Abstract
Background
Serine hydroxymethyltransferase (SHMT) is the enzyme that catalyzes the reversible conversion of serine to glycine and tetrahydrofolate-bound one-carbon unit. Upregulation of SHMT2 has been observed in a variety of cancers, but the expression profile and clinical value of SHMT2 in gastric cancer (GC) are still unknown.
Material/Methods
In this study, SHMT2 expression was assessed in 130 patients with GC by immunohistochemistry (IHC). mRNA of SHMT2 in GC tissues and normal gastric epithelium was compared with qRT-PCR results. The correlations between SHMT2 and the clinicopathologic factors were analyzed with the chi-square test. Univariate analysis with Kaplan-Meier method was used to estimate the correlations between survival rate and clinicopathologic factors, including SHMT2. The independent prognostic biomarkers were confirmed by multivariate analysis using the Cox-regression hazard model. The function of SHMT2 in progression of GC was assessed by in vitro experiments.
Results
The percentages of low and high expression of SHMT2 were 46.92% and 53.08%, respectively. SHMT2 mRNA in GC tissue was significantly higher than mRNA in the patient-paired adjacent tissues. In the clinical analysis, SHMT2 expression was notably associated with positive lymphatic invasion. High SHMT2 was also demonstrated to independently predict poor prognosis of GC. After silencing SHMT2, we proved that SHMT2 can promote proliferation and invasion of GC cells.
Conclusions
High SHMT2 promoted progression and was an independent prognostic biomarker of GC, suggesting that SHMT2 detection would be helpful for stratification of high-risk patients and thus directing personalized treatment.
MeSH Keywords: Aminomethyltransferase, Lymphatic Metastasis, Prognosis, Stomach Neoplasms
Background
Gastric cancer (GC) is an aggressive and common malignancy ranking 5th in morbidity and 3rd in cancer-associated deaths throughout the world, with over 1 million new cases and an estimated 783 000 deaths in 2018 [1]. GC imposes a severe burden on global health finance, especially East Asia and particularly in China. The most radical treatment of GC is surgery. The postoperative 5-year overall survival rate is 90% for early-stage patients and just 10% for those in advanced stage [2]. Patients with advanced GC are generally treated with surgery followed by chemotherapy [3], but relapse and metastasis are common. Therefore, effective biomarkers that can identify patients at high risk are urgently needed.
Serine hydroxymethyltransferase (SHMT) is the enzyme that catalyzes the reversible conversion of serine to glycine and tetrahydrofolate-bound one-carbon unit. Upregulation in the synthesis and consumption of serine and glycine was observed in transformed cells and cancers [4–6]. In the human genome, there are 2 kinds of SHMT proteins – SHMT1 and SHMT2 [7] – with different functions and expression profiles. SHMT1 is a cytoplasmic isozyme involved in the de novo synthesis of thymidylate, and SHMT2 is expressed in mitochondria and regulates the synthesis of mitochondrial thymidine monophosphate (dTMP) [8]. It is interesting to note that SHMT2 and its downstream mitochondrial enzyme – 5,10-methylene-tetrahydrofolate dehydrogenase (MTHFD2) – is significantly overexpressed in a variety of cancers, including colorectal, brain, central nervous system (CNS), kidney, and bladder cancers [9–11]. However, the expression profiles of SHMT2 in GC are still unknown.
In the present study, we assessed SHMT2 expression in 130 GC patients by immunohistochemistry (IHC), and 15 fresh GCs and their patient-paired normal tissues with quantitative real-time polymerase chain reaction (qRT-PCR) for the first time. The clinical value of SHMT2 was assessed by analyzing the association between SHMT2 and other clinicopathologic factors. In addition, the prognostic significance of SHMT2 was investigated using univariate analysis (log-rank test) and multivariate analysis (Cox regression model).
Material and Methods
Specimens and follow-up
The primary cohort consisted of 364 patients who underwent radical surgery and were pathologically diagnosed as having GC at the Sixth People’s Hospital of Qingdao and the Yidu Central Hospital of Weifang from 2008 to 2016. From the primary cohort, a final cohort comprising 130 cases was enrolled using the following inclusion criteria: (1) no preoperative chemotherapy or radiotherapy before radical surgery, (2) available follow-ups and tissues for IHC, (3) no severe complication and a follow-up >3 months, and (4) no other malignancies. The final cohort was composed of 49 female patients and 81 male patients, with an average follow-up of 46.6 months. Moreover, 15 cases of GC and their patient-paired normal tissues were obtained during the operation and stored in liquid nitrogen and used for mRNA extraction. The study was approved and supervised by the Ethics Committees of the Sixth People’s Hospital and Yidu Central Hospital. All the specimens were collected with written consent of the patients. The TNM stage in this study was determined according to the 8th American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system.
