24(6) (1998) 994–1001. [DOI] [PubMed] [Google Scholar]
- [143].Garcia-Talavera J, Catovsky D, Fuentes Castellano J, [Membrane receptors in acute monocytic leukaemia (author’s transl)], Sangre (Barc) 23(3) (1978) 282–90. [PubMed] [Google Scholar]
- [144].Davis JM, Auten RL, Maturation of the antioxidant system and the effects on preterm birth, Semin Fetal Neonatal Med 15(4) (2010) 191–5. 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- [145].Jankov RP, Negus A, Tanswell AK, Antioxidants as therapy in the newborn: some words of caution, Pediatr Res 50(6) (2001) 681–7. 10.1203/00006450-200112000-00009. [DOI] [PubMed] [Google Scholar]
- [146].Loui A, Raab A, Maier RF, Bratter P, Obladen M, Trace elements and antioxidant enzymes in extremely low birthweight infants, J Trace Elem Med Biol 24(2) (2010) 111–8. 10.1016/j.jtemb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- [147].Many A, Roberts JM, Increased xanthine oxidase during labour--implications for oxidative stress, Placenta 18(8) (1997) 725–6. [DOI] [PubMed] [Google Scholar]
- [148].Stipek S, Mechurova A, Crkovska J, Zima T, Platenik J, Lipid peroxidation and superoxide dismutase activity in umbilical and maternal blood, Biochem Mol Biol Int 35(4) (1995) 705–11. [PubMed] [Google Scholar]
- [149].Georgeson GD, Szony BJ, Streitman K, Varga IS, Kovacs A, Kovacs L, Laszlo A, Antioxidant enzyme activities are decreased in preterm infants and in neonates born via caesarean section, Eur J Obstet Gynecol Reprod Biol 103(2) (2002) 136–9. [DOI] [PubMed] [Google Scholar]
- [150].Walther FJ, David-Cu R, Lopez SL, Antioxidant-surfactant liposomes mitigate hyperoxic lung injury in premature rabbits, Am J Physiol 269(5 Pt 1) (1995) L613–7. 10.1152/ajplung.1995.269.5.L613. [DOI] [PubMed] [Google Scholar]
- [151].Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD, A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia, Am J Respir Crit Care Med 167(1) (2003) 57–64. 10.1164/rccm.200203-232OC. [DOI] [PubMed] [Google Scholar]
- [152].Padmanabhan RV, Gudapaty R, Liener IE, Schwartz BA, Hoidal JR, Protection against pulmonary oxygen toxicity in rats by the intratracheal administration of liposome-encapsulated superoxide dismutase or catalase, Am Rev Respir Dis 132(1) (1985) 164–7. 10.1164/arrd.1985.132.1.164. [DOI] [PubMed] [Google Scholar]
- [153].Davis JM, Rosenfeld WN, Sanders RJ, Gonenne A, Prophylactic effects of recombinant human superoxide dismutase in neonatal lung injury, J Appl Physiol (1985) 74(5) (1993) 2234–41. 10.1152/jappl.1993.74.5.2234. [DOI] [PubMed] [Google Scholar]
- [154].Koo HC, Davis JM, Li Y, Hatzis D, Opsimos H, Pollack S, Strayer MS, Ballard PL, Kazzaz JA, Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia, Am J Physiol Lung Cell Mol Physiol 288(4) (2005) L718–26. 10.1152/ajplung.00456.2003. [DOI] [PubMed] [Google Scholar]
- [155].Perez M, Wedgwood S, Lakshminrusimha S, Farrow KN, Steinhorn RH, Hydrocortisone normalizes phosphodiesterase-5 activity in pulmonary artery smooth muscle cells from lambs with persistent pulmonary hypertension of the newborn, Pulm Circ 4(1) (2014) 71–81. 10.1086/674903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Perez M, Lakshminrusimha S, Wedgwood S, Czech L, Gugino SF, Russell JA, Farrow KN, Steinhorn RH, Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn, Am J Physiol Lung Cell Mol Physiol 302(6) (2012) L595–603. 10.1152/ajplung.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kim M, Stepanova A, Niatsetskaya Z, Sosunov S, Arndt S, Murphy MP, Galkin A, Ten VS, Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury, Free Radic Biol Med 124 (2018) 517–524. 10.1016/j.freeradbiomed.2018.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Aceti A, Beghetti I, Martini S, Faldella G, Corvaglia L, Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk, Oxid Med Cell Longev 2018 (2018) 7397659 10.1155/2018/7397659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Muzykantov VR, Delivery of antioxidant enzyme proteins to the lung, Antioxid Redox Signal 3(1) (2001) 39–62. 10.1089/152308601750100489. [DOI] [PubMed] [Google Scholar]
