Abstract
Taurine, a nonprotein amino acid, is widely distributed in almost all animal tissues. Ingestion of taurine helps to improve obesity and its related metabolic disorders. However, the molecular mechanism underlying the protective role of taurine against obesity is not completely understood. In this study, it was found that intraperitoneal treatment of mice with taurine alleviated high-fat diet (HFD)-induced obesity, improved insulin sensitivity, and increased energy expenditure and adaptive thermogenesis of the mice. Meanwhile, administration of the mice with taurine markedly induced the browning of inguinal white adipose tissue (iWAT) with significantly elevated expression of PGC1α, UCP1, and other thermogenic genes in iWAT. In vitro studies indicated that taurine also induced the development of brown-like adipocytes in C3H10T1/2 white adipocytes. Knockdown of PGC1α blunted the role of taurine in promoting the brown-like adipocyte phenotypes in C3H10T1/2 cells. Moreover, taurine treatment enhanced AMPK phosphorylation in vitro and in vivo, and knockdown of AMPKα1 prevented taurine-mediated induction of PGC1α in C3H10T1/2 cells. Consistently, specific knockdown of PGC1α in iWAT of the HFD-fed mice inhibited taurine-induced browning of iWAT, with the role of taurine in the enhancement of adaptive thermogenesis, the prevention of obesity, and the improvement of insulin sensitivity being partially impaired. These results reveal a functional role of taurine in facilitating the browning of white adipose tissue, which depends on the induction of PGC1α. Our studies also suggest a potential mechanism for the protective role of taurine against obesity, which involves taurine-mediated browning of white adipose tissue.
Keywords: peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) (PPARGC1A), obesity, adipose tissue, energy metabolism, metabolic regulation, AMP-activated kinase (AMPK), adaptive thermogenesis, browning of adipose tissue, insulin resistance, taurine, UCP1
Introduction
Obesity and its related metabolic disorders have become an escalating global pandemic because people's diet and lifestyle have changed in the 20th century (1, 2). Obesity leads to many common and serious illnesses, including type 2 diabetes, cardiovascular diseases, fatty liver, and even the increased risk of several cancers (3, 4). Obesity develops when the energy intake surpasses the energy expenditure. While restricting energy intake either by decreasing fat absorption or by suppressing appetite is the first line of defense against obesity, enhancing energy expenditure in key metabolic organs, such as adipose tissue, is emerging as an appealing strategy to combat obesity in recent years (5–7).
Taurine, or 2-aminoethylsulfonic acid, accounts for about 0.1% of the human body weight, making it one of the most abundant amino acids (8). Taurine is endogenously synthesized from methionine and cysteine and is also provided by diet (9). Many studies have shown that taurine is involved in a diverse array of biological and physiological functions, such as membrane stabilization, salt conjugation, immunomodulation, anti-oxidation, and so on (10, 11). Recent and accumulating evidence has revealed an anti-obesity effect of taurine (8, 12–14). Obese people have a lower content of taurine in their bodies (15). People who consume a lot of seafood, which contains taurine in abundance, are less likely to develop metabolic diseases such as obesity, diabetes, dyslipidemia, and hypertension (16–18). Used as a supplement, taurine can reduce plasma levels of inflammatory and oxidative markers and increase plasma adiponectin levels in humans (15). Animal studies have also shown that taurine supplementation effectively reduces or delays obesity in mice fed a high-fat diet (HFD)3 (19, 20). The studies above suggest that taurine deficiency could be involved in the development and progression of metabolic dysfunctions such as obesity and diabetes, and taurine is a beneficial substance that could help to preserve metabolic health. Thus, it is important to elucidate the molecular mechanism underlying the anti-obesity effect of taurine, which is still incompletely understood.
In mammals, adipose tissue can be broadly divided into white and brown adipose tissue (WAT and BAT) (21). WAT is characterized by large unilocular lipid droplets containing white adipocytes, and it stores energy in the form of triglycerides during periods of excessive caloric intake for later use when energy demand exceeds intake (22). In contrast, BAT is composed of multilocular lipid droplets and large numbers of mitochondria containing uncoupling protein-1 (UCP1), a protein critical for thermogenic respiration. BAT consumes “stored triglycerides” to generate energy in the form of heat, most notably when environmental temperature falls (23). Promotion of BAT activities may help to combat obesity. Rodents and other small mammals have copious BAT, but larger mammals often lose prominent BAT after infancy (24). However, clusters of UCP1-positive multilocular adipocytes with thermogenic capacity can be developed in WAT in response to some stimuli, which is known as the browning of WAT (25). These brown-like adipocytes in WAT have been designated as “beige” or “brite” cells, and studies have revealed that adult humans also have significant deposits of this type of cells that can be detected by positron emission tomography–scanning methods (26–28). Enhancing the BAT function and inducing the browning of WAT provide a potential approach for enhancing energy expenditure and decreasing obesity.
In this study, we investigated the anti-obesity effect of taurine by using intraperitoneal administration of it to HFD-fed C57BL6 mice. It was also found that taurine treatment induced the browning of WAT, and this effect depends on AMPK signaling–mediated induction of PGC1α in white adipocytes. Furthermore, we demonstrated that taurine-induced browning of WAT could contribute to the anti-obesity role of taurine in mice. Our data reveal a potential mechanism by which taurine inhibits the metabolic dysfunction.
Results
Taurine reduces obesity and improves insulin sensitivity in HFD-fed mice
The metabolic effects of taurine were examined by intraperitoneal (i.p.) administration of taurine into HFD-fed C57BL6 male mice. The 6-week-old mice were fed a HFD for 14 weeks. During the last 5 weeks of HFD feeding, mice were treated with taurine or PBS as a control once every day. As shown in Fig. 1A, the mice were obviously protected from weight gain after 5 weeks of taurine treatment. This was associated with significantly decreased adiposity and increased percentage of lean body mass (Fig. 1B). Consistent with the reduced fat mass content, mice treated by taurine had smaller adipocytes in inguinal white adipose tissue (iWAT, the subcutaneous fat), in which the size of lipid droplets was reduced (Fig. 1C). The amount of iWAT was also significantly decreased by the treatment of taurine (Fig. 1D). Similar results were observed in epididymal white adipose tissue (eWAT, the visceral fat) as shown in Fig. 1, E and F. Histological analyses also showed that taurine treatment slightly decreased the size of lipid droplets in BAT and alleviated fatty liver (Fig. S1, A and B, respectively). Because obesity is usually associated with elevated plasma free fatty acid (FFA) levels and impaired glucose homeostasis, we analyzed whether taurine treatment could improve them. The plasma FFA levels of taurine-treated mice were significantly lower than those of PBS-treated mice (Fig. 1G). When challenged with an i.p. glucose load, mice treated with taurine displayed significantly improved glucose tolerance (Fig. 1H). The i.p. insulin tolerance tests also showed improved insulin sensitivity in mice treated with taurine (Fig. 1I). Our results demonstrate that taurine reduced the body weight and improved glucose tolerance and insulin sensitivity in HFD-fed mice.
Figure 1.
