Abstract
Previous studies have shown that some plants in the genus of Ferula (Apiaceae) have antidiabetic effects. The present work was aimed to evaluate effects of Ferula gummosa oleo-resin in a rat model of streptozotocin-induced diabetes. Male Wistar rats were randomized into five groups (n = 6): normal control, diabetic control, diabetic rats treated with insulin (3 IU/day), and diabetic rats treated with 100 or 400 mg/kg/day of an ethanolic extract of the oleo-resin. After 4 weeks, blood samples were collected for measuring fasting blood glucose (FBG), lipid profile, aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase, blood urea nitrogen, and creatinine. In addition, levels of lipid peroxidation, thiol groups, and superoxide dismutase (SOD) activity were evaluated in the liver and kidney. At the end of the fourth week, the level of FBG in rats treated with 100 mg/kg of the extract was lower than that in diabetic control rats (273 ± 39 mg/dL vs 471 ± 32 mg/dL). Administration of insulin and the extract had no significant effects on the serum lipids. Insulin and both doses of the extract significantly reduced the activity of ALT. In addition, the extract inhibited lipid peroxidation in the kidney and restored the elevated level of SOD in the liver and kidneys. Ferula gummosa oleo-resin has the potential to prevent or delay the complications of diabetes by inhibiting the progression of hyperglycemia and attenuating oxidative stress-induced damage in the liver and kidneys.
Keywords: Diabetes, Ferula gummosa, Glucose, Lipid, Sesquiterpene coumarins
INTRODUCTION
Despite considerable progresses in the understanding of the pathogenesis of diabetes and in the control of blood glucose with currently available hypoglycemic drugs, diabetic complications appear in a large number of the patients (1,2). Studies on the pathophysiological mechanisms of diabetic complications suggest hyperglycemia, dyslipidemia, oxidative stress, and inflammation as the main contributing factors (3,4). In recent decades, investigations on finding new antidiabetic agents from medicinal plants with potential anti-hyperglycemic, hypolipidemic, antioxidant, and anti-inflammatory effects are continued (5,6).
Experimental studies reported that some plants in the genus of Ferula (Apiaceae) such as F. asafetida and F. hermonis show anti-hyperglycemic and hypolipidemic effects (7,8). F. gummosa Boiss. is one of the plants of this genus that is commonly known as “Barijeh” and “Ghasni” in Iran (9). In Iranian traditional medicine it is used for the treatment of wounds, neurological disorders, nephropathy, and liver damage (10,11,12).
Nevertheless, no academic study has so far evaluated the beneficial effects of F. gummosa on diabetic changes in the serum, liver, and kidneys. Many biological activities of the genus Ferula such as neuroprotective, nephroprotective, and anti-inflammatory effects are attributed to sesquiterpene coumarin compounds (13,14,15,16). These compounds have also shown to attenuate the formation of advanced glycation end products (17). The present work was aimed to evaluate possible beneficial effects of ethanolic extract of F. gummosa oleo-resin in rat model of streptozotocin (STZ)-induced diabetes.
MATERIALS AND METHODS
Chemicals and reagents
Pyrogallol, tetraethoxypropane and ethylene diamene tetraacetate (EDTA) were purchased from Merck (Damstadt, Germany). STZ, trichloroacetic acid (TCA), thiobarbituric acid (TBA), 2,20-dinitro-5,50-dithiodibenzoic acid (DTNB), and 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium (MTT) were obtained from Sigma (St. Louis, USA).
Plant material and extraction
The oleo-resin of F. gummosa (Boiss.) was collected from 4-6 years old plants in Esfarayen mountains (North-Khorasan Province, I.R. Iran) during June-September 2017. The plant was identified at the herbarium of Ferdowsi University of Mashhad (Voucher specimen No. 34577). The alcoholic extract of the oleo-resin was prepared by maceration method. The plant material (200 g) was suspended in 2 L of ethanol (96% v/v) for 24 h at 40 °C under gentle shaking (18). The extracted solution was centrifuged at 1500 rpm for 5 min to remove insoluble particles. The soluble fraction was dried in an oven at 40 °C to give an yield of 100 g (50%, w/w). The dried extract was kept at 4 °C until use.