IHC
The expression and location of SHMT2 were estimated with IHC by the streptavidin peroxidase complex method according to the method described in a previous study [12]. In brief, after being deparaffinized and rehydrated with xylene and graded alcohol, tissues were incubated in boiled 0.01 M citrate buffer (pH=6.0) for the best antigen retrieval. We used 3% H2O2 to inactivate the endogenous peroxidase. Following the blockage of unspecific binding by 5% bovine serum albumin (BSA), tissues were incubated in primary antibody of SHMT2 at 1: 100 (Abcam, Cambridge, MA, USA, cat. no. EPR3198) at 1: 100 dilution at 4°C overnight. After rinsing in phosphate-buffered saline 3 times, tissues were incubated in HRP-labeled secondary antibody (ZSBio, Beijing, China) at room temperature for 30 min. Finally, the complex reagent of streptavidin peroxidase (ZSBio, Beijing, China) was used, and 3,3′-diaminobenzidine solution (ZSBio, Beijing, China) was applied for final visualization of the antigen.
Evaluation of IHC result
The IHC results were semi-quantified by calculating IHC score according to the method described in a previous study [13]. The IHC score were evaluated by 2 senior pathologists blinded to the clinical data. The IHC score consisted of 2 aspects: the percentage of positive cells and the staining intensity. The scores for positive cell percentage were set as: 0 points represents <10% positive cells; 1 point represents 10–25% positive cells; 2 points represents 25–50% positive cells, and 3 points represents >50% positive cells. The scores for staining intensity were set as: 0 points represents the negative staining, 1 point represents weak staining, 2 points represents the moderate staining, and 3 points represents strong staining. The final IHC scores were the product of the score (positive cell percentage) multiplied by the score (staining intensity), ranging from 0 to 9. The cut-off was set as the point with highest sum of specificity and sensitivity in the receiver operating characteristic curve [14], and this was used to separate the final cohort into subgroups with high SHMT2 and low SHMT2 expression. In our study, the cut-off of SHMT2 was 3.5, meaning that patients with scores ≥4 were defined as SHMT2 high expression and those with scores ≤3 were defined as SHMT2 low expression.
Cell culture
The gastric cancer cell line MKN-28 was purchased from Shanghai Cell Bank (Shanghai, China). MKN28 cells were cultured in RPMI-1640 medium (HyClone, USA) supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 ug/ml streptomycin (HyClone, USA) in 5% CO2 resuscitation.
qRT-PCR
SHMT2 mRNA in GCs and paired normal tissues was estimated by qRT-PCR referring to a previous study [15]. In brief, total mRNAs were extracted with Trizol reagent (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s instructions, and reverse transcription was performed using a QuantiTect Reverse Transcription Kit (Qiagen, Venlo, Netherlands). The StepOnePlus™ RT-PCR system (Thermo Fisher, Waltham, MA, USA) was used for quantitative real-time PCR with SYBR Green qPCR Master Mix (Thermo Scientific, Waltham, MA, USA). GAPDH was used as a control to standardize SHMT2, and results were calculated by 2−ΔΔCt method. The primer sequence of SHMT2 and GAPDH was as follows:
SHMT2, forward: 5′-CGAGTTGCGATGCTGTACTT-3′;
reverse: 5′-CTGCGTTGCTGTGCTGAG-3′;
GAPDH, forward: 5′-GGGAAGGTGAAGGTCGGAGTC-3′,
reverse: 5′-CCATGGGTGGAATCATATTGGAA-3′
RNA knockdown
The RNA knockdown of SHMT2 was performed by transfection of siRNA. Two independent siRNAs and a scrambled siRNA of SHMT2 were purchased from Santa Cruz Biotech. Transfection of siRNA was performed using Lipofectamine 2000 according to the user guide.
