Abstract
The introduction of immunotherapy into the treatment of cancer patients has revolutionised the oncological approach and significantly improved patient survival. The key drugs are immune checkpoint inhibitors (CPIs), whose mechanism of action is to elicit immune response against cancer cell antigens. Three types of CPIs are currently used and approved: an anti-CTLA-4 antibody, ipilimumab; anti-PD-1 antibodies, nivolumab and pembrolizumab; and anti-PD-L1 antibodies: atezolizumab, avelumab and durvalumab. CPIs have been widely used in metastatic and adjuvant melanoma settings, metastatic lung cancer, Hodgkin’s lymphoma, renal cancer, bladder cancer, head and neck tumours, and Merkel cell carcinoma. However, side effects of CPIs differ from toxicities of other oncological drugs. According to literature data, in 10–30% of patients CPIs are responsible for immune-related adverse events (irAE) associated with excessive activation of the immune system. Systemic irAEs include enterocolitis, pneumonitis, hepatitis, nephritis, hypophysitis, and autoimmune thyroid disease. However, the most common irAEs of checkpoint inhibitors are dermatologic toxicities ranging from pruritus and mild dermatoses to severe reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis. Each irAE can become serious if not early diagnosed and appropriately treated. In the article we present different types of skin irAEs related to CPIs together with the recommended therapies.
Keywords: skin toxicities, immune checkpoint antibody
Introduction
The use of immunotherapy in the treatment of cancer patients has revolutionised modern oncology and significantly improved the outcomes of cancer treatment. However, a distinction should be made between immunotherapy with monoclonal antibodies to surface receptors of cancer cells, and treatment with antibodies against immune checkpoints (immune checkpoint inhibitors – ICIs). The former, classical passive immunotherapy antibodies, work by blocking receptors for intracellular conduction pathways, which inhibits proliferation, induces apoptosis of target cells and, consequently, significantly impairs the proliferation of cancer cells. This group of antibodies includes, among others, trastuzumab, pertuzumab, panitumumab and cetuximab [1]. On the other hand, the activity of checkpoint inhibitors consists in inducing their own immune response against various neoplastic antigens by blocking receptors responsible for lymphocyte inactivation. The purpose of the patient’s own immune response is to destroy cancer cells with specific antigens and to induce long-term immune response of the body (through lymphocytes) by providing immunological memory (the so-called inhibition of immunotolerance) [2, 3]. Immune checkpoints include CTLA-4, PD-1 and PD-L1. Cytotoxic T-lymphocyte antigen 4 (CTLA-4), located on T cells, is the primary regulator of the activity of these lymphocytes. An antibody binding to CTLA-4 immune checkpoint blocks the inhibitory signalling for T cells, induced by the CTLA-4 pathway, leading to an increase in the number of activated effector T cells that mobilise T cells for a direct immune attack against tumour cells. Another immunoreceptor is the programmed cell death 1 (PD-1) receptor, which undergoes induced expression on T cells as well as B cells. It is a negative regulator of immune response. Through interactions with its ligands, PD-L1 and PD-L2, expressed in many types of tissues, PD-1 is responsible for maintaining peripheral tolerance by limiting the activation, proliferation and effector functions of T cells. The expression of PD-1 and its ligands has been reported in many types of tumours, where they are important in modelling the microenvironment, and may also be associated with the escape of tumour cells from immune surveillance. Antibodies to PD-1/PD-L1, by inhibiting the binding of PD-1 with PD-L1 and PD-L2, support the activity of T cells, including the anticancer response [4, 5].
The introduction of checkpoint inhibitors in the form of anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies significantly prolonged the survival of patients with metastatic forms of melanoma and lung cancer. These agents have also been used in the adjuvant treatment of melanoma patients and in the treatment of Hodgkin’s lymphoma, advanced kidney cancer, bladder cancer, Merkel cell cancer, head and neck cancer, and other solid tumours [6–16]. Six ICIs have been approved by the US Food and Drug Administration (FDA) in the United States and by the European Medicines Agency (EMA) in Europe for use in various cancers: a CTLA-4 inhibitor, ipilimumab; PD-1 inhibitors, nivolumab and pembrolizumab; PD-L1 inhibitors, atezolizumab, avelumab and durvalumab.