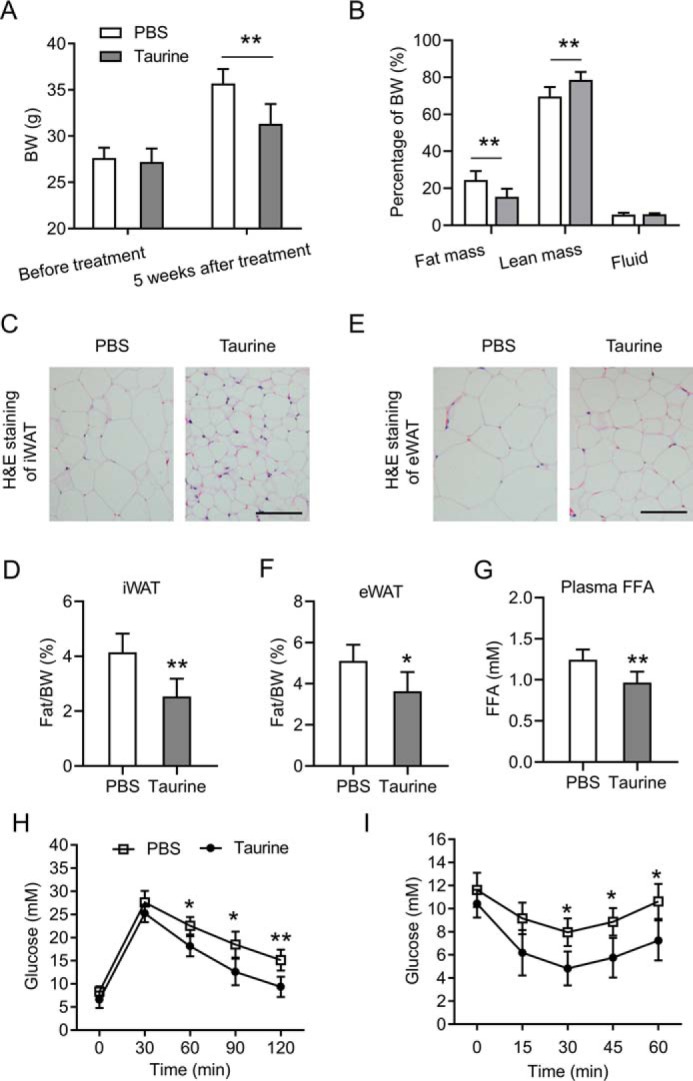
Taurine reduces obesity and improves insulin sensitivity in HFD-fed mice. The 6-week-old C57BL6 male mice were fed a HFD for 14 weeks before being sacrificed for analyses. During the last 5 weeks of HFD feeding, mice were administered taurine intraperitoneally (150 mg/kg per mouse, once a day) or PBS as a control. A, body weights (BW) of the mice before taurine treatment and 5 weeks after taurine treatment. B, body composition of the mice after 5 weeks of taurine treatment. C, representative H&E staining images of iWAT of the mice. Scale bar, 50 μm. D, percentage of iWAT weight relative to the whole-body weight of the mice. E, representative H&E staining images of eWAT of the mice. Scale bar, 50 μm. F, percentage of eWAT weight relative to the whole-body weight of the mice. G, plasma FFA levels of the mice after overnight fasting. H and I, glucose concentrations during an i.p. glucose tolerance test (H) or an insulin tolerance test (I). For statistical analysis, two-way analysis of variance and Bonferroni's post hoc tests were performed in A, B, H, and I, and unpaired two-tailed Student's t tests were performed in D, F, and G. For statistical analysis in D, F–I, data were compared between the PBS group and the taurine group. All values are represented as means with error bars representing S.D. *, p < 0.05; **, p < 0.01. n = 6 for each group.
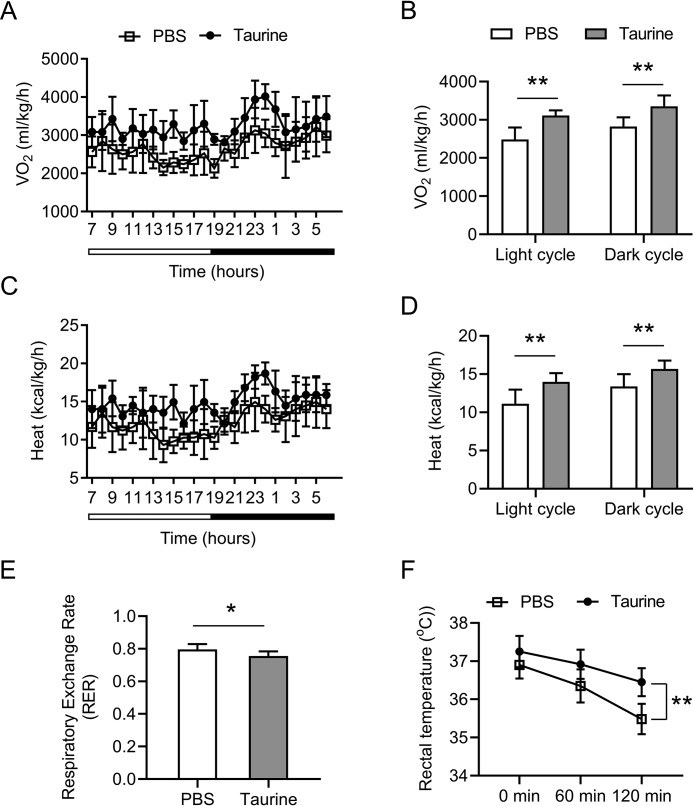
Taurine increases energy expenditure and adaptive thermogenesis
We then analyzed the actions of taurine on energy expenditure using a comprehensive laboratory animal-monitoring system. As shown in Fig. 2, A and B, basal oxygen consumption rates of taurine-treated mice were significantly higher than those of PBS-treated mice through a 12-h light/dark cycle. The mice that received taurine showed a significant increase in the whole-body energy expenditure through a 12-h light/dark cycle (Fig. 2, C and D). The respiratory exchange ratio (RER) is an indicator of the relative use of carbohydrates versus lipids as a source of energy (29). Taurine treatment significantly decreased RER, demonstrating a shift of the fuel preference toward fatty acids by taurine (Fig. 2E). To further investigate the differences in energy expenditure among these animals, we performed a cold tolerance test to study the adaptive thermogenesis, another important component of energy expenditure (6, 7). During 2 h of cold exposure, the body temperature of taurine-treated mice dropped a significantly less extent than that of PBS-treated mice (Fig. 2F), suggesting that taurine is able to increase body adaptation to cold exposure by generating more heat in mice.
Figure 2.
Taurine increases energy expenditure and adaptive thermogenesis. Mice were treated as indicated in Fig. 1. A and B, whole-body oxygen consumption rate (VO2) of the mice during a 12-h light/12-h dark cycle measured in a metabolic cage (A) and the average values for 12-h light/12-h dark periods were shown in B. C and D, heat production of the mice during a 12-h light/12-h dark cycle was calculated and shown in C, and the average values for 12-h light/12-h dark periods are shown in D. E, average values of RER in the mice for a 24-h cycle were calculated from the metabolic cage data. F, rectal temperature of the mice was recorded at the indicated time points after cold exposure (4 °C). For statistical analysis, two-way analysis of variance and Bonferroni's post hoc tests were performed in B, D, and F, and unpaired two-tailed Student's t tests were performed in E. All values are represented as means with error bars representing S.D. *, p < 0.05; **, p < 0.01. n = 6 for each group.
