Abstract
Background
The initiation of atherosclerosis (AS) is attributed to the dysfunction of endothelial cells (ECs) via the inhibition of g protein-coupled estrogen receptor (GPER). In the current study, we assessed the potential of Ginsenoside Rb1 (Rb1) to attenuate the dysfunction of ECs via GPER-mediated PI3K/Akt pathway.
Material/Methods
AS was induced in rabbits and then the AS rabbits were treated with Rb1. Thereafter, the ECs were isolated from AS and healthy rabbits, and treated with Rb1. The effect of Rb1 on blood lipid levels in AS rabbits and on apoptosis, inflammatory response, and GPER/PI3K/Akt axis activity in ECs was detected. Furthermore, the activities of GPER and PI3K were modulated to verify the key role of the axis in the anti-AS effect of Rb1.
Results
The levels of total cholesterol, low-density lipoprotein (LDL), and triglyceride in AS rabbits were suppressed by Rb1 while the high-density lipoprotein (HDL) level was increased. In in vitro assays, Rb1 administration inhibited apoptosis process and the production of pro-inflammation cytokines in AS ECs. The expression levels of GPER, p-PI3K, and p-Akt were upregulated by Rb1, associated with the increased level of Bcl-2 and reduced level of Bax. When the activity of GPER was inhibited by GP-15 in AS ECs, the treatment effect of Rb1 was blocked. However, the activation of PI3K could restore the protective effect of Rb1 after the inhibition of GPER.
Conclusions
The anti-AS potential of Rb1 was exerted by restoring the regular function of ECs via the activation of GPER-mediated PI3K/Akt signaling.
MeSH Keywords: Endothelial Cells, Ginsenosides, Phosphatidylinositol 3-Kinases
Background
Endothelial cells (ECs) are both the source for the development of atherosclerosis (AS) and the target for the treatment of the disorder [1]. The cells are the major component forming the inner surface of arteries [2]. Under normal physiological conditions, ECs play important roles in regulating inflammation, vascular smooth muscle cell growth, vasomotion, platelet function, and other key biological processes by producing a number of vasoactive and trophic substances [3,4]. However, once the balance between vasodilating and vasoconstricting substances produced by ECs is impaired, atherosclerotic plaque will gradually aggregate and induce atherogenesis [5,6]. The process is also referred to as EC dysfunction, which has been identified as a central event in the early stage of AS development.
The EC dysfunction process can be induced by multiple factors, including suppressed nitric oxide generation, oxidative stress, and induced production of adhesion molecules [7,8]. In previous reports, it is found that the initiation of AS is associated with increased production of VCAM-1, ICAM-1, E-selectin, and reactive oxygen species (ROS), and decreased secretion of NO [9–11], which supports the theory that EC dysfunction is key to the progression of AS. Moreover, a study by Pircher et al. also indicates that the dysfunctional ECs can futher increase the production of ROS and promote the inflamamtory and thrombosis processes associated with AS [12]. Therefore, restoring the regular function of ECs has been proposed as a promsing treatment strategy for managing AS in recent years [13,14].
Multiple molecular alterations involved in the initiation of EC dysfunction are being identified, of which G protein-coupled estrogen receptor (GPER) is predominantly expressed in ECs and vascular smooth muscle cells [15] and has been proved to be a key factor related to the development of AS [14,16]. For example, Kong et al. showed that GPER is involved in the protective effect of protocatechuic aldehyde against EC dysfunction [14]. As a typical receptor of estrogen, the activation of GPER is conceived to be the central factor contributing the anti-atherogenic effect of estrogen [14,1, 17]. GPER is the upstream regulator of numerous pathways that are critically involved in EC dysfunction and AS progression, including PI3K/Akt and NF-κB [13,18,19]. Regarding the role of the PI3K/Akt pathway in the dysfunction of ECs, it is proved that the activation of the pathway can protect ECs from impairments associated with the dysfunction [20,21]. These findings show that the specific activation of GPER might be a potential strategy to restore EC function and to treat AS.
Ginseng is a traditional herbal medicine that has been widely used in Eastern Asia for its restorative properties [22]. Regarding its effect on AS, previous studies have already shown that the most abundant active component of ginseng, Ginsenoside Rb1 (Rb1), reduces lipid accumulation in macrophage foam cells and enhances atherosclerotic plaque stability [22]. In addition, the close interaction between Rb1 and the PI3K/Akt pathway also supports the anti-atherogenic function of the compound [23]. Therefore, it was reasonable to assess the effect of Rb1 on dysfunctional ECs as well as on the activity of GPER in those cells, which would not only explain the mechanism driving the anti-atherogenic function of Rb1, but also provide a novel treatment agent against EC dysfunction.
To fulfill the research purpose, in the current study, AS was induced in rabbits using high-fat diet plus balloon catheter method and treated with Rb1 at different doses. Then, the ECs were isolated from AS and healthy rabbits to detect the effect of Rb1 on the apoptotic and inflammatory processes in ECs as well as on GPER and PI3K/Akt pathways. Moreover, the effect of GPER modulation on the treatment effect of Rb1 was also validated in dysfunctional ECs. The current study demonstrated that Rb1 attenuated AS progression in rabbits and inhibited inflammation and apoptosis in dysfunctional ECs in a GPER activation-dependent manner.
