Abstract
Microcirculation accounts for about 99% of blood vessels in adults and mediates between the arterial and venous parts of the cardiovascular system, both structurally and functionally. Skin microcirculation consists of two vascular plexuses: superficial and deep. Microcirculation includes vessels with a diameter of less than 150 μm, i.e. arteries, small veins, lymphatic vessels and arteriovenous anastomoses, which build the microcirculation unit. Skin microcirculation may be affected both in systemic pathologies and specific skin disorders. Several non-invasive techniques are available to assess the skin microcirculation. Methods used in clinical practice were presented previously in Advances in Dermatology and Allergology. The current article describes methods that may be used in clinical research.
Keywords: skin microcirculation, methods
Photoplethysmography
Photoplethysmography (PPG) measures volumetric changes in blood in peripheral microcirculation. PPG is simple, painless and inexpensive and it does not require much experience of the person who conducts the test [1]. PPG allows only for a point assessment of microcirculation and the result of the test is not given in absolute units. PPG involves a probe equipped with a source of infrared light and a set of optic sensors. The infrared light is absorbed by the examined tissue. Depending on the volume of blood at the moment of the test in the examined skin fragment, more or less light is absorbed. In consequence, the amount of the light reflected relates to the point changes in the blood volume. Thus, the alterations in blood flow can be registered as alterations in light intensity [2]. The measurement may be performed on fingers and toes on both hands and feet. The test is conducted in a silent room at > 20ºC temperature, allowing time for the end of the vascular response, connected to the locomotive muscle tension and heat adaptation.
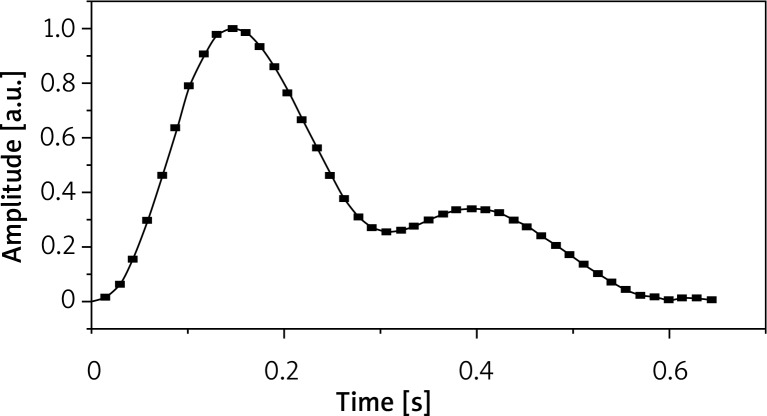
The PPG shows alterations in blood flow in the form of a chart presenting point alterations in the volume of the flowing blood. Its waveform consists of two phases: the rapid anacrotic phase and the slow catacrotic phase (Figures 1, 2). The anacrotic phase is primarily related to systole, while the catacrotic phase is related to diastole and reflexes from the periphery. Typically in the subjects with healthy artery compliance we see a dicrotic notch in the catacrotic phase [3]. Reduction in microcirculation flow detected in the course of the photoplethysmography exam can show growth retardation, rounded top and a very slow decline without the dicrotic notch [4].
Figure 1.
The photoplethysmography waveform: scheme phases
Figure 2.
The photoplethysmography waveform (healthy person)
Photoplethysmography is currently the most commonly used method of diagnosis of chronic venous insufficiency. It allows for the assessment of the functioning of valves in the superficial and deep vein systems by the measurement of the venous refilling time (VRT). The photoplethysmographic test can also be applied in diagnostics of peripheral arterial occlusive disease. Allen et al. have demonstrated the clinical value of photoplethysmography pulse measurements on the toes of both feet in the diagnostics of the disease in the lower limbs [5]. PPG may be also used in the diagnostics of alopecia areata (AA) in order to confirm the vascular aetiology of this disease. Sudnik has demonstrated alterations in the photoplethysmographic curve denoting mild flow distortions in 63.5% and severe flow distortions in 36.5% of AA patients [2].