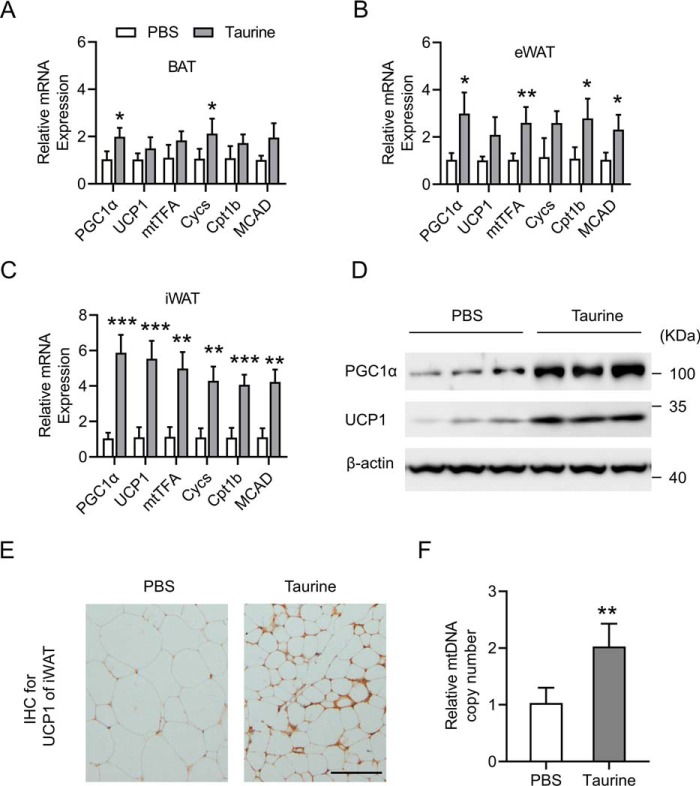
Taurine induces the browning of iWAT and activates a thermogenic program in iWAT
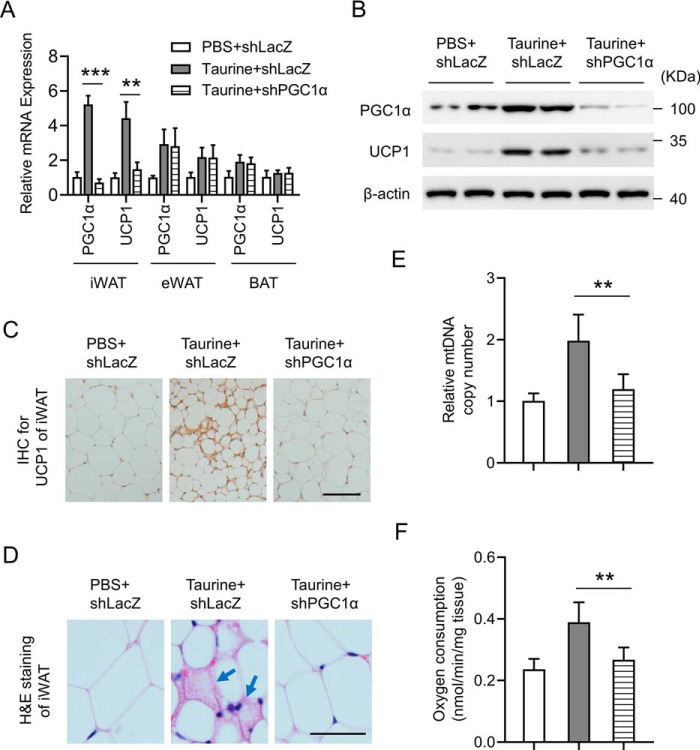
The decreased fat accumulation (Fig. 1, B–F) and increased adaptive thermogenesis (Fig. 2F) in mice treated with taurine prompted us to investigate the possible role of taurine in regulating the thermogenic program in adipose tissue. A network of genes controlling energy expenditure and thermogenic program were examined in BAT, eWAT, and iWAT. Peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) is regarded as one of the key inducers of brown adipocyte development. PGC1α transactivates the expression of uncoupling protein 1 (UCP1), a BAT marker gene, and has been shown to be a potent activator of mitochondrial biogenesis and fatty acid oxidation (FAO) (30). The expression levels of mitochondrial cytochrome c (Cycs) and mitochondrial transcription factor A (mtTFA) reflect the mitochondrial biogenesis level, although the expression levels of carnitine palmitoyl-CoA:transferase 1b (Cpt1b) and medium-chain acyl-CoA dehydrogenase (MCAD) indicate the FAO activity (29). Although to different extents, taurine treatment induced the expression of the above genes in BAT (Fig. 3A), eWAT (Fig. 3B), and iWAT (Fig. 3C), with its most significant effect in iWAT (Fig. 3C). Fractionated and differentiated primary BAT adipocytes and primary iWAT adipocytes were also treated with taurine. The mRNA levels of the above genes and the protein levels of PGC1α and UCP1 were increased by taurine treatment both in BAT adipocytes and in iWAT adipocytes, with more robust effect in iWAT adipocytes (Fig. S2). The role of taurine in iWAT was then subjected to further analyses. Western blot analysis further confirmed that the protein levels of PGC1α and UCP1 in iWAT were obviously elevated by the taurine treatment (Fig. 3D). Immunohistochemistry (IHC) staining assays also showed that taurine led to the elevation of UCP1 expression in iWAT, with smaller size of adipocytes than that in the control PBS group (Fig. 3E). Furthermore, the mitochondrial copy number in iWAT was significantly increased by the taurine treatment (Fig. 3F), suggesting enhanced mitochondrial biogenesis. Collectively, these results above demonstrated that taurine activated the browning of white adipose tissue, especially in subcutaneous fat (iWAT) of the mice.
Figure 3.
Browning of iWAT and activation of a thermogenic program by taurine. Mice were treated as indicated in Fig. 1. A–C, mRNA levels of the indicated genes were determined by RT-qPCR in BAT, eWAT, and iWAT, respectively. D, Western blot analysis of the protein levels in iWAT by using the indicated antibodies. β-Actin serves as an internal control. E, IHC for UCP1 protein (brown stain) in iWAT sections of the mice. Scale bar, 50 μm. F, mtDNA copy number of iWAT. Data were normalized to the PBS-treated group. For statistical analysis, two-way analysis of variance and Bonferroni's post hoc tests were performed in A–C, and unpaired two-tailed Student's t tests were performed in F. All groups were compared with the PBS group. All values are represented as means with error bars representing S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001. n = 6 for each group.
Taurine induces a brown adipocyte-like phenotype in CH10T1/2 adipocytes in vitro
To better investigate the role of taurine in the browning of adipocytes and its underlying mechanism, in vitro studies were further performed. C3H10T1/2 cells are a mesenchymal stem cell line that can be induced to undergo adipogenic development and become mature adipocytes (31). Taurine treatment of C3H10T1/2 adipocytes up-regulated the mRNA levels of the genes involved in the activation of energy expenditure and thermogenesis, including PGC1α, UCP1, mtTFA, and Cpt1b (Fig. 4A), in a taurine dose-dependent manner. Western blot analysis further confirmed that taurine dose-dependently increased the protein levels of PGC1α and UCP1 (Fig. 4B). Enhanced mitochondrial biosynthesis was observed in taurine-treated C3H10T1/2 adipocytes as shown by MitoTracker Green (MTG) staining (Fig. 4C). Moreover, oxygen consumption rate (OCR) was also determined, which demonstrated that taurine treatment promoted the basal OCR in C3H10T1/2 adipocytes (Fig. 4D). Thus, these in vitro results, together with the primary iWAT adipocytes results (Fig. S2, C and D), indicate that taurine can directly activate the browning of adipocytes.
Figure 4.
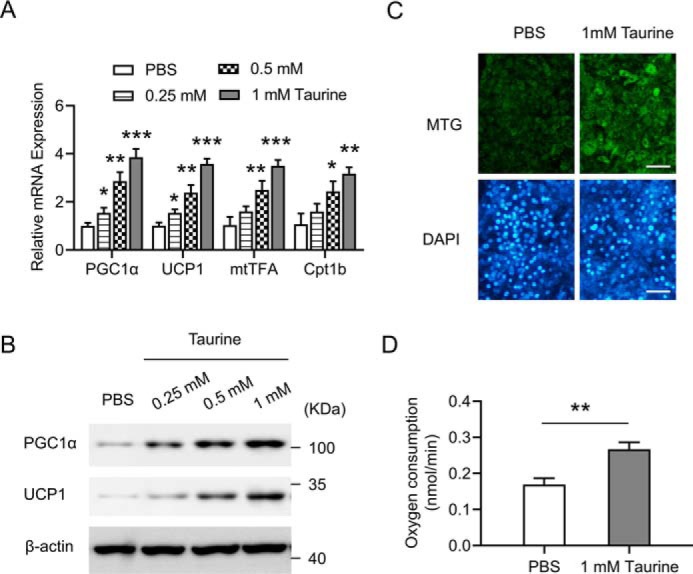
Taurine induces a brown adipocyte-like phenotype in C3H10T1/2 white adipocytes in vitro. C3H10T1/2 white adipocytes were treated with taurine at the indicated dose or with PBS as a control. After 24 h of treatment, cells were harvested for analyses. A, mRNA levels of the indicated genes were determined by using RT-qPCR. Data were normalized to the PBS group. B, cell lysates were then subjected to Western blotting by using the indicated antibodies. β-Actin serves as an internal control. C, cells were stained with MitoTracker Green (MTG) and 4′,6-diamidino-2-phenylindole (DAPI), and representative images are shown. Scale bar, 100 μm. Staining was performed after 24 h of treatment with PBS or 1 mm taurine. D, basal OCR was measured and shown. For statistical analysis, two-way analysis of variance and Bonferroni's post hoc tests were performed in A, and unpaired two-tailed Student's t tests were performed in D. All groups were compared with the PBS group. All values are represented as means with error bars representing S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001. n = 5 for each group.
