Abstract
Triazoles and quinazolinones are important heterocyclic structures with diverse biological properties including cytotoxic, antibacterial, antifungal and anticonvulsant activities. Due to valuable cytotoxic effects of both triazole and quinazoline derivatives, in this study a series of quinazolinone-triazole hybrids were synthesized in a multiple-step reaction procedure. 3-Amino-quinazolinone derivatives were treated with chloroacetyl chloride in the presence of dichloromethane/triethylamine to afford 2-chloro -N-(4-oxo-2- quinazolin3 (3H)-yl) acetamide derivatives. The reaction of resultants with 4-mehyl-4-H-1, 2, 4-triazole-3- thiol in dry acetone and potassium carbonate led to the formation of final products. Synthesized compounds were evaluated for their cytotoxic effects against MCF-7 and Hela cell lines using MTT colorimetric assay. Amongst tested compounds, 6a showed the highest cytotoxic activity against MCF7 cell line at all tested concentrations while compounds 6b and 6c indicated mild cytotoxic effects against Hela cell line at highest tested concentration reducing cell viability about 40%. The IC50 values of tested compounds revealed that the MCF-7 is more susceptible to the compound 6a.
Keywords: Cytotoxicity, Hybrid, Quinazolinone, Triazole
INTRODUCTION
Cancer, as a major health problem, has been identified by high proliferation of abnormal cells. Although there have been great advances in the treatment of cancer, due to the complications associated with clinical use of anticancer drugs such as drug resistance, side effects, and systemic toxicity, discovering various chemical structures with high efficacy and minimum undesirable side effects is very important (1). Quinazolinone as an important pharmacophore in medicinal chemistry has drawn much attention due to its wide range of biological effects, which include antibacterial, antifungal, anticancer, anti-inflammatory, and antihypertensive activities (2,3,4,5,6,7). Different mechanisms such as inhibition of the DNA repair enzyme, inhibition of epidermal growth factor receptor (EGFR), thymidylate enzyme inhibition and inhibitory effects for tubulin polymerize have been proposed for anticancer activities of quinazolinone-based structures (8,9). Triazole is another important heterocyclic structure, which can be incorporate in different templates to improve their activities (10). This five membered ring heterocycle is available with three nitrogen atoms in the form of two isomers 1,2,3-triazole and 1,2,4-triazole. Biological activities of 1,2,4-triazole such as antibacterial, antifungal, antiviral, anticancer, anticonvulsant, and anti-tubercular have been explored in many investigations (11,12,13,14). The known systems fused to triazoles are pyridines, pyridazines, quinazolines, pyrazines and triazines(15). Triazoles have been linked to quinazolinone structure to synthesis novel hybrid structure with different biological effects (15,17). Triazoloquinazolines constitute an important class of compounds in medicinal chemistry (15).
In view of valuable cytotoxic effects of both triazoles and quinazolinones and in continuation of our previous research on quinazolinone derivatives (2,3,4,5,6,7,8,9), it seemed most interesting to synthesize derivatives of quinazolinone bearing triazole group as enhancing moiety for cytotoxic activity.
MATERIALS AND METHODS
All starting materials, reagents, and solvents were purchased from commercial suppliers Merck (Germany) and Aldrich (USA) companies. Merck silica gel 60 F254 plates (Germany) were applied for analytical thin layer chromatography (TLC) (Germany). Proton nuclear magnetic resonance (HNMR) spectra were recorded using a Bruker 400 MHz (Germany). Spectrometer and chemical shifts are expressed as ppm with tetramethylsilane (TMS) as internal standard.
IR (KBr discs) spectra were recorded with a WQF-510 FT-IR spectrophotometer (China). Melting points were determined using electrothermal 9200 melting point apparatus (England) and are uncorrected. All cell lines were supplied from Pasteur Institute of Iran (Tehran, I.R. Iran).
General procedure for synthesis of compounds
3-Amino-quinazolinone derivatives were prepared according to the procedures shown in Scheme 1 (18). Then quinazoline derivatives were treated with chloroacetyl chloride in the presence of dichloromethane/triethylamine to afford the 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives. Final products were synthesized by the reaction of 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives with 4-mehyl-4-H-1, 2, 4-triazole- 3-thiol in dry acetone and potassium carbonate (19,20).
Scheme 1.