High-performance liquid chromatography
Quantitative reverse-phase high-performance liquid chromatography (HPLC) analysis was carried out using a Knauer HPLC system (Berlin, Germany) equipped with K-1001 pumps, K 2600 UV detector, and EZChrom Elite software. The oleo-resin extract was prepared at concentrations of 5 and 6 mg/mL and filtered through a 0.45 μm nylon filter prior to the analysis. The chromatographic separation was done on a C18 (4.6 × 250 mm, 5 μm) column using a mobile phase consisting of 20-100% methanol in deionized water (including 0.05% trifluoroacetic acid). The volume of the sample injection and flow rate was 20 μL and 1 mL/min, respectively. The UV absorbance was set at 320 nm. The amount of sesquiterpene coumarins in the extract was quantified using a standard calibration curve (y = 37.87x-0.086, R2 = 0.999) prepared for mogoltacin (0, 0.125, 0.25, and 0.5 mg/mL).
Animals and experimental design
Adult male Wistar rats weighing 210-270 g were used in this study. The investigation was approved by the Animal Ethical Committee of Mashhad University of Medical Sciences (ethics committee agreement code: IR.MUMS.REC.1395.50). A total of 30 rats were randomly divided into five experimental groups (n = 6) as follows: group I, normal control rats; group II, diabetic control rats; group III, diabetic rats treated with 3 IU/day of neutral protamine Hagedorn (NPH) insulin for 4 weeks (19); group IV, diabetic rats treated with the oleo-resin extract of F. gummosa at dose of 100 mg/kg per day for 4 weeks; and group V, diabetic rats treated with 400 mg/kg of the extract for 4 weeks.
Considering body surface area for dose translation, 100 and 400 mg/kg in the rat are approximately equivalent to the doses of 16 and 64 mg/kg in human, respectively (20,21).
These doses were chosen for the present work because in traditional medicine the oleo-resin is typically consumed at about 1-3 g/day (i.e., 12-50 mg/kg/day for people weighing 60-80 kg), which is equivalent to 6-25 mg/kg/day of the oleo-resin extract (yield of the extract was 50%). For administration of F. gummosa, the food of animals was supplemented with the oleo-resin extract (doses of the extract were adjusted weekly based on the body weight and amount of food intake).
Diabetes was induced by intraperitoneal injection of STZ (65 mg/kg). After three days, fasting blood glucose (FBG) was checked by glucometer using glucostrips (ACCU-CHEK, Roch, Germany) after taking blood sample from tail vein. The animals with FBG level of 200 mg/dL or higher were considered diabetic.
Serum biochemical analysis
At the end of the 4th week of treatment, fasting blood samples were collected by cardiac puncture after euthanasia with carbon dioxide. The levels of blood urea nitrogen (BUN), glucose, triglyceride, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were measured using Parsazmun company kits (Karaj, I.R. Iran).
Measurement of total thiol groups
A sample of the homogenized tissues or serum (50 μL) was added to the tris-EDTA buffer (1 mL, pH 8.6) and optical density was determined at 412 nm against tris-EDTA buffer alone (A1). Then, 20 μL of DTNB reagent (10 mmol/L in methanol) was added to the mixture and after 10 min the absorbance was determined again (A2). The absorbance of DTNB reagent was also recorded (B). The concentration of total thiol was calculated from the following equation:
Thiol concentration (mmol/L) = (A2 - A1 - B) × 1.07/(0.05×13.6) (1)
Superoxide dismutase activity assay
The activity of superoxide dismutase (SOD) in the serum and samples of the liver and left kidney was evaluated by a calorimetric method based on the inhibition of production of superoxide anion due to auto-oxidation of pyrogallol and MTT oxidation, as described previously (22). SOD activity was expressed as unit/mg protein.