Material and Methods
Chemical and agents
Rb1 (purity >98%) was purchased from ChromaDex Corp. (USA). Antibodies against GPER (ab188790) were purchased from Abcam (USA). Antibodies against PI3K (bs-2067R) and phosphorylated PI3K (p-PI3K) (bs-10657R) were purchased from Bioss (Beijing, China). Antibodies against Akt (sc-8312), p-Akt (sc-135651), and β-actin (sc-47778) were purchased from Santa Cruz Biotechnology, Inc. (CA, USA). Antibodies against Bax (BA0315-2) and Bcl-2 (BA0412) were purchased from Boster (Wuhan, China). Enzyme-linked immunosorbent assay (ELISA) kits for detection of IL-6 (H007), IL-1β (H002), and TNF-α (H052) were obtained from Nanjing Jiancheng Bioengineering Institute. Secondary goat anti-rabbit (A0208), goat anti-mouse IgG-HRP (A0216), and cy3-labled goat anti-mouse IgG-HRP (A0521) antibodies were purchased from Beyotime (Shanghai, China). Total Protein Extraction Kits (P0027) were purchased from Beyotime (Shanghai, China). PI/Annexin V-FITC Apoptosis Detection Kits were purchased from JingMei Biotech (Beijing, China). Antagonist (G-15) (3678) for GPER and agonist (740 Y-P) for PI3K (1983) were obtained from R&D system (USA).
Animals
Male New Zealand rabbits (weighing 2.0 to 2.5 kg) were obtained from Experimental Animal Center of Southern Medical University. The rabbits were housed individually in stainless steel cages at 20±3°C with a 12-h light/dark cycle and with free access to food and water. All the experiments with animals were performed following the Institutional Animal Ethics Committee and Animal Care Guidelines of the Affiliated Hospital of Jining Medical University.
High-cholesterol diet plus balloon catheter-injured rabbit model
To ensure the induction of EC dysfunction, we used the high-cholesterol diet (HCD) plus balloon catheter injury method to establish an AS model. All animals were fed standard chow diet for 2 weeks before balloon injury: briefly, a 3F Fogarty catheter was inserted into the right femoral artery and advanced to a position just below the diaphragm. The balloon was inflated and the catheter was pulled 3 times until the bifurcation of the iliac arteries was reached, then the balloon was deflated and the catheter withdrawn. During the next 8 weeks after the surgery, injured rabbits received a high-fat (HF) diet. Upon completion of the experiments, the histological changes in rabbit arteries were detected by HE staining and morphological observations (Supplementary Figure 1). The concentrations of blood lipids [including total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride] in model rabbits were measured [24]. Moreover, the induction of the AS model and the treatment effect of Rb1 against AS were also assessed by comparing the intima-media thickness of abdominal aorta and hemodynamics parameters of model rabbits with health control rabbits using the algorithms of the Philips iE33 ultrasound system (Philips Ultrasound, Bothell, WA).
For Rb1 administration, 25 rabbits were randomly divided into 5 groups (5 in each group): 1) Control group, health rabbits; 2) HCD group, rabbits underwent high-fat diet plus balloon catheter-injured rabbit model; 3) HCD+L group, rabbits underwent high-fat diet plus balloon catheter-injured rabbit model and the diet containing low dose of Rb1 (5 mg/kg body weight) [21]; 4) HCD+M group, rabbits underwent high-fat diet plus balloon catheter-injured rabbit model and the diet containing medium dose of Rb1 (10 mg/kg body weight) [21]; 5) HCD+H group, rabbits underwent high-fat diet plus balloon catheter-injured rabbit model and the diet containing high dose of Rb1 (20 mg/kg body weight) [21].
Isolation of rabbit abdominal aortic endothelial cells
Model rabbits were anesthetized with intraperitoneal injection of pentobarbital sodium (10 mg/mL), and then the midline of the abdomen was incised and the thorax was opened to exposure the heart and lungs. The aortic endothelial cells were isolated from the abdominal aorta following the standard procedures reported by Kobayashi et al. [25]: the abdominal aorta was cut, washed with PBS, ligated at proximal portion, and infused with 0.1% collagenase. After being incubated at 37°C for 15 min, ECs were collected from the aorta by flushing with DMEM containing 20% fetal bovine serum (FBS). ECs were identified by immunofluorescence detection of surface antigen CD31 (Supplementary Figure 2) and cultured in RPMI1640 medium. Cells from second to fifth passages were used to subsequent treatments.
For Rb1 administration, the cells were divided into 5 groups: 1) Control group, ECs isolated from health rabbits; 2) HCD group, ECs isolated from HCD rabbits; 3) HCD+L group, ECs isolated from HCD rabbits administrated with low concentration of Rb1 (20 μM) for 24 h; 4) HCD+M group, ECs isolated from HCD rabbits administrated with medium concentration of Rb1 (40 μM) for 24 h; 5) HCD+H group, ECs isolated from HCD rabbits administrated with high concentration of Rb1 (80 μM) for 24 h. The incubation time for Rb1 administration was selected by subjecting ECs to Rb1 (80 μM) for 6, 12, 24, and 48 h (Supplementary Figure 3).
Activities of the GPER and PI3K/Akt signaling in ECs were modulated to determine the role of GPER in the treatment of Rb1 on EC dysfunction. The cells were divided into 4 groups: 1) HCD group, ECs isolated from HCD rabbits; 2) HCD+H group, ECs isolated from HCD rabbits administrated with low concentration of Rb1 (80 μM) for 24 h; 3) HCD+H+G-15 group, ECs isolated from HCD rabbits were co-administrated with high concentration of Rb1 (80 μM) and G-15 (100 nM) for 24 h; 4) HCD+H+G-15+740 Y-P group, ECs isolated from HCD rabbits were co-administrated with high concentration of Rb1 (80 μM), G-15 (100 nM), and 740 Y-P (25 mg/mL) for 24 h.