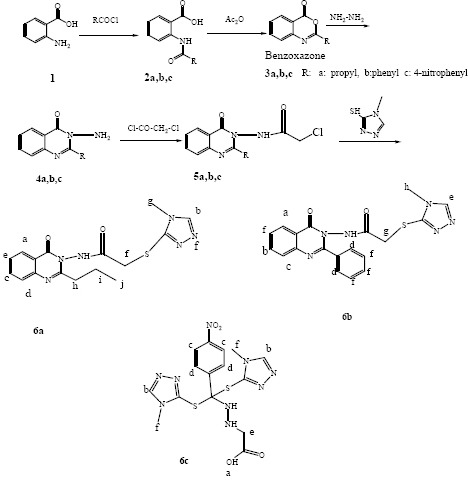
Synthesis of the target compounds (6a-6c).
Cytotoxicity assay
Sample and culture media preparation
MCF-7 (breast cancer) and HeLa (cervical cancer) cells were purchased from pasture institute of Iran (Tehran, I.R. Iran) and maintained at 37 °C in a humidified atmosphere (90%) containing 5% CO2. Cells were cultured in RPMI 1640 completed with 5% v/v fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. After 2-3 subcultures, 180 μL of the cell suspension (5 × 104 cells/mL) were seeded in 96-well plates and incubated for 24 h.
The stock solutions of compounds (10 mM, 1 mL) were prepared in DMSO and diluted with the medium to obtain 10, 100, 1000 μM concentrations.
After 24 h incubation, cells were treated with different concentrations of the derivatives (1, 10, 100 μM) for 48 h. Doxorubicin was used as positive control. Then 20 μL of MTT solution (5 mg/mL in phosphate buffer solution) was added to each well and incubated for 4 h. Afterwards, the media in each well was gently replaced with 150 μL DMSO to dissolve formazan crystals. The absorbance of each well was measured at 540 nm using an ELISA plate reader. Each experiment was repeated three times. Analysis of variance (ANOVA) followed by Tukey test was used to determine the differences between various groups. The significance level was set at P < 0.05. Cell viability was calculated using following equation:
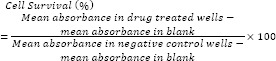
RESULTS
Synthesis of 2- amido-benzoic acid derivatives (2a, 2b, 2c)
Acyl chloride (butyryl chloride, benzoyl chloride, and 4-nitrobenzoyl chloride) (0.045 mol) was added dropwise to a solution of (0.04 mol) anthranilic acid (1) in dimethyl formamide (35 mL) and stirred at room temperature for 3 h. The mixture was poured into water and the precipitate was collected by filtration, washed with water, and dried under reduced pressure to give 2a-c (Scheme 1).
Synthesis of benzoxazone derivatives (3a, 3b, 3c)
Compound 2a-c (0.01 mol) was dissolved in acetic anhydride (30 mL) and heated for 1 h with vigorous stirring. The solvent was removed by distillation under reduced pressure to give 3a-c (Scheme 1).
Synthesis of 3-aminoquinazolinone derivatives (4a, 4b, 4c)
A mixture of benzoxazone derivatives 3a-c (0.01 mol) and hydrazine hydrate (0.02 mol) in ethanol was refluxed for 3 h. After the reaction was completed, the mixture was cooled and the separated solid was collected by filtration and recrystallized from ethanol or isopropanol.
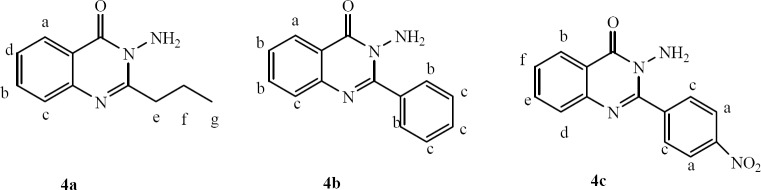
3-Amino-2-propylquinazolin-4 (3H)-one (4a)
1HNMR δ: (400 MHz; CDCl3), 8.14 (1H, dd, J = 8Hz, J = 4 Hz, Ha), 7.65 (1H, t, J = 8 Hz, Hb) 7.58 (1H, d, J = 8 Hz, Hc), 7.35 (1H, t, J = 8 Hz, Hd), 4.8 (2H, s, NH2), 2.92 (2H, t, J = 8 Hz, CH2 e),1.77 (2H, m, CH2 f), 1.0 (3H, t, J = 8 Hz, CH2 g) (Fig. 1)
Fig. 1.
