The differential diagnostics of non-pigmented nodular dermatological lesions pose a challenge even for an experienced dermatologist. We present the case of a female patient qualified for urgent excision of a skin tumour located in the nasal apex area, for which dermoscopy suggested the diagnosis of amelanotic melanoma.
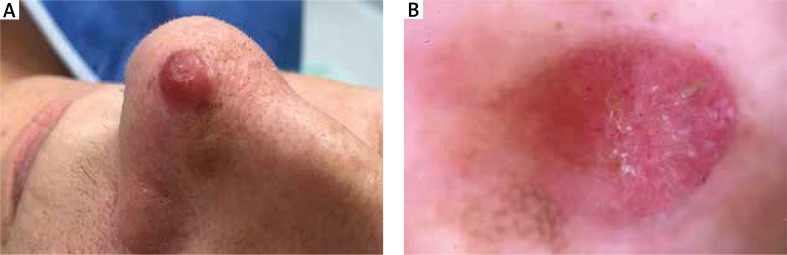
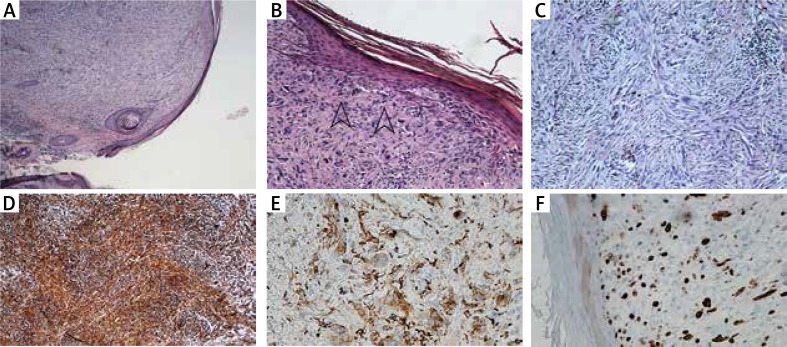
The 64-year-old female patient was admitted to the Dermatology Department for surgical treatment of a tumour located in the skin of the nasal apex. The patient reported the occurrence of the lesion 6 months before. Dermoscopic examination performed by an experienced dermatologist confirmed existence of the lesion in the form of a well-bordered, cohesive, pink skin tumour, with a diameter of 8 mm. The examination demonstrated presence of single, irregular, dot and linear type blood vessels. Exfoliation and white structureless areas were observed on the surface of the lesion. Residues of the regular pigment network were visible on the circumference of the lesion (Figure 1). Considering absence of a pathological vascularisation, white structureless areas, residues of the pigment network, and a pink colour of the tumour, amelanotic melanoma was suspected [1, 2]. The tumour was surgically excised and skin defect was closed with a full thickness skin graft from the left preauricular area. The excised skin fragment with the lifted nodule was fixed in 10% buffered formalin and sent to the Department of Pathology. Microscopically, the tumour was well circumscribed, dome shaped skin nodule composed of atypical spindle cells arranged in a fascicular and haphazard pattern (Figure 2 A). There was no grenz zone of uninvolved dermis seen between the tumour and epidermis. Frequent mitotic figures were present. Atypical spindled cells were polymorphic, some of them had prominent nucleoli, other had large, hyperchromatic nuclei (Figures 2 B, C). The immunohistochemistry stains were viewed under a light microscope. There was no expression of S100, melan A and HMB45. Also there was no expression of desmin, CK5/6 and CD34 in tumour cells. The expression of vimentin and CD68 was present (Figures 2 D, E). Ki67 index was approximately 20% (Figure 2 F). According to microscopic and immunohistochemical manifestation of the entity, atypical fibroxanthoma was diagnosed.
Figure 1.
A – Clinical presentation of a pink tumour located in the skin of the nasal apex. B – Dermoscopic tumour examination (DermLite® Cam, polarized light) revealing mainly pathological vessels and white structureless areas
Figure 2.