- [160].Stabler SP, Morton RL, Winski SL, Allen RH, White CW, Effects of parenteral cysteine and glutathione feeding in a baboon model of severe prematurity, Am J Clin Nutr 72(6) (2000) 1548–57. 10.1093/ajcn/72.6.1548. [DOI] [PubMed] [Google Scholar]
- [161].Watts JL, Milner R, Zipursky A, Paes B, Ling E, Gill G, Fletcher B, Rand C, Failure of supplementation with vitamin E to prevent bronchopulmonary dysplasia in infants less than 1,500 g birth weight, Eur Respir J 4(2) (1991) 188–90. [PubMed] [Google Scholar]
- [162].Darlow BA, Winterbourn CC, Inder TE, Graham PJ, Harding JE, Weston PJ, Austin NC, Elder DE, Mogridge N, Buss IH, Sluis KB, The effect of selenium supplementation on outcome in very low birth weight infants: a randomized controlled trial. The New Zealand Neonatal Study Group, J Pediatr 136(4) (2000) 473–80. [DOI] [PubMed] [Google Scholar]
- [163].Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, Carlo WA, National Institute of Child, H., Human Development Neonatal Research, N., Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months, Pediatrics 115(3) (2005) e249–54. 10.1542/peds.2004-1812. [DOI] [PubMed] [Google Scholar]
- [164].Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, Fanaroff AA, Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network, N Engl J Med 340(25) (1999) 1962–8. 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- [165].Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, North American Recombinant Human CuZn, S. O. D. S. G., Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase, Pediatrics 111(3) (2003) 469–76. [DOI] [PubMed] [Google Scholar]
- [166].Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O, N-acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants, Biol Neonate 86(4) (2004) 275–9. 10.1159/000080089. [DOI] [PubMed] [Google Scholar]
- [167].Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, Jonsbo F, Esberg G, Stovring S, Kjartansson S, Stiris T, Lossius K, Virkola K, Fellman V, N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial, J Pediatr 143(6) (2003) 713–9. 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- [168].Sokol GM, Konduri GG, Van Meurs KP, Inhaled nitric oxide therapy for pulmonary disorders of the term and preterm infant, Semin Perinatol 40(6) (2016) 356–369. 10.1053/j.semperi.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Cole FS, Alleyne C, Barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, Gregory K, Johnston MV, Kramer M, Mitchell C, Neu J, Pursley DM, Robinson W, Rowitch DH, NIH consensus development conference: Inhaled nitric oxide therapy for premature infants, NIH Consens State Sci Statements 27(5) (2010) 1–34. [PubMed] [Google Scholar]
- [170].Askie LM, Ballard RA, Cutter GR, Dani C, Elbourne D, Field D, Hascoet JM, Hibbs AM, Kinsella JP, Mercier JC, Rich W, Schreiber MD, Wongsiridej PS, Subhedar NV, Van Meurs KP, Voysey M, Barrington K, Ehrenkranz RA, Finer NN, Meta-analysis of Preterm Patients on Inhaled Nitric Oxide, C., Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials, Pediatrics 128(4) (2011) 729–39. 10.1542/peds.2010-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hasan SU, Potenziano J, Konduri GG, Perez JA, Van Meurs KP, Walker MW, Yoder BA, Newborns Treated With Nitric Oxide Trial, G., Effect of Inhaled Nitric Oxide on Survival Without Bronchopulmonary Dysplasia in Preterm Infants: A Randomized Clinical Trial, JAMA Pediatr 171(11) (2017) 1081–1089. 