Abstract
Similar to many other biological molecules, RNA is vulnerable to chemical insults from endogenous and exogenous sources. Noxious agents such as reactive oxygen species or alkylating chemicals have the potential to profoundly affect the chemical properties and hence the function of RNA molecules in the cell. Given the central role of RNA in many fundamental biological processes, including translation and splicing, changes to its chemical composition can have a detrimental impact on cellular fitness, with some evidence suggesting that RNA damage has roles in diseases such as neurodegenerative disorders. We are only just beginning to learn about how cells cope with RNA damage, with recent studies revealing the existence of quality-control processes that are capable of recognizing and degrading or repairing damaged RNA. Here, we begin by reviewing the most abundant types of chemical damage to RNA, including oxidation and alkylation. Focusing on mRNA damage, we then discuss how alterations to this species of RNA affect its function and how cells respond to these challenges to maintain proteostasis. Finally, we briefly discuss how chemical damage to noncoding RNAs such as rRNA, tRNA, small nuclear RNA, and small nucleolar RNA is likely to affect their function.
Keywords: RNA, RNA modification, oxidative stress, translation, ribosome, ubiquitin, stress, Alzheimer disease, alkB, alkylation, mRNA surveillance, quality control, RNA damage, RNA repair
Introduction
Biomolecules possess unique chemical properties that are essential for their function. Yet, the overabundance of reactive species in both the environment and within living cells constantly threatens these properties (1, 2). Unwanted modifications to the chemical structure of nucleic acids, proteins, lipids, or carbohydrates often result in drastic effects on the ability of these molecules to carry out these functions. Changes to proteins, for example, are known to cause misfolding and aggregation, and the inability of cells to degrade these aberrant protein products has been associated with disease states (3, 4). Similarly, lipid peroxidation contributes to the pathogenicity of several diseases (5). Because of the reactivity of the oxygen and nitrogen atoms on the nucleobase, nucleic acids are especially susceptible to certain types of chemical damage from sources such as reactive oxygen species (ROS),2 UV light (UV), and alkylating agents (Fig. 1A) (1). The oxygen atoms of the ribose and the phosphodiester backbone are also vulnerable to chemical damage (Fig. 1B). In the case of DNA, and most RNA species, small changes have the ability to severely affect their structure and hence their function (6). This is because, by–and–large, nucleic acids require strict Watson-Crick base pairs between the nucleobases.
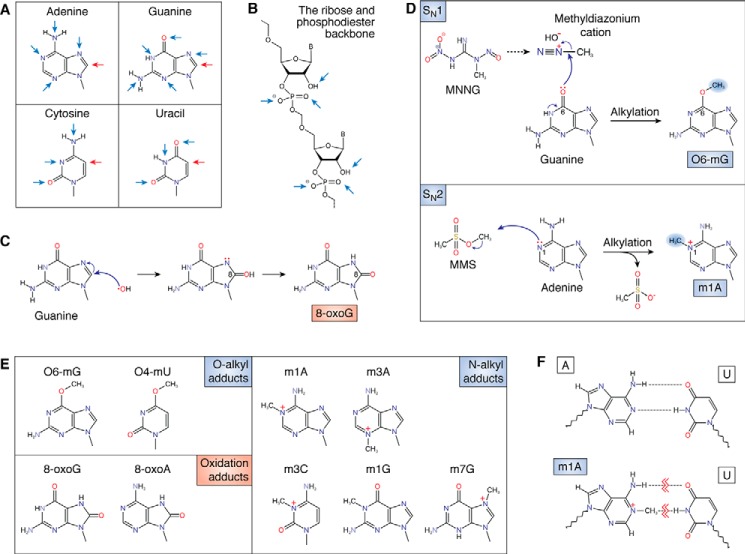
Figure 1.
Effects of alkylation and oxidation on the chemical structure of RNA. A, structures of the four RNA nucleobases with the location of common oxidation sites (red arrows) and alkylative damage sites (blue arrows) marked. B, targets of chemical insults (mainly alkylative damage) to the phosphodiester backbone and 2′-OH of the ribose. C, reaction between a hydroxyl radical and guanosine, forming the 8-oxoG adduct. D, reaction between MNNG and guanosine, which forms the O-alkyl adduct O6-mG (top). The bottom shows the reaction between methyl methanesulfonate (MMS) and adenosine forming the N-alkyl adduct m1A. E, common modified bases that result from chemical insults. Alkylative adducts, grouped by the position of modification, are as follows: O-alkyl adducts: O6-methylguanosine (O6-mG) and O4-methyluridine (O4-mU); N-alkyl adducts: N1-methyladenosine (m1A), N3-methyladenosine (m3A), N3-methylcytidine (m3C), N1-methylguanosine (m1G), and N7-methylguanosine (m7G); oxidative adducts: 8-oxo-7,8-dihydroguanosine (8-oxoG) and 8-oxo-7,8-dihydroadenosine (8-oxoA). F, damaged nucleobases disrupt base pairing. The base pairing between adenosine and uridine (top) is disrupted by the formation of the alkylative adduct m1A (bottom).