A – Dome-shaped skin nodule composed of spindle cells arranged in a fascicular pattern. B – Atypical spindle cells arranged in a haphazard pattern, some with mitotic figures (arrows). No grenz zone of uninvolved dermis. C – Large, atypical, spindled, polymorphic cells with prominent nucleoli arranged in a haphazard pattern. D – Tumour cells were vimentin (mesenchymal marker) positive in immunohistochemical staining. E – Tumour cells were CD68 (histiocyte marker) positive in immunohistochemical staining. F – Ki67 index (proliferation index) was about 20% in tumour cells
Atypical fibroxanthoma (AFX) is a rare and rapidly growing skin tumour. It belongs to rare mesenchymal tumours [3]. Its name comes from the characteristic histopathological presentation of xanthomatous cells, and a variable number of fibrous cells in the tumour pattern [4]. AFX usually appears on the skin chronically exposed to sunlight, mostly in elderly males [4]. There are reports of AFX occurring in a burn scar [5] and in patients in course of cancer treatment with Sonic Hedgehog pathway inhibitors [6]. It may seem that risk factors for the tumour are similar to those for squamous cell carcinoma. Despite its rapid growth, the prognosis of confirmed AFX is favourable. In 2015, Koch et al. analysed 18 patients with 21 foci of AFX treated in the authors’ centre, and 2,912 patients with 2,939 foci of the tumour, selected from reports published in 1962–2014 [7]. In the study group from the authors’ centre, in all patients the lesion was radically excised with safety margins. A local recurrence was observed in 25% of cases, and metastases to the parotid gland in 5%. The 10-year survival rate was 100% [7]. Therefore, AFX requires further patient monitoring, despite the excellent prognosis. According to statistics, AFX is correctly diagnosed in just 43% of cases [7].
From the point of view of histopathology, AFX needs to be differentiated from undifferentiated pleomorphic sarcoma [8]. The histopathological diagnosis is often the exclusion diagnosis. It is necessary to perform a range of immunohistochemical stainings in order to exclude tumours of epithelial and melanocytic origin. From the clinical point of view, AFX needs to be differentiated from the most common non-pigmented skin tumours, including basal cell carcinoma, squamous cell carcinoma, and other tumours that may be localised in the face and neck area, including dermatofibroma, Merkel cell carcinoma, amelanotic/hypomelanotic melanoma, metastases of other tumours to the skin, leiomyosarcoma, and angiosarcoma [8–10].
The differential diagnosis is mostly based on the clinical examination and dermoscopy. Clinically, AFX is usually a single, rapidly growing, pink tumour with a central ulceration covered with crust or exfoliation. Most commonly it is localised on the skin of the scalp, face, ears and neck [8]. The dermoscopic presentation of AFX is not specific. Red and white structureless areas and a pathological pattern of vascularisation of the tumour are particularly notable. The Bugatti report of 2009 mentioned several types of vessels: “linear, dotted, hairpin, arborescent and highly tortuous” [11]. That presentation of blood vessels may be observed in cases of basal cell carcinoma, squamous cell carcinoma, and amelanotic melanoma as well. On the other hand, white areas and elements of tumour may have forms described in 2014 by Pitarch. Among shiny white structures the author distinguished “chrysalis structures, shiny white areas or rosettes” [12]. The dermoscopic attribute that may suggest the diagnosis of AFX is, among others the so-called dermoscopic rainbow pattern, consisting in occurrence of various rainbow colours in well-vascularised lesions, when inspected under dermoscope with polarised light [12, 13]. In 2018, Moscarella et al. analysed 40 cases of AFX and concluded that the most common dermoscopic attributes of AFX are red and white structureless areas and irregular linear vessels [10]. The authors analysed dermoscopic attributes that would allow differentiation between AFX and basal cell carcinoma and squamous cell carcinoma. They concluded that AFX is difficult to discriminate from basal cell carcinoma based on the dermoscopic examination, but differentiation from a well or moderately differentiated squamous cell carcinoma is more probable. However, the most challenging is the dermoscopic differentiation between AFX and poorly differentiated squamous cell carcinoma [10].