Immunofluorescent detection
The expression and distribution of CD31 and GPER were detected by immunofluorescent staining. Cells were seeded in 14-well chambers. After the cells grew into a monolayer, they were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 30 min. Then, the cells were blocked with 10% goat serum for 15 min and incubated with primary rabbit polyclonal antibodies against CD31 (1: 100) and GPER (1: 400) overnight at 4ºC. The fluorescein isothiocyanate-labeled secondary antibody was then added to cells and incubated for 1 h. After being washed with PBS, the cells were stained with 4,6-diamino-2-phenyl indole (DAPI) for 5 min at room temperature. After another 3 cycles of 5-min washes with PBS, the cells were imaged with a fluorescent microscope at 400× magnification.
Flow cytometry
After completion of the treatments, 5 μl Annexin V was added to the ECs in different groups. After incubation with Annexin V for 10 min at room temperature, the cells were re-suspended with 1×binding buffer and incubated with 5 μl propidium iodide (PI). Then, the apoptosis rates of the cells under different treatments were detected with a flow cytometer (C6, BD, USA). The apoptosis cell rate (UR+LR-all apoptosis cell percentage) was equal to the sum of the late apoptosis rate (UR, upper right quadrant-advanced stage apoptosis cell percentage) and the early apoptosis rate (LR, lower right quadrant-prophase apoptosis cell percentage).
Enzyme-linked immunosorbent assay (ELISA)
Production of IL-6, IL-1β, and TNF-α (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu) in supernatant of EC cultures was measured using ELISA kits according to the manufacturers’ instructions.
Western blot analysis
Total protein in different samples was extracted using the Total Protein Extraction Kit according to the manufacturer’s instructions (WLA019, Wanleibio, China). β-actin was used as the internal reference protein. All the extracts were boiled in loading buffer for 5 min and then subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels. Then, targeted proteins were transferred onto polyvinylidene difluoride sheets. The membranes were washed with TBST 3 times for 20 min for each time. Then, primary antibodies against different proteins [GPER (1: 400), p-Akt (1: 200), Akt (1: 200), p-PI3K (1: 200), PI3K (1: 200), Bcl-2 (1: 400), Bax (1: 400), β-actin (1: 1000)] were incubated with the membranes overnight at room temperature. After another 3 washes, secondary antibodies (1: 5000) were added and incubated with the membranes for 5 h. After another 3 washes, the blots were developed using Beyo ECL Plus reagent and the results were detected in the gel imaging system. The relative expression levels of different proteins were calculated using Bio-Rad Quantity One software.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was conducted and post hoc multiple comparisons were performed with Duncan method. All statistical analyses were conducted using SPSS version 19.0, with a significance level of 0.05 (IBM, Armonk, NY, USA).
Results
Administration of Rb1 suppressed the levels of blood lipids in AS rabbits
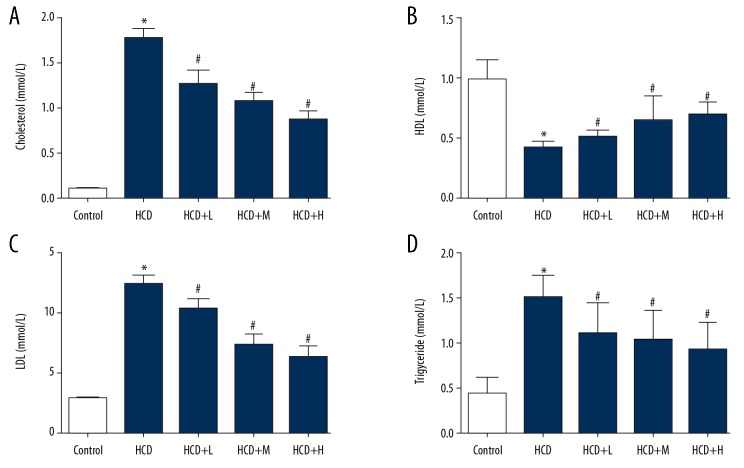
Successful induction of the AS model was assessed by detecting the pathological changes in arteries and the atherosclerotic plaques and thinned artery surface were detected (unpublished data). Then, the levels of blood lipids were measured. As shown in Figure 1, the levels of total cholesterol, LDL, and triglyceride were all increased in HCD rabbits, while the level of HDL was suppressed, and the differences compared with health rabbits were statistically significant (P<0.05). Additionally, the body weight, intima-media thickness of abdominal aorta (ITAA), interventricular septal thickness (IVS), and left ventricular posterior wall thickness (LVPW) were increased by the model induction, while left atrium diameter (LA), left ventricular internal diameter (LV), right atrium diameter (RV), and aortic diameter (AO) were decreased (Supplementary Table 1), which confirmed the successful establishment of the AS model. After the rabbits were administered Rb1, the levels of blood lipids were reversed and the effect of Rb1 on lipid production was exerted in a dose-dependent manner (Figure 1), confirming the anti-AS effect of Rb1.
Figure 1.
Administration of Rb1 attenuated lipid metabolism disorder induced by high-fat diet plus balloon catheter-injured model. Rabbits were subjected to high-fat diet plus balloon catheter-injured surgery and treated with Rb1 of 5, 10, and 20 mg/kg body weight. (A) Quantitative analysis results of ELISA detection of blood total cholesterol. (B) Quantitative analysis results of ELISA detection of blood HDL. (C) Quantitative analysis results of ELISA detection of blood LDL. (D) Quantitative analysis results of ELISA detection of blood triglyceride. * P<0.05 vs. Control group. # P<0.05 vs. HCD group. Each assay was performed 5 times.