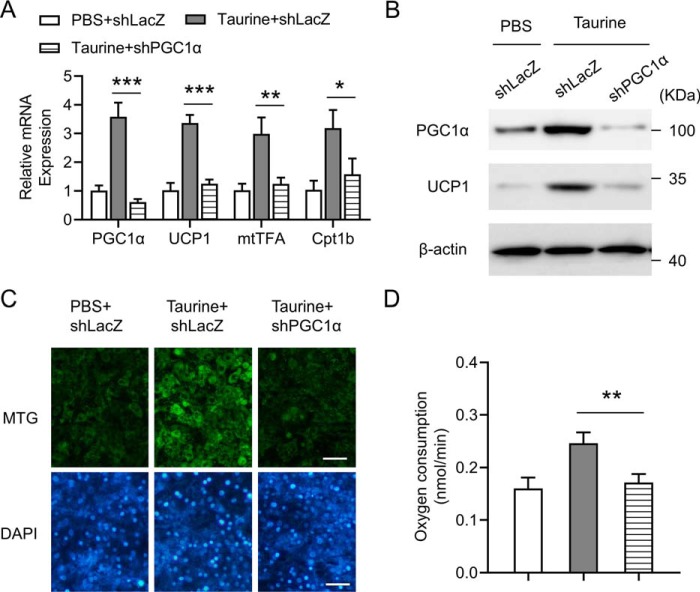
Knockdown of PGC1α blunts the role of taurine in promoting the brown adipocyte-like features in C3H10T1/2 adipocytes
Because PGC1α is one of the key factors promoting thermogenic program in adipocytes, the role of PGC1α in taurine-mediated browning of adipocytes was investigated in vitro. Adenovirus harboring the short hairpin RNA (shRNA) against LacZ (shLacZ) or shRNA against PGC1α (shPGC1α) was used to infect C3H10T1/2 adipocytes. The adenovirus-mediated knockdown of PGC1α in C3H10T1/2 adipocytes obviously decreased the PGC1α mRNA level and significantly impaired taurine-mediated induction of UCP1, mtTFA, and Cpt1b mRNAs (Fig. 5A). Western blot analysis further confirmed that knockdown of PGC1α blunted taurine-mediated up-regulation of PGC1α and UCP1 protein levels (Fig. 5B). MTG staining showed that the effect of taurine treatment on promoting mitochondrial biosynthesis was blocked by the knockdown of PGC1α (Fig. 5C). Furthermore, down-regulation of PGC1α expression significantly impaired the ability of taurine to enhance the OCR in C3H10T1/2 adipocytes (Fig. 5D). The small interfering RNAs (siRNAs) targeting another two independent sequences of the PGC1α gene were also applied to knock down PGC1α in C3H10T1/2 adipocytes, with the results further confirming the above shRNA data (Fig. S3). Taken together, these data demonstrate an important role of PGC1α in taurine-induced browning of adipocytes.
Figure 5.
Knockdown of PGC1α blunts the role of taurine in promoting the brown adipocyte-like features in C3H10T1/2 adipocytes. C3H10T1/2 white adipocytes were infected with adenovirus harboring the shRNA against LacZ (shLacZ) or shRNA against PGC1α (shPGC1α). After 48 h of adenovirus infection, cells were treated with taurine at a final concentration of 1 mm or with PBS as a control. After 24 h of treatment, cells were harvested for analyses. A, mRNA levels of the indicated genes were determined by using RT-qPCR. Data were normalized to PBS + shLacZ group. B, cell lysates were then subjected to Western blotting by using the indicated antibodies. β-Actin serves as an internal control. C, cells were stained with MTG and DAPI, and representative images are shown. Scale bar, 100 μm. D, basal OCR was measured and shown. For statistical analysis, two-way analysis of variance and Bonferroni's post hoc tests were performed in A, and one-way analysis of variance plus Bonferroni's post hoc tests were carried out in D. All values are represented as means with error bars representing S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001. n = 5 for each group.
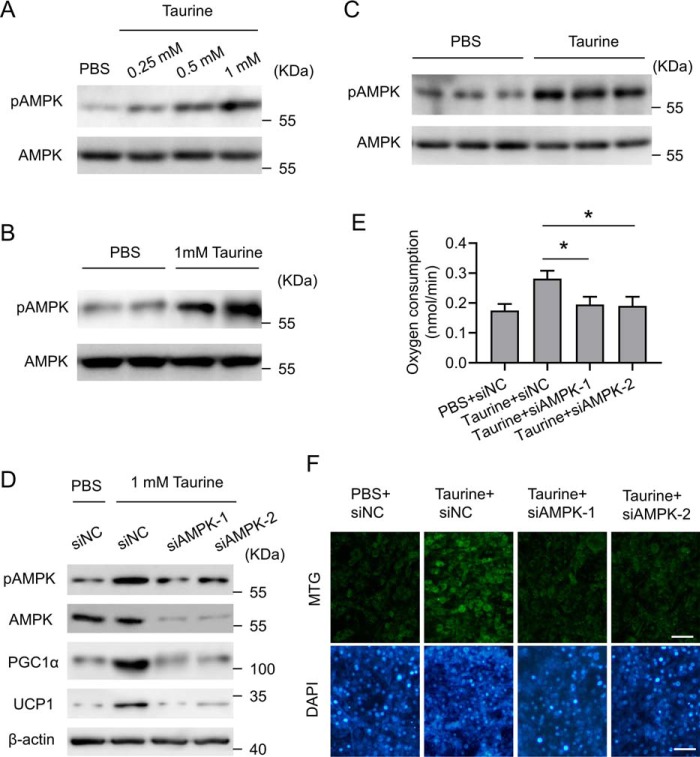
Taurine-regulated PGC1α expression is AMPK signaling-dependent
AMPK is a key regulator of energy metabolism and mitochondrial biogenesis (32, 33). The mechanism by which AMPK modulates energy balance is not completely elucidated, but it seems to require PGC1α. AMPK could either increase the expression of PGC1α or directly phosphorylate PGC1α (34, 35). Moreover, it has been shown that AMPK signaling was activated by taurine in skeletal muscle and liver of the mice (19, 36), which prompted us to investigate the involvement of AMPK in taurine-mediated effects in adipocytes. Taurine induced the phosphorylation of AMPK both in C3H10T1/2 adipocytes (Fig. 6A) and in fractionated and differentiated primary iWAT adipocytes (Fig. 6B). Up-regulation of AMPK phosphorylation by taurine treatment was also observed in iWAT of the HFD-fed mice (Fig. 6C). The siRNA-mediated knockdown of the α1 subunit of AMPK (AMPKα1) was performed in C3H10T1/2 adipocytes to show that down-regulation of AMPK signaling blunted the role of taurine in inducing the expression of PGC1α and UCP1 (Fig. 6D), promoting OCR (Fig. 6E) and enhancing mitochondrial biosynthesis (Fig. 6F). These results suggest a taurine/AMPK/PGC1α pathway that promotes the browning of adipocytes.
Figure 6.
Taurine-regulated PGC1α expression is AMPK signaling-dependent. A, C3H10T1/2 white adipocytes were treated with taurine at the indicated dose or with PBS as a control. After 24 h of treatment, cells were harvested for analyses. Cell lysates were subjected to Western blotting by using the indicated antibodies. B, fractionated and differentiated primary iWAT adipocytes were treated with taurine at a final concentration of 1 mm or with PBS as a control. After 24 h of treatment, cells were harvested for analyses. Cell lysates were subjected to Western blotting by using the indicated antibodies. C, mice were treated as indicated in Fig. 1. The tissue lysates of iWAT were subjected to Western blotting by using the indicated antibodies. D, C3H10T1/2 white adipocytes were transfected with the control siRNA (siNC) or two separate siRNAs (siAMPK1/2) against AMPKα1. 48 h post-transfection, cells were treated with taurine at a final concentration of 1 mm or with PBS as a control. After 24 h of treatment, cells were harvested for analyses. Cell lysates were subjected to Western blotting by using the indicated antibodies. E, cells were treated as in D, and OCR was measured. F, cells were treated as in D and then stained with MTG and DAPI. Representative images are shown. Scale bar, 100 μm. For statistical analysis, one-way analysis of variance plus Bonferroni's post hoc tests were carried out in E. All values are represented as means with error bars representing S.D. *, p < 0.05. n = 5 for each group.