Unfortunately, the use of ICIs is also associated with the risk of new, previously unknown, adverse events, so-called immune-related adverse events (irAEs), which are often severe and unpredictable.
Immune complications associated with excessive activation of the immune system may affect virtually any system and organ and may occur at different stages of treatment (often even after the end of immunotherapy), which adds to this problem. Mild initial symptoms may suddenly become significantly worse and severe; therefore, it is extremely important to diagnose irAEs correctly, to determine their severity, and use the appropriate treatment as soon as possible after the onset of an irAE. The most common irAEs include skin complications, which may already occur at the beginning of treatment.
This article presents various types of irAEs affecting the skin, and the recommendations for prophylactic and therapeutic management in case they occur.
Frequency and time of onset of skin lesions during therapy with checkpoint inhibitors
The most common immune-related adverse events (irAEs) associated with the use of ICIs include the following skin symptoms: maculopapular rash, dermatitis, itching and vitiligo-like depigmentation of the skin. Immune-related complications affecting the skin have been reported in 45–65% of ipilimumab-treated patients and 30–40% of patients treated with anti-PD-1 inhibitors [17–20]. In most cases, they are mild (grade 1/2) according to Common Terminology Criteria for Adverse Events (CTCAE) [21]. Severe symptoms of dermal toxicity are rare (< 3% cases with anti-PD-1 monotherapy and < 5 % in combined therapy with anti-CTLA-4 and anti-PD-1) and usually do not require discontinuation of immunotherapy. Skin symptoms, other than vitiligo, appear relatively early after the beginning of therapy, usually during the first weeks of treatment (3–6 weeks).
They usually resolve in a few weeks and (with the exception of vitiligo) are reversible. In most cases, their severity depends on the dose of the immunological drug. Vitiligo is usually associated with a better response to anti-PD-1 therapy in patients with melanoma [17–20, 22].
In the case of combination therapy with ipilimumab and an anti-PD-1 inhibitor, skin complications were reported in 62% of cases, most frequently in the form of pruritus, rash, dermatitis, urticaria, vitiligo, bullous pemphigoid and lichenoid dermatitis. Grade 3/4 dermal toxicities were reported in 1% of cases during nivolumab therapy and in 1.5% during pembrolizumab therapy. These complications usually occurred earlier during therapy with nivolumab (4th–8th week of therapy) compared to pembrolizumab (23rd week of therapy). Serious (grade 3/4) skin irAEs are rare.
It is very difficult to accurately describe the prevalence and clinical nature of skin irAEs (the lesions are usually treated collectively as rash) due to the relatively short duration of use of ICIs and the lack of precise dermatological terms in most published studies.
Description of the most common skin lesions during ICI therapy
In terms of histology, skin lesions associated with the use of ICIs can be classified into four groups: inflammatory lesions as a manifestation of acute, subacute or chronic dermatitis, immune-related bullous diseases, keratinocyte disorders, immune-related melanocytic disorders. The classification is presented in Table 1 [18].
Table 1.