Administration of Rb1 suppressed apoptosis and inflammatory response in ECs
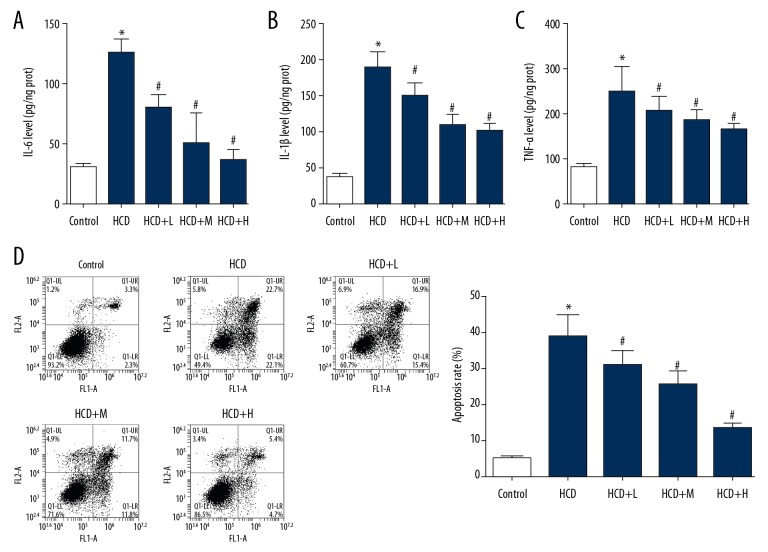
The major purpose of the current study was to assess the potential of Rb1 to restore the regular function of ECs. Thus, the cells were isolated from AS and health rabbits. Based on flow cytometry and ELISA assays, ECs isolated from AS rabbits showed higher levels of apoptosis (Figure 2A) and higher production level of pro-inflammation cytokines (Figure 2B). In cells treated with Rb1, the apoptosis was suppressed (Figure 2C) and the levels of IL-6, IL-1β, and TNF-α were all reduced (Figure 2D). The effect of Rb1 on EC cells was also exerted in a dose-dependent manner, which was similar to its effect on lipid production in AS rabbits.
Figure 2.
Administration of Rb1 inhibited inflammatory response and apoptosis in dysfunctional ECs. ECs were isolated from AS and health rabbits and administrated with Rb1 of 20, 40, and 80 μM. (A) quantitative analysis results of ELISA detection of blood IL-6. (B) Quantitative analysis results of ELISA detection of blood IL-1β. (C) Quantitative analysis results of ELISA detection of blood TNF-α. (D) Representative images and quantitative analysis of flow cytometry detection of apoptosis. * P<0.05 vs. Control group. # P<0.05 vs. HCD group. Each assay was performed 5 times.
Administration of Rb1 activated GPER-mediated PI3K/Akt pathway in ECs
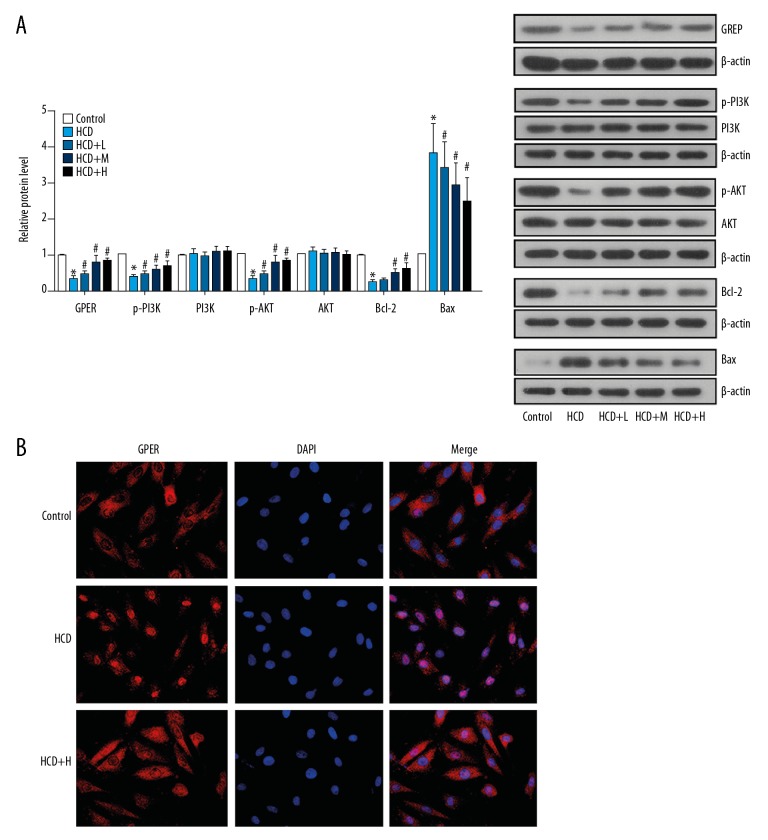
In the current study, we also assessed the effect of Rb1 on GPER and its downstream PI3K/Akt pathway to elucidate the anti-AS mechanism of the agent. As shown Figure 3A, the expression levels of GPER, p-PI3K, p-Akt, and Bcl-2 were all reduced in AS-derived ECs, while the level of Bax was increased. However, the administration of Rb1 at all 3 concentrations significantly reversed the activity of the GPER-mediated PI3K/Akt pathway; the expression level of GPER and the phosphorylation levels of PI3K and Akt were all upregulated by Rb1 in dysfunctional ECs (Figure 3A). At the same time, the level of anti-apoptosis factor Bcl-2 was increased, while the level of pro-apoptosis factor Bax was inhibited by Rb1. The effect of Rb1 on the expressions of the above indicators was dose-dependent. Moreover, the expression and distribution of GPER in dysfunctional ECs under the administration of high concentration of Rb1 was also determined by immunofluorescence detection, and the results were similar to that of the Western blot analysis (Figure 3B).