Proliferation assay
CCK8 assay was used to detect the influence of SHMT2 on proliferation. In brief, cells transfected with SHMT2 siRNAs or scrambled siRNA were plated into 96-well plates (5×103 cells/well) and cultured for 0 to 72 h. After incubation, CCK8 (Dojindo, Japan) was used to incubate cells at 37°C for 2 h. The optical density at 450 nm was detected using a spectrophotometer (Molecular Devices Company, San Jose, CA, USA).
Invasion assay
Pre-coated 8.0-μm pore Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA) were used to evaluate the invasion ability of MKN28 cells. After starvation in serum-free medium for 6 h, 104 cells were plated into each upper chamber. The lower chambers contained medium with 5% FBS to facilitate the invasion of cells. After incubation for 12 h, cells in the lower surface were fixed with methanol and stained with 0.1% crystal violet (Beyotime, Beijing, China). The invaded cells were counted using a microscope.
Statistical analysis
All data were analyzed with SPSS 22.0 software (IBM cooperation, Chicago, USA). The chi-square test was used to evaluate the correlation between the SHMT2 expression and the clinicopathologic factors. The Kaplan-Meier method was performed to create a survival curve and the log-rank test was used to evaluate the difference between variable clinicopathologic factors and the survival rates. Multivariate analysis with Cox regression model was carried out for identification of independent prognostic factors. P values less than 0.05 were defined as statistically significant.
Results
The expression of SHMT2 in gastric cancers and normal gastric tissues
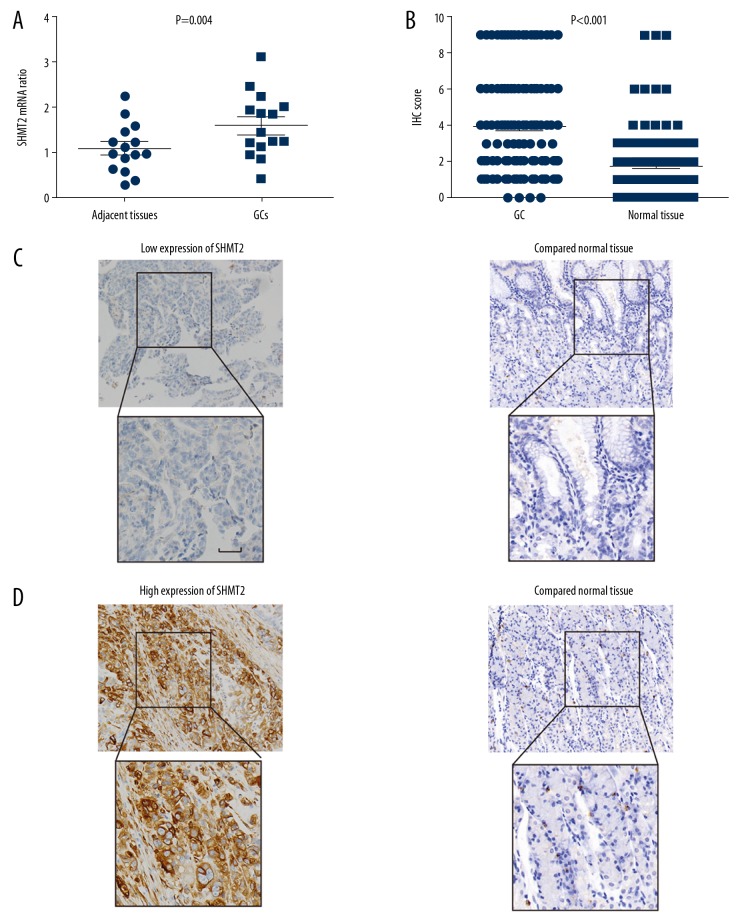
The mRNA expression of SHMT2 in GCs and patient-paired normal tissues were compared by qRT-PCR. In the 15 pairs of GC tissues and corresponding adjacent tissues were collected, the SHMT2 mRNA in GCs was substantially higher than in patient-paired normal gastric tissues (Figure 1A), which suggested that SHMT2 may participate in the oncogenesis of GC. The expression and subcellular location of SHMT2 were then investigated by IHC. In the stained 130 cases of GC and adjacent tissues, GCs had significantly higher IHC score than in adjacent tissues (Figure 1B). As an essential enzyme that catalyzes the reversible conversion of serine to glycine, the SHMT2 was mainly expressed in the cytoplasm of GC tissues. The 130 cases were divided into different subgroups according to the expression level of SHMT2. The percentage of low and high expression of SHMT2 accounted for 46.92% and 53.08%, respectively (Table 1). The representative IHC images of low and high SHMT2 expression were shown in Figure 1C and 1D.