Because DNA damage can lead to permanent alteration of genomic information, it is not surprising that organisms from Escherichia coli to humans evolved exquisite and conserved pathways to repair and respond to it. Given their importance in maintaining genomic integrity together with their role in cancer biology, DNA–repair pathways have been extensively studied for more than half a century (7–9). Similar to DNA, RNA is vulnerable to the same chemical insults and, as we shall see later, is damaged to a much larger extent (1). However, until recently the processes by which cells might cope with this type of damage was largely ignored likely because of the assumption that the transient nature of RNA means that damage to it does not matter. This, for example, is true for bacteria, whose mRNAs are known to turnover rapidly with half-lives measured in minutes (10, 11). In contrast, functional RNAs, like tRNAs and rRNAs, are long lived (10, 12), and as a result, any alteration to their chemical structure could have long-lasting effects. Furthermore, many mRNAs in mammals, and especially in certain cell types like neurons, are very stable with day-long half-lives (13). Therefore, modifications to the mRNA that affect its base-pairing properties are likely to lead to defects in protein synthesis. Unless cells have pathways to recognize and degrade/repair mRNAs, chemical damage to mRNA is highly likely to result in the accumulation of aberrant protein products and/or stall ribosomes. Indeed, emerging evidence strongly suggests that quality control for damaged RNA is vital to cell survival and should not be ignored (2, 14). To this end, the accumulation of damaged RNA has been correlated with the development of several neurodegenerative diseases (1, 2, 15, 16).
This review is divided into two main sections: the first section is focused on the chemistry of different types of RNA damage, and how they affect its chemical and functional properties; the second section is focused on the cellular mechanisms that are likely to respond to damaged RNA. In particular, we discuss ribosome-dependent and -independent mechanisms of quality control of damaged mRNA as well as RNA-repair pathways.
Chemistries of RNA damage
Reactive oxygen species
The highly-reactive superoxide anion (O2˙̄) is a key component of ROS and is produced in eukaryotes under normal physiological conditions by a number of cytosolic enzymes and membrane-bound ones (17). It is essential for stress responses, maintenance of the redox state, inflammation, autophagy, and signal transduction (18). However, the levels of O2˙̄ need to be carefully monitored as high levels of superoxide are known to cause cellular damage and even death (16). High levels of O2˙̄ are produced through side reactions between molecular oxygen (O2) and reduced nicotinamide adenine dinucleotide (NADH) and FAD (FADH2) in the electron-transport chain in the mitochondria (19). Thus, the mitochondria, and hence cellular respiration, are considered as the major endogenous source of ROS production, including both O2˙̄ and hydrogen peroxide (H2O2). In addition to the mitochondria, recent studies on human liver cells reported that other organelles, such as peroxisomes, microsomes, and the endoplasmic reticulum, are also capable of producing high levels of ROS (20, 21).
The highly-reactive nature of O2˙̄ makes it exceptionally harmful because as it reacts it generates even more ROS such as hydroxyl radical (•OH), perhydroxyl radical (HO2·), and hydrogen peroxide H2O2 (22). Because of their ability to severely disrupt the integrity of biological molecules, organisms evolved multiple pathways to rid cells of ROS (23). These include superoxide dismutase, which catalyzes the dismutation of O2˙̄ into molecular oxygen and hydrogen peroxide (24), and catalase, which catalyzes the decomposition of hydrogen peroxide to water and molecular oxygen (25). Even with the presence of these mechanisms, disruption of the normal redox state of cells by exposure to oxidative agents or aging often leads to the accumulation of free radicals and peroxides resulting from an imbalance between the production and detoxification of ROS (26). Under these conditions, pathways such as Haber-Weiss, the Fenton reaction, or diethylnitrosamine (DEN) metabolism generate the highly-reactive •OH radical (27). Reactions with •OH have been suggested to be the main source of oxidative damage to nucleic acids. Besides •OH, H2O2 is known to elicit damage (28); whereas singlet-oxygen species (1O2), which are produced by wounding or photooxidative stresses, oxidize both RNA and DNA (29). In addition to these endogenous sources, exogenous factors, including X-rays and gamma-rays, UV radiation, and toxic compounds from air pollution, tobacco, and xenobiotics such as drugs, environmental agents, and natural compounds, are known to generate ROS (30).
Oxidation adducts
The reaction of •OH with free nucleobases, nucleosides, nucleotides, and nucleic acids abstracts H from C–H bonds (16, 31). This results in the production of numerous distinct modifications on nucleic acids such as 8-oxo-7,8-dihydroguanosine (8-oxoG) (Fig. 1C), 8-oxo-7,8-dihydroadenosine (8-oxoA), 5-hydroxyuridine, 5-hydroxycytidine, and cytosine glycol (16, 21, 31). Furthermore, products such as 2,6-diamino-4-hydroxy-5-formamidoguanine and 4,6-diamino-5-formamidoadenine are also formed as intermediates during the process of generating 8-oxoG and 8-oxoA (2, 32). Oxidation of other cellular components such as lipid peroxidation is also known to introduce oxidative damage to the nucleobase and generate etheno-adducts 1,N6-ethenoadenosine and 3,N4-ethenocytidine (33).