Figure 3.
Administration of Rb1 activated GPER/PI3K/Akt axis dysfunctioned in ECs. ECs were isolated from AS and health rabbits and administrated with Rb1 of 20, 40, and 80 μM for 24 h. (A) Representative images and quantitative analysis of western blotting detection of molecule expressions in GPER/PI3K/Akt axis. (B) Representative images of immunofluorescence detection of GPER expression and distribution. Magnification, 400×. * P<0.05 vs. Control group. # P<0.05 vs. HCD group. Each assay was performed 5 times.
The anti-AS function of Rb1 depended on activation of the GPER-mediated PI3K/Akt pathway
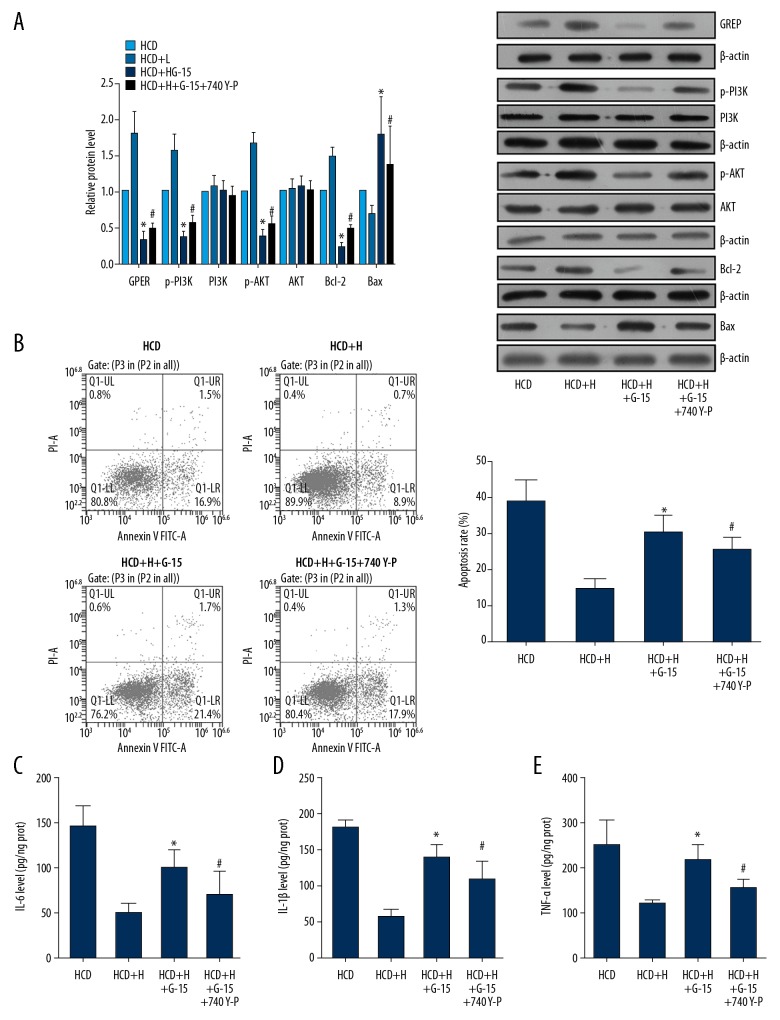
The above results suggested that Rb1 administration restores the function of ECs, which was associated with activation of the GPER-mediated PI3K/Akt pathway. However, whether the pathway is the central signaling transduction mediating the effect of Rb1 needed further exploration. Thus, dysfunctional ECs were further subjected to the co-administration of Rb1 of high concentration and GPER antagonist G-15 to determine the role of GPER in the anti-AS effect of Rb1. As shown in Figure 4A, when treated with G-15, ability of Rb1 to induce activation of the GPER-mediated PI3K/Akt pathway was blocked; the expressions of GPER, p-PI3K, and p-Akt were all inhibited by G-15, even when treated with Rb1. In addition, the apoptosis and production of pro-inflammation cytokines in dysfunctional ECs were all increased (Figure 4B–4E). ECs with GPER suppression were further subjected to administration of the PI3K agonist 740 Y-P, and the results showed that activation of the PI3K/Akt pathway (Figure 4A) maintained the protective effect of Rb1 on ECs by inhibiting apoptosis (Figure 4B) and inflammatory response (Figure 4C–4E). Thus, it was concluded that the effect of Rb1 in restoring the regular function of ECs depended on activation of the GPER-mediated PI3K/Akt pathway; during the treatment, the Rb1 induced the function of GPER, which exerted its anti-apoptosis and anti-inflammation functions by activating the downstream PI3K/Akt signaling.
Figure 4.