Types of dermatologic toxicities reported with anti-CTLA-4, anti-PD-1 and PDL-1 antibody therapy [18]
| Category | Dermatologic toxicity | Clinical presentation | Histologic feature | Anti-CTLA-4 | Anti-PD-1 | Anti-PDL-1 |
|---|---|---|---|---|---|---|
| Inflammatory | Acneiform | Not reported | Not reported | Yes | Yes | Yes |
| AGEP (acute generalized exanthematous pustulosis) | Oedematous, erythematous pustules | Subcorneal neutrophils with eosinophils | Yes | Yes | Yes | |
| CD30 lymphomatoid reaction | 3 to 6-mm pink papules coalescing into plaques on the abdomen and back | CD30+ lymphoid infiltrate with overlying epidermal hyperplasia | Yes | No | No | |
| Dermatomyositis | Photo-distributed, erythematous eruption on the face and upper chest, erythematous papules over the dorsal hand, nail fold and eyelid erythema, muscle weakness | Not performed | Yes | No | No | |
| DHR (dermal hypersensitivity reaction) | Maculopapular eruption on the trunk and extremities | Perivascular lymphocytic inflammation with ± eosinophils and spongiosis | Yes | Yes | Yes | |
| DRESS (drug reaction with eosinophilia and systemic symptoms) | Diffuse maculopapular rash with erythroderma | Not performed | Yes | No | No | |
| Eczema/spongiotic | No | Yes | Yes | |||
| Erythema/erythematous | No | Yes | Yes | |||
| Exfoliative | No | Yes | Yes | |||
| Lichenoid/interface | Not reported | Not reported | Yes | Yes | Yes | |
| Maculopapular | No | Yes | Yes | |||
| Pityriasis lichenoides (PL)-like skin lesions | No | Yes | Yes | |||
| Photosensitivity | Erythematous macules on sun-exposed sites with subsequent plaques on the scalp, trunk and extremities | Spongiotic dermatitis with eosinophils and parakeratosis and acanthosis | Yes | Yes | Yes | |
| Psoriasiform | No | Yes | Yes | |||
| Pyoderma gangrenosum | Ulcerated, erythematous nodule | Ulcer with neutrophilic dermal inflammation | Yes | No | No | |
| Radiation-associated dermatitis | Blisters within the radiated area | Not performed | Yes | Yes | Yes | |
| Sweet syndrome | Erythematous, tender papules and plaques on the face, trunk and extremities | Papillary dermal oedema and neutrophilic inflammation | Yes | No | No | |
| SJS/TEN (Stevens–Johnson syndrome/ toxic epidermal necrolysis) | Not specified | Skin necrosis and vasculitis | Yes | Yes | Yes | |
| Vasculopathic | No | Yes | Yes | |||
| Immunobullous | Bullous pemphigoid | No | Yes | Yes | ||
| Dermatitis herpetiformis | Pink papules, grouped near the elbows, back and buttocks | Collection of neutrophils in the papillary dermis, IgA deposits in the dermal papillae | Yes | No | No | |
| Alteration of keratinocytes | Actinic keratosis | No | Yes | Yes | ||
| Basal cell carcinoma | No | Yes | Yes | |||
| Grover’s disease | Papulokeratotic eruption on the trunk | Acantholytic dyskeratosis | Yes | Yes | Yes | |
| Prurigo nodularis | Not reported | Not performed | Yes | No | No | |
| Seborrheic keratosis | No | Yes | Yes | |||
| Squamous cell carcinoma | No | Yes | Yes | |||
| Alteration of melanocytes | Regression of melanocytic naevi | Unremarkable clinically, DELM, alteration in pigment with areas of hyperpigmentation | Lichenoid lymphocytic inflammation of CD8+ lymphocytes associated with naevus cells | Yes | Yes | Yes |
| Tumoral melanosis | Multiple purple, black papules and nodules coalescing into plaques | Nodular aggregates of pigmented macrophages in dermis, absence of viable melanoma cells | Yes | No | No | |
| Vitiligo | Hypopigmentation | Dead melanocytes along dermal-epidermal junction with associated dermal lymphocytic inflammation | Yes | Yes | Yes |
Maculopapular rash
During ipilimumab therapy, it usually occurs in the 3rd–4th week after the beginning of treatment [9] and mainly affects the skin of the body and extremities (Figure 1) [23]. It has the form of small, vivid red papulae merging into larger plaque lesions, often accompanied by desquamation [17, 19]. During treatment with anti-PD-1 agents, it may also occur at the beginning of therapy; however, in patients treated with nivolumab, maculopapular rash occurs after 3 weeks to 2 years, and in those treated with pembrolizumab, between 6 and 20 weeks of therapy [17]. Skin lesions usually have the form of numerous, scattered maculopapular lesions (sometimes accompanied by desquamation), with or without itching. They are located mainly in the upper part of the body and on the skin of the limbs, with a predominance of the upper limbs. Histopathological examination reveals perivascular eosinophilic infiltrates and lymphocytic infiltrates, often coexisting with peripheral eosinophilia [17].
Figure 1.
