Abstract
B-cell lymphoma 2 (Bcl-2) is a regulator protein involved in apoptosis. In the past few decades, this protein has been demonstrated to have high efficacy in cancer therapy, and several approaches targeting Bcl-2 have been tested clinically (e.g., oblimersen, ABT-737, ABT-263, obatoclax mesylate, and AT-101). This review reports potential Bcl-2 inhibitors according to current information on their underlying mechanism and the results of clinical trials. In addition, the function and mechanisms of other potentially valuable Bcl-2 inhibitors that did not show efficacy in clinical studies are also discussed. This summary of the development of Bcl-2 inhibitors provides worthwhile viewpoints on the use of biomedical approaches in future cancer therapy.
1. Introduction
In current gene and cell immunotherapy techniques, cellular pathways are popular and effective targets for curing cancer. It is important to discover a switch or inhibitor of a cellular pathway that is expressed not only in leukemia but also in solid tumors because solid tumors remain a critical barrier to cell therapy due to their size and the protective effects of the microenvironment [1]. Therefore, the identification of cellular pathway inhibitors is a valuable research topic for destroying the tumor microenvironment from the inside.
In the last few decades, researchers have tried to induce tumor regression by blocking cellular pathways to enhance the efficiency of therapeutic treatments. One such candidate is B-cell lymphoma 2 (Bcl-2) protein, a potential inhibitor of apoptosis that belongs to the Bcl-2 family, members of which are involved in several cellular pathways such as DNA damage/p53 pathway, survival/NF-κB pathway, estrogen pathway, STAT pathway, and PI-3 kinase/AKT pathway [2]. Bcl-2 was the first identified mammalian regulator of apoptosis [3] and consists of four conserved domains (BH4, BH3, BH1, and BH2), which differentiate it from other Bcl-2 family members, (e.g., Bim, Bid, Puma, Noxa, Bad, Hrk, Bmf, and Bik) [4]. Among these homology motifs, BH3, BH1, and BH2 are the most commonly targeted in clinical approaches.
2. Clinical Approaches
In the past 30 years, efforts have been made to identify methods to cure Bcl-2 protein-related diseases, including molecular antibodies, small molecule drugs, and antisense oligonucleotides. In currently reported cases, the efficiency and safety of some clinical drugs targeting Bcl-2 protein have been demonstrated in hematologic carcinoma.
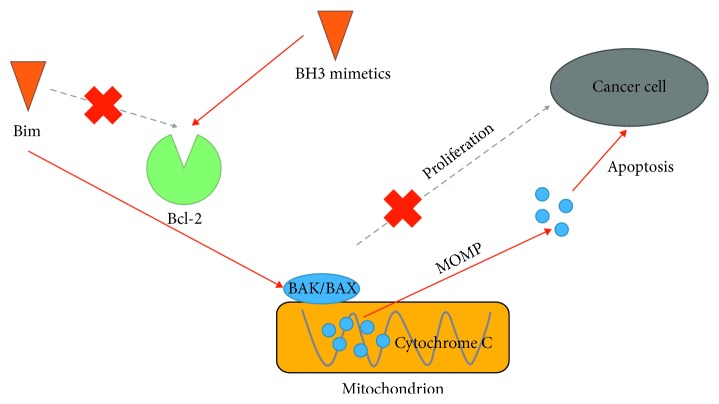
The mechanism of action of Bcl-2 inhibitors has been widely studied. Most Bcl-2 inhibitors block the binding of BH3-only proteins (mostly Bim) to Bcl-2 via a small molecule that mimics the BH3 domain. This process allows free Bim to activate Bak/Bax on the surface of mitochondria and induces mitochondrial outer membrane permeabilization (MOMP) to release cytochrome C, which leads to the death of cancer cells. The proliferation of specific cancer cells is also inhibited in the presence of cytochrome C [5] (Figure 1).
Figure 1.
Bcl-2 inhibitors inducing apoptosis of cancer cells.