3-Aminoquinazolinone derivatives (4a, 4b, 4c).
3-Amino-2-phenylquinazolin-4 (3H)-one (4b)
1HNMR δ: (400 MHz; CDCl3), 8.26 (1H, dd, J = 8 Hz, J = 4 Hz, Ha), 7.71-7.74 (4H, m, Hb), 7.44-7.47 (4H, m, Hc), 4.94 (2H, s, NH2) (Fig1).
3- Amino - 2 - (4-nitrophenyl) quinazolin -4 (3H)-one (4c)
1HNMR δ: (400 MHz; CDCl3), 8.30 (2H, d, J = 8 Hz, Ha), 8.28 (1H, d, J = 8 Hz, Hb), 7.95 (2H, d, J = 8 Hz, Hc), 7.7-7.8 (2H, m, Hd, He), 7.5 (1H, t, J = 8 Hz, Hf), 4.8 (2H, s, NH2) (Fig 1).
Synthesis of 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives (5a, 5b, 5c)
3-Amino-quinazoline derivatives 4a-4c (0.01 mol) was dissolved in dry dichloromethane (20 mL). To this mixture, triethylamine (0.01 mol) and chloroacetylchloride (0.01 mol) were added and stirred at room temperature for 30 min. Then the reaction mixture was poured in to ice-water and extracted with dichloromethane and ethyl acetate. The extracted ethyl acetate was washed with 3 % sodium bicarbonate solution and dried over anhydrous magnesium sulfate, which upon evaporation afforded the product 5a-5c.
Synthesis of quinazolinone-triazole hybrid derivative (6a, 6b, 6c)
A mixture of 2-chloro -N-(4-oxo-2- quinazolin3 (3H)-yl) acetamide derivatives 5a-5c (0.01 mol),4-mehyl-4-H-1,2,4-triazole- 3-thiol(0.01 mol) and anhydrous potassium carbonate (0.01 mmol) was refluxed in dry acetone (20 mL) for 6 h. The reaction mixture was filtered while hot and the residue was washed with hot acetone. The organic solution was concentrated and purified by preparative thin layer chromatography.
2-(4-Methyl-4H-1, 2, 4-triazol-3-ylthio)-N-(4- oxo-2-propylquinazolin-3(4H)-yl) acetamide (6a)
Yield: 30%, m.p.128-129 °C, IR νmax, 3428 (NH), 2844 (C-H),1696 (C=O) cm-1. 1HNMR δ: (400 MHz; CDCl3), 10 (b, NH), 8.05-8.15 (2H, m, Ha , Hb), 7.65 (1H, t, J = 8 Hz, Hc),7.58 (1H, d, J = 8 Hz, Hd),7.32(1H, t, J = 8 Hz, He), 4.14-4.17 (1H, d, J = 12 Hz, CH2 f), 3.82-3.85 (1H, d, J = 12 Hz, CH2 f), 3.62 (3H, s, CH3 g),2.65 (2H, m, J = 8 Hz, CH2 h), 1.73 (2H, m, CH2 i), 0.93(3H, t, J = 8 Hz, CH3 J).
2-(4-Methyl-4H-1, 2, 4-triazol-3-ylthio)-N-(4- oxo-2-phenylquinazolin-3(4H)-yl) acetamide (6b)
Yield: 33%, m.p.190-191 °C, IR νmax, 3353 (NH), 3050 (C-H, Ar), 1690 (C=O) cm-1. 1HNMR δ: (400MHz; DMSO), 8.27 (1H, d, J = 8 Hz, Ha), 8.0 (1H, t, J = 8 Hz, Hb), 7.84 (1H, d, J = 8 Hz, Hc ), 7.68-7.72 (3H, m, Hd, He), 7.54-7.61 (4H, m, Hf), 7.15(1H, s, NH), 4.23-4.26 (1H, d, J = 12 Hz, CH2 g), 4.18-4.21(1H, d, J = 12 Hz, CH2 g), 3.61 (3H, s, CH3 h).