Maculopapular rash (CTCEA grade 2) in a patient treated with anti-PD-1, located in the neckline, back and shoulders, with onset in the 8th week of treatment
The occurrence of maculopapular rash during anti-PD-1 inhibitor therapy is the most common adverse event and usually correlates with histopathological image typical of lichenoid dermatitis or interface dermatitis [17]. In some patients, papular lesions tend to spontaneously resolve approximately 8 weeks after the beginning of treatment, whereas lichenoid lesions may occur as late as a few months after the beginning of therapy with anti-PD-1 and anti-CTLA-4 inhibitors.
Vitiligo-like depigmentation
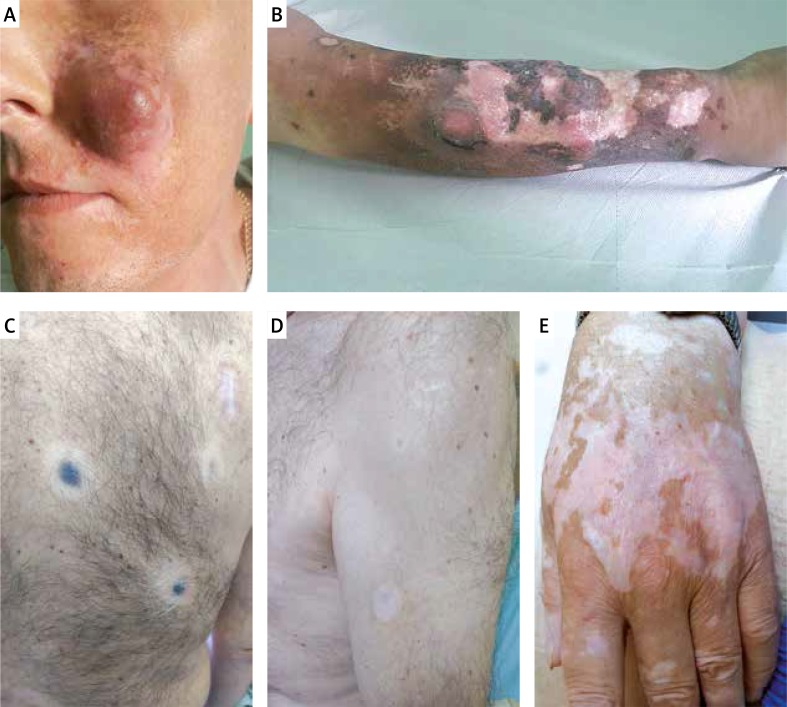
Vitiligo-like depigmentation is relatively common, and has been reported in more than 25% of patients with stage III or IV advanced melanoma receiving nivolumab or pembrolizumab [24]. It has been hypothesised that PD-1 inhibitors induce vitiligo-like depigmentation in melanoma patients via the immune response by targeting healthy melanocytes owing to overlapping antigen expression. This side effect is observed after a few weeks of CPI therapy and in the majority of cases it does not resolve after interruption or discontinuation of treatment. Recently published data show that vitiligo-like depigmentation has been associated with a favourable response to treatment, especially in patients with metastatic melanoma receiving pembrolizumab [24] or nivolumab [25]. The limitations of these studies include relatively small cohorts of enrolled patients; therefore, these findings require validation in larger studies. The evaluation of other cancer immunotherapies (vaccines, antibody-based or adoptive transfer treatments) has shown a strong association between vitiligo-like depigmentation and survival (data from a meta-analysis of 137 studies) [26]. Clinically, vitiligo-like depigmentation may be located within metastatic melanoma lesions in the skin (Figure 2 A), often in the area of a scar after excised melanoma with local recurrence (Figure 2 B), sometimes not only within metastatic lesions throughout the body (Figure 2 C), but also with a tendency to form white halo surrounding benign melanocytic naevi (Figure 2 D). Moreover, they can resemble classic forms of vitiligo with discolouration in typical locations – over the dorsal hands, or segmentally on the trunk (Figure 2 E).
Figure 2.