Of the oxidized adducts, oxidation of the guanine base to form 8-oxoG is most notable mainly because of its abundance and its effect on the structure of DNA and RNA. The accumulation of 8-oxoG in RNA and DNA is driven by the low reduction potential of G among all five bases (2, 21). Under normal physiological conditions, the frequency of 8-oxoG in RNA has been estimated to be 1 per 105 of unmodified G (34). This ratio increases dramatically, as much as 10-fold, under only mild oxidative stress such as that experienced during conditions of inflammation (34, 35). In addition to its abundance, the modification has a profound effect on the structure of the nucleotide and its ability to form a Watson-Crick base pair. The steric clash between the oxygen at carbon 8 and the phosphate group at carbon 5 forces the nucleotide to form the less favorable syn conformation (36). In this conformation, base pairs with A is the basis of its mutagenic potential during DNA replication (36, 37). As we shall see later, 8-oxoG severely disrupts RNA–RNA interactions and has a drastic effect on decoding during translation.
Alkylating agents
Similar to oxidizing agents, alkylating chemicals are widespread; they are found endogenously, as a result of cellular metabolism, and exogenously in the environment (38). Endogenous alkylating agents include the universal methyl donor S-adenosylmethionine (SAM), nitrosated amines, and bile acids. SAM is notable due to its relatively high cellular concentration, in the millimolar range (39), which overcomes its weak nonenzymatic reactivity with biomolecules. In vitro, SAM reacts with nucleic acids to form a number of adducts (40). In contrast to SAM, nitrosated amines and bile acids result from enzymatic side reactions (41). In starving bacterial cells, nitrosation of glycine and its derivatives forms N-alkyl–N-nitroso compounds that readily react with G to form O6-methylguanosine (O6-mG) (Fig. 1D) (42, 43). In the intestine, nitrosation of bile acids forms highly-reactive O-alkylating agents and has been associated with elevated levels of methylated bases (44).
Among environmental alkylating chemicals, halogenated hydrocarbons are the most abundant. For example, chloromethane is produced naturally by plants and fungi and artificially through industrial processes (45–47). In contrast to chloromethane, which is found predominantly in the atmosphere, bromomethane and iodomethane are mainly found in marine environments (48). These compounds readily react with nucleic acids (49), but their contribution to RNA damage is unknown given their overall low concentrations. Unlike halocarbons, N-nitroso compounds are not abundant in the environment, but they pose the most significant risk to humans because of their association with tobacco smoke and certain food compounds. Tobacco smoke contains many nitrosamines, which when metabolized by cells are activated to form potent alkylating agents (50). Food products—especially those that are overcooked, smoked, and cured—contain nitrosoamines and nitrosating agents that are carcinogenic through their mutagenic effect on DNA (51). Finally, some of the most well-known alkylating agents are those used as chemotherapeutic agents, and their reactivity with nucleic acids is the precise reason they are used to treat cancers (52). These include cyclophosphamide, streptozotocin, and Temodar, which are all known to modify RNA in addition to DNA (53). The efficacy of many of these drugs in treating cancer to a large extent relies on their ability to modify RNA and alter its function.
Alkylative adducts
Nucleobases, sugars, and the phosphate backbone of DNA and RNA harbor oxygen and nitrogen atoms that are all susceptible to modification by alkylating agents. In general, O-alkyl adducts are highly mutagenic and genotoxic (54) because they alter the base-pairing preference of the nucleobase. This is best exemplified by O6-alkylguanosine, such as O6-mG, and O4-alkylthymidine or O4-alkyluridine, such as O4-mU. O6-mG readily base pairs with U, whereas O4-mU readily bp with G (Fig. 1E) (55, 56). The geometry of both bp is indistinguishable from that of Watson-Crick ones explaining their highly mutagenic nature (57). N-Alkylation, in contrast, is generally cytotoxic because it disrupts base pairing during template-driven processes. Cytotoxic modifications include N1- and N3-alkyl adducts of A (e.g. m1A and m3A), and N3-alkyl adducts of C (e.g. m3C). These modifications alter either the Watson-Crick face of the nucleobase and introduce a bulky group, which prevents base pairing from taking place, or the minor groove, which disrupts the mechanism by which polymerases and likely the ribosome monitor the bp geometry (Fig. 1F). Interestingly, one of the most prevalent modifications that results from alkylation stress is m7G (Fig. 1E), which neither disrupts hydrogen bonding required for base pairing nor the minor groove of the bp (58). However, this modification affects the binding of proteins that recognize the major groove of helices (59) and also accelerates the depurination of the nucleotide (60). As such, m7G is likely to be detrimental to RNA function.