Determination of lipid peroxidation level
A sample of 0.5 mL of the homogenized tissues (liver and kidney) was mixed with 0.5 mL of deionized water, and 0.5 mL of TCA reagent (TCA 15% and TBA 0.37%, HCl 0.25 N). The reaction mixture was incubated for 60 min at 95 °C and after cooling, 25 μL of HCl and 1.5 mL of n-butanol were added to the mixture. After centrifuging at 1000 rpm for 10 min, the fluorescence of the supernatant was measured using fluorescent plate reader (PerkinElmer VICTOR X5, USA) at an excitation of 485 nm and an emission of 535 nm. Tetraethoxypropane was used to prepare a standard curve at concentration ranges between 0.01- 0.2 mmol/L.
Statistical analysis
Comparisons between groups were performed using one-way analysis of variance followed by the LSD post hoc test for multiple comparisons. Data obtained before and after treatment were compared by paired-sample t-test. The results were presented as mean ± SEM and probability level of less than 0.05 was considered significant.
RESULTS
HPLC analysis of the oleo-resin extract of Ferula gummosa
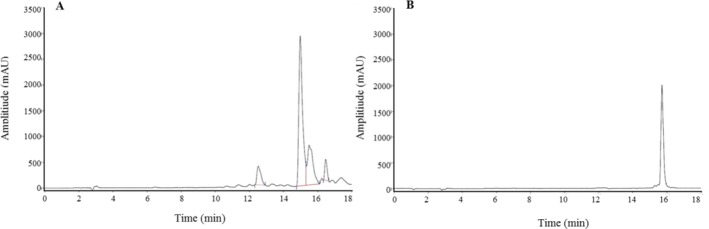
The HPLC chromatograms of ethanol extract of F. gummosa oleo-resin and standard are shown in Fig. 1. The retention time of mogoltacin, as the standard for sesquiterpene coumarin compounds, was 15.7 min. Using the standard curve, the content of sesquiterpene coumarins in the extract was estimated to be 370 mg/g.
Fig. 1.
HPLC chromatograms of ethanolic extract of the (A) oleo-resin of F. gummosa and (B) mogoltacin as internal standard.
Effects of the oleo-resin extract on the body weight, food, and water intakes
Table 1 indicates that the initial body weight was not statistically different between the experimental groups. At the end of the study, normal animals showed an increased body weight compared to their initial weight (P < 0.01). On the other hand, the diabetic control group showed a significant decrease in the body weight (P < 0.01) despite increased food and water intakes (P < 0.01). While, treatment with insulin suppressed diabetes-induced weight loss and polydipsia, the effect of the oleo-resin extract on the body weight, food consumption, and water intake remained statistically insignificant.
Table 1.
Effects of Ferula Gummosa oleo-resin on the body weight, food intake, water intake, and the weight of liver and kidneys of diabetic rats. Values are expressed as mean ± SEM (n = 6). Significant differences were observed (*P < 0.01 and ***P < 0.001, respectively) in comparison with normal control; # and ## show significant differences (P < 0.05 and P < 0.01, respectively) versus diabetic control; and × shows significant differences (P < 0.01) versus day 1 in the corresponding group.
| Parameters | Time | Normal control | Diabetic control | Insulin-treated diabetes | F. gummosa-treated diabetes (100 mg/kg) | F. gummosa-treated diabetes (400 mg/kg) |
|---|---|---|---|---|---|---|
| Body weight (g) | Day 1 | 253 ± 7 | 230 ± 11 | 237 ± 5 | 248 ± 4 | 242 ± 8 |
| Week 4 (weight gain) | 303 ± 8× (+20%) | 187 ± 13*× (-19%) | 278 ± 6##× (+17%) | 213 ± 4*#× (-14%) | 210 ± 10*× (-13%) | |
| Food intake (g/24 h) | Week 4 | 21 ± 1 | 37 ± 2.5* | 33 ± 2.5* | 39 ± 2* | 38 ± 1.5* |
| Water intake (mL/24 h) | Week 4 | 56 ± 4 | 122 ± 10* | 98 ± 10*# | 105 ± 5* | 100 ± 13* |
| Liver weight (g) | Week 4 | 11.2 ± 0.6 | 8.1 ± 0.4*** | 11.8 ± 0.84## | 10.1 ± 0.2 | 10.4 ± 0.5# |
| Kidney weight (g) | Week 4 | 1 ± 0.04 | 1.02 ± 0.06 | 1.06 ± .06 | 1.15 ± .03 | 1.08 ± 0.06 |
The weight of the liver was significantly lower in the diabetic control rats than the normal control animals at the end of the study. Treatment with insulin and F. gummosa (400 mg/kg) significantly increased the liver weight when compared to the diabetic control group (P < 0.05).