Figure 1.
Expression of SHMT2 in gastric cancer tissues and normal gastric tissues. (A) The mRNA level of SHMT2 in GCs and paired normal gastric tissues was evaluated with qRT-PCR. The P value was evaluated with the paired t test. (B) The IHC scores of GC tissues were higher than the scores of adjacent normal tissues. The P value was evaluated with the paired t test. (C, D) Representative images of low (C) and high (D) immunohistochemical staining of SHMT2 in GC tissues and paired normal tissues. Scale bar: 50 um.
Table 1.
Basic information on the patients with GC.
| Variables | Number | Percentage |
|---|---|---|
| Sex | ||
| Female | 49 | 37.69% |
| Male | 81 | 62.31% |
| Age | ||
| ≤60 | 44 | 33.85% |
| >60 | 86 | 66.15% |
| Tumor size (cm) | ||
| ≤5 cm | 51 | 39.23% |
| >5 cm | 79 | 60.77% |
| Histopathological grade | ||
| I | 33 | 25.38% |
| II+III | 97 | 74.62% |
| T stage | ||
| T1+T2 | 19 | 14.62% |
| T3+T4 | 111 | 85.38% |
| Lymphatic invasion | ||
| Negative | 32 | 24.62% |
| Positive | 98 | 75.38% |
| Distant metastasis | ||
| Negative | 120 | 92.31% |
| Positive | 10 | 7.69% |
| TNM stage | ||
| I–II | 47 | 36.15% |
| III–IV | 83 | 63.85% |
| SHMT2 | ||
| Low | 61 | 46.92% |
| High | 69 | 53.08% |
SHMT2 is the abbreviation of serine hydroxymethyltransferase 2.
SHMT2 expression was notably associated with positive lymphatic invasion
The association between all the clinicopathologic factors and SHMT2 expression was analyzed with the chi-square test (Table 2). SHMT2 expression was shown to be significantly associated with lymphatic invasion. Patients with high SHMT2 expression had high risk of positive lymphatic invasion (P=0.042), indicating the possible role of SHMT2 in GC invasion. Moreover, patients with high SHMT2 seemed to be more likely to have larger tumor size (P=0.068) and advanced histological grade (P=0.068), although the differences were not statistically significant. These results all suggest that SHMT2 is involved in progression of GC.
Table 2.
The association between SHMT2 and clinicopathologic factors.
| Variables | SHMT2 | P* | |
|---|---|---|---|
| Low | High | ||
| Sex | |||
| Female | 21 | 28 | 0.587 |
| Male | 40 | 41 | |
| Age | |||
| <60 | 22 | 22 | 0.711 |
| ≥60 | 39 | 47 | |
| Tumor size | |||
| ≤5 cm | 29 | 22 | 0.068 |
| >5 cm | 32 | 47 | |
| Histopathological grade | |||
| I | 20 | 13 | 0.068 |
| II+III | 41 | 56 | |
| T stage | |||
| T1+T2 | 10 | 9 | 0.626 |
| T3+T4 | 51 | 60 | |
| Lymphatic invasion | |||
| Negative | 20 | 12 | 0.042 |
| Positive | 41 | 57 | |
| Distant metastasis | |||
| Negative | 56 | 64 | 0.839 |
| Positive | 5 | 5 | |
| TNM stage | |||
| I–II | 29 | 18 | 0.149 |
| III–IV | 32 | 51 | |
SHMT2 is the abbreviation of serine hydroxymethyltransferase 2.
Calculated by the χ2 test.