Oblimersen (G3139, Genasense) was the first developed drug that utilized Bcl-2 inhibitors and was produced by Genta Inc. It utilizes an 18-mer short sequence RNA to inactivate mRNA via hybridization to inhibit the production of Bcl-2 protein and the proliferation of lymphoma cells. Unfortunately, no difference was found in the 5-year survival rate of patients in phase III clinical trials of oblimersen between the test and reference groups, so oblimersen has been rejected twice for approval by the US Food and Drug Administration (FDA) [6]. Most clinical studies using only oblimersen ended before 2010, and it is unknown whether it decreased cancer cells by an antisense drug mechanism or the induction of interferon release via its own CpG motif. After the failure of single-use oblimersen, the antitumor efficacy of oblimersen combined with other drugs has been examined. Clinical trials of Bcl-2 inhibitors conducted from 2010 to 2019 are shown in Table 1. In 2010, Raab et al. reported a phase I trial of oblimersen combined with cisplatin and 5-fluorouracil in 15 patients with advanced esophageal, gastroesophageal junction, or gastric carcinoma, which resulted in 1 case with complete remission and 2 with a partial response [7]. In 2011, Galatin et al. indicated that 5 of 16 patients with refractory and advanced malignancies had stable disease after combined treatment with oblimersen and gemcitabine [8]. In 2013, Ott et al. reported that treatment of patients with advanced melanoma with oblimersen combined with temozolomide and albumin-bound paclitaxel resulted in 2 cases with complete remission, 11 with a partial response, and 11 with stable disease [9].
Table 1.
2010–2019 Clinical trials of Bcl-2 inhibitors.
| Bcl-2 inhibitor | Combined therapy with other drugs | Targeted cancer type | No. of patients | Complete remission | Partial response | Stable disease | Phase | Researchers |
|---|---|---|---|---|---|---|---|---|
| Oblimersen | Cisplatin and 5-fluorouracil | Advanced esophageal, gastroesophageal junction, and gastric carcinoma | 15 | 1 (metastatic gastric carcinoma with pulmonary metastases) | 2 (1 gastric carcinoma, 1 squamous cell carcinoma of the esophagus) | I | Raab et al. [7] | |
| Gemcitabine | Refractory and advanced malignancies | 16 | 0 | 0 | 5 | I | Galatin et al. [8] | |
| Temozolomide and albumin-bound paclitaxel | Advanced melanoma | 32 | 2 | 11 | 11 | I | Ott et al. [9] | |
|
| ||||||||
| Navitoclax (ABT-263) | None | SCLC or pulmonary carcinoid | 47 | 0 | 1 (SCLC) | 8 (5 SCLC, 3 atypical pulmonary carcinoid) | I | Gandhi et al. [10] |
| None | Relapsed SCLC | 39 | 0 | 1 | 9 | II | Rudin et al. [11] | |
| None | Relapsed or refractory CLL | 29 | 0 | 9 | 7 | I | Roberts et al. [12] | |
| Irinotecan | Advanced solid tumors | 31 | 0 | 2 (1 Merkel cell carcinoma, 1 colon carcinoma) | 6 | I | Tolcher et al. [13] | |
| Erlotinib | Advanced solid tumors | 11 | 0 | 0 | 3 | I | Tolcher et al. [14] | |
| Rituximab | Relapsed or refractory CD20+ lymphoid malignancies | 29 | 6 (1 diffuse large B-cell lymphoma, 5 follicular lymphoma) | 10 (5 CLL/SLL, 4 follicular lymphoma, 1 lymphoma/Waldenström's macroglobulinemia) | I | Roberts et al. [15] | ||
| Rituximab | Previously untreated B-cell CLL | 78 | 2 | 47 | 25 | II | Kipps et al. [16] | |
|
| ||||||||
| Venetoclax (ABT-199) | Obinutuzumab | CLL and coexisting conditions | 216 | 107 | 76 | III | Fischer et al. [17] | |
| Ibrutinib | CLL | 80 | 25 | 1 | II | Jain et al. [18] | ||
| Ibrutinib | CLL | 91 | 8 | 48 | 22 | II | Jones et al. [19] | |
| None | Relapsed or refractory non-Hodgkin lymphoma | 106 | 14 | 33 | 32 | I | Davids et al. [20] | |
| None | Acute myelogenous leukemia | 32 | 6 (2 CR, 4 CRi) | 0 | 6 | II | Konopleva et al. [21] | |
| Decitabine or azacitidine | Acute myeloid leukemia | 57 | 35 | 1 | 0 | I | DiNardo et al. [22] | |
| Bendamustine and obinutuzumab | CLL | 66 | 5 | 55 | II | Cramer et al. [23] | ||
|
| ||||||||
| Obatoclax mesylate (GX15-070) | Bortezomib | Relapsed or refractory mantle cell lymphoma | 13 | 3 | 1 | 6 | I/II | Goy et al. [24] |