It is also worth noting that the chemotherapeutic drugs of doxorubicin, 5-fluorouracil and cisplatin, do not alkylate RNA directly but modify RNA through their reactions. This reactivity with RNA appears to be important for their effectiveness in treating cancer. Doxorubicin intercalates into RNA helices, which affects the activity of many functional RNAs (61). 5-Fluorouracil is metabolized by the cell into a nucleotide triphosphate that is utilized by RNA polymerases and, as a result, is incorporated into RNA (62). Cisplatin forms cross-links on the ribosome and inhibits translation (63). Recent structural studies of cisplatin-induced Pt–RNA adducts on the ribosome offered some clues about how the drug inhibits translation (64). Many of the sites on the ribosome that are modified by cisplatin encompass functionally important regions that include the GTPase center and the mRNA channel of the ribosome. Therefore, not only is RNA damage likely to pose significant challenges to organisms, but it can also be utilized to treat tumors.
Susceptibility of RNA to damage
Although RNA undergoes analogous biochemical reactions to those documented to occur between DNA and ROS or alkylating agents (2), it has been long predicted that RNA molecules, especially mRNAs, are more prone to chemical damage than DNA for various reasons. 1) mRNA is on average 30–40% single-stranded; as a result, the nucleobase on RNA is more vulnerable to chemical modification due to its unprotected Watson-Crick face. In DNA, this is protected because it participates in hydrogen bonding with the complementary strand (65–67). 2) In eukaryotic cells, unlike DNA, mRNA typically lacks the protection afforded by proteins such as histones (2, 66). 3) The nuclear compartmentation in eukaryotic cells also offers protection of DNA from exogenous agents that have to travel through the cytoplasm to access it (2, 66); in fact, the close proximity of RNA to mitochondria in the cytosol has been proposed as a potential source of oxidative damage to the RNA pool (68). 4) The pool size of ribonucleotides is hundreds of times larger than that of 2′-deoxyribonucleotides (69), and as a result modifications are more likely to be incorporated into RNA during transcription relative to DNA incorporation during replication; to this end, oxidized and alkylated NTPs have been measured to accumulate to much higher levels relative to modified dNTPs (68, 70, 71).
In support of these predictions, findings from numerous reports have revealed that the same damage to a nucleobase accumulates to much higher levels in RNA relative to DNA under both oxidative and alkylative conditions (66, 67). For instance, a recent study showed that human lung epithelial cells accumulate the oxidative adduct 8-oxoG to levels that are 14–25 times higher than 8-oxodG when challenged with hydrogen peroxide (67). Similarly, UV-A radiation induces approximately a 7-fold higher degree of RNA oxidation over DNA in human skin fibroblast cells, whereas the hepatocarcinogen 2-nitropropane leads to considerably more RNA oxidation over DNA (70, 72).
Among the different types of RNA species, the degree to which they associate with proteins and their secondary structure appears to be important to the extent that a certain RNA is vulnerable to chemical damage (73). As a case in point, a recent report indicates that mRNA harbors 5-fold higher 8-oxoG levels when compared with total RNA, which consists mainly of rRNA and tRNA that adopt complex tertiary structures and are bound by proteins (14). Interestingly, even among mRNAs and within an mRNA itself, the extent of damage varies significantly. Certain transcripts and regions within them appear to be more vulnerable to oxidative damage than others, in an abundance-independent manner (74–76). Many factors have been speculated to contribute to these differences (77). For instance, sequence identity and context play important roles, especially given that the oxidation of guanosine relies heavily on the neighboring nucleotides (77). Additionally, the level of association with RNA-binding proteins, translation efficiency, and secondary-structural differences affect the reactivity between the mRNA and damaging agents (2).
Damaged mRNA and the ribosome
A priori, chemical modification to the mRNA could have three distinct effects on its function during translation (Fig. 2). 1) Modifications that do not alter base pairing between the codon and anticodon interaction are expected to have no effect on protein synthesis. 2) Modifications that change the base-pairing preference of nucleotides are expected to result in miscoding and production of proteins with errors. 3) Modifications that disrupt base pairing altogether are expected to stall the ribosome and lead to the production of abortive protein products. Obviously the latter two are detrimental to proteostasis and potentially necessitate the evolution of pathways to cope with them. This is especially true for the last one, which, in addition to producing truncated protein products, sequesters valuable ribosomes and prevents them from carrying out their function (Fig. 2).