2- (2 - bis (4 - methyl-4H - 1,2,4triazole – 3 - nitrophenyl)methyl)hydrazinyl)aceticacid) (6c)
Yield: 35%, m.p.208-209 °C, IR νmax, 3200 (NH), 2993 (CH), 1691(C=O), 1536, 1345 (NO2) cm-1. 1HNMR δ: (400MHz; DMSO),10.62 (1H, s ,Ha), 9.39 (2H, s , Hb), 8.38 (2H, d, J = 8 Hz, Hc), 8.22 (2H, d, J = 8 Hz, Hd), 4.32(2H, s ,He), 4.23 (1H, NH), 3.86 (1H, NH),3.75 (6H, s, Hf).
Cytotoxic effects of the derivatives (6a-c)
The cytotoxicity of compounds were evaluated against HeLa and MCF7 cell lines at different concentrations of 1, 10, and 100 μM using MTT assay. Results are shown in Figs. 2, 3, and Table 1.
Fig. 2.
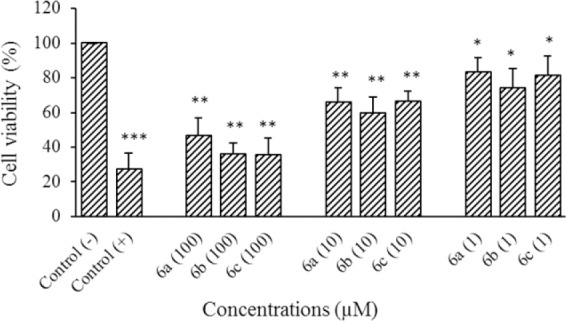
Cytotoxic effect of compounds 6a-6c on HeLa cells following exposure to different concentrations (1, 10, and 100 μM). Data are presented as mean ± SD, (SD for negative control is 0.06), n = 3.* Shows significant differences (*P < 0.05 and **P < 0.01) in comparison with negative control group; doxorubicin used as positive control.
Fig. 3.
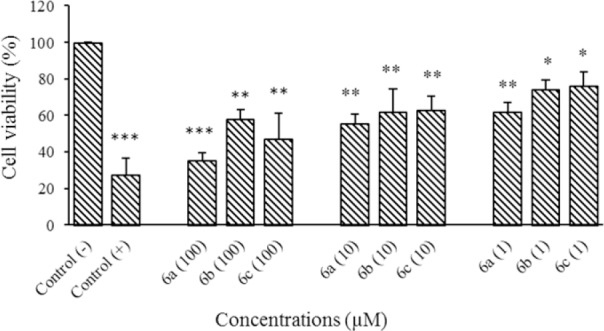
Cytotoxic effect of compounds 6a-6c on MCF7 cells following exposure to different concentrations (1, 10, and 100 μM). Data are presented as mean ± SD, (SD for negative control is 0.06), n = 3. * Shows significant differences (*P < 0.05, **P < 0.01, and ***P < 0.001) in comparison with negative control group; doxorubicin used as positive control.
Table 1.
The IC50 (μM) of tested compounds against MCF7 and Hela cell lines.
| Target compounds | R | MCF-7 cells | HeLa cells |
|---|---|---|---|
| 6a | Propyl | 38.385 ± 4.5 | 85.58 ± 9 |
| 6b | Phenyl | 159.42 ± 8.5 | 49.30 ± 7.5 |
| 6c | Nitro phenyl | 87.77 ± 9 | 54.11 ± 7.4 |
The obtained results from cytotoxic tests against HeLa cell line indicated that all three compounds 6a-c exhibited significant cytotoxic effects (P < 0. 05 at 1 μM and P < 0. 01 at 10 and 100 μM) in comparison with the negative control. Although significant differences between different compounds in the same concentrations were not observed (6a-6c), compounds 6b and 6c at highest tested concentrations demonstrated mild cytotoxic effects against Hela cell line
Cytotoxic assessment also indicated that compounds 6b and 6c had significant cytotoxic activities against MCF7 cell line (P < 0. 05 at 1 μM and P < 0. 01 at 10 and 100 μM) compared to the negative control. Compound 6a, however, showed more considerable cytotoxicity (P < 0. 01 at 10 μM, 1 μM and P < 0. 001 at 100 μM) against MCF7 cell line compared to the negative control.