| None | Myelodysplastic syndromes with anemia or thrombocytopenia | 24 | 0 | 0 | 17 | II | Arellano et al. [25] | |
|
| ||||||||
| AT-101 | Paclitaxel and carboplatin | Several types of solid tumors (most common tumor type was prostate, N = 11) | 24 | 1 (esophageal cancer) | 4 (1 NSCLC, 3 prostate) | I | Stein et al. [26] | |
| Docetaxel | Recurrent, locally advanced, or metastatic head and neck cancer | 22 | 0 | 3 | 12 | II | Swiecicki et al. [27] | |
| Cisplatin and etoposide | Extensive-stage SCLC | 27 | 0 | 10 | 11 | I | Schelman et al. [28] | |
CLL, chronic lymphocytic leukemia; CRi, complete response with incomplete blood count recovery; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; and SLL, small lymphocytic leukemia.
After oblimersen, Petros et al., comprising the Abbott team (Abbott Laboratories, USA), devised a new method called fragment-based drug discovery that could be used in the development of Bcl-2 inhibitors [29]. Initially, Abbott focused on Bcl-xL protein, but the high structural homology between Bcl-xL and Bcl-2 proteins (with a difference of only four amino acids in the active site) meant that the new drug was suitable for Bcl-2 and Bcl-xL [30]. Abbott divided the Bcl-xL binding site into two fragments, based on the previous discovery of two small chemical compounds (I and II) from a library of molecules specifically binding to the Bcl-xL BH3 domain. The study data indicated that modified compound I was able to enter the P2 binding pocket, while compound II could readily enter the P4 pocket [31, 32]. Combination of compounds I and II together with modified compound II resulted in an inhibitor with potent affinity [33]. Further developing the findings of Petros et al., an antibody targeting Bcl-2 protein appeared in 2005, when Oltersdorf et al. reported their candidate antibody ABT-737 and provided preclinical evidence for targeting Bcl-2 in treating solid tumors [34]. Even though this discovery was established using a mouse model, it led to the development of the orally administered drug navitoclax (ABT-263) by Tse et al. 3 years after the first report of ABT-737. Navitoclax showed effective tumor regression in patients with small-cell lung cancer and acute lymphocytic leukemia, but there was a large decrease in the number of platelets that encouraged the development of a new application with higher safety [35]. However, Zhang et al. suggested that Bcl-xL is crucial for the survival of mature platelets in vivo, so the previous two applications are not suitable drugs for patients [36]. Reports from 2012 and 2015 also confirmed the low efficacy of navitoclax [10–16]. AbbVie pharmaceuticals then introduced venetoclax (ABT-199) targeting BH3-binding sites only on Bcl-2 that maintains efficiency while protecting the survival of platelets, and improved treatment of chronic lymphocytic leukemia was reported [37]. In 2016, the FDA approved the use of venetoclax. In recent years, venetoclax has been tried in different combinations with other antitumor monoclonal antibodies or small molecule drugs; however, these attempts have mostly focused on hematologic carcinoma [17–23].
Obatoclax mesylate (GX15-070), another drug targeting the Bcl-2 family, has also shown efficacy in phase III clinical trials. This drug is a BH3 mimetic, so it can potentially bind to most Bcl-2 family members (including Bcl-2 protein). However, the main mechanism of this inhibitor is binding to Mcl-1 as a supplemental therapy in Bcl-xLlow or Bcl-xL− cancer cells or cells with resistance to Bcl-2 inhibitors [38]. Bcl-2 protein is reportedly crucial for sustaining hematopoietic stem cells, but this antiapoptotic factor is also expressed at a high level in acute myeloid leukemia and acute lymphocytic leukemia [39]. Therefore, obatoclax mesylate provides an additional option for patients who need a Bcl-2 inhibitor. Unfortunately, the developers of obatoclax mesylate suddenly terminated their clinical trials in 2012, and no further details have been released since 2014 [24, 25].