Anti-AS function of Rb1 depended on the activation GPER- mediated PI3K/Akt pathway. ECs were isolated from AS and health rabbits and administrated with 80 μM Rb1, 100 nM G-15, and 25 mg/mL 740 Y-P for 24 h. (A) Representative images and quantitative analysis of western blotting detection of molecule expressions in GPER/PI3K/Akt axis. (B) Representative images and quantitative analysis of flow cytometry detection of apoptosis. (C) Quantitative analysis results of ELISA detection of blood IL-6. (D) Quantitative analysis results of ELISA detection of blood IL-1β. (E) Quantitative analysis results of ELISA detection of blood TNF-α. * P<0.05 vs. HCD+H group. # P<0.05 vs. HCD+H+G-15 group. Each assay was performed 5 times.
Discussion
Atherosclerosis (AS) is a chronic inflammatory disease of the injured arterial wall, which forms the pathological basis of cardiovascular diseases (CVDs) [26,27]. It is well-recognized that during the initiation of AS, the deposition of atherogenic lipoproteins, especially LDL, in the large arteries gradually induces an imbalance between vasodilating and vasoconstricting substance and causes the dysfunction of ECs, which promotes progression of the disease [5,6]. Therefore, restoring the regular function of ECs has been proposed as a promising treatment strategy for AS. In the current study, we employed a therapeutically relevant rabbit model that resembles incipient atherogenesis, which was characterized by fatty streaks and early atheroma formation. Then, we assessed the potential of Rb1 to attenuate AS progression and EC dysfunction by focusing on the activity of the GPER/PI3K/Akt pathway. We demonstrated that Rb1 administration reversed the production of blood lipids in AS rabbits and inhibited apoptosis and inflammatory response in ECs in a GPER/PI3K/Akt-activation-dependent manner.
Ginseng has been widely used as a general tonic medicine in Asian countries for centuries [28]. According to a previous study, the majority of pharmacological properties of ginseng are attributed to ginsenosides [29]. Currently, more than 20 ginsenosides has been identified, and based on the chemical characteristics, most ginsenosides can be classified into 2 major group: panaxadiols, including Rb1, Rb2, Rg3, Rh2 and Rh3; and panaxatriols, including Re, Rg1, Rg2, and Rh1 [29]. The different ginsenosides exhibit distinct pharmacological functions. For example, Rh2 and Rg3 have antineoplastic and immunomodulatory effects [30, 31], and Rg1 and Re benefit learning and memory [32,33]. Of the various ginsenosides, Rb1 is the most abundant and has the anti-obesity potential and can increase insulin sensitivity [34, 35]. A recent study by Qiao et al. the demonstrate that Rb1 can enhance atherosclerotic plaque stability by improving autophagy and lipid metabolism, which is indicative of the anti-AS potential of Rb1 [22]. Consistent with that study, we also proved the anti-AS effect of Rb1 in an AS rabbit model, showing that the administration of Rb1 suppressed the production of total cholesterol, LDL, and triglyceride, and increased the level of HDL, all in a dose-dependent manner, representing the attenuation of AS in model animals. To further support the protective effect of Rb1 against AS, the ability of Rb1 to restore the regular function of ECs was also validated by our data: the apoptosis and inflammatory response in ECs derived from AS rabbits were inhibited by Rb1. Moreover, the treatment effect of Rb1 on AS ECs was associated with activation of the GPER/PI3K/Akt pathway, which might be the central signaling transduction mediating the effect of Rb1.
GPER is a typical receptor of estrogen, and the previous studies demonstrated that the anti-AS effect of estrogen depends on the activation of GPER [16]. Kong et al. reported that GPER plays a central role in the protective effect of protocatechuic aldehyde against EC dysfunction [14]. GPER also exhibits an anti-inflammation function by regulating the activation of eNOS via PI3K/Akt and NF-κB signaling [19,36]. Consistent with these previous studies, we found that the activation of GPER/PI3K/Akt signaling by Rb1 led to decreased levels of pro-inflammatory cytokines. Activation of GPER/PI3K/Akt signaling also inhibited apoptosis in AS ECs. The inducing effect of Rb1 on the activation of GPER/PI3K/Akt probably resulted in an increased expression of Bcl-2 and suppressed expression of Bax, representing the inhibition of apoptosis in ECs as well. The central function of GPER/PI3K/Akt in the protective effect of Rb1 on EC function was further validated by subjecting AS ECs to GPER antagonist, which inhibited activity of the PI3K/Akt pathway and led to enhancement of apoptosis and inflammatory response. Generally, signaling associated with GPER is transduced via transactivation of epidermal growth factor receptor (EGFR) and involvement of the Src family [37]. Through this mechanism, the stimulation of GPER activates metalloproteinases and induces the release of EGF, which leads to activation of the downstream PI3K/Akt pathway [38,39]. The potentially central role of PI3K/Akt signaling in the EC-protection effect of GPER activation was also validated in the current study. When the GPER-inhibited ECs were treated with PI3K agonist, the apoptotic and inflammatory processes were inhibited again, the same as those in ECs solely treated with Rb1. Our results demonstrate that the ability of Rb1 to restore the regular function of ECs depends on activation of GPER-mediated PI3K/Akt.
Conclusions
Collectively, the current study confirmed the anti-AS potential of Rb1, which was exerted by restoring the regular function of ECs via the activation of GPER-mediated PI3K/Akt signaling. Moreover, the effect of Rb1 on the phenotype and molecular alterations in ECs was shown in a dose-dependent manner; thus, the application of the component in clinical practice should be carefully handled based on the individual patient’s conditions. However, the current study failed to eliminate the possible toxic dose on ECs, and more comprehensive work needs to be performed with model animals and cells to facilitate the development of Rb1-dependent therapies.
Supplementary Data
Supplementary Table 1.