DISCUSSION
Quinazolinone are important scaffolds for new drug development with diverse biological properties particularly anticancer activity. While many of the naturally bioactive quinazolinones have rigid structures, the synthetic derivatives such as 2 or 4-substituted are more flexible (21). It is also reported that quinazoline derivatives that contain aromatic rings or some nitrogenated heterocycles in their structures are potential antitumor agents (5,22,23). 2-(2thieno)-6- iodo-3-phenylamino-3,4-dihydro-quinzolin - 4- one, 3-benzyl-2-cinnamylthio - 6 - (methyl or nitro)quinazolin-4(3H)-ones,2-(2-Aminothiazol- 5-ylthio)-3-phenyl -6-methyl-quinazolin-4(3H)- ones and 2- (4 - chlorophenyl)-6-nitro-3- (2 - (2phenylthiazole - 4 - yl)ethyl)quinazolin - 4(3H)one are derivatives whose anticancer activities are mediated via dihydrofolate reductase (DHFR) inhibition (8,24-26).
Triazole is a unique template which can conjugate to different heterocyclic to improve their activities (10,27). The known systems fused to triazoles are pyridines, pyridazines, quinazolines, pyrazines and triazines(15). Hybrid structures of quinazolinone-triazole have been reported with anticancer (28), antibacterial (15,16,29), anti-malaria (30) and anticonvulsant (31) activities.
In this study, 3-aminoquinazoline-4-(3H)- one derivatives were synthesized in three steps starting from anthranilic acid. Fairly unstable compounds 5a-5c were prepared by the reaction between 3-aminoquinazoline-4-(3H)- one derivatives and chloroacetyl chloride in dry dichloromethane in the presence of triethylamine. Final compounds 6a-6b were obtained through the nucleophilic displacement of the chloride with thiol of 4-mehyl-4-H-1, 2,4-triazole-3-thiol using acetone and potassium carbonate in reflux condition. Compound 6c was produced according to proposed mechanism in Scheme 2. Reaction between 3-aminoquinazoline-4-(3H)- one derivatives and chloroacetyl chloride in dry dichloromethane in the presence of triethylamine produced compound 1 as an undesirable product. Triazole acted as a nucleophile and attacked the carbonyl group of molecule 1, which resulted in the ring opening to afford the intermediate 2. Subsequently two molecules of triazole attacked to the C=N and carbon of intermediate 2 and 3, respectively. Exiting the derivative of anthranilic acid-triazole from the intermediate 3 produced compound 6c (Scheme 2).
Scheme 2.
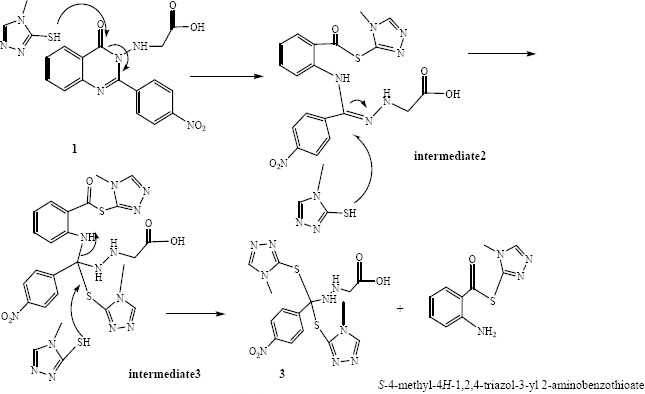
The probable mechanism to produce compound 6C.
Among tested compounds, 6a showed the highest cytotoxic activity against MCF7 cell line at all tested concentrations while compounds 6b and 6c indicated mild cytotoxic effects against Hela cell line at highest tested concentrations. The IC50 values of tested compounds revealed that the MCF-7 is more susceptible to the compound 6a. Substitution of propyl group at 2 position of quinazolinone improved the cytotoxic activity against MCF7 due to electronic effect. The cell growth inhibitory activity of the compounds may be attributed to substituent property at position 2 of quinazolinone ring.
CONCLUSIONS
In this study, quinazolinones as a pharmacophor structure were conjugated with triazole ring in a multi-step procedure. The final compounds were tested against two cell lines. Compound 6a showed the highest cytotoxic activities against MCF7 cell line. Compounds 6b and 6c indicated mild cytotoxic effects against HeLa cells at highest tested concentrations.
ACKNOWLEDGMENT
This work was partially supported (Grant No. 395904) by Vice Chancellery of Research of Isfahan University of Medical Sciences.