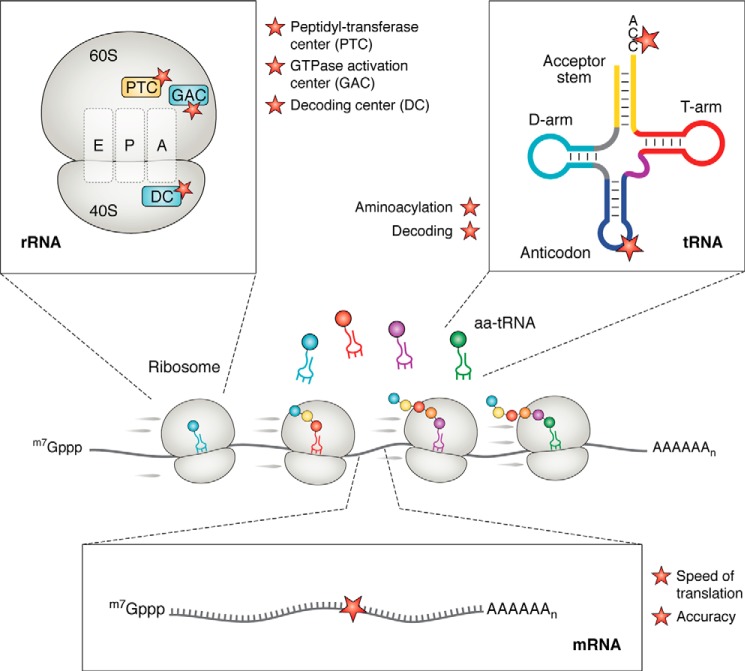
Figure 2.
Chemical damage to RNA could affect multiple steps of translation. At the center is a schematic highlighting a eukaryotic mRNA being translated. Damage might alter the structure of the rRNA, the tRNA, and the mRNA. On the rRNA, modifications could affect important functional sites of the ribosome. Shown are the PTC, the GTPase activation center (GAC), and the decoding center (DC). On the tRNA, modifications to the anticodon and acceptor stem, for example, could affect decoding and aminoacylation, respectively. On the mRNA, modifications to the coding sequence could affect the speed and accuracy of translation during elongation.
Although one could learn a lot about how modifications to the nucleobase affect translation from equivalent studies conducted on DNA polymerases, the fact that the ribosome reads three nucleotides at a time makes the decoding process somewhat different from replication. Furthermore, the RNA-rich decoding center and the mechanism by which it recognizes the codon–anticodon minihelix is quite different from the active site of polymerases, which is made entirely of amino acids (78). These distinctions between the ribosome and DNA polymerases are best exemplified by studies characterizing the effect of 8-oxoG on translation. During DNA replication, the oxidative adduct 8-oxoG is highly mutagenic, when it adopts a syn conformation and bp with A (37). During translation, the modification stalls translation and causes little to no miscoding (14). Indeed, initial studies by Shan et al. (79) found that the yield of protein synthesis from RNAs containing 8-oxoG is significantly lower than from those that do not harbor the modification. RNAs treated with hydrogen peroxide and transfected into cell culture produce significantly less functional proteins at a transcript level–independent manner (75). These observations suggest that oxidation of mRNA disrupts protein synthesis and likely stalls elongation by the ribosome. Consistent with these ideas, studies by Tanaka et al. (81) showed that oxidized mRNAs associate with polysomes but produce much less protein products. Because RNA oxidation results in a myriad of modifications, it is difficult to assess which one of these modifications is responsible for stalling the ribosome. Later studies by our group showed that 8-oxoG reduces the rate of peptide-bond formation by up to 4 orders of magnitude using a well-defined in vitro system (14). Interestingly, this effect on decoding is independent of the position of the adduct in the codon. In particular, the third position, which allows for unusual base pairing between the codon and the anticodon, is equally sensitive to the presence of the modification. Based on these observations from multiple groups, it is now generally accepted that 8-oxoG—contrary to its effect during DNA replication—does not cause miscoding, but instead it is highly disruptive to translation and leads to the generation of truncated protein products.
In contrast to RNA oxidation, which has been investigated by a number of groups, only a couple of studies have explored the effect of alkylation damage to mRNA on translation. In one study from our group, we examined the effect of O6-mG on decoding using a bacterial reconstituted system (82). This adduct is notable as it is highly mutagenic during DNA replication, whereby the O6-mG:T bp is almost indistinguishable from a normal Watson-Crick bp such that mismatch repair is unable to recognize it (57). Instead, organisms dedicate specialized “suicide” methyltransferases to recognize and repair it (83). Similarly, on the ribosome, O6-mG causes miscoding by mispairing with U but only at the first position of the codon. At the second position, the adduct was found to reduce peptide-bond formation by almost 3 orders of magnitude (82). Again, these observations highlight not only the differences between the ribosome and DNA polymerases but also the position-dependent effects of these modifications on decoding.
N-Alkyl adducts, in contrast to O-alkyl ones, tend to be more cytotoxic and less genotoxic on DNA as they lead to stalled polymerases (84). Whether these modifications stall the ribosome during translation was unknown until recently. In particular, You et al. (85) examined the effect of three N-alkyl modifications on translation efficiency and fidelity using bacterial and eukaryotic extracts. As expected, based on its deleterious effect on base pairing, m1A was found to severely reduce protein-synthesis yield regardless of its position within the codon (85). The severity of the effect, especially at the third position, highlights the deleterious effect of the substitution on the codon–anticodon interaction. Surprisingly, the modification appears to have no effect on the fidelity of translation. Interestingly, m1G inhibits protein synthesis only when placed at the first and second position of the codon (85). At the third position, the modification has no apparent effect on protein-synthesis yield, which is consistent with the promiscuity of tRNA selection at this position. Why m1A and m1G affect decoding disparately at the third position, even though the methyl group is added to same N1 of the purine, can be explained by the introduction of a positive charge in the case of m1A and not m1G. These observations suggest that, in addition to the effect on hydrogen-bonding interactions, other parameters, like charge differences, need to be taken into account when assessing how modifications might affect tRNA selection. Finally, N-alkyl modifications that do not interfere with Watson-Crick base-pairing interactions have a modest effect on protein-synthesis yield and fidelity (85). These include “unintentional” modifications, which are not known to occur naturally in mRNAs such as m2G, and “intentional” ones, which occur naturally in mRNAs such as m6A (83, 86).