Effects of the oleo-resin extract on serum glucose and lipids
As shown in Table 2, the FBG level significantly increased in STZ-diabetic rats as compared to normal control rats (P < 0.001). The diabetic control rats displayed a further increase in FBG after 4 weeks (35%).
Table 2.
Effects of Ferula Gummosa oleo-resin on the levels of FBG and serum lipids of diabetic rats. Values are expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus normal control; #P < 0.05 versus diabetic control.
| Parameters | Normal control | Diabetic control | Insulin-treated diabetes | F. gummosa-treated diabetes (100 mg/kg) | F. gummosa-treated diabetes (400 mg/kg) |
|---|---|---|---|---|---|
| FBG Day 1 (mg/dL) | 99 ± 5 | 346 ± 39*** | 393 ± 20*** | 300 ± 21*** | 326 ± 23*** |
| FBG Week 4 (mg/dL) | 97 ± 11 | 421 ± 56*** | 299 ± 65* | 275 ± 60*# | 349 ± 57** |
| Changes related to day 1 | -2% | +22% | -24% | -8% | +7% |
| Triglyceride (mg/dL) | 56 ± 11 | 72 ± 15 | 55 ± 16 | 55 ± 15 | 60 ± 12 |
| Total cholesterol (mg/dL) | 65 ± 4 | 96 ± 8** | 88 ± 5** | 76 ± 6 | 82 ± 7* |
| LDL (mg/dL) | 14 ± 1 | 26 ± 9* | 21.5 ± 3.5 | 16.5 ± 1.3 | 18 ± 1.5 |
| HDL (mg/dL) | 45 ± 4 | 38 ± 2 | 44 ± 2 | 42 ± 2 | 45 ± 3 |
F. gummosa, Ferula Gummosa; FBG, fasting blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Administration of insulin inhibited the progression of hyperglycemia though nonsignificant. At the end of the fourth week, the level of FBG in rats treated with 100 mg/Kg of the oleo-resin extract (but not 400 mg/kg) was significantly lower than that in diabetic control rats. However, the change in FBG level related to day 1 was not statistically significant in groups treated with insulin or the oleo-resin extract.
A significant increase in the levels of serum total cholesterol (P < 0.01) and LDL (P < 0.05) was observed in the diabetic control group when compared to normal control group. Neither insulin nor the oleo-resin extracts significantly reduced the levels of total cholesterol or LDL. The level of triglyceride was not significantly increased in diabetic animals after 4 weeks of STZ injection.
Effects of the oleo-resin extract on parameters of kidney and liver functions
Serum level of the BUN was significantly increased in the diabetic control group compared to normal control animals (P < 0.001, Table 3). Treatment with insulin significantly reduced the BUN levels with respect to the diabetic control group (P < 0.01). Such reduction was not observed in the groups treated with the oleo-resin extract. Induction of diabetes also resulted in a significant increase in the activities of serum ALT and ALP (P < 0.001). Insulin treatment could reduce the activities of these enzymes (P < 0.01). Similarly, the activity of ALT was recovered significantly in the group treated with 100 mg/kg of the oleo-resin extract (P < 0.05).
Table 3.