10.1001/jamapediatrics.2017.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Askie LM, Ballard RA, Cutter G, Dani C, Elbourne D, Field D, Hascoet JM, Hibbs AM, Kinsella JP, Mercier JC, Rich W, Schreiber MD, Srisuparp P, Subhedar NV, Van Meurs KP, Voysey M, Barrington K, Ehrenkranz RA, Finer N, Meta-Analysis of Preterm Patients on inhaled Nitric Oxide, C., Inhaled nitric oxide in preterm infants: a systematic review and individual patient data meta-analysis, BMC Pediatr 10 (2010) 15 10.1186/1471-2431-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Dixon F, Ziegler DS, Bajuk B, Wright I, Hilder L, Abdel Latif ME, Somanathan A, Oei JL, Treatment with nitric oxide in the neonatal intensive care unit is associated with increased risk of childhood cancer, Acta Paediatr 107(12) (2018) 2092–2098. 10.1111/apa.14436. [DOI] [PubMed] [Google Scholar]
- [174].Manzoni P, Guardione R, Bonetti P, Priolo C, Maestri A, Mansoldo C, Mostert M, Anselmetti G, Sardei D, Bellettato M, Biban P, Farina D, Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial, Am J Perinatol 30(1) (2013) 25–32. 10.1055/s-0032-1321494. [DOI] [PubMed] [Google Scholar]
- [175].Mitchell K, Lyttle A, Amin H, Shaireen H, Robertson HL, Lodha AK, Arginine supplementation in prevention of necrotizing enterocolitis in the premature infant: an updated systematic review, BMC Pediatr 14 (2014) 226 10.1186/1471-2431-14-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Aly H, Elmahdy H, El-Dib M, Rowisha M, Awny M, El-Gohary T, Elbatch M, Hamisa M, El-Mashad AR, Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study, J Perinatol 35(3) (2015) 186–91. 10.1038/jp.2014.186. [DOI] [PubMed] [Google Scholar]
- [177].Robertson NJ, Martinello K, Lingam I, Avdic-Belltheus A, Meehan C, Alonso-Alconada D, Ragab S, Bainbridge A, Sokolska M, Tachrount M, Middleton B, Price D, Hristova M, Golay X, Soliani Raschini A, Aquino G, Pelizzi N, Facchinetti F, Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: A translational study, Neurobiol Dis 121 (2019) 240–251. 10.1016/j.nbd.2018.10.004. [DOI] [PubMed] [Google Scholar]
- [178].Sodhi C, Richardson W, Gribar S, Hackam DJ, The development of animal models for the study of necrotizing enterocolitis, Dis Model Mech 1(2–3) (2008) 94–8. 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Hsia CC, Hyde DM, Ochs M, Weibel ER, Structure, A. E. J. T. F. o. Q. A. o. L., An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure, Am J Respir Crit Care Med 181(4) (2010) 394–418. 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Gordon PV, Swanson JR, MacQueen BC, Christensen RD, A critical question for NEC researchers: Can we create a consensus definition of NEC that facilitates research progress?, Semin Perinatol 41(1) (2017) 7–14. 10.1053/j.semperi.2016.09.013. [DOI] [PubMed] [Google Scholar]
- [181].Jobe AH, Bancalari E, Bronchopulmonary dysplasia, Am J Respir Crit Care Med 163(7) (2001) 1723–9. 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- [182].Bancalari E, Jain D, Bronchopulmonary Dysplasia: Can We Agree on a Definition?, Am J Perinatol 35(6) (2018) 537–540. 10.1055/s-0038-1637761. [DOI] [PubMed] [Google Scholar]
- [183].ClinicalTrials.gov [Internet], Identifiers: premature infants, antioxidants or BPD and antioxidants., National Institutes of Health 2000-present. [Google Scholar]
- [184].Saugstad OD, Oxygenation of the Immature Infant: A Commentary and Recommendations for Oxygen Saturation Targets and Alarm Limits, Neonatology 114(1) (2018) 69–75. 10.1159/000486751. [DOI] [PubMed] [Google Scholar]
- [185].Saugstad OD, Oei JL, Lakshminrusimha S, Vento M, Oxygen therapy of the newborn from molecular understanding to clinical practice, Pediatr Res (2018). 10.1038/s41390-018-0176-8. [DOI] [PubMed] [Google Scholar]