A – Vitiligo-like depigmentation after 6 months of anti-PD-1 therapy (CTCEA grade 1) within metastatic melanoma tumours on the face. B – Vitiligo-like depigmentation after 6 months of anti-PD-1 therapy (CTCEA grade 1) within the scar after excised primary melanoma lesion on the anterior surface of the left lower leg. C – Vitiligo-like depigmentation lesions within metastatic melanoma of the skin after a few months of anti-PD-1 treatment for metastatic melanoma. D – Vitiligo-like depigmentation lesions within selected melanocytic naevi after a few months of anti-PD-1 treatment for metastatic melanoma. E – Classical vitiligo-like depigmentation within the dorsal hands (CTCAE grade 1) that occurred after a few months of anti-PD-1 treatment for metastatic melanoma
Pseudoprogression
The assessment of response to CPI treatment is challenging and should be performed by experienced oncologists. A substantial proportion of patients treated with ICIs do not respond to treatment, while a small proportion of patients have a survival benefit regardless of the initially observed treatment failure (pseudoprogression). Some melanoma patients treated with ipilimumab, who experienced an initial increase in tumour size with subsequent decrease of tumour burden, have biopsy-confirmed inflammatory cell infiltrates or necrosis in metastatic lesions [27]. Pseudoprogression and immune-related patterns of mixed response pose a growing clinical challenge. In contrast to classical chemotherapy, patients may continue immunotherapy even in the presence of tumour enlargement or new tumour lesions on imaging scans based on irRECIST criteria. The decision problem is to discontinue treatment in select patients who have true disease progression. Continuation of CPI therapy postpones the alternative treatment options and increases the risk of immune-related adverse events.
Inflammation
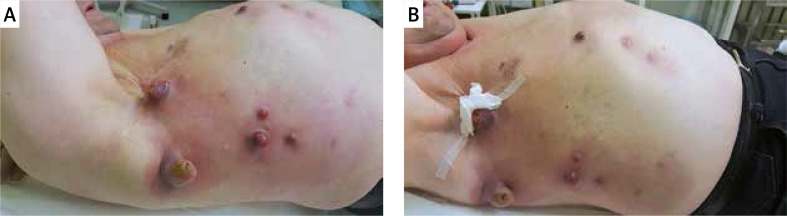
The immune activation and inflammation induced by CPIs can also affect tissues adjacent to metastatic lesions. Based on the authors’ clinical observation, with early beginning of CPI therapy, acute inflammation may occur without any other symptoms. In most cases, these adverse events remain self-limiting and easily manageable. General principles revolve around managing mild toxicity with observation only, and considering local steroids in cases of moderate toxicity. There is no need to add an antibiotic, unless bacterial/fungal infection is confirmed. For severe toxicity, systemic steroids may be required. Clinically, erythema occurs within metastatic melanoma lesions in the skin, as well as in the surrounding skin area (Figures 3 A, B).
Figure 3.
A – Acute inflammation (CTCEA grade 1) in the skin around metastatic lesions after the second dose of anti-PD-1 therapy. B – After 3 weeks the inflammation partially resolved without any therapy
Pruritus
During treatment with ipilimumab, pruritus is usually associated with irritation, dryness and rash, and is an expression of increased activity of the immune system [28]. Persistent pruritus during therapy with anti-PD-1 and anti-PD-L1 inhibitors always requires dermatological consultation in order to exclude bullous pemphigoid in the so-called non-bullous form [29]. In contrast to the drug-induced variant of bullous pemphigoid, which usually disappears after discontinuation of treatment, the duration of the variant associated with the use of anti-PD-1 and anti-PD-L1 inhibitors may persist for up to several months after discontinuation of treatment [29].
Other rare skin complications during therapy with anti-PD-1, anti-PD-L1 and anti-CTLA-4 inhibitors
The following other rare skin complications may occur during combined anti-PD-1 and anti-CTLA-4 therapy: erythrodysaesthesia syndrome, i.e. the so-called hand-foot syndrome, urticaria, toxic epidermis necrolysis and hypersensitivity reactions to UV radiation [30]. Stomatitis, alopecia, hyperhidrosis, and epidermal peeling have been reported in patients treated with nivolumab [13, 31]. Patients receiving pembrolizumab may also experience dry skin, change of hair colour, and alopecia [17, 32, 33].