Effects of Ferula gummosa oleo-resin on the serum parameters of kidneys and liver functions in diabetic rats. Values are expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 versus normal control; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus diabetic control.
| Parameters | Normal control | Diabetic control | Insulin-treated diabetes | F. gummosa-treated diabetes (100 mg/kg) | F. gummosa-treated diabetes (400 mg/kg) |
|---|---|---|---|---|---|
| BUN (mg/dL) | 50 ± 5 | 102 ± 11*** | 63 ± 6## | 99 ± 8*** | 98 ± 6*** |
| Creatinine (mg/dL) | 0.67 ± 0.02 | 0.63 ± 0.03 | 0.67 ± 0.02 | 0.58 ± 0.03 | 0.60 ± 0.02 |
| AST (U/L) | 130 ± 7 | 180 ± 19 | 200 ± 37 | 162 ± 12 | 174 ± 19 |
| ALT (U/L) | 55 ± 3 | 221 ± 37*** | 119 ± 15*## | 153 ± 23**# | 136 ± 27*# |
| ALP (U/L) | 371 ± 25 | 1700 ± 181*** | 546 ± 97### | 1816 ± 152*** | 1621 ± 283*** |
Effects of the oleo-resin extract on parameters of oxidative stress
As shown in Table 4, a significant decrease in the content of thiol groups was observed in the serum and renal and hepatic tissues of rats in the diabetic control group when compared with normal control group (P < 0.05). Insulin treatment increased the thiol content in serum and in the tissues but this effect was statistically significant only in the liver (P < 0.01). The oleo-resin extract of F. gummosa had no significant effect on the content of thiol groups in the serum and tissues.
Table 4.
Effects of Ferula Gummosa (F. gummosa) oleo-resin on the levels of thiol groups, superoxide dismutase (SOD), and malondialdehyde (MDA) in the serum, kidneys, and liver in diabetic rats. Values are expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus normal control; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus diabetic control.
| Parameters | Specimens tested | Normal control | Diabetic control | Insulin-treated diabetes | Diabetic F. gummosa (100 mg/kg) | Diabetic F. gummosa (400 mg/kg) |
|---|---|---|---|---|---|---|
| Thiol groups (μmol/mg protein) | Serum | 2.8 ± 0.7 | 0.7 ± 0.5** | 1.8 ± 0.6 | 0.6 ± 0.2** | 1.5 ± 0.4 |
| Kidney | 28.7 ± 9 | 9.2 ± 4.5* | 13.7 ± 3.2 | 5 ± 1.2** | 8 ± 0.8* | |
| Liver | 25 ± 4.5 | 5 ± 0.3** | 26 ± 6## | 3.5 ± 1.3** | 8 ± 0.7 | |
| SOD (Unit/mg protein) | Serum | 32 ± 5 | 50 ± 9 | 36 ± 9 | 35 ± 4.4 | 27 ± 3.5# |
| Kidney | 108 ± 8 | 223 ± 26*** | 116 ± 17### | 183 ± 23 | 79 ± 14### | |
| Liver | 98 ± 7.4 | 240 ± 27*** | 142 ± 46# | 143 ± 20## | 159 ± 13# | |
| MDA (nmol/mg protein) | Kidney | 365 ± 56 | 1687 ± 105*** | 248 ± 32### | 1090 ± 220## | 736 ± 136### |
| Liver | 925 ± 253 | 1010 ± 105 | 528 ± 380 | 1191 ± 225 | 1142 ± 132 |
Induction of diabetes significantly increased the levels of SOD and MDA in the renal tissue (P < 0.001). In diabetic groups treated with insulin and the oleo-resin extract, the levels SOD and MDA significantly reduced in the kidney (P < 0.01). Similarly, both insulin and the oleo-resin extract could decrease the elevated level of SOD in the hepatic tissue of diabetic rats (P < 0.05).
DISCUSSION
The oleo-resin of F. gummosa is traditionally used to treat diabetes in north-east Iran. Also, previous studies reported that some plants in the genus of Ferula have anti-hyperglycemic and hypolipidemic effects and prevent progression of diabetic complications (7,8). Therefore, the present study was designed to evaluate if F. gummosa has any beneficial effects on diabetic changes in STZ model of hyperglycemic rats. In this study model, the oleo-resin extract of F. gummosa could inhibit the progression of hyperglycemia and attenuated tissue oxidative stress. Considering that chronic hyperglycemia and oxidative stress play significant roles in the pathophysiology of diabetic complications, F. gummosa may be a complementary agent for preventing or delaying the complications of diabetes.