AT-101 is an orally active pan-Bcl-2 inhibitor that consists of gossypol, a natural compound derived from the cotton plant [40]. In preclinical trials, it induced a strong apoptotic response in leukemic cells [41]. However, phase II trial data of AT-101 posted in 2010 indicated that it did not generate the expected response (NCT00286780), so the company stopped the clinical development of this drug until the founders decided to continue research in China. Recently, clinical trials using AT-101 in combination with other drugs for the treatment of solid tumors have been reported, such as its combination with docetaxel to treat head and neck cancer [27], in combination with docetaxel and prednisone to treat castration-resistant prostate cancer [42], in combination with topotecan to treat small-cell lung cancer [26], in combination with paclitaxel and carboplatin for different carcinoma types [43], and in combination with cisplatin and etoposide for extensive-stage small-cell lung cancer [28]. Given the reported findings, it is reasonable to believe that AT-101 could be a potent inhibitor of Bcl-2.
3. Other Valuable Candidates
In 2007, Mohammad et al. introduced another Bcl-2 inhibitor named TW-37, which is a small molecule inhibitor of Bcl-2/Bcl-XL/MUC-1 that could prevent Bcl-2 overexpression [44]. TW-37 has a higher affinity and selectivity for Bcl-2 compared with Bcl-xL protein. According to in vitro tests, TW-37 acts in lymphoma cells from patients, but not in normal peripheral blood cells [44]. In a mouse model, the combination of TW-37 with an MEK inhibitor was found to prevent the growth of melanoma cells [45]. Considering the features of TW-37, it should be a potent drug for inhibiting Bcl-2; however, due to unknown reasons, this inhibitor was not examined in clinical trials. Besides TW-37, several Bcl-2 inhibitors are in the preclinical development stage, such as HA14-1, sabutoclax, S55746 (S055746, BCL201), and gambogic acid. Among these compounds, S55746 shows similar high affinity to Bcl-2 as ABT-737 and ABT-199 [46].
4. Conclusion
With regard to Bcl-2 protein inhibitors, two main streams have been utilized in clinical approaches, namely, antibodies and small molecule drugs. Techniques have been developed to inhibit the expression and function of Bcl-2 and its family proteins at the gene and protein levels. According to current data, approximately 19 different Bcl-2 inhibitors are the subjects of preclinical or clinical studies and various combinations of therapeutic methods are being assessed for Bcl-2-positive cancer. Results from the clinical trials conducted in the last few decades suggest that the combination of Bcl-2 inhibitors with other antitumor drugs or RNA/DNA inhibitors will be more effective than their single use.
Acknowledgments
This work was supported by grants from the Administration of Traditional Chinese Medicine of Guangdong Province (no. 20192073); the Guangzhou Science and Technology Project (201904010044); the Guangzhou Medicine and Health Care Technology Project (nos. 20191A011119, 20192A011027); the Guangzhou Health and Family Planning Commission Program (no. 20181A011118); the National Natural Science Foundation of Guangdong Province (no. 2018A0303130191); the Medical and Health Science and Technology Project of Panyu District, Guangzhou (nos. 2017-Z04-18, 2018-Z04-59); and the Science and Technology Planning Project of Guangdong Province (no. 2017ZC0372).
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Authors' Contributions
Zeping Han & Jiening Liang contributed equally to this work.