Management of skin toxicities during treatment with checkpoint inhibitors
The treatment of skin complications that occur during therapy with immune checkpoint inhibitors depends on the severity of these symptoms. The severity is determined based on the Common Terminology Criteria for Adverse Events (CTCAE) (Table 2) [21]. The extent of skin lesions can be assessed by estimation of the total body surface area affected (< 10%, 10–30%, > 30%) using the Lund-Browder chart (so-called rule of nines), which so far has been used in the assessment of the extent of burns (Table 3) [28].
Table 2.
The severity of skin irAEs based on the Common Terminology Criteria for Adverse Events (CTCAE) [21]
| Grade | Description |
|---|---|
| G1 | Skin lesions cover < 10% of the body surface area with or without symptoms (e.g. itching, burning, etc.) |
| G2 | Skin lesions cover 10–30% of the body surface area with or without symptoms (e.g. itching, burning, etc.), limited daily activities |
| G3 | Skin lesions cover > 30% of the body surface area or G2 with significant clinical symptoms, limited self-care |
| G4 | Epidermal detachment and necrosis, skin lesions cover > 30% of the body surface area, accompanying symptoms (erythema, purpura, epidermal detachment) |
Table 3.
Body surface area – classification based on the rule of nines to assess the surface of affected skin in skin toxicities (adapted from [28])
| Body area | % of total body surface area |
|---|---|
| Head and neck | 9 |
| Upper limb | 9 |
| Front of the body (anterior surface of the chest and abdomen) | 18 |
| Back of the body (back of the chest and lumbar region) | 18 |
| Lower limbs | 18 |
| Perineal area | 1 |
In case of grade 1 skin lesions, immunotherapy (anti-PD-1/PD-L1, anti-CTLA-4) can be continued. However, the following actions should be taken: physical examination with comprehensive assessment of the patient’s skin, advice on preventive measures to avoid skin irritation and exposure to UV radiation, regular application of emollients, and the use of high photoprotection. In addition, mild local glucocorticosteroids are recommended once daily, and in case of pruritus, oral or topical antihistamines may be considered; moreover, other dermatoses that may suggest skin toxicity (viral, bacterial, fungal infections or other drug-induced lesions) should be excluded.
In grade 2 skin lesions, the procedure is similar to those in grade 1. However, if no improvement is achieved after one week of treatment, therapy with anti-PD-1/PD-L1 or anti-CTLA-4 should be discontinued. Dermatological consultation and biopsy of the skin lesion for histopathological evaluation should be considered. In the case of grade 2 (or higher) skin irAEs with a clinical manifestation that is nonspecific and difficult to assess, especially with combined therapy (ipilimumab with nivolumab), dermatological consultation is necessary to exclude dermatological conditions that could be exacerbated as a result of treatment (psoriasis, toxic epidermis necrolysis, lichenoid dermatitis, bullous pemphigoid and scleroderma-like reactions).
If skin lesions do not improve or worsen despite the treatment, or grade 3 skin complications occur, immunological treatment (anti-PD-1/PD-L1, anti-CTLA-4) should always be stopped. The primary treatment in this case is systemic glucocorticosteroids. In mild to moderate lesions, oral prednisone should be used at a dose of 0.5–1 mg/kg bw/day (or equivalent). In case of severe lesions, intravenous glucocorticosteroids should be used: methylprednisolone 0.5–1 mg/kg bw 1–2 times a day (or another product at an equivalent dose). If the lesions improve, oral glucocorticosteroids should be used. In case of good response to glucocorticosteroids, a gradual dose reduction and withdrawal within 2–4 weeks is recommended. In patients with grade 3 skin manifestations, dermatological consultation and photographic documentation of the skin lesions are recommended. Excision biopsy of a skin lesion for histopathological evaluation can be considered. Restart of immunotherapy is only possible if the severity of skin lesions is reduced to grade 1 or 2 (mild). In any case, further treatment options, potential benefits of immunotherapy and the risk of further complications should be discussed with both the patient and the consulting dermatologist.