STZ is a cytotoxic agent that leads to degeneration of pancreatic beta cells and therefore induces symptoms of type-1 diabetes, i.e., hypoinsulinemia, hyperglycemia, polydipsia, polyuria, and weight loss (23,24). In the present study, as expected, insulin could inhibit weight loss and reduced water intake in diabetic rats. While untreated diabetic rats showed a further increase in FBG at week 4 compared to day 1, insulin inhibited the progression of hyperglycemia in insulin-treated animals. Nevertheless, the FBG levels in the insulin-treated group were not statistically different from those of diabetic control group, in part, due to improper blood sampling time. Blood sample was taken 24 h after the last insulin dosing while the average duration of action for NPH insulin is about 10-15 h (25). Likewise, although the oleo-resin extract of F. gummosa inhibited further increase in FBG levels, it did not improved hyperglycemia considerably. This sugar lowering effect of F. gummosa was seen at lower dose of oleo-resin (100 mg/kg), but not at higher dose (400 mg/kg), indicating that the antihyperglycemic effect of the extract is dose-independent between 100 and 400 mg/kg. One of the limitations of the present study is that we did not evaluate glucose tolerance test after administration of the extract. It is possible that F. gummosa can reduce postprandial hyperglycemia, which should be investigated in future studies. Also, both insulin and F. gummosa were unable to significantly reduce the serum levels of total cholesterol and LDL after 4 weeks of treatment. Therefore, effects of F. gummosa on long-term (> 4 weeks) diabetes-induced dyslipidemia should be studied in future research.
The pathophysiological mechanism that underlines microvascular and macrovascular complications of diabetes suggests oxidative stress as one of the main pathogenic factors. The increased oxidative stress is mostly a result of hyperglycemia and dyslipidemia enhancing susceptibility of damage to lipids, proteins, and DNA (4,26). In the present study, administration of insulin attenuated oxidative stress in the liver and kidneys and reduced the elevated levels of serum ALT and ALP. Elevated levels of aminotransferases are a common indicator of liver injury and are observed more frequently among diabetic patients than healthy individuals (27). Similar to insulin, administration of F. gummosa significantly decreased the levels of ALT and restored the liver weight in diabetic rats. In addition, F. gummosa significantly reduced the elevated levels of lipid peroxidation and SOD activity in the kidneys of diabetic groups. In agreement with our findings, antioxidant and anti-inflammatory effects of F. gummosa have been shown in different tissues such as the kidneys and liver (10,12). For example, it has been shown that F. gummosa reduces acetaminophen-induced liver damage through attenuation of oxidative stress (12). Also, hydroalcoholic extract of F. gummosa was shown to protect kidneys against nitric oxide deficiency-induced inflammation and oxidative stresses (10).
In the present study, induction of diabetes significantly increased SOD activity in the serum, kidneys, and liver. In the STZ model of diabetes, the level of this enzyme has been reported to decrease, increase, or unchanged by various investigators. For instance, Ogunyinka et al. reported that STZ significantly reduced hepatic SOD activity in diabetic rats (28). Aliciguzel et al. observed no changes in the activity of SOD in the liver, brain, and kidneys after the induction of diabetes (29). On the other hand, Huang et al. showed an increase in both gene expression and activity of SOD in STZ-diabetic rats (30). Also, some clinical studies reported higher activity of extracellular and erythrocyte SOD among diabetic patients compared to non-diabetic subjects (31,32). It has been suggested that the level of serum SOD may be a marker of hyperglycemia-induced vascular injury (33). Since F. gummosa has been able to maintain SOD activity in diabetic rats close to that of normal control rats, it is probably able to reduce hyperglycemia-induced oxidative damage to the vascular endothelium, which should be confirmed in future studies.
Many beneficial effects of the genus Ferula are attributed to sesquiterpene coumarin compounds such as feselol, conferone, umbelliprenin, ferulsinaic acid, and mogoltacin (13,14,15,17,34). Although most studies have focused on the anticancer effects of these compounds (13), in some studies their beneficial effects in diabetes have also been taken into consideration.