Orthogonal polarization spectroscopy
Orthogonal polarization spectroscopy (OPS) is a relatively new technique of human microcirculation imaging without using fluorescent dyes. OPS imaging shows a wide range of clinical applications with a strong emphasis on establishing the precise diagnosis, prognosis and treatment implementation [6]. Polarized light with wavelength of 548 nm, which is reflected from the examined tissue, is captured by a camera and used to obtain the optimal microcirculation imaging in OPS. The light scattered by the superficial layer of tissue is blocked in the process of second polarization. As a consequence, only the light returning from the deeper layers of tissue passes second polarization and improves visibility of erythrocytes. Computer analysis of erythrocyte movement enables assessment of perfusion, diameter of vessels and capillary density [6].
The orthogonal polarization spectroscopy technique can be applied not only for real-time studying of microcirculation in skin vessels, but also for submucosal microcirculation. The test is usually conducted in the sublingual area and skin of newborns. Moreover, OPS technology allows for examination of tissues covered by a thin epithelial layer and internal organs during surgical operation [6]. The obtained measurements have rather a quantitative character, however, OPS results are susceptible to artefacts due to organ movement and changes in blood pressure. De Backer et al. using the OPS technique have proved significant changes in skin microcirculation in patients with sepsis and septic shock [7]. OPS proved to give a more objective visualization of microcirculation in critically ill patients compared to videocapillaroscopy and laser Doppler techniques [8]. Orthogonal polarization spectroscopy has been also applied in monitoring of hypovolemic patients and in term and preterm infants as an effective non-invasive method of tissue perfusion assessment. Further development of the OPS is bound to bring a broader clinical application of this method, especially in the field of surgery, mainly neurosurgery and plastic surgery, as well as neonatology, anaesthesiology and dermatology.
Near-infrared spectroscopy
Near-infrared spectroscopy (NIRS) is a specific form of tissue reflectance spectrophotometry. This method uses infrared light of 700–1000 nm which easily penetrates soft tissues and is subsequently partly diffused and partly absorbed by chromophores such as haemoglobin, myoglobin and cytochrome aa3. On this basis the tissue haemoglobin oxygen saturation (tHbO2) assessment is performed [9].
This technique may be used for assessment of various organs, mostly for the measurement of blood flow through the brain and muscle tissue. Advantages of this method are the simplicity of use and short time of examination. The devices for clinical measurement which are currently available are easy to operate. Unfortunately, NIRS does not allow for measurements of absolute values of tHbO2. In addition, the resolution of the signal is poor and the exact depth of light tissue penetration is not known [9]. Originally this method was used for continuous monitoring of tissue haemoglobin oxygen saturation in the brain during carotid endarterectomy [9].
NIRS technique is also currently recognized as a good tool for assessing superficial tissues, including skin microcirculation. NIRS is used primarily in critically ill patients for monitoring oxygen supply to the skeletal muscles in ICUs. Lima and Bakker proved that monitoring of circuit perfusion may show early indications of tissue hypoperfusion [10]. The NIRS method has been reported to assess skin microcirculation in patients with sepsis and in septic shock, however its prognostic value has not been confirmed yet [11]. Hartwig et al. in a pilot research successfully used this technique for the assessment of microcirculatory dysfunction in systemic sclerosis both in static and dynamic conditions. They also demonstrated clinical use of NIRS in assessment of skin microcirculation in connection with vascular occlusion testing [12]. NIRS, similarly to laser Doppler flowmetry and transcutaneous oxygen tension, may be applied in the assessment of microcirculation in patients with diabetic foot [13]. The assessment of microvasculature may help to make a therapeutic decision [14].
Tissue reflectance spectrophotometry
Tissue reflectance spectrophotometry (TRS) is based on the detection of backscattered light in specific wavelength spectra for oxygenated haemoglobin at two peaks of 542 nm and 577 nm and deoxygenated haemoglobin at a peak of 556 nm. This technique allows for the assessment of haemoglobin oxygen saturation and haemoglobin concentration in capillaries so that it reflects skin microcirculation functioning in real time [9]. Measurements may be taken from the surface of any organ, although for obvious reasons it is most commonly performed on the surface of the skin and gastric mucosa. A short time of examination, expression of the results in absolute units and the possibility to perform repetitive measurements are the main advantages of TRS. Limitations include the fact that the result depends on presence of tissue chromophores other than haemoglobin i.e. melanin or cytochromes [9]. Erroneous results in measuring blood content and oxygenation might occur in persons of significant skin pigmentation as melanin in the epidermis reduces the amount of backscattered light and has a characteristic absorption spectrum, different in every person [15]. Thus, TRS is presently applied predominantly for assessment of tissue healing.