Specific knockdown of PGC1α in iWAT blocks taurine-induced browning of iWAT, with the anti-obesity effect of taurine being partially impaired
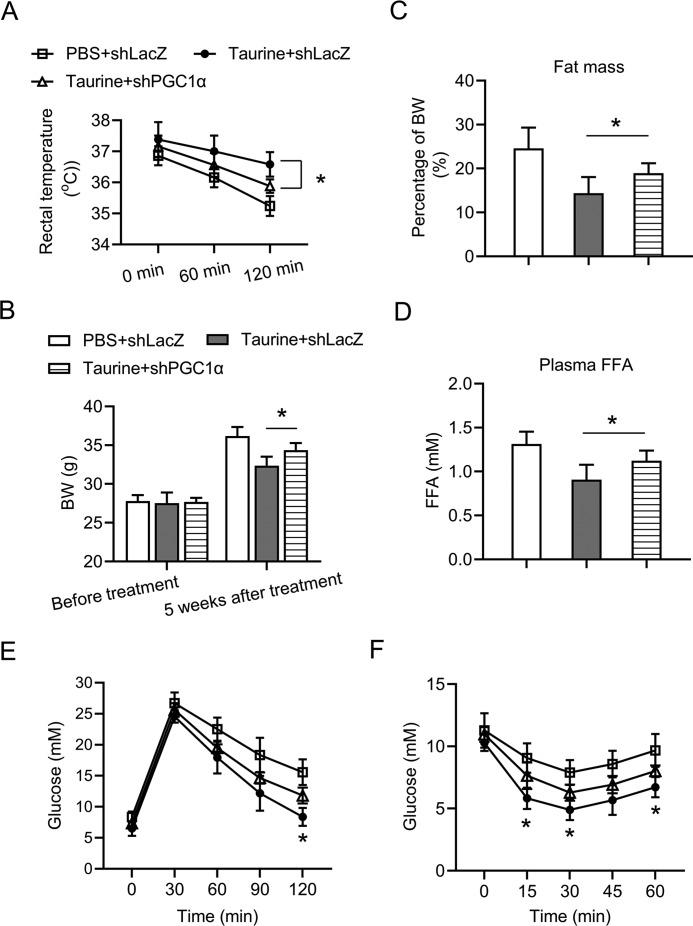
The role of PGC1α in taurine-mediated browning of WAT and the anti-obesity effect was further evaluated in vivo. Adenovirus harboring the short hairpin RNA (shRNA) against LacZ (shLacZ) or shRNA against PGC1α (shPGC1α) was injected subcutaneously adjacent to iWAT on both sides of the mice. The above method led to significant knockdown of PGC1α in iWAT, but it had no effect on the expression level of PGC1α in other tissues, which indicates a specific knockdown of PGC1α in iWAT (Fig. S4). Then, the above method was applied in HFD-fed mice, with or without taurine treatment. As shown in Fig. 7A, significant knockdown of PGC1α was observed in iWAT, but not in eWAT or BAT. Consistently, knockdown of PGC1α in iWAT blocked the effect of taurine treatment on inducing UCP1 expression in iWAT (Fig. 7, A–C) but not in eWAT or BAT (Fig. 7A). Taurine treatment caused a morphological transformation of the adipocytes from a unilocular morphology to a multilocular appearance (Fig. 7D), which is a BAT-like phenotype. This morphological transformation was abolished by the knockdown of PGC1α (Fig. 7D). Moreover, the ability of taurine to increase mitochondrial copy number and OCR in iWAT was also blunted by the knockdown of PGC1α (Fig. 7, E and F). Aside from the effects on iWAT, knockdown of PGC1α in iWAT partially impaired the role of taurine treatment in promoting adaptive thermogenesis (Fig. 8A), reducing body weight gain (Fig. 8B), decreasing fat mass content (Fig. 8C) and plasma FFA (Fig. 8D), and improving glucose tolerance (Fig. 8E) and insulin sensitivity (Fig. 8F) of the HFD-fed mice. Collectively, these results demonstrate an important role of PGC1α in taurine-induced browning of white adipose tissue and its beneficial effects on metabolic health.
Figure 7.
Specific knockdown of PGC1α in iWAT blocks taurine-induced browning of iWAT. The 6-week-old C57BL6 male mice were fed a HFD for 14 weeks before being sacrificed for analyses. During the last 5 weeks of HFD feeding, mice were administered taurine intraperitoneally (150 mg/kg per mouse, once a day) or PBS as a control. During the last 4 weeks of HFD feeding, adenoviruses harboring the shRNA against LacZ (shLacZ) or shRNA against PGC1α (shPGC1α) were injected subcutaneously adjacent to iWAT on both sides of the mouse once a week. A, mRNA levels of the indicated genes in the indicated tissues were determined by using RT-qPCR. Data were normalized to PBS + shLacZ group. B, iWAT tissue lysates were then subjected to Western blotting by using the indicated antibodies. β-Actin serves as an internal control. C, IHC for UCP1 protein (brown stain) in iWAT sections of the mice. Scale bar, 50 μm. D, representative H&E staining images of iWAT of the mice. Scale bar, 20 μm. Arrows indicate the adipocytes with multilocular morphology. E, mtDNA copy number of iWAT. Data were normalized to the PBS + shLacZ group. F, OCR of the iWAT. For statistical analysis, one-way analysis of variance plus Bonferroni's post hoc tests were carried out in E and F, and two-way analysis of variance plus Bonferroni's post hoc tests were carried out in A. **, p < 0.01; ***, p < 0.001. All values are represented as means with error bars representing S.D. n = 5 for each group.
Figure 8.
Anti-obesity effect of taurine was partially impaired upon the specific knockdown of PGC1α in iWAT. The 6-week-old C57BL6 male mice were fed a HFD for 14 weeks before being sacrificed for analyses. During the last 5 weeks of HFD feeding, mice were administered taurine intraperitoneally (150 mg/kg per mouse, once a day) or PBS as a control. During the last 4 weeks of HFD feeding, adenoviruses harboring the shRNA against LacZ (shLacZ) or shRNA against PGC1α (shPGC1α) were injected subcutaneously adjacent to iWAT on both sides of the mice once a week. A, rectal temperature of the mice was recorded at the indicated time points after cold exposure (4 °C). B, body weights (BW) of the mice before taurine treatment and 5 weeks after taurine treatment. C, fat mass levels of the mice after 5 weeks of taurine treatment. D, plasma FFA levels of the mice after overnight fasting. E and F, glucose concentrations during an i.p. glucose tolerance test (E) or an insulin tolerance test (F). For statistical analysis, one-way analysis of variance plus Bonferroni's post hoc tests were carried out in C and D, and two-way analysis of variance plus Bonferroni's post hoc tests were carried out in A, B, E, and F. For the statistical analysis in E and F, data were compared between taurine + shLacZ group and taurine + shPGC1α group. *, p < 0.05. All values are represented as means with error bars representing S.D. n = 5 for each group.
Discussion
Taurine is abundant in seafood and has a variety of physiological and pharmacological functions, including the alleviation of obesity and its related metabolic diseases (19, 20, 37–39). In this study, we confirmed the anti-obesity effects of taurine by intraperitoneal treatment of the HFD-fed mice with taurine. Mechanistic studies revealed that taurine was able to promote the browning of white adipose tissue, which contributed to the role of taurine in ameliorating obesity in mice.
In this paper, we showed that intraperitoneal administration with taurine inhibited the weight gain, reduced fat mass content, decreased plasma FFA levels, and improved glucose tolerance and insulin sensitivity in HFD-fed mice (Fig. 1). In addition, taurine treatment increased oxygen consumption and adaptive thermogenesis in HFD-fed mice (Fig. 2), suggesting that taurine prevents obesity with increased energy expenditure. Further studies indicated that taurine treatment activated the thermogenic program in adipose tissues, with the most obvious impact on iWAT (Fig. 3). By using the primary iWAT adipocytes and the cell model of C3H10T1/2 adipocytes, we demonstrated that taurine directly induced the browning of adipocytes in vitro (Fig. 4 and Fig. S2). Moreover, it was shown that PGC1α was required for the above role of taurine because knockdown of PGC1α in C3H10T1/2 adipocytes blunted the role of taurine in the induction of browning features in adipocytes (Fig. 5 and Fig. S3), and knockdown of PGC1α in iWAT abolished taurine-mediated browning in iWAT (Fig. 7) and partially impaired the anti-obesity effects of taurine (Fig. 8) in HFD-fed mice. Thus, our results reveal a potential new mechanism by which taurine exerts anti-obesity effects.
Targeting energy expenditure is believed to be an attractive concept for combating obesity. Thermogenesis improves metabolic homeostasis by dissipating energy in the form of heat. This process is achieved through the activation of UCP1, a protein unique to thermogenic adipocytes, including brown and beige adipocytes, serving as a functional marker for these cells. It might be a therapeutic strategy for treating obesity and its complications by promoting thermogenic capacity in BAT or obtaining BAT-like characteristics in WAT (23). Our data unveiled a functional role of taurine in the initiation of thermogenic program in adipocytes, which is shown to be involved in the increased energy expenditure and anti-obesity function of taurine. It has been reported that taurine inhibited the differentiation of human preadipocytes into adipocytes in a dose-dependent manner (13, 40). Our results indicated that differentiated C3H10T1/2 adipocytes could be activated by taurine to express more UCP1 and acquire brown adipocyte-like phenotypes. Thus, it is suggested that taurine could drive a white to brown feature switch through the direct transformation of the mature adipocytes, besides affecting the differentiation of preadipocytes.