CONCLUSION
Results of the present study suggest that ethanolic extract of F. gummosa oleo-resin has the potential to prevent or delay the complications of diabetes by inhibiting the progression of hyperglycemia and attenuating oxidative stress-induced damage in the liver and kidneys.
ACKNOWLEDGMENTS
This study was financially supported (Grant No. 941316) by the Vice-Chancellor of Research and Technology Center, Mashhad University of Medical Sciences, Mashhad, I.R. Iran.
REFERENCES
- 1.Gregg EW, Li Y, Wang J, Rios Burrows N, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731–b4739. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domingueti CP, Dusse LM, Carvalho Md, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Ghorbani A. Best herbs for managing diabetes: a review of clinical studies. Braz J Pharm Sci. 2013;49(3):413–422. [Google Scholar]
- 6.Hosseini A, Shafiee-Nick R, Ghorbani A. Pancreatic beta cell protection/regeneration with phytotherapy. Braz J Pharm Sci. 2015;51(1):1–16. [Google Scholar]
- 7.Azizian H, Rezvani ME, Esmaeilidehaj M, Bagheri SM. Anti-obesity, fat lowering and liver steatosis protective effects of Ferula asafoetida gum in type 2 diabetic rats: possible involvement of leptin. IJDO. 2012;4(3):120–126. [Google Scholar]
- 8.Raafat K, El-Lakany A. Acute and subchronic in-vivo effects of Ferula hermonis L. and Sambucus nigra L and their potential active isolates in a diabetic mouse model of neuropathic pain. BMC Complement Altern Med. 2015;15(1):257–270. doi: 10.1186/s12906-015-0780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amiri MS, Joharchi MR. Ethnobotanical knowledge of Apiaceae family in Iran: A review. Avicenna J Phytomed. 2016;6(6):621–635. [PMC free article] [PubMed] [Google Scholar]
- 10.Moosavi SJ, Habibian M, Peeri M, Azarbayjani MA, Nabavi SM, Nabavi SF, et al. Protective effect of Ferula gummosa hydroalcoholic extract against nitric oxide deficiency-induced oxidative stress and inflammation in rats renal tissues. Clin Exp Hypertens. 2015;37(2):136–141. doi: 10.3109/10641963.2014.913609. [DOI] [PubMed] [Google Scholar]
- 11.Mahboubi M. Ferula gummosa, a traditional medicine with novel applications. J Diet Suppl. 2016;13(6):700–718. doi: 10.3109/19390211.2016.1157715. [DOI] [PubMed] [Google Scholar]
- 12.Dadkhah A, Khalaj GR, Fatemi F, Dini S, Hesaraki S, Naij S, et al. Evaluation the role of Ferula gummosa essential oil against the hepatoxicity induced by acetaminophen in animal model. Journal of Medicinal Plants. 2016;4(60):14–23. [Google Scholar]
- 13.Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25(3):315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 14.Xing Y, Li N, Zhou D, Chen G, Jıao K, Wang W, et al. Sesquiterpene coumarins from Ferula sinkiangensis act as neuroinflammation inhibitors. Planta Med. 2017;83(1-02):135–142. doi: 10.1055/s-0042-109271. [DOI] [PubMed] [Google Scholar]
- 15.Sayed AA. Ferulsinaic acid attenuation of diabetic nephropathy. Eur J Clin Invest. 2013;43(1):56–63. doi: 10.1111/eci.12015. [DOI] [PubMed] [Google Scholar]
- 16.Sayed AAR. Ferulsinaic acid modulates SOD, GSH, and antioxidant enzymes in diabetic kidney. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/580104. Article ID:580104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayed AA. Ferulsinaic acid attenuation of advanced glycation end products extends the lifespan of Caenorhabditis elegans. J Pharm Pharmacol. 2011;63(3):423–428. doi: 10.1111/j.2042-7158.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 18.Jalali HT, Ebrahimian ZJ, Evtuguin DV, Neto CP. Chemical composition of oleo-gum-resin from Ferula gummosa. Ind Crops Prod. 2011;33(2):549–553. [Google Scholar]