References
- 1.Cordaro T. A., de Visser K. E., Tirion F. H., et al. Tumor size at the time of adoptive transfer determines whether tumor rejection occurs. European Journal of Immunology. 2000;30(5):1297–1307. doi: 10.1002/(sici)1521-4141(200005)30:5<1297::aid-immu1297>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Cory S., Adams J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nature Reviews Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 3.Leverson J. D., Sampath D., Souers A. J., et al. Found in translation: how preclinical research is guiding the clinical development of the BCL2-selective inhibitor venetoclax. Cancer Discovery. 2017;7(12):1376–1393. doi: 10.1158/2159-8290.cd-17-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams J. M., Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Mondello P., Erazo T., et al. NOXA genetic amplification or pharmacologic induction primes lymphoma cells to BCL2 inhibitor-induced cell death. Proceedings of the National Academy of Sciences. 2018;115(47):12034–12039. doi: 10.1073/pnas.1806928115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien S., Moore J. O., Boyd T. E., et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. Journal of Clinical Oncology. 2009;27(31):5208–5212. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raab R., Sparano J. A., Ocean A. J., et al. A phase I trial of oblimersen sodium in combination with cisplatin and 5-fluorouracil in patients with advanced esophageal, gastroesophageal junction, and gastric carcinoma. American Journal of Clinical Oncology. 2010;33(1):61–65. doi: 10.1097/COC.0b013e3181a31ad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galatin P. S., Advani R. H., Fisher G. A., et al. Phase I trial of oblimersen (genasense®) and gemcitabine in refractory and advanced malignancies. Investigational New Drugs. 2011;29(5):971–977. doi: 10.1007/s10637-010-9416-4. [DOI] [PubMed] [Google Scholar]
- 9.Ott P. A., Chang J., Madden K., et al. Oblimersen in combination with temozolomide and albumin-bound paclitaxel in patients with advanced melanoma: a phase I trial. Cancer Chemotherapy and Pharmacology. 2013;71(1):183–191. doi: 10.1007/s00280-012-1995-7. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi L., Camidge D. R., Ribeiro de Oliveira M., et al. Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. Journal of Clinical Oncology. 2011;29(7):909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudin C. M., Hann C. L., Garon E. B., et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clinical Cancer Research. 2012;18(11):3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts A. W., Seymour J. F., Brown J. R., et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of Clinical Oncology. 2012;30(5):488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolcher A. W., LoRusso P., Arzt J., et al. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with irinotecan: results of an open-label, phase 1 study. Cancer Chemotherapy and Pharmacology. 2015;76(5):1041–1049. doi: 10.1007/s00280-015-2882-9. [DOI] [PubMed] [Google Scholar]
- 14.Tolcher A. W., LoRusso P., Arzt J., et al. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology. 2015;76(5):1025–1032. doi: 10.1007/s00280-015-2883-8. [DOI] [PubMed] [Google Scholar]
- 15.Roberts A. W., Advani R. H., Kahl B. S., et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. British Journal of Haematology. 2015;170(5):669–678. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kipps T. J., Eradat H., Grosicki S., et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leukemia & Lymphoma. 2015;56(10):2826–2833. doi: 10.3109/10428194.2015.1030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer K., Al-Sawaf O., Bahlo J., et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. New England Journal of Medicine. 2019;380(23):2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 18.Jain N., Keating M., Thompson P., et al. Ibrutinib and venetoclax for first-line treatment of CLL. New England Journal of Medicine. 2019;380(22):2095–2103. doi: 10.1056/NEJMoa1900574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones J. A., Mato A. R., Wierda W. G., et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. The Lancet Oncology. 2018;19(1):65–75. doi: 10.1016/S1470-2045(17)30909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davids M. S., Roberts A. W., Seymour J. F., et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. Journal of Clinical Oncology. 2017;35(8):826–833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konopleva M., Pollyea D. A., Potluri J., et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discovery. 2016;6(10):1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiNardo C. D., Pratz K. W., Letai A., et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. The Lancet Oncology. 2018;19(2):216–228. doi: 10.1016/s1470-2045(18)30010-x. [DOI] [PubMed] [Google Scholar]
- 23.Cramer P., von Tresckow J., Bahlo J., et al. Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. The Lancet Oncology. 2018;19(9):1215–1228. doi: 10.1016/S1470-2045(18)30414-5. [DOI] [PubMed] [Google Scholar]