Ribosome-based quality control of aberrant mRNAs that stall translation
As highlighted earlier, damage to mRNA, depending on its type, is highly detrimental to its decoding capacity, and unless dealt with, it could lead to the production of toxic protein products. Even more detrimental is the ability of damaged mRNA to drastically affect ribosome homeostasis through stalling. In particular, because multiple ribosomes typically occupy one mRNA during translation, a single stalling event, such as the presence of a single 8-oxoG modification, is likely to lead to queuing of multiple ribosomes (Fig. 2), sequestering them from the translating pool. This, in turn, would lead to greatly diminished translation capacity for cells, especially those under oxidative stress. Therefore, the inability of cells to recognize and resolve translational-stalling events is detrimental to proteostasis and could even lead to disease development (86, 87). In this section, we discuss the role of the ribosome in recognizing and targeting defective mRNAs for degradation.
Recent studies have identified conserved processes in eukaryotes that evolved to resolve these stalling events and rescue stuck ribosomes (Fig. 3) (for review see Refs. 88–90). In addition, these processes are coupled to the degradation of the aberrant mRNA and incomplete peptide product. As a result, the ribosome appears to be at the center of quality control of aberrant mRNAs and the encoded protein products (88–90). We note that although these processes have almost exclusively been studied in the context of artificial reporters that harbor genetically-encoded stalling sequences, one could easily infer that they are also likely to be activated in response to chemically-modified mRNAs. Indeed, the inactivation of some of these processes leads to the accumulation of damaged mRNA and renders cells sensitive to chemical insults (14).
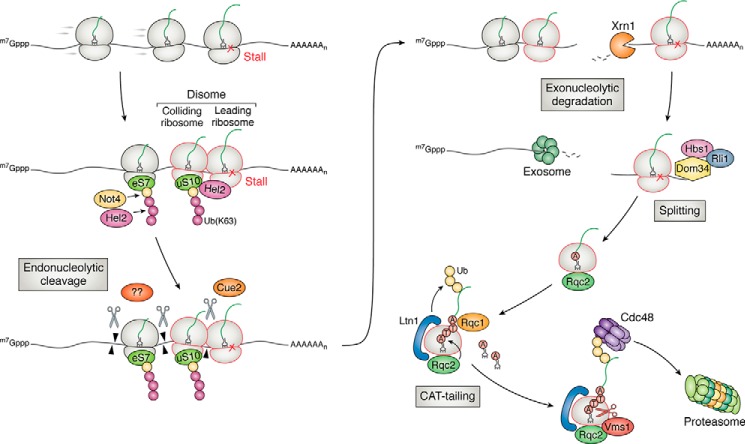
Figure 3.
Overview of ribosome-based quality control of aberrant mRNAs in yeast. Ribosomes stall on an aberrant transcript (such as a damaged one), resulting in ribosome collisions. The unique structural feature of the collided ribosomes is recognized by the E3 ligase Hel2 for ubiquitination of multiple targets, including uS10 and eS7 on the 40S subunit. The ubiquitinated ribosomes are recognized by a number of factors, which are hypothesized to recruit an unknown endonuclease to cleave the mRNA and initiate NGD. In a secondary branch of NGD, Cue2 cleaves the mRNA in the A site of the collided ribosome. The cleaved transcript is degraded by Xrn1 and the exosome. The resulting ribosomes are rescued by Dom34, Hbs1, and Rli1. The incomplete peptide attached to the peptidyl-tRNA on the 60S subunit is recognized and ubiquitinated by another E3 ligase Ltn1. C-terminal alanine and threonine residues (CAT tails) are added to the nascent peptide by Rqc2 to help expose lysine residues to the active site of Ltn1 and/or mark the nascent peptide for degradation in an Ltn1-independent manner. Released from the ribosome by Vms1, the ubiquitinated polypeptides are presented to the proteasome for degradation by Cdc48 as facilitated by Rqc1.
In eukaryotes, quality-control processes that are triggered by stalling events accomplish three tasks to alleviate stalling-induced translational stresses: 1) degradation of the aberrant mRNA; 2) degradation of the nascent peptide; and 3) ribosome rescue (Fig. 3). mRNA degradation proceeds through a process called no-go decay (NGD), which initiates with an endonucleolytic cleavage of the mRNA (91, 92). Protein degradation is mediated by a process termed ribosome-quality control (RQC), which involves ubiquitination of the incomplete peptide and subsequent proteolysis by the proteasome (93–95). Ribosome rescue is carried out by three factors: Dom34 in yeast (Pelo in mammals) and Hbs1 and Rli1 (ABCE1 in mammals) that bind the A site of the ribosome and catalyze the dissociation of the two subunits (96–98).