- 19.Ghorbani A, Omrani GR, Hadjzadeh MA, Varedi M. Proinsulin C-peptide inhibits lipolysis in diabetic rat adipose tissue through phosphodiestrase-3B enzyme. Horm Metab Res. 2013;45(3):221–225. doi: 10.1055/s-0032-1323764. [DOI] [PubMed] [Google Scholar]
- 20.Ghorbani A, Mohebbati R, Jafarian AH, Vahedi MM, Hosseini SM, Soukhtanloo M, et al. Toxicity evaluation of hydroalcoholic extract of Ferula gummosa root. Regul Toxicol Pharmacol. 2016;77:35–41. doi: 10.1016/j.yrtph.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 22.Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35(3):184–188. [PubMed] [Google Scholar]
- 23.Merzouk H, Madani S, Sari DC, Prost J, Bouchenak M, Belleville J. Time course of changes in serum glucose, insulin, lipids and tissue lipase activities in macrosomic offspring of rats with streptozotocin-induced diabetes. Clin Sci. 2000;98(1):21–30. doi: 10.1042/cs0980021. [DOI] [PubMed] [Google Scholar]
- 24.Rath D, Kar DM, Panigrahi SK, Maharana L. Antidiabetic effects of Cuscuta reflexa Roxb. in streptozotocin induced diabetic rats. J Ethnopharmacol. 2016;192:442–449. doi: 10.1016/j.jep.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Lucidi P, Porcellati F, Andreoli AM, Carriero I, Candeloro P, Cioli P, et al. Pharmacokinetics and pharmacodynamics of NPH insulin in type 1 diabetes: the importance of appropriate resuspension before subcutaneous injection. Diabetes Care. 2015;38(12):2204–2210. doi: 10.2337/dc15-0801. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 27.Malenica M, Prnjavorac B, Causevic A, Dujic T, Bego T, Semiz S. Use of databases for early recognition of risk of diabetic complication by analysis of liver enzymes in type 2 diabetes mellitus. Acta Inform Med. 2016;24(2):90–93. doi: 10.5455/aim.2016.24.90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunyinka BI, Oyinloye BE, Osunsanmi FO, Opoku AR, Kappo AP. Protective effects of Parkia biglobosa protein isolate on streptozotocin-induced hepatic damage and oxidative stress in diabetic male rats. Molecules. 2017;22(10):E1654. doi: 10.3390/molecules22101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliciguzel Y, Ozen I, Aslan M, Karayalcin U. Activities of xanthine oxidoreductase and antioxidant enzymes in different tissues of diabetic rats. J Lab Clin Med. 2003;142(3):172–177. doi: 10.1016/S0022-2143(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 30.Huang WC, Juang SW, Liu IM, Chi TC, Cheng JT. Changes of superoxide dismutase gene expression and activity in the brain of streptozotocin-induced diabetic rats. Neurosci Lett. 1999;275(1):25–28. doi: 10.1016/s0304-3940(99)00704-1. [DOI] [PubMed] [Google Scholar]
- 31.Domínguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998;21(10):1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- 32.Adachi T, Nakamura M, Yamada H, Futenma A, Kato K, Hirano K. Quantitative and qualitative changes of extracellular-superoxide dismutase in patients with various diseases. Clin Chim Acta. 1994;229(1-2):123–131. doi: 10.1016/0009-8981(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 33.Kimura F, Hasegawa G, Obayashi H, Adachi T, Hara H, Ohta M, et al. Serum extracellular superoxide dismutase in patients with type 2 diabetes: relationship to the development of micro-and macrovascular complications. Diabetes Care. 2003;26(4):1246–1250. doi: 10.2337/diacare.26.4.1246. [DOI] [PubMed] [Google Scholar]
- 34.Rassouli FB, Matin MM, Iranshahi M, Bahrami AR, Neshati V, Mollazadeh S, et al. Mogoltacin enhances vincristine cytotoxicity in human transitional cell carcinoma (TCC) cell line. Phytomedicine. 2009;16(2-3):181–187. doi: 10.1016/j.phymed.2008.06.011. [DOI] [PubMed] [Google Scholar]