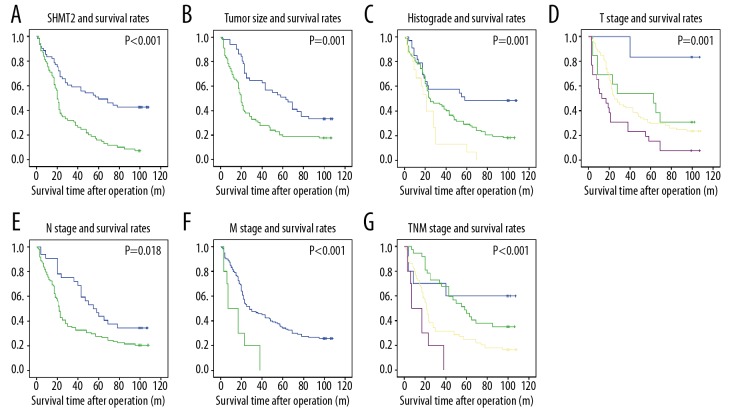
SHMT2 expression is correlated with low survival rates
The associations among SHMT2 expression, clinicopathologic factors, and survival time were further evaluated with univariate analysis (Table 3). The 5-year survival rates of high-SHMT2 patients was 15.9%, which was remarkably lower than for patients with low SHMT2 expression (49.2%, P<0.001) (Figure 2A). Moreover, large tumor size (P=0.001), high histological grade (P=0.001), more severe tumor filtration (P=0.001), positive lymphatic invasion (P=0.018), positive metastasis (P<0.001), and advanced TNM stage (P<0.001) were all correlated with unfavorable prognosis (Figure 2B–2G).
Table 3.
Prognostic significance of clinicopathologic factors including SHMT2 was analyzed with univariate analysis.
| Variables | 5-year survival rate | P* |
|---|---|---|
| Sex | ||
| Female | 28.6 | 0.333 |
| Male | 33.3 | |
| Age | ||
| <60 | 25 | 0.577 |
| ≥60 | 34.9 | |
| Tumor size(cm) | ||
| ≤5 | 51 | 0.001 |
| >5 | 19 | |
| Histopathological grade | ||
| I | 48.5 | 0.001 |
| II | 29.3 | |
| III | 6.7 | |
| T stage | ||
| T1 | 83.3 | 0.001 |
| T2 | 53.8 | |
| T3 | 29.4 | |
| T4 | 15.4 | |
| Lymphatic invasion | ||
| Negative | 46.9 | 0.018 |
| Positive | 27.6 | |
| Distant metastasis | ||
| Negative | 34.2 | <0.001 |
| Positive | 0 | |
| TNM stage | ||
| I | 60 | <0.001 |
| II | 45.9 | |
| III | 24.7 | |
| IV | 0 | |
| SHMT2 | ||
| Low | 49.2 | <0.001 |
| High | 15.9 | |
SHMT2 is the abbreviation of serine hydroxymethyltransferase 2.
Calculated by log-rank test.
Figure 2.
The correlation between survival rates and SHMT2 expression, tumor size, histopathological grade, T stage, lymphatic invasion, distant metastasis, and TNM stage. The overall survival rates were stratified into groups according to the SHMT2 expression (A), tumor size (B), histological grade (C), tumor filtration (D), lymphatic invasion (E), metastasis (F) and TNM stage (G).
SHMT2 independently predicts prognosis of GC
All the significant prognostic valuables in the log-rank test were entered into the Cox regression hazard model for identification of prognostic factors (Table 4). TNM stage was ruled out because of its natural interaction with other factors. In the multivariate analysis, SHMT2 was confirmed as an independent prognostic biomarker of GC (P<0.001), which suggested that high SHMT2 expression can predict poor prognosis by itself. The hazard ratio of SHMT2 was 2.52, indicating that the possibility of cancer-associated death of high-SHMT2 patients was approximately 2.5 times higher compared with those who had low SHMT2 expression. In addition, positive distant metastasis (HR=2.52, 95% CI=1.25–5.07, P=0.010) and high histological grade (HR = 3.45, 95% CI 1.60–7.44, P=0.002) were also independent prognostic factors of GC.
Table 4.
Multivariate analysis.
| Variables | HR | 95%CI | P* |
|---|---|---|---|
| Tumor size (cm) | |||
| ≤5 | 1 | ||
| >5 | 1.22 | 0.75–1.97 | 0.419 |
| Histopathological grade | |||
| I | 1 | ||
| II | 1.45 | 0.82–2.57 | 0.197 |
| III | 3.45 | 1.60–7.44 | 0.002 |
| T stage | |||
| T1+T2 | 1 | ||
| T3+T4 | 1.86 | 0.93–3.69 | 0.078 |
| Lymphatic invasion | |||
| Negative | 1 | ||
| Positive | 1.23 | 0.73–2.05 | 0.441 |
| Distant metastasis | |||
| Negative | 1 | ||
| Positive | 2.52 | 1.25–5.07 | 0.010 |
| SHMT2 | |||
| Low | 1 | ||
| High | 2.52 | 1.60–3.95 | <0.001 |
SHMT2 is the abbreviation of serine hydroxymethyltransferase 2.