REFERENCES
- 1.Akbarzadeh T, Noushini S, Taban S, Mahdavi M, Khoshneviszadeh M, Saeedi M, Saeed Emami S, et al. Synthesis and cytotoxic activity of novel poly-substituted imidazo[2,1-c][1,2,4]triazin-6-amines. Mol Divers. 2015;19(2):273–281. doi: 10.1007/s11030-015-9566-6. [DOI] [PubMed] [Google Scholar]
- 2.Hassanzadeh F, Jafari E, Hakimelahi GH, Rahmani Khajouei M, Jalali M, Khodarahmi GA. Antibacterial, antifungal and cytotoxic evaluation of some new quinazolinone derivatives. Res Pharm Sci. 2012;7(2):87–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Hassanzadeh F, Rahmani Khajouei M, Hakimelahi GH, Jafari E, Khodarahmi GA. Synthesis of some new 2,3-disubstituted-4(3H)quinazolinone derivatives. Res Pharm Sci. 2012;7(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Jafari E, Khodarahmi GA, Hakimelahi GH, Tsai FY, Hassanzadeh F. Synthesis of some new tricyclic 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2011;6(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Khodarahmi GA, Rahmani Khajouei M, Hakimelahi GH, Abedi D, Jafari E, Hassanzadeh F. Antibacterial, antifungal and cytotoxic evaluation of some new 2,3-disubstituted 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2012;7(3):151–158. [PMC free article] [PubMed] [Google Scholar]
- 6.Khodarahmi GH, Jafari E, Hakimelahi GH, Abedi D, Rahmani Khajouei M, Hassanzadeh F. Synthesis of some new quinazolinone derivatives and evaluation of their antimicrobial activities. Iranian J Pharm Res. 2012;11(3):789–797. [PMC free article] [PubMed] [Google Scholar]
- 7.Rezaee Nasab R, Mansourian M, Hassanzadeh F. Synthesis, antimicrobial evaluation and docking studies of some novel quinazolinone Schiff base derivatives. Res Pharm Sci. 2018;13(3):213–221. doi: 10.4103/1735-5362.228942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafari E, Rahmani Khajouei M, Hassanzadeh F, Hakimelahi GH, Khodarahmi GA. Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res Pharm Sci. 2016;11(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Vaseghi G, Jafari E, Hassanzadeh F, Haghjooy- Javanmard S, Dana N, Rafi eian-Kopaei M. Cytotoxic evaluation of some fused pyridazino- and pyrrolo-quinazolinones derivatives on melanoma and prostate cell lines. Adv Biomed Res. 2017;6:76. doi: 10.4103/2277-9175.209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbot V, Sharma P, Dhıman S, Noolvi MN, Patel HM, Bhardwaj V. Small hybrid heteroaromatics: resourceful biological tools in cancer research. RSC Adv. 2017;7:28313–28349. [Google Scholar]
- 11.Shneine JK, Alaraji YH. Chemistry of 1, 2, 4- triazole: a review article. Int J Sci Res. 2016;5(3):1411–1423. [Google Scholar]
- 12.Ali KA, Ragab EA, Farghaly TA, Abdalla MM. Synthesis of new functionalized 3-substituted [1,2,4]triazolo [4,3-a]pyrimidine derivatives: potential antihypertensive agents. Acta Pol Pharm. 2011;68(2):237–247. [PubMed] [Google Scholar]
- 13.Kumar R, Yar MS, Chaturvedi S, Srivastava A. Triazole as pharmaceuticals potentials. Int J Pharmtech Res. 2013;5(4):1844–1869. [Google Scholar]
- 14.Asif M. Recent advancement of potentially significant Triazole derivatives. Discovery Chem. 2015;1(1):18–32. [Google Scholar]
- 15.Nazeer A, Parveen N, Aslam S, Ain Khan M, Munawar MA. Synthesis and antibacterial activity of 2-phenyl-5-aryl-4, 5, 6, 7, 8, 9- hexahydro- 1,2.4- triazolo[1,5-A] quinazolines. AFINIDAD. 2013;70(563):220–225. [Google Scholar]
- 16.Krastina G, Ravina I, Mierina I, Zicane D, Turks M, Tetere Z, et al. Synthesis of novel quinazolinone- 1,2,3-triazole conjugates. J Chem Pharm Res. 2014;6(12):6–14. [Google Scholar]