Taurine appeared to induce the expression of many genes associated with the function of the brown/beige adipocytes (Figs. 3 and 4). PGC1α is a strong regulator of energy metabolism in adipose tissues, and its expression level can be up-regulated by cold exposure (41). PGC1α has been shown to switch the cells from an energy-storage state to an energy-expenditure phenotype, inducing mitochondrial biogenesis, fatty acid oxidation, and adaptive thermogenesis (30, 42). The ectopic expression of PGC1α in white adipocytes induces the acquisition of BAT features, including the up-regulation of mitochondrial genes, fatty acid oxidation, and thermogenic genes (42). Down-regulation of PGC1α is associated with obesity and increased risk of diabetes mellitus in the human population (43). We found that shRNA-mediated knockdown of PGC1α in iWAT impaired the role of taurine in the induction of iWAT browning and anti-obesity effects. This finding supports the notion that PGC1α could be a key molecule downstream of taurine in ameliorating metabolic disorders.
AMPK is an energy sensor that plays a key role in regulating complex signaling networks of energy metabolism and mitochondrial biogenesis (32, 33). Emerging evidence indicates that PGC1α is an important downstream effector of AMPK (34, 35). Previous studies have shown that taurine could activate AMPK signaling pathway in skeletal muscle and liver of the mice (19, 36). Here, we demonstrate that taurine was also able to enhance the phosphorylation of AMPK in murine white adipose tissue and adipocytes (Fig. 6). Furthermore, down-regulation of AMPK signaling by the knockdown of AMPKα1 abolished taurine-mediated induction of PGC1α and browning of adipocytes in C3H10T1/2 cells (Fig. 6). Together, these results suggest that AMPK might be an important mediator of taurine effects.
In conclusion, we demonstrate that taurine could activate the thermogenic program in adipose tissue, which is involved in its defense against obesity. We also reveal the molecular target and mechanism by which taurine induces the browning of white adipose tissue. These results suggest an important role for taurine in regulating adipose tissue thermogenesis and white adipose plasticity toward BAT, providing a mechanistic insight into the protective role of taurine against obesity.
Experimental procedures
Animals
Our animal protocol has been reviewed and approved by the Animal Care and Use Committee of the Fudan University Shanghai Medical College (no. 20180302-010). All studies involving animal experimentation follow the National Institutes of Health guidelines on the care and use of animals. Male C57BL/6J mice were purchased from the Model Animal Research Center of Nanjing University. Mice were housed at 22 ± 2 °C, 55 ± 5% relative humidity and maintained in 12-h light/dark cycles. To produce diet-induced obesity, 6-week-old C57BL/6J mice were fed a high-fat diet (D12492, Research Diets) for 14 weeks. For cold acclimation, mice were maintained in a temperature-controlled chamber at 4 °C for the indicated time. Body fat and lean mass were measured using an NMR analyzer (Minispec LF90II, Bruker Optics). The plasma FFA levels of the mice were measured by using FFA assay kit from Abcam.
Cell culture and reagents
C3H10T1/2 cells were obtained from the American Type Culture Collection (Manassas, VA). Primary BAT and iWAT stromal vascular cells (SVFs) were fractionated by collagenase digestion according to the published methods as described elsewhere (6, 44). C3H10T1/2 cells and primary SVFs were maintained in Dulbecco's modified Eagle's medium (DMEM) at 37 °C in a 5% CO2 environment. Adipocyte differentiation was induced in preadipocyte cultures by treating confluent cells for 48 h in medium containing 10% (v/v) fetal bovine serum (FBS, Invitrogen), 1 μg/ml insulin, 1 μm dexamethasone, 0.5 mm 3-isobutyl-1-methylxanthine, and 1 μm rosiglitazone until day 2. The cells then received DMEM supplemented with 10% (v/v) FBS, 1 μg/ml insulin, and 1 μm rosiglitazone for another 2 days. From day 4, the adipocytes were given DMEM containing 10% (v/v) FBS, which was changed every other day. On day 6, cells were treated with taurine and transfected with siRNAs or infected with adenoviruses for the indicated times. The primary antibodies used in this study are as follows: anti-UCP1 (Abcam, ab209483); anti-PGC1α (Abcam, ab54481); anti-AMPK (Cell Signaling, 5832, detecting AMPKα1); anti-phospho-AMPK (Cell Signaling, 2535, Thr-172 of AMPKα); and anti-β-actin (Santa Cruz Biotechnology, sc-47778). Taurine was purchased from Sigma (T8691) and dissolved in PBS. 4′,6-Diamidino-2-phenylindole (DAPI) was from Sigma (D9542), and MitoTracker Green (MTG) was from Beyotime Biotechnology (C1048).
Western blotting
The cells and tissues were harvested and homogenized with lysis buffer containing 1% SDS, 50 mm Tris-HCl (pH 6.8), 10 mm DTT, 10% glycerol, 0.002% bromphenol blue, 1 mm sodium fluoride (Sigma), 1 mm sodium orthovanadate (Sigma), and protease inhibitor mixture (Roche Applied Science). Equal amounts of protein were resolved on SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA), immunoblotted with primary antibodies, and visualized with horseradish peroxidase-coupled secondary antibodies.
RNA extraction and reverse transcription (RT)-qPCR
Total RNA from cells and tissues was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol as described before (45, 46). Complementary DNA (cDNA) was synthesized from total RNA with PrimeScript reverse transcriptase and random primers (TaKaRa Bio, Otsu, Japan). cDNAs were amplified with Power SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA) and Prism 7500 instrument (Applied Biosystems), with 18S rRNA as an endogenous control. The qPCR was done in triplicate and repeated at least three times. The primers for qPCR are as follows: 5′-CGCCGCTAGAGGTGAAATTCT-3′ (18S rRNA-forward); 5′-CATTCTTGGCAAATGCTTTCG-3′ (18S rRNA-reverse); 5′-ACAGGAACAGCAGCAGAG-3′ (PGC1α-forward); 5′-TAAGGTTCGCTCAATAGTC-3′ (PGC1α-reverse); 5′-CACCTTCCCGCTGGACACT-3′ (UCP1-forward); 5′-TCGGCAATCCTTCTGTTT-3′ (UCP1-reverse); 5′-GTCTGTATTCCGAAGTGTTT-3′ (mtTFA-forward); 5′-TTGCATCTGGGTGTTTAG-3′ (mtTFA-reverse); 5′-AGGATACCCTGATGGAGT-3′ (Cycs-forward); 5′-ATTAGGTCTGCCCTTTCT-3′ (Cycs-reverse); 5′-ATGTATCGCCGCAAACTG-3′ (Cpt1b-forward); 5′-CCTGGGATGCGTGTAGTG-3′ (Cpt1b-reverse); 5′-ATGCCTGTGATTCTTGCT-3′ (MCAD-forward); and 5′-TAACATACTCGTCACCCTTC-3′ (MCAD-reverse).
Construction of adenoviral expression vectors and infection
Adenoviral expression vector pBlock-it (Invitrogen) encoding shRNA against lacZ or PGC1α were constructed according to the manufacturer's protocol. The sequences (5′ to 3′) for successful shRNAs were as follows: shLacZ, CTACACAAATCAGCGATTT, and shPGC1α, GGTGGATTGAAGTGGTGTAGC. Adenovirus vectors were amplified and purified with Sartorius Adenovirus Purification kits. Viral titers were determined by the tissue culture infectious dose 50 (TCID50) method using 293A cells. For in vivo studies, 1 × 109 plaque-forming units/mouse of adenoviruses harboring shLacZ or shPGC1α were diluted to 200 μl with PBS, and then 100 μl of adenovirus solution was injected adjacently to the subcutaneous fat pads at each side, once a week for 2 or 4 weeks as indicated. For in vitro studies, adenoviruses harboring shLacZ or shPGC1α at a multiplicity of infection of 70 were added to the cell culture medium. Then, cells were cultured with the viruses for 24 h before replacement with fresh culture medium. Experiments were performed 48 h after adenovirus infection.
siRNA-mediated knockdown assay
The target sequences for successful siRNAs were as follows: siAMPK-1, CCTTCCGAAGTATCTCTTT; siAMPK-2, GCAATCAAGCAGTTGGATT; siPGC1α-1, TCGTGTTCCCGATCACCATAT; siPGC1α-2, TAACTATGCAGACCTAGATAC; and siNC, TTCTCCGAACGTGTCACGT. The siAMPK1 and siAMPK2 are the siRNAs targeting the gene of AMPKα1. C3H10T1/2 adipocytes (day 6 post-induction) were transfected with siRNAs using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. Further experiments were performed after 48 h of siRNA transfection.