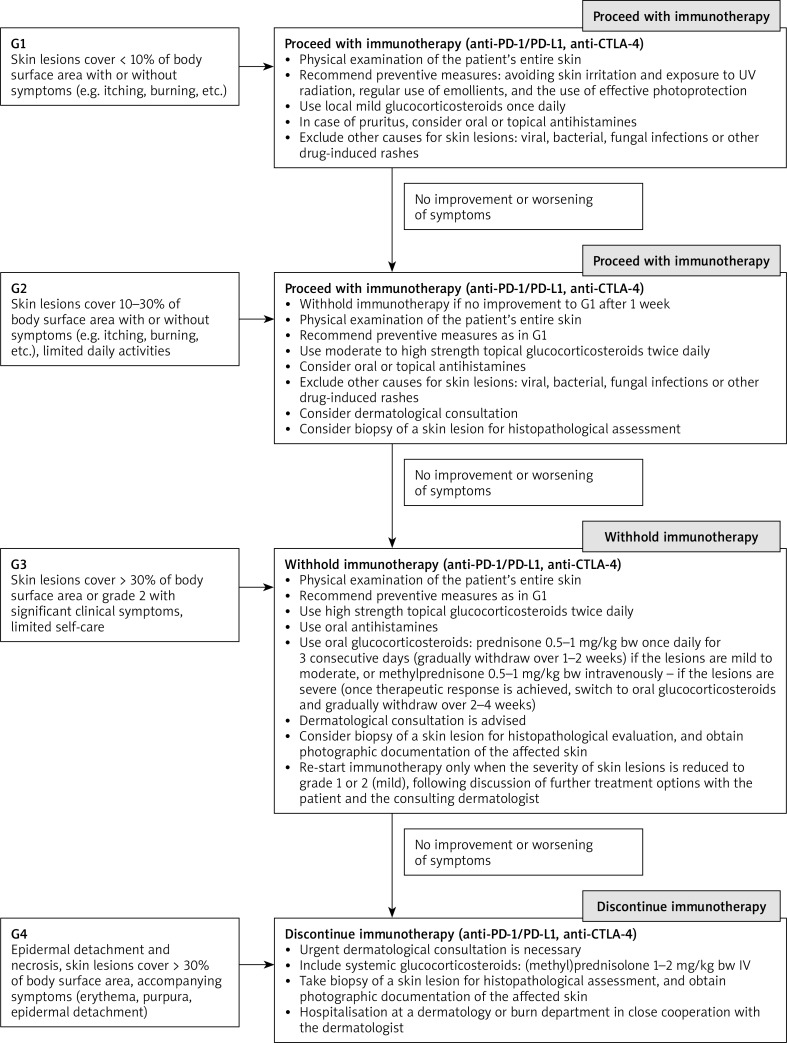
In case of grade 4 skin complications, immunological treatment (anti-PD-1/PD-L1, anti-CTLA-4) should be definitely discontinued. There is always a need for urgent dermatological consultation and the use of systemic glucocorticosteroids: (methyl)prednisolone 1–2 mg/kg bw IV 1–2 times daily. Biopsy of a skin lesion should be taken for histopathological evaluation, and photographic documentation of the affected skin should be obtained. Hospitalisation at a dermatology or burn department is advised in close cooperation with the dermatologist. In the case of a good response to glucocorticosteroid treatment, it can be switched to oral drugs with gradual dose reduction (within a few to several weeks, due to the possibility of recurrence of skin lesions in the case of too rapid withdrawal of glucocorticosteroids) [19, 22, 23, 28, 34–39]. The proposed algorithm for the prophylaxis and treatment of dermal toxicity is presented in Figure 4.
Figure 4.
An algorithm for prophylactic and therapeutic management of skin toxicities depending on the severity of skin irAEs according to the Common Terminology Criteria for Adverse Events (CTCAE) of the ESMO guidelines in the authors’ modification [19, 39]
Whenever skin irAEs occur, diagnostic workup is advised for irAEs affecting other organs (e.g. the lungs, liver, gastrointestinal tract, endocrine system, etc.).
With long-term use of steroids, consideration should be given to prophylaxis of gastric ulcer disease (proton pump inhibitors), prophylaxis of osteoporosis (calcium, vitamin D3), management of electrolyte disturbances (potassium level), and prophylaxis of Pneumocystis jirovecii infection.