- 24.Goy A., Hernandez-Ilzaliturri F. J., Kahl B., Ford P., Protomastro E., Berger M. A phase I/II study of the pan Bcl-2 inhibitor obatoclax mesylate plus bortezomib for relapsed or refractory mantle cell lymphoma. Leukemia & Lymphoma. 2014;55(12):2761–2768. doi: 10.3109/10428194.2014.907891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arellano M. L., Borthakur G., Berger M., Luer J., Raza A. A phase II, multicenter, open-label study of obatoclax mesylate in patients with previously untreated myelodysplastic syndromes with anemia or thrombocytopenia. Clinical Lymphoma Myeloma and Leukemia. 2014;14(6):534–539. doi: 10.1016/j.clml.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Stein M. N., Goodin S., Gounder M., et al. A phase I study of AT-101, a BH3 mimetic, in combination with paclitaxel and carboplatin in solid tumors. Investigational New Drugs. 2019 doi: 10.1007/s10637-019-00807-2. [DOI] [PubMed] [Google Scholar]
- 27.Swiecicki P. L., Bellile E., Sacco A. G., et al. A phase II trial of the BCL-2 homolog domain 3 mimetic AT-101 in combination with docetaxel for recurrent, locally advanced, or metastatic head and neck cancer. Investigational New Drugs. 2016;34(4):481–489. doi: 10.1007/s10637-016-0364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schelman W. R., Mohammed T. A., Traynor A. M., et al. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Investigational New Drugs. 2014;32(2):295–302. doi: 10.1007/s10637-013-9999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petros A. M., Medek A., Nettesheim D. G., et al. Solution structure of the antiapoptotic protein Bcl-2. Proceedings of the National Academy of Sciences. 2001;98(6):3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendt M. D. Protein-Protein Interactions. Topics in Medicinal Chemistry. Vol. 8. Berlin, Germany: Springer; 2012. The discovery of navitoclax, a Bcl-2 family inhibitor. [DOI] [Google Scholar]
- 31.Park C.-M., Bruncko M., Adickes J., et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. Journal of Medicinal Chemistry. 2008;51(21):6902–6915. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- 32.Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 1996;274(5292):1531–1534. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 33.Lamoree B., Hubbard R. E. Current perspectives in fragment-based lead discovery (FBLD) Essays in Biochemistry. 2017;61(5):453–464. doi: 10.1042/EBC20170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oltersdorf T., Elmore S. W., Shoemaker A. R., et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 35.Tse C., Shoemaker A. R., Adickes J., et al. ABT-263: a potent and orally bioavailable bcl-2 family inhibitor. Cancer Research. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Nimmer P. M., Tahir S. K., et al. Bcl-2 family proteins are essential for platelet survival. Cell Death & Differentiation. 2007;14(5):943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 37.Cang S., Iragavarapu C., Savooji J., Song Y., Liu D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. Journal of Hematology & Oncology. 2015;8(1):p. 129. doi: 10.1186/s13045-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen M., Marcellus R. C., Roulston A., et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proceedings of the National Academy of Sciences. 2007;104(49):19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi R., Lartigue L., Perkins G. Targeting Mcl-1 and other Bcl-2 family member proteins in cancer therapy. Pharmacology & Therapeutics. 2019;195:13–20. doi: 10.1016/j.pharmthera.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Wang G., Nikolovska-Coleska Z., Yang C.-Y., et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. Journal of Medicinal Chemistry. 2006;49(21):6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 41.Zerp S. F., Stoter R., Kuipers G., et al. AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis. Radiation Oncology. 2009;4(1):p. 47. doi: 10.1186/1748-717X-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macvicar G. R., Kuzel T. M., Curti B. D., et al. An open-label, multicenter, phase I/II study of AT-101 in combination with docetaxel (D) and prednisone (P) in men with hormone refractory prostate cancer (HRPC) Journal of Clinical Oncology. 2008;26(15) doi: 10.1200/jco.2009.27.15s.5062.16043 [DOI] [Google Scholar]
- 43.Heist R. S., Fain J., Chinnasami B., et al. Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. Journal of Thoracic Oncology. 2010;5(10):1637–1643. doi: 10.1097/JTO.0b013e3181e8f4dc. [DOI] [PubMed] [Google Scholar]
- 44.Mohammad R. M., Goustin A. S., Aboukameel A., et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clinical Cancer Research. 2007;13(7):2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 45.Verhaegen M., Bauer J. A., Martin de la Vega C., et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Research. 2006;66(23):11348–11359. doi: 10.1158/0008-5472.CAN-06-1748. [DOI] [PubMed] [Google Scholar]
- 46.Casara P., Davidson J., Claperon A., et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget. 2018;9(28):20075–20088. doi: 10.18632/oncotarget.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]