Hematoxylin and Eosin (H&E) staining IHC
Tissues were fixed in 4% paraformaldehyde were sectioned after being paraffin-embedded. Paraffin-embedded tissues were cut, deparaffinized, and hydrated, and H&E staining and IHC were performed as described previously (47, 48). The anti-UCP1 (Abcam, ab209483) antibody was used for IHC. Images were captured using a charge-coupled device camera, and representative images were shown.
MitoTracker Green staining
Cultured cells were incubated in PBS with MitoTracker Green (final concentration 200 nm, Beyotime Biotechnology, C1048) for 10 min in a 37 °C incubator. The cells were observed and photographed under an Axioskop 2 microscope (Carl Zeiss) with a DP70 charged-coupled device system (Olympus). DAPI was used to mark the cell nuclei.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
Levels of blood glucose were measured using a glucometer monitor (Roche Applied Science). For GTT, mice were injected intraperitoneally with d-glucose (2 mg/g body weight) after overnight fasting, and tail blood glucose levels were monitored every 0.5 h. For ITT, mice were injected intraperitoneally with human insulin (Lilly; 0.75 milliunits/g body weight) after 4 h of fasting, and tail blood glucose levels were monitored every 15 min.
Cold tolerance test
For cold tolerance test, mice were maintained in a temperature-controlled chamber (MMM Friocell, Germany) at 4 °C without access to food or water. The rectal temperature was measured at the indicated time after exposure to the cold.
mtDNA content quantification by quantitative real-time PCR
Mouse tissues were homogenized and digested with proteinase K overnight in a lysis buffer for DNA extraction by using the DNeasy blood and tissue kit (Qiagen) following the manufacturer's instructions. The results were calculated from the difference in the threshold cycle (ΔCT) values for mtDNA and nuclear-specific gene via the amplification method of quantitative real-time PCR. The data are expressed as mtDNA-specific 16S rRNA normalized to nuclear-specific gene hexokinase as described previously (6).
Metabolic studies
Mice were housed and monitored individually in metabolic cages (Columbia Instruments) with free access to regular chow and drinking water for 48 h. Mice were maintained at 24 °C under a 12-h light/dark cycle. The animals were acclimated to the system for 24 h, and measurement of VO2 and VCO2 was performed during the next 24 h. Each cage was monitored for metabolic parameters (including oxygen consumption and carbon dioxide production) at 25-min intervals throughout the 48-h period. Parameters of oxygen consumption (ml/kg/h), carbon dioxide production (ml/kg/h), heat (kcal/kg/h), and RER (VCO2/VO2) were calculated for each mouse divided by its body weight.
Cellular oxygen consumption rate
The cellular OCR was measured by using an OxygenMeter (Strathkelvin Instruments) with a Mitocell (MT200) mixing chamber. Cells (1 × 105) were suspended in 1 ml of culture medium, and the oxygen concentration was recorded for 2 min. To measure the OCR of iWAT tissue, around 100 mg of iWAT tissue was cut into small pieces and then subjected to the OCR test as described above. The OCR was calculated using software (782 Oxygen System version 4.0).
Statistics
Results are expressed as means with error bars representing S.D. Comparisons between groups were made using unpaired two-tailed Student's t tests. For comparison of three or more independent groups with only one variable, one-way analyses of variance plus Bonferroni's post hoc tests were performed. For comparison of two or more independent groups with two variables, two-way analysis of variance plus Bonferroni's post hoc tests were carried out. The statistical analyses were also indicated in the legends of each figure, with p < 0.05 being considered statistically significant. All experiments were repeated a minimum of three times, and representative data are shown.
Author contributions
Y.-Y. G. and L. G. validation; Y.-Y. G. and L. G. writing-original draft; L. G. and Q.-Q. T. project administration; L. G. and Q.-Q. T. supervision; L. G. and Q.-Q. T. funding acquisition; L. G., Y.-Y. G., B.-Y. L., W.-Q. P. and Q.-Q. T. investigation; L. G. writing-review and editing.
Supplementary Material
This work was supported by National Key R&D Program of China Grant 2018YFA0800401 (to Q.-Q. T.) and National Natural Science Foundation of China (NSFC) Grants 31871435 and 31370027 (to L. G.) and 81730021 and 31571471 (to Q.-Q. T.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
- HFD
- high-fat diet
- AMPK
- adenosine 5′-monophosphate (AMP)-activated protein kinase
- PGC1α
- peroxisome proliferator-activated receptor γ coactivator 1-α
- Cycs
- cytochrome c
- mtTFA
- mitochondrial transcription factor A
- Cpt1b
- carnitine palmitoyl-CoA:transferase 1b
- MCAD
- medium-chain acyl-CoA dehydrogenase
- FAO
- fatty acid oxidation
- BAT
- brown adipose tissue
- WAT
- white adipose tissue
- iWAT
- inguinal white adipose tissue
- eWAT
- epididymal white adipose tissue
- OCR
- oxygen consumption rate
- RER
- respiratory exchange ratio
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- qPCR
- quantitative PCR
- SVF
- stromal vascular cell
- FFA
- free fatty acid
- IHC
- immunohistochemistry
- MTG
- MitoTracker Green
- DAPI
- 4′,6-diamidino-2-phenylindole
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- H&E
- hematoxylin and eosin.
References
- 1. Smith K. B., and Smith M. S. (2016) Obesity statistics. Prim. Care 43, 121–135 10.1016/j.pop.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 2. Zobel E. H., Hansen T. W., Rossing P., and von Scholten B. J. (2016) Global changes in food supply and the obesity epidemic. Curr. Obes. Rep. 5, 449–455 10.1007/s13679-016-0233-8 [DOI] [PubMed] [Google Scholar]
- 3. Bhupathiraju S. N., and Hu F. B. (2016) Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 118, 1723–1735 10.1161/CIRCRESAHA.115.306825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grundy S. M. (2016) Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 64, 1082–1086 10.1136/jim-2016-000155 [DOI] [PubMed] [Google Scholar]
- 5. Tseng Y. H., Cypess A. M., and Kahn C. R. (2010) Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 9, 465–482 10.1038/nrd3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Z., Zhang H., Li B., Meng X., Wang J., Zhang Y., Yao S., Ma Q., Jin L., Yang J., Wang W., and Ning G. (2014) Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 5, 5493 10.1038/ncomms6493 [DOI] [PubMed] [Google Scholar]
- 7. Lu P., Zhang F. C., Qian S. W., Li X., Cui Z. M., Dang Y. J., and Tang Q. Q. (2016) Artemisinin derivatives prevent obesity by inducing browning of WAT and enhancing BAT function. Cell Res. 26, 1169–1172 10.1038/cr.2016.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murakami S. (2015) Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food Res. 59, 1353–1363 10.1002/mnfr.201500067 [DOI] [PubMed] [Google Scholar]
- 9. Ide T., Kushiro M., Takahashi Y., Shinohara K., and Cha S. (2002) mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 51, 1191–1197 10.1053/meta.2002.34036 [DOI] [PubMed] [Google Scholar]
- 10. Huxtable R. J. (1992) Physiological actions of taurine. Physiol. Rev. 72, 101–163 10.1152/physrev.1992.72.1.101 [DOI] [PubMed] [Google Scholar]
- 11. Lambert I. H., Kristensen D. M., Holm J. B., and Mortensen O. H. (2015) Physiological role of taurine–from organism to organelle. Acta Physiol. 213, 191–212 10.1111/apha.12365 [DOI] [PubMed] [Google Scholar]
- 12. Cao P. J., Jin Y. J., Li M. E., Zhou R., and Yang M. Z. (2016) PGC-1α may associated with the anti-obesity effect of taurine on rats induced by arcuate nucleus lesion. Nutr. Neurosci. 19, 86–93 10.1179/1476830514Y.0000000153 [DOI] [PubMed] [Google Scholar]
- 13. Kim K. S., Jang M. J., Fang S., Yoon S. G., Kim I. Y., Seong J. K., Yang H. I., and Hahm D. H. (2019) Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids 51, 245–254 10.1007/s00726-018-2659-7 [DOI] [PubMed] [Google Scholar]