The preliminary diagnosis of AFX based on the dermoscopic presentation does not exempt from the obligation of surgical excision and histopathological examination to make the final diagnosis. Despite the lack of valid recommendations for the treatment and observation of patients, surgical excision of the tumour using the micrographic Mohs’ method results is a lower percentage of local recurrence [14] and should be used in centres offering that type of treatment. Dermoscopy is useful but as an indirect diagnostic tool in recognition of skin tumours. It is obligatory to perform histologic examination for the final diagnosis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Stojkovic-Filipovic J, Kittler H. Dermatoscopy of amelanotic and hypomelanotic melanoma. J Dtsch Dermatol Ges. 2014;12:467–72. doi: 10.1111/ddg.12368. [DOI] [PubMed] [Google Scholar]
- 2.Rajczykowski M, Kaminska-Winciorek G, Nowara E, et al. Dermoscopic assessment of skin toxicities in patients with melanoma during treatment with vemurafenib. Adv Dermatol Allergol. 2018;35:39–46. doi: 10.5114/ada.2018.73163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693–6. doi: 10.1111/j.1524-4725.2007.33145.x. [DOI] [PubMed] [Google Scholar]
- 4.Fretzin DF, Helwig EB. Atypical xanthoma of the skin. A clinopathologic study of 140 cases. Cancer. 1973;31:1541–52. doi: 10.1002/1097-0142(197306)31:6<1541::aid-cncr2820310635>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Fix LN, Khanna T, Lewin JM. Atypical fibroxanthoma arising in a burn scar treated with Mohs micrographic surgery. Dermatol Surg. 2018;44:1229–31. doi: 10.1097/DSS.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 6.Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86–88. doi: 10.1111/ced.13736. [DOI] [PubMed] [Google Scholar]
- 7.Koch M, Freundl AJ, Agaimy A, et al. Atypical fibroxanthoma – histological diagnosis, immunohistochemical markers and concepts of therapy. Anticancer Res. 2015;35:5717–35. [PubMed] [Google Scholar]
- 8.López L, Vélez R. Atypical fibroxanthoma. Arch Pathol Lab Med. 2016;140:376–9. doi: 10.5858/arpa.2014-0495-RS. [DOI] [PubMed] [Google Scholar]
- 9.Mentzel T, Requena L, Brenn T. Atypical fibroxanthoma revisited. Surg Pathol Clin. 2017;10:319–35. doi: 10.1016/j.path.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Moscarella E, Piana S, Specchio F, et al. Dermoscopy features of atypical fibroxanthoma: a multicenter study of the International Dermoscopy Society. Australas J Dermatol. 2018;59:309–14. doi: 10.1111/ajd.12802. [DOI] [PubMed] [Google Scholar]
- 11.Bugatti L, Filosa G. Dermatoscopic features of cutaneous atypical fibroxanthoma: three cases. Clin Exp Dermatol. 2009;34:e898–900. doi: 10.1111/j.1365-2230.2009.03670.x. [DOI] [PubMed] [Google Scholar]
- 12.Pitarch G. Dermoscopic rainbow pattern in atypical fibroxanthoma. Actas Dermosifiliogr. 2014;105:97–9. doi: 10.1016/j.ad.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Kunz M, Svensson H, Paoli J. Dermoscopic rainbow pattern: a clue to diagnosing aneurysmal atypical fibroxanthoma. JAAD Case Rep. 2018;4:292–4. doi: 10.1016/j.jdcr.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolkachjov SN, Kelley BF, Alahdab F, et al. Atypical fibroxanthoma: systematic review and meta-analysis of treatment with Mohs micrographic surgery or excision. J Am Acad Dermatol. 2018;79:929–34.e6. doi: 10.1016/j.jaad.2018.06.048. [DOI] [PubMed] [Google Scholar]