Conclusions
In the aspect of dynamic development of new melanoma therapies, including the use of checkpoint inhibitors, the occurrence of selected skin toxicities significantly impairs the patient’s quality of life, and in some cases it is necessary to immediately discontinue therapy or adjust the dose, which reduces the chances for improving the patient’s overall survival.
In this aspect, knowledge of proper therapeutic management of selected skin toxicities is an essential element of knowledge in the work of every clinician – dermatologist, oncologist, and surgeon.
Conflict of interest
B. Cybulska-Stopa received honoraria for speaker, consultancy or advisory role from: MSD, BMS, Novartis, Roche, Pierre Fabre. Other authors declare no conflict of interest.
References
- 1.Krawczyk P, Wojas-Krawczyk K. Monoclonal antibodies against immune checkpoints in immunotherapy of cancer patients. Onkol Prak Klin. 2015;11:76–86. [Google Scholar]
- 2.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thallinger C, Füreder T, Preusser M, et al. Review of cancer treatment with immune checkpoint inhibitors: current concepts, expectations, limitations and pitfalls. Wien Klin Wochenschr. 2018;130:85–91. doi: 10.1007/s00508-017-1285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrov DA, Shi W, Schwartz JC, et al. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290:816–9. doi: 10.1126/science.290.5492.816. [DOI] [PubMed] [Google Scholar]
- 5.Swatler J, Kozłowska E. Immune checkpoint-targeted cancer immunotherapies. Postepy Hig Med Dosw. 2016;70:25–42. doi: 10.5604/17322693.1192926. [DOI] [PubMed] [Google Scholar]
- 6.Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. Clin Oncol. 2015;33:1191–6. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18:611–22. doi: 10.1016/S1470-2045(17)30231-0. [DOI] [PubMed] [Google Scholar]
- 11.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. Clin Oncol. 2015;33:2013–20. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 14.Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–57. doi: 10.1016/j.ejca.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158–76. doi: 10.1111/cup.12858. [DOI] [PubMed] [Google Scholar]
- 19.Haanen JBAG, Carbonnel F, Robert C, et al. ESMO Guidelines Committee Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 20.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. U.S. Department of Health and Human Services National Institutes of Health, National Cancer Institute; published: May 28, 2009 U.S. [Google Scholar]
- 22.Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161–9. doi: 10.1016/j.jaad.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 23.de Golian E, Kwong BY, Swetter SM, et al. Cutaneous complications of targeted melanoma therapy. Curr Treat Options Oncol. 2016;17:57. doi: 10.1007/s11864-016-0434-0. [DOI] [PubMed] [Google Scholar]
- 24.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 25.Freeman-Keller M, Kim Y, Cronin H. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–94. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–81. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 27.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fecher LA, Agarwala SS, Hodi FS, et al. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–43. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383–9. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. doi: 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer J, Reckamp KL, Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert C, Schachter J, Long GV, et al. KEYNOTE-006 investigators Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 33.Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Kumar V, Chaudhary N, Garg M. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemech C, Arkenau HT. Novel treatments for metastatic cutaneous melanoma and the management of emergent toxicities. Clin Med Insights Oncol. 2012;6:53–66. doi: 10.4137/CMO.S5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med. 2016;4:272. doi: 10.21037/atm.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peuvrel L, Dréno B. Dermatological toxicity associated with targeted therapies in cancer: optimal management. Am J Clin Dermatol. 2014;15:425–44. doi: 10.1007/s40257-014-0088-2. [DOI] [PubMed] [Google Scholar]
- 38.Świtaj T, Wysocki P, Wojtukiewicz M, et al. Ipilimumab – progress in therapy of advanced melanoma. Onkol Prakt Klin. 2011;7:231–45. [Google Scholar]
- 39.Ziobro M, Cybulska-Stopa B, Kamińska-Winciorek G, et al. Bezpieczeństwo immunoterapii – zasady postępowania profilaktyczno-terapeutycznego w przypadku wystąpienia działań niepożądanych. Gdańsk: Via Medica; 2018. [Google Scholar]