- 14. Murakami S. (2017) The physiological and pathophysiological roles of taurine in adipose tissue in relation to obesity. Life Sci. 186, 80–86 10.1016/j.lfs.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 15. Rosa F. T., Freitas E. C., Deminice R., Jordão A. A., and Marchini J. S. (2014) Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur. J. Nutr. 53, 823–830 10.1007/s00394-013-0586-7 [DOI] [PubMed] [Google Scholar]
- 16. Yamori Y., Liu L., Ikeda K., Miura A., Mizushima S., Miki T., Nara Y., and WHO-Cardiovascular Disease and Alimentary Comprarison (CARDIAC) Study Group). (2001) Distribution of twenty-four h urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: results from the WHO-CARDIAC study. Hypertens. Res. 24, 453–457 10.1291/hypres.24.453 [DOI] [PubMed] [Google Scholar]
- 17. Yamori Y., Taguchi T., Mori H., and Mori M. (2010) Low cardiovascular risks in the middle aged males and females excreting greater 24-hour urinary taurine and magnesium in 41 WHO-CARDIAC study populations in the world. J. Biomed. Sci. 17, Suppl. 1, S21 10.1186/1423-0127-17-S1-S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sagara M., Murakami S., Mizushima S., Liu L., Mori M., Ikeda K., Nara Y., and Yamori Y. (2015) Taurine in 24-h urine samples is inversely related to cardiovascular risks of middle aged subjects in 50 populations of the world. Adv. Exp. Med. Biol. 803, 623–636 10.1007/978-3-319-15126-7_50 [DOI] [PubMed] [Google Scholar]
- 19. Batista T. M., Ribeiro R. A., da Silva P. M., Camargo R. L., Lollo P. C., Boschero A. C., and Carneiro E. M. (2013) Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol. Nutr. Food Res. 57, 423–434 10.1002/mnfr.201200345 [DOI] [PubMed] [Google Scholar]
- 20. Lin S., Hirai S., Yamaguchi Y., Goto T., Takahashi N., Tani F., Mutoh C., Sakurai T., Murakami S., Yu R., and Kawada T. (2013) Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol. Nutr. Food Res. 57, 2155–2165 10.1002/mnfr.201300150 [DOI] [PubMed] [Google Scholar]
- 21. Wang W., and Seale P. (2016) Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 10.1038/nrm.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosen E. D., and Spiegelman B. M. (2014) What we talk about when we talk about fat. Cell 156, 20–44 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartelt A., and Heeren J. (2014) Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36 10.1038/nrendo.2013.204 [DOI] [PubMed] [Google Scholar]
- 24. Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W. D., Hoeks J., Enerbäck S., et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenwald M., Perdikari A., Rülicke T., and Wolfrum C. (2013) Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 10.1038/ncb2740 [DOI] [PubMed] [Google Scholar]
- 26. Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., and Kahn C. R. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., and Teule G. J. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- 28. Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., and Nuutila P. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- 29. Qian S. W., Tang Y., Li X., Liu Y., Zhang Y. Y., Huang H. Y., Xue R. D., Yu H. Y., Guo L., Gao H. D., Liu Y., Sun X., Li Y. M., Jia W. P., and Tang Q. Q. (2013) BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. U.S.A. 110, E798–E807 10.1073/pnas.1215236110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J., Wu H., Ma S., Jing F., Yu C., Gao L., and Zhao J. (2018) Transcription regulators and hormones involved in the development of brown fat and white fat browning: transcriptional and hormonal control of brown/beige fat development. Physiol. Res. 67, 347–362 10.33549/physiolres.933650 [DOI] [PubMed] [Google Scholar]
- 31. Tang Q. Q., and Lane M. D. (2012) Adipogenesis: from stem cell to adipocyte. Annu. Rev. Biochem. 81, 715–736 10.1146/annurev-biochem-052110-115718 [DOI] [PubMed] [Google Scholar]
- 32. Cantó C., and Auwerx J. (2009) PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20, 98–105 10.1097/MOL.0b013e328328d0a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cantó C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., and Auwerx J. (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jäger S., Handschin C., St-Pierre J., and Spiegelman B. M. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. U.S.A. 104, 12017–12022 10.1073/pnas.0705070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suwa M., Nakano H., and Kumagai S. (2003) Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 95, 960–968 10.1152/japplphysiol.00349.2003 [DOI] [PubMed] [Google Scholar]
- 36. Cheong S. H., and Chang K. J. (2013) Antidiabetic effect of taurine in cultured rat skeletal l6 myotubes. Adv. Exp. Med. Biol. 775, 311–320 10.1007/978-1-4614-6130-2_26 [DOI] [PubMed] [Google Scholar]
- 37. Tsuboyama-Kasaoka N., Shozawa C., Sano K., Kamei Y., Kasaoka S., Hosokawa Y., and Ezaki O. (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147, 3276–3284 10.1210/en.2005-1007 [DOI] [PubMed] [Google Scholar]
- 38. Kim K. S., Oh D. H., Kim J. Y., Lee B. G., You J. S., Chang K. J., Chung H. J., Yoo M. C., Yang H. I., Kang J. H., Hwang Y. C., Ahn K. J., Chung H. Y., and Jeong I. K. (2012) Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp. Mol. Med. 44, 665–673 10.3858/emm.2012.44.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gomez R., Caletti G., Arbo B. D., Hoefel A. L., Schneider R. Jr., Hansen A. W., Pulcinelli R. R., Freese L., Bandiera S., Kucharski L. C., and Barros H. M. (2018) Acute intraperitoneal administration of taurine decreases the glycemia and reduces food intake in type 1 diabetic rats. Biomed. Pharmacother. 103, 1028–1034 10.1016/j.biopha.2018.04.131 [DOI] [PubMed] [Google Scholar]
- 40. Kim K. S., Ji H. I., Chung H., Kim C., Lee S. H., Lee Y. A., Yang H. I., Yoo M. C., and Hong S. J. (2013) Taurine chloramine modulates the expression of adipokines through inhibition of the STAT-3 signaling pathway in differentiated human adipocytes. Amino Acids 45, 1415–1422 10.1007/s00726-013-1612-z [DOI] [PubMed] [Google Scholar]
- 41. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., and Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 10.1016/S0092-8674(00)81410-5 [DOI] [PubMed] [Google Scholar]
- 42. Tiraby C., Tavernier G., Lefort C., Larrouy D., Bouillaud F., Ricquier D., and Langin D. (2003) Acquirement of brown fat cell features by human white adipocytes. J. Biol. Chem. 278, 33370–33376 10.1074/jbc.M305235200 [DOI] [PubMed] [Google Scholar]
- 43. Patti M. E., Butte A. J., Crunkhorn S., Cusi K., Berria R., Kashyap S., Miyazaki Y., Kohane I., Costello M., Saccone R., Landaker E. J., Goldfine A. B., Mun E., DeFronzo R., Finlayson J., Kahn C. R., and Mandarino L. J. (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U.S.A. 100, 8466–8471 10.1073/pnas.1032913100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soukas A., Socci N. D., Saatkamp B. D., Novelli S., and Friedman J. M. (2001) Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J. Biol. Chem. 276, 34167–34174 10.1074/jbc.M104421200 [DOI] [PubMed] [Google Scholar]
- 45. Liu Y., Peng W. Q., Guo Y. Y., Liu Y., Tang Q. Q., and Guo L. (2018) Kruppel-like factor 10 (KLF10) is transactivated by the transcription factor C/EBPβ and involved in early 3T3-L1 preadipocyte differentiation. J. Biol. Chem. 293, 14012–14021 10.1074/jbc.RA118.004401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo L., Guo Y. Y., Li B. Y., Peng W. Q., and Tang Q. Q. (2019) Histone demethylase KDM5A is transactivated by the transcription factor C/EBPβ and promotes preadipocyte differentiation by inhibiting Wnt/β-catenin signaling. J. Biol. Chem. 294, 9642–9654 10.1074/jbc.RA119.008419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo L., Zhou S. R., Wei X. B., Liu Y., Chang X. X., Liu Y., Ge X., Dou X., Huang H. Y., Qian S. W., Li X., Lei Q. Y., Gao X., and Tang Q. Q. (2016) Acetylation of mitochondrial trifunctional protein α-subunit enhances its stability to promote fatty acid oxidation and is decreased in nonalcoholic fatty liver disease. Mol. Cell Biol. 36, 2553–2567 10.1128/MCB.00227-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo L., Zhang P., Chen Z., Xia H., Li S., Zhang Y., Kobberup S., Zou W., and Lin J. D. (2017) Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J. Clin. Invest. 127, 4449–4461 10.1172/JCI96324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.