- 17.Ouahrouch A, Taourirte M, Engels JW, Benjelloun S, Lazrek HB. Synthesis of new 1,2,3-triazol-4-yl-quinazoline nucleoside and acyclonucleoside analogues. Molecules. 2014;19:3638–3653. doi: 10.3390/molecules19033638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atia AJKh, Al-Mufrgeiy SS. Synthesis and antibacterial activities of new3-amino-2-methyl-quinazolin-4 (3h)-one derivative. American J Chem. 2012;2(3):150–156. [Google Scholar]
- 19.Alagarsamy V, Solomon VR, Sulthana MT, Vijay MS, Narendhar B. Design and synthesis of quinazolinyl acetamide for their analgesic and anti-inflammatory activities.Z. Naturforsch. 2015;70(8):1–8. [Google Scholar]
- 20.Abbas SE, Awadallah FM, Ibrahin NA, Said EG, Kamel GM. New quinazolinone-pyrimidine hybrids: synthesis, anti-inflammatory, and ulcerogenicity studies. E J Med Chem. 2012;53:141–149. doi: 10.1016/j.ejmech.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Marzaro G, Guiotto A, Chilin A. Quinazoline derivatives as potential anticancer agents: apatent review (2007 - 2010) Expert Opin Ther Pat. 2012;22(3):223–252. doi: 10.1517/13543776.2012.665876. [DOI] [PubMed] [Google Scholar]
- 22.Raffa D, Edler MC, Daidone G, Maggio B, Merickech M, Plescia S, et al. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2- styrylquinazolinones. Eur J Med Chem. 2004;39(4):299–304. doi: 10.1016/j.ejmech.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Yang ZY, Hour MJ, Kuo SC, Xia P, Bastow KF, et al. Antitumor agents. Part 204: synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg Med Chem Lett. 2001;11(9):1193–1196. doi: 10.1016/s0960-894x(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 24.Al-Obaid AM, Abdel-Hamide SG, El-Kashef HA, Abdel-Aziz AM, El-Azab AS, Al-Khamees HA, et al. Substituted quinazolines, part 3.Synthesis, invitro antitumor activity and molecular modelingstudy of certain 2-thieno-4(3H)- quinazolinone analogs. Eur J Med Chem. 2009;44(6):2379–2391. doi: 10.1016/j.ejmech.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Al-Omary FA, Abou-zeid LA, Nagi MN, Habib ESE, Abdel-Aziz AA, El-Azab AS, et al. Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2,6- substituted-quinazolin-4-ones. Bioorg Med Chem. 2010;18(8):2849–2863. doi: 10.1016/j.bmc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinzadeh L, Aliabadi A, Kalantari M, Mostafavi A, Rahmani Khajouei M. Synthesis and cytotoxicity evaluation of some new 6-nitro derivatives of thiazole-containing 4-(3H)-quinazolinone. Res Pharm Sci. 2016;11(3):210–218. [PMC free article] [PubMed] [Google Scholar]
- 27.Singhal N, Sharma PK, Dudhe R, Kumar N. Recent advancement of triazole derivatives and their biological significance. J Chem Pharm Res. 2011;3(2):126–133. [Google Scholar]
- 28.Safavi M, Ashtari A, Khalili F, Mirfazli SS, Saeedi M, Ardestani SK, et al. Novel quinazolin-4(3H)-one linked to 1,2,3-triazoles: synthesis and anticancer activity. Chem Biol Drug Des. 2018;92(1):1373–1381. doi: 10.1111/cbdd.13203. [DOI] [PubMed] [Google Scholar]
- 29.Sahoo S, Patwari KP, Kumar MCB, Settyd CM. Synthesis and biological activity of certain mannich bases derivatives from 1, 2, 4-triazoles. Iranian J Pharm Sci. 2013;9(4):51–60. [Google Scholar]
- 30.Havaldar FH, Patil AR. Syntheses of 1, 2, 4 triazole derivatives and their biological activity. E-J Chem. 2008;5(2):347–354. [Google Scholar]
- 31.Kamel MM, Zaghary WA, Al-wabli RI, Anwar MM. Synthetic approaches and potential bioactivity of different functionalized quinazoline and quinazolinone scaffolds. Egyptian Pharm J. 2016;15(3):98–131. [Google Scholar]