Calculated by Cox regression model.
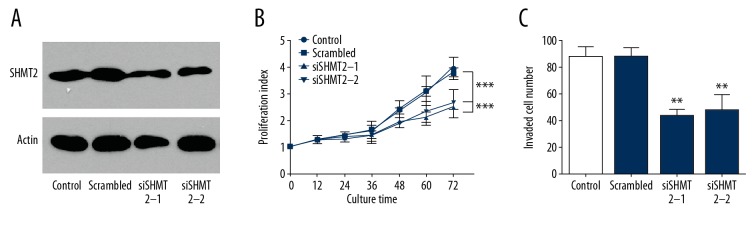
SHMT2 promotes proliferation and invasion of GC cells
Function assays were performed to evaluate the effect of SHMT2 on GC cells. Two independent siRNAs of SHMT2 was transfected into GC cell MKN28 for SHMT2 knockdown (Figure 3A). After successful knockdown, MKN28 was cultured for 3 days and the proliferation was detected by CCK-8 assay. Consequently, SHMT2 knockdown significantly decreased the proliferation of MKN28 (Figure 3B). Transwell assay was carried out to evaluate the influence of SHMT2 on GC invasion (Figure 3C). Silencing SHMT2 also notably attenuated the invasion of GC cells. All of the above results indicate an important role of SHMT2 in GC cell progression.
Figure 3.
SHMT2 promoted the proliferation and invasion of GC cells. (A) Successful knockdown of SHMT2 in MKN-28 cells by siRNA was detected with Western blot. (B) CCK-8 assay was used to detect the proliferation of MKN-28 cells. SHMT2 knockdown notably attenuated the proliferation of MKN-28 cells. (C) Cell invasion was detected by Matrigel Transwell assay. SHMT2 knockdown decreased the invasion of MKN-28 cells.
Discussion
The folate-dependent one-carbon (1C) metabolism is essential in progression and representative metabolic change in cancer growth and proliferation, just like enhanced glucose uptake and aerobic glycolysis [16,17]. In the most rapidly proliferating cells, such as tumor cells, 1C units generated from serine catabolism in the mitochondria can be exported to the cytosol as formate, and then re-assimilated into folates to support nucleotide synthesis [18–20]. The antagonism of 1C metabolic enzymes has been used in chemotherapy for over 60 years, and since then, the important role of 1C metabolism in tumorigenesis has been extensively studied [21]. Many drugs, including the common clinical agents pemetrexed, 5-fluorouracil, and methotrexate, are therapeutically targeted at 1C metabolism [22]. Between the only 2 SHMT members, expression of SHMT2 is significantly upregulated in a variety of cancers compared with SHMT1 [9,23]. In a previous study, SHMT2 was identified as a potential cancer driver gene by comparative oncogenomics [9]. Our identification of SHMT2 as a prognostic biomarker in GC is an important addition to the oncogenic study of SHMT2, and provides insight into the significance of SHMT2 as a drug target.
Along with the trend of personalized management, new applications are being tried to treat patients based on the molecular profile of the tumor. For example, a recent clinical trial demonstrated that immune checkpoint blockade therapy can suppress the progression in 12 types of tumors only if they have mismatch repair deficiency [24].
However, the clinical application of molecularly guided personalized treatment in GC remains stagnant. The extensive use of gene sequencing has resulted in discovery of many new biomarkers or genetic alterations of gastric cancer. For example, the Cancer Genome Atlas Research Network has published the results of full genomic profiling of 295 primary gastric adenocarcinomas [25]. Unfortunately, only a few of these genetic findings lead to clinical trials [26]. One reason for this is that final protein expression was not only altered by genetic alteration, but also by other cellular procedures like lncRNA and post-transcriptional modification. One great breakthrough in gastric biomarkers is the discovery of human epidermal growth factor receptor-2(HER2). Approximately 12–20% of gastric adenocarcinomas are HER2-positive [27], and previous studies reported that HER2-positive patients have worse prognoses, necessitating use of Herceptin in the treatment of gastric cancer [28]. Therefore, discovery of effective biomarkers with high potency to be drug targets in gastric cancer is still urgently needed. In our study, we investigated the potential biomarker of GC by IHC, which could directly affect the expression level of the potential biomarkers. This increased the efficiency from biomarker identification to potential drug target.