These three processes are triggered by the same originating signal of ribosome stalling, but how this signal is propagated to activate them and coordinate these activities remained poorly understood until recently. Initial clues came out of studies showing a ubiquitin E3 ligase (Hel2 in yeast and Znf598 in mammals) is required for stalling (99, 100). In particular, its deletion results in ribosomes reading through stall sequences (99, 101). Hel2 was later found to add Lys-63–linked ubiquitin chains to a number of ribosomal proteins, and this activity requires the presence of the ribosome-bound factor Asc1 in yeast (or RACK1 in mammals) (102, 103). Some of the first studies of Hel2 suggested that it recognizes an unusual conformation that the ribosome adopts during stalling (104). However, a later study by our group showed that ubiquitination by the factor requires ribosomes to collide into each other, which is also critical for NGD to occur (105). Subsequent cryoEM structures from Hegde and co-workers (106) and Inada and co-workers (107) provided a molecular rationale for these observations. Collided ribosomes provide an interface that can be recognized by the E3 ligase to ubiquitinate its target proteins. In agreement with these observations, Znf598 and Hel2 appear to prefer higher-order structures of polysomes during their ubiquitination reactions (106, 107).
How ubiquitination mediates the downstream events of NGD, RQC, and ribosome rescue is not fully understood. However, we are beginning to learn about how certain ribosomal proteins ubiquitination contributes to these processes. In particular, in a recent study, Inada and co-workers showed that ubiquitination is required for the activation of both NGD and RQC (107). Deletion of HEL2 saves the aberrant mRNA and prevents the nascent peptide from degradation. NGD, in contrast, is divided into two branches depending on the identity of the ubiquitinated ribosomal protein (107). The first branch called the RQC-dependent branch requires the ubiquitination of ribosomal protein uS10 (107). In this branch of NGD, the cleavage occurs in the vicinity of the lead ribosome. The second branch of NGD, whose efficiency depends on whether the first one takes place or not, is called RQC-independent, and it requires monoubiquitination of ribosomal protein eS7 by another E3 ligase, Not4, and subsequent Lys-63 ubiquitination by Hel2. In this branch of NGD, cleavage takes place well-upstream of the lead ribosome (Fig. 3) (107). These findings strongly suggest cleavage of the mRNA is mediated by at least two endonucleases. How these ubiquitination reactions recruit the endonucleases is currently unknown.
As stated before, NGD is initiated by endonucleolytic cleavage reactions in the vicinity of stalled ribosomes (Fig. 3). This leads to the production of at least two mRNA fragments: a 5′-end one that lacks a poly(A) tail and is rapidly degraded by the cytoplasmic exosome, and a 3′-end one that lacks a cap structure and is rapidly degraded by the major 5′-3′ exonuclease Xrn1 (91, 92). The identity of the endonucleases remained unknown for a long time, but in a very recent study, D'Orazio et al. (108) identified the conserved factor Cue2 as one of the endonucleases that contributes to NGD. Using a reverse-genetic screen, overexpression of Cue2 was found to reduce the level of protein production from a stalling reporter suggesting that it contributes to NGD. The overexpression screen was necessary to identify Cue2's activity because of the two branches of NGD and the potential existence of a second endonuclease. In particular, in the absence of Cue2, NGD still occurs in a process that depended on Xrn1 (108). Only in the absence of Xrn1 does the Cue2-dependent branch dominate (108). Arguably, what makes Cue2 interesting is its domain architecture. The factor contains CUE (coupling of ubiquitin to ER degradation) ubiquitin-binding domains and putative-UBA (ubiquitin-associated domain) domains, which could allow it to bind ubiquitin and hence be recruited to ubiquitinated-stalled ribosomes. Furthermore, the C-terminal domain of the enzyme contains a small MutS-related (SMR) hydrolase domain. Some SMR-containing plant proteins exhibit an endonucleolytic activity. Indeed, D'Orazio et al. (108) showed conserved residues in this domain are important for NGD in vivo. Perhaps most interesting about Cue2's SMR domain is its likelihood to adopt a structure similar to the bacterial initiation factor 3 (IF3) based on modeling studies. IF3 binds the ribosome near the P and A site close to the mRNA-entry channel. In sum, Cue2 has ubiquitin-binding domains and harbors an SMR domain with a putative endonuclease activity that could bind the ribosome close to where the mRNA would reside. Indeed, ribosome profiling by the same group showed the factor to cleave mRNA in the A site of the collided ribosome (108).