Optical coherence tomography
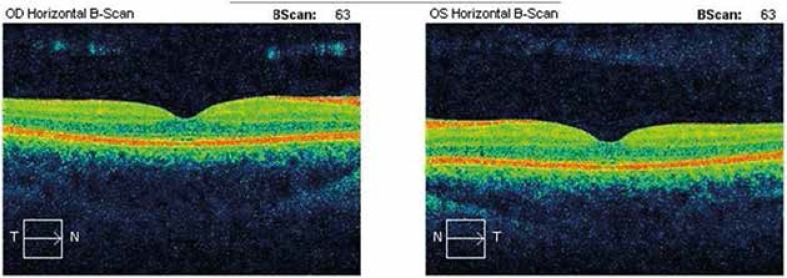
Optical coherence tomography (OCT) is a developing technique of imaging, mainly used in medical diagnostics. This method allows for non-invasive penetration into the examined tissue, which is why it is also called optical biopsy [16]. Optical coherence tomography uses the phenomenon of interferometry of light with the tissues. In the OCT method, sources of light of special coherence and spectrum of considerable spectral width. Light used in this method has an intensity lesser than a couple of milliwatts. Thus, the OCT examination is entirely non-invasive and it may be safely applied repeatedly in many places. This technique is analogous to the ultrasonographic technique, with the use of light instead of sound [17]. The most important advantage of this method is high resolution of 10–20 μm, allowing for obtaining images comparable to histopathology. Other advantages include the simplicity of use of the device, imaging in real time and capability of continuous registration. The OCT does not require prior preparation of a patient. Unfortunately, OCT is most effective for optically transparent tissue assessment. It covers a relatively small surface and is limited to imaging only 1–2 mm into the surface of the tissue (Figure 3).
Figure 3.
The optical coherence tomography scan of normal retina (with the permission of L. Glasner MD, Medical University of Gdansk, Department of Ophthalmology)
The OCT is a constantly developing method and is applicable in many fields of medicine. In ophthalmology it is regarded as the most modern technique of imaging in diseases of retina and anterior segment of the eye. In patients with diabetic retinopathy, OCT is used as an objective monitoring technique of the macular thickening before and after therapy as well as for vitreous assessment [18, 19]. In interventional cardiology it is applied in the coronary artery disease diagnostics. In dermatology, OCT is used to assess the extent of tissue damage, to measure pathologic skin lesions such as angioma and for precise assessment of scars and wrinkles. Comparison of correlation mapping optical coherence tomography (cmOCT) performed on the forearm of healthy persons and psoriasis patients demonstrates characteristic microcirculatory alterations in the presence of psoriasis (Table 1).
Table 1.