Most gastric cancers are gastric adenocarcinomas, but are highly heterogeneous with respect to architecture and growth, cell differentiation, histogenesis, and molecular pathogenesis [29]. This heterogeneity makes the investigation of molecular classification based on different biomarkers of genetic amplification essential. In our study, we demonstrated that SHMT2 is an independent prognostic biomarker of GC, with a very remarkably high statistical significance (P<0.001). This result indicates that SHMT2 detection after surgery would be helpful for stratification of patients with high risk, and may thus direct personalized treatment. Moreover, the correlation between SHMT2 and GC progression indicated that SHMT2 may be a potential drug target, which may stimulate pharmacological industry interest in SHMT2 and help develop new targeted drugs to treat GC. It is especially important to define the value of SHMT2 as a potential drug target in GC, because small-molecule dual inhibitors of SHMT1/2 have been developed in recent studies [30]. However, the function of the specific inhibitor of SHMT2 in suppressing tumor progression remains unclear, and the potency of SHMT2 inhibitor as a possible clinical drug needs more trials and experiments. Clearly, the clinical effects of the inhibitors need clinical trials because one-carbon donor formate generally rescues cells from SHMT inhibition.
Conclusions
In this study, we assessed the expression of SHMT2 in 130 patients with GC by immunohistochemistry (IHC) and evaluated its clinical and prognostic significance for the first time. Consequently, we showed that SHMT2 expression was notably correlated with lymphatic invasion, and that SHMT2 was an independent prognostic biomarker of GC. Our results suggest that SHMT2 detection would be helpful for stratification of high-risk patients and thus direct personalized treatment. SHMT2 may be a potential drug target of GC, and anti-SHMT2 drugs may be promising in therapy for GC treatment.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hamashima C, Shabana M, Okada K, et al. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015;106:1744–49. doi: 10.1111/cas.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dikken JL, van de Velde CJ, Coit DG, et al. Treatment of resectable gastric cancer. Therap Adv Gastroenterol. 2012;5:49–69. doi: 10.1177/1756283X11410771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locasale JW, Grassian AR, Melman T, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–74. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Possemato R, Marks KM, Shaul YD, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–50. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–44. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Z, Song J, Wang G, et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun. 2018;9:4468. doi: 10.1038/s41467-018-06812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebbring SJ, Chai Y, Ji Y, et al. Serine hydroxymethyltransferase 1 and 2: Gene sequence variation and functional genomic characterization. J Neurochem. 2012;120:881–90. doi: 10.1111/j.1471-4159.2012.07646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee GY, Haverty PM, Li L, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res. 2014;74:3114–26. doi: 10.1158/0008-5472.CAN-13-2683. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Sahra I, Hoxhaj G, Ricoult SJH, et al. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–33. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson R, Jain M, Madhusudhan N, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu YF, Liu HD, Liu ZL, et al. Sprouty2 suppresses progression and correlates to favourable prognosis of intrahepatic cholangiocarcinoma via antagonizing FGFR2 signalling. J Cell Mol Med. 2018;22:5596–606. doi: 10.1111/jcmm.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26:13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Xu YF, Liu ZL, Pan C, et al. HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene. 2019;38:868–80. doi: 10.1038/s41388-018-0485-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–73. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 17.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducker GS, Chen L, Morscher RJ, et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 2016;24:640–41. doi: 10.1016/j.cmet.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Lewis CA, Parker SJ, Fiske BP, et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell. 2014;55:253–63. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13:37–50. doi: 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
- 22.Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–57. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- 23.Ye J, Fan J, Venneti S, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4:1406–17. doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aberle MR, Burkhart RA, Tiriac H, et al. Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br J Surg. 2018;105:e48–60. doi: 10.1002/bjs.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–46. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 28.Gravalos C, Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–29. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8:4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ducker GS, Ghergurovich JM, Mainolfi N, et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2017;114:11404–9. doi: 10.1073/pnas.1706617114. [DOI] [PMC free article] [PubMed] [Google Scholar]