Raw data of body weight, intima-media thickness of abdominal aorta, and hemodynamics parameters.
| No. | Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight | ITAA | LA | AO | IVS | LV | LVPW | RA | RV | ||
| Control | 1 | 2 | 0.27 | 7*9*8 | 5*5.6 | 2 | 12 | 2 | 68 | 8*7 |
| 2 | 2 | 0.28 | 8*10*9 | 5*6 | 2 | 13 | 1.8 | 69 | 9*7 | |
| 3 | 2 | 0.31 | 7*10*9 | 4*7 | 2 | 15 | 2 | 63 | 10*8 | |
| 4 | 1.9 | 0.3 | 7*9*8 | 5*6 | 2 | 14 | 2 | 68 | 10*8 | |
| 5 | 1.8 | 0.3 | 9*10*9 | 5.5*6 | 1.8 | 13 | 1.8 | 70 | 9*7 | |
| HF | 1 | 2.6 | 0.72 | 8.8*9.8*7.9 | 8.9 | 2.5 | 13 | 2 | 10.8*7.6 | 5.4 |
| 2 | 2.2 | 0.7 | 7.3*9.9*8.1 | 8.2 | 2.6 | 13.6 | 2.4 | 9.3*7.9 | 7.1 | |
| 3 | 2.6 | 0.61 | 9.5*9.9*8.4 | 9.5 | 2.5 | 17 | 2 | 9.9*7.6 | 7 | |
| 4 | 2.6 | 0.62 | 9.4*9.6*8.3 | 8.9 | 2.6 | 15 | 2.2 | 9.7*8.3 | 5.4 | |
| 5 | 2.1 | 0.85 | 8.5*10*8.5 | 8.5 | 2.5 | 15 | 2.3 | 10*8 | 7.5 | |
ITAA – intima-media thickness of abdominal aorta; LA – left atrium diameter; AO – aortic diameter; IVS – interventricular septal thickness; LV – left ventricular internal diameter; LVPW – left ventricular posterior wall thickness; RA – right atrium diameter; RV – right ventricular internal diameter.
Illustration of morphological changes in arteries after HCD Plus Balloon Catheter Injury model induction. (A) Morphological changes in artery wall. (B) H&E detection of artery wall. Magnification, 200×.
Immunofluorescence detection of CD31 in ECs isolated from abdominal aortas. Magnification, 400×.
Determination of the incubation time of Rb1 on ECs isolated from abdominal aortas. ECs were incubated with 80 μM Rb1 for 6, 12, 24, and 48 h to determine the best incubation time. * P<0.05 vs. 12 h
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Furchgott RF. Introduction to EDRF research. J Cardiovasc Pharma. 1993;22(Suppl 7):S1–2. [PubMed] [Google Scholar]
- 2.Galili O, Versari D, Sattler KJ, et al. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol-Heart C. 2007;292:H904. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 3.Barton M, Haudenschild CC. Endothelium and atherogenesis: Endothelial therapy revisited. J Cardiovasc Pharma. 2001;38(Suppl 2):S23. doi: 10.1097/00005344-200111002-00007. [DOI] [PubMed] [Google Scholar]
- 4.Traupe T, Ortmann J, Münter K, Barton M. Endothelial therapy of atherosclerosis and its risk factors. Curr Vasc Pharmacol. 2003;1:111–21. doi: 10.2174/1570161033476763. [DOI] [PubMed] [Google Scholar]
- 5.Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015;25:44–50. doi: 10.1111/bpa.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med. 2014;6:1105–20. doi: 10.15252/emmm.201404156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endemann DH, Schiffrin EL. Endothelial dysfunction. Hosp Med. 1998;59:1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 8.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Szmitko PE, Wang CH, Weisel RD, et al. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108:1917–23. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 10.Uemura S, Matsushita H, Li W, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001;88:1291–98. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 11.Khan BV, Harrison DG, Olbrych MT, et al. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA. 1996;93:9114–19. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pircher A, Treps L, Bodrug N, Carmeliet P. Endothelial cell metabolism: A novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis. 2016;253:247–57. doi: 10.1016/j.atherosclerosis.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Yang K, Zhang H, Luo Y, et al. Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: Insight into the ERα-mediated PI3K/Akt pathway. Int J Mol Sci. 2017;18:77. doi: 10.3390/ijms18020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong BS, Cho YH, Lee EJ. G protein-coupled estrogen receptor-1 is involved in the protective effect of protocatechuic aldehyde against endothelial dysfunction. PLoS One. 2014;9:e113242. doi: 10.1371/journal.pone.0113242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isensee J, Meoli L, Zazzu V, et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–30. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 16.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–26. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: Clinical application of the timing hypothesis. J Steroid Biochem. 2014;142:68–75. doi: 10.1016/j.jsbmb.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang ZF, Pan ZY, Xu CS, Li ZQ. Activation of G-protein coupled estrogen receptor 1 improves early-onset cognitive impairment via PI3K/Akt pathway in rats with traumatic brain injury. Biochem Bioph Res Commun. 2016;482:948–53. doi: 10.1016/j.bbrc.2016.11.138. [DOI] [PubMed] [Google Scholar]
- 19.Xing D, Nozell S, Chen YF, et al. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–95. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan X, Li W, Yang L, et al. MiR-135a protects vascular endothelial cells against ventilator-induced lung injury by inhibiting PHLPP2 to activate PI3K/Akt pathway. Cell Physiol Biochem. 2018;48:1245–58. doi: 10.1159/000492010. [DOI] [PubMed] [Google Scholar]