Ribosome rescue is most likely to proceed after the initial cleavage as the ribosome runs to the end of the mRNA and ends up displaying an empty A site and has a peptidyl-tRNA in the P site. As we shall see, later dissociation of the two subunits needs to be completed for RQC to occur. Two pieces of evidence suggest that rescue occurs after the initial cleavage. First, structural and biochemical studies have shown a requirement for little to no mRNA downstream of the P site in order for the dissociation reaction to take place efficiently (96, 97). The N-terminal domain of the GTPase Hbs1, which is required for the rescue reaction, binds in the mRNA-entry channel and would sterically clash with the mRNA if it were present (109). Additionally, a steric clash between Dom34 and the mRNA would exist, preventing both factors from binding unless the mRNA is cleaved (110). Second, deletion of Dom34 results in multiple secondary cleavage reactions that take place further upstream of the initial stall sequence (105, 111). Hence, inhibition of subunit dissociation leads to a ribosome pile up, which results in spreading of ubiquitination by Hel2 and more cleavage reactions by Cue2 and the second unknown endonuclease (Fig. 3).
Following ribosome rescue, the incomplete peptide attached to the peptidyl-tRNA remains associated with the large ribosome subunit (60S) (Fig. 3). The peptidyl-tRNA–bound 60S constitutes an unusual complex that is easily recognized by components of RQC (94, 95). The E3 ligase Ltn1 (Listerin in humans) recognizes this atypical form of the 60S subunit and, in addition to preventing the reassociation of the 40S subunit, adds Lys-48–linked ubiquitin to the peptidyl-tRNA (93, 94). Additional specificity is afforded by Rqc2, which along with making contacts with the tRNA binds an interface on the 60S subunit that would otherwise be used to bind the 40S subunit (Fig. 3). Rqc2 exhibits an unusual activity, for which it adds untemplated alanine and threonine residues (112, 113). The role of this so-called CAT (C-terminal alanine threonine) tagging is controversial. One group suggested that it is used to promote protein aggregation, which in turn is used to signal stress (114). Another group suggested that it is used to move the peptide outside the exit tunnel to place lysine residues of the nascent peptide substrate near the active site of LTN1 (115). In a more recent study, Sitron and Brandman (116) suggested that in addition to aiding Ltn1 in recognizing structured proteins, CAT tails serve as a degradation signal for incomplete peptides and as a failsafe for peptides that escape Ltn1 (116). Regardless of the role of CAT tailing, the peptidyl-tRNA, once ubiquitinated, needs to be released from the ribosome and presented to the proteasome. This is accomplished by Vms1 in yeast (AZKF1 in mammals) (Fig. 3). Initial data suggested that the factor acts like a peptidyl hydrolase, hydrolyzing the ester bond between the peptide and the tRNA (117). More recent biochemical data, however, showed that the factor instead cleaves a phosphodiester bond in the acceptor stem of the tRNA, functioning as an endonuclease (118). Following this cleavage reaction, the ubiquitinated peptide is presented to the proteasome for degradation by Cdc48 in a process that requires Rqc1 (Fig. 3) (95, 119). How the tRNA remnant is removed from the peptide is currently unknown.
Ribosome-independent recognition of damaged mRNA
As detailed above, the ribosome plays a critical role in recognizing aberrant mRNAs and targeting them for degradation, which makes sense because it is the only machine that sees every single mRNA. Nevertheless, it appears that organisms may have evolved secondary mechanisms to recognize and degrade modified mRNA (1, 2). The notion that certain RNA-binding proteins are able to recognize a single modification on an RNA is best exemplified by the YTHDF class of proteins, which recognize m6A (120). For example, YTHDF2 binds m6A-decorated mRNAs and delivers them to P bodies, where they are hypothesized to be degraded (121, 122). It should be noted that this modification is not a damage mark, but instead it serves a regulatory role for gene expression (for review see Refs. 123–125).
Some of the first studies aimed at identifying ribosome-independent factors that recognize damaged adducts focused on 8-oxoG. These studies revealed that bacteria and eukaryotes possess factors that bind 8-oxoG–containing mRNAs and target them for degradation. For instance, in bacteria, the 3′–5′-exonuclease polynucleotide phosphorylase was reported to have a preference for oxidized RNAs to target them for rapid degradation (126, 127). In humans, the RNA-binding protein YB-1 displays a specificity for 8-oxoG–containing mRNAs and is likely to promote their degradation (128). In agreement with a role for this factor in damaged mRNA quality control, its overexpression renders bacteria more resistant to oxidative stress (128). However, the protein is known to sequester RNAs into stress granules, which could offer a protective environment from chemical insults (127). As a result, whether the protective phenotype induced by YB-1 overexpression is due to an mRNA-insulating or mRNA quality-control activity is unknown. Regardless of how YB-1 functions in the quality control of damaged RNA, these observations suggest that compartmentalization of RNA during stress is likely to serve as an important mechanism for protecting it from chemical insults.
More recent studies, using 8-oxoG–containing RNAs as bait, identified a new class of proteins that bind oxidized mRNA (129). The most interesting quality-control candidate to come out of this study was AUF1 (also called HNRNPD) (130). Not only does AUF1 bind 8-oxoG–containing mRNA specifically, in its absence oxidized mRNAs are stabilized, suggesting that the factor might be responsible for their degradation (130).The same group that identified