Non-invasive methods of assessment of microcirculation
| Method | Measuring principle | Measured parameters | Examined tissues | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Photoplethysmography | Measurement of small variations in the intensity of the reflected infrared light associated with changes in tissue perfusion | Blood volume changes | SaO2, blood pressure on the toes, vascular reactivity in the cooling test | Non-invasive, the signal is strong and robust; the electronic circuit is fairly simple and small | Sensitive to motion artefacts |
| Orthogonal spectral polarization | Microcirculation assessment – polarized light 548 nm | Blood vessel diameter, blood flow rate, capillary density | Sublingual, neonatal skin, internal organs – surgery | Short measurement time, a variety of local blood flow | The penetration depth of only 1 mm, time-consuming, semi-quantitative assessment, offline |
| Near-infrared spectroscopy | Infrared light (700–1000 nm) absorbed by chromophores such as Hb, myoglobin, cytochrome aa3 | Oxygenation of haemoglobin | The surface of organs especially the brain | Easy to use, short time | Unknown depth of light penetration, does not measure absolute values |
| Tissue reflectance spectrophotometry | Recording of reflected light in a defined spectrum of length (oxygenated Hb – 542 nm and 577 nm, deoxygenated Hb – 556 nm) | Oxygenated haemoglobin saturation, concentration of haemoglobin in capillaries | All surfaces of the organ, the skin and gastric mucosa | Simple technique, absolute values, repeatedly measured in a short time | Spectrum of wavelengths depending on the content of other tissue chromophores (melanin, cytochromes) |
| Optical coherence tomography | Uses the phenomenon of interferometry of light with the tissues | E.g. macular and nerve fibre layer thickness | When used in vivo and in vitro it enables imaging of organ systems inside the body | High resolution of 10–20 µm, allowing for obtaining image comparable to histopathology, simplicity of use of the device, imaging in real time and capability of continuous registration, does not require prior preparation of a patient | Works best for optically transparent tissues, it covers a relatively little surface and is limited to imaging only 1–2 mm into the surface of the tissue |
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 2.Sudnik W. Rola selektyn E, L i P w patomechanizmie łysienia plackowatego. Poznan: Poznan University of Medical Sciences; 2012. PhD thesis. [Google Scholar]
- 3.Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012;8:14–25. doi: 10.2174/157340312801215782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinert JM, Gupta A. Pulse volume recording. Hand Clin. 1993;9:13–46. [PubMed] [Google Scholar]
- 5.Allen J, Oates CP, Lees TA, Murray A. Photoplethysmography detection of lower limb peripheral arterial occlusive disease: a comparison of pulse timing, amplitudę and shape characteristics. Physiol Meas. 2005;26:811–21. doi: 10.1088/0967-3334/26/5/018. [DOI] [PubMed] [Google Scholar]
- 6.Cerný V, Turek Z, Parízková R. Orthogonal polarization spectral imaging. Physiol Res. 2007;56:141–7. doi: 10.33549/physiolres.930922. [DOI] [PubMed] [Google Scholar]
- 7.De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 8.De Backer D, Dubois MJ. Assessment of the microcirculatory flow in patients in the intensive care unit. Curr Opin Crit Care. 2001;7:200–3. doi: 10.1097/00075198-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Knotzer H, Hasibeder WR. Microcirculatory function monitoring at the bedside – a view from the intensive care. Physiol Meas. 2007;28:R65–86. doi: 10.1088/0967-3334/28/9/R01. [DOI] [PubMed] [Google Scholar]
- 10.Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005;31:1316–26. doi: 10.1007/s00134-005-2790-2. [DOI] [PubMed] [Google Scholar]
- 11.Lima A, van Bommel J, Sikorska K, et al. The relation of near-infrared spectroscopy with changes in peripheral circulation in critically ill patients. Crit Care Med. 2011;39:1649–54. doi: 10.1097/CCM.0b013e3182186675. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig V, Marinelli M, Rocco F, L’Abbate A. Assessment of microvascular function using near-infrared spectroscopic 2D imaging of whole hand combined with vascular occlusion test. J Med Biol Engineering. 2016;36:87–95. [Google Scholar]
- 13.Cobb J, Claremont D. Noninvasive measurement techniques for monitoring of microvascular function in the diabetic foot. Int J Lower Extrem Wounds. 2002;1:161–9. doi: 10.1177/153473460200100303. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RH, Nadeau KP, Jaworski FB, et al. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J Biomed Opt. 2015;20:030901. doi: 10.1117/1.JBO.20.3.030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson-Pell M, Hagisawa S. An empirical technique to compensate for melanin when monitoring skin microcirculation using reflectance spectrophotometry. Med Eng Phys. 1995;17:104–10. doi: 10.1016/1350-4533(95)91880-p. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature Biotechnol. 2003;21:1361–7. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2:9–25. doi: 10.1038/sj.neo.7900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikorski BL, Malukiewicz G, Stafiej J, et al. The diagnostic function of OCT in diabetic maculopathy. Mediators Inflamm. 2013;2013:434560. doi: 10.1155/2013/434560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457–73. doi: 10.1007/s00403-011-1152-x. [DOI] [PubMed] [Google Scholar]