- 21.Lin XP, Cui HJ, Yang AL, et al. Astragaloside IV improves vasodilatation function by regulating the PI3K/Akt/eNOS signaling pathway in rat aorta endothelial cells. J Vasc Res. 2018;55:169–76. doi: 10.1159/000489958. [DOI] [PubMed] [Google Scholar]
- 22.Qiao L, Zhang X, Liu M, et al. Ginsenoside Rb1 enhances atherosclerotic plaque stability by improving autophagy and lipid metabolism in macrophage foam cells. Front Pharmacol. 2017;8:727. doi: 10.3389/fphar.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang T, Miao Y, Zhang T, et al. Ginsenoside Rb1 inhibits autophagy through regulation of Rho/ROCK and PI3K/mTOR pathways in a pressure-overload heart failure rat model. J Pharm Pharmacol. 2018;70(6):830–38. doi: 10.1111/jphp.12900. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Jayachandran M, Sumi D, et al. Physiological concentration of 17β-estradiol retards the progression of severe atherosclerosis induced by a high-cholesterol diet plus balloon catheter injury. Arterioscler Thromb Vasc Biol. 2000;20:1613–21. doi: 10.1161/01.atv.20.6.1613. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Inoue K, Warabi E, et al. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–42. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 26.Barrettconnor E. Menopause, atherosclerosis, and coronary artery disease. Curr Opin Pharmacol. 2013;13:186–91. doi: 10.1016/j.coph.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: Physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–73. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 28.Dong M, Yang X, Lim S, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013;18:118. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 30.Tode T, Kikuchi Y, Kita T, et al. Inhibitory effects by oral administration of ginsenoside Rh2 on the growth of human ovarian cancer cells in nude mice. J Cancer Res Clin Oncol. 1993;120:24–26. doi: 10.1007/BF01200720. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki M, Yoo YC, Matsuzawa K, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 32.Takemoto Y, Ueyama T, Saito H, et al. Potentiation of nerve growth factor-mediated nerve fiber production in organ cultures of chicken embryonic ganglia by ginseng saponins: Structure – activity relationship. Chem Pharm Bull. 1984;32:3128–33. doi: 10.1248/cpb.32.3128. [DOI] [PubMed] [Google Scholar]
- 33.Salim KN, McEwen BS, Chao HM. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Brain Res Mol Brain Res. 1997;47:177–82. doi: 10.1016/s0169-328x(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y, Shen L, Liu KJ, et al. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010;59:2505–12. doi: 10.2337/db10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen L, Xiong Y, Wang DQ, et al. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–38. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revankar CM, Cimino DF, Sklar LA, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 37.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 38.Prenzel N, Zwick E, Daub H. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 2009;402:884–88. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 39.Edwin F, Wiepz GJ, Singh R, et al. A historical perspective of the EGF receptor and related systems. Methods Mol Biol. 2006;327:1–24. doi: 10.1385/1-59745-012-x:1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Raw data of body weight, intima-media thickness of abdominal aorta, and hemodynamics parameters.
| No. | Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight | ITAA | LA | AO | IVS | LV | LVPW | RA | RV | ||
| Control | 1 | 2 | 0.27 | 7*9*8 | 5*5.6 | 2 | 12 | 2 | 68 | 8*7 |
| 2 | 2 | 0.28 | 8*10*9 | 5*6 | 2 | 13 | 1.8 | 69 | 9*7 | |
| 3 | 2 | 0.31 | 7*10*9 | 4*7 | 2 | 15 | 2 | 63 | 10*8 | |
| 4 | 1.9 | 0.3 | 7*9*8 | 5*6 | 2 | 14 | 2 | 68 | 10*8 | |
| 5 | 1.8 | 0.3 | 9*10*9 | 5.5*6 | 1.8 | 13 | 1.8 | 70 | 9*7 | |
| HF | 1 | 2.6 | 0.72 | 8.8*9.8*7.9 | 8.9 | 2.5 | 13 | 2 | 10.8*7.6 | 5.4 |
| 2 | 2.2 | 0.7 | 7.3*9.9*8.1 | 8.2 | 2.6 | 13.6 | 2.4 | 9.3*7.9 | 7.1 | |
| 3 | 2.6 | 0.61 | 9.5*9.9*8.4 | 9.5 | 2.5 | 17 | 2 | 9.9*7.6 | 7 | |
| 4 | 2.6 | 0.62 | 9.4*9.6*8.3 | 8.9 | 2.6 | 15 | 2.2 | 9.7*8.3 | 5.4 | |
| 5 | 2.1 | 0.85 | 8.5*10*8.5 | 8.5 | 2.5 | 15 | 2.3 | 10*8 | 7.5 | |
ITAA – intima-media thickness of abdominal aorta; LA – left atrium diameter; AO – aortic diameter; IVS – interventricular septal thickness; LV – left ventricular internal diameter; LVPW – left ventricular posterior wall thickness; RA – right atrium diameter; RV – right ventricular internal diameter.
Illustration of morphological changes in arteries after HCD Plus Balloon Catheter Injury model induction. (A) Morphological changes in artery wall. (B) H&E detection of artery wall. Magnification, 200×.
Immunofluorescence detection of CD31 in ECs isolated from abdominal aortas. Magnification, 400×.
Determination of the incubation time of Rb1 on ECs isolated from abdominal aortas. ECs were incubated with 80 μM Rb1 for 6, 12, 24, and 48 h to determine the best incubation time. * P<0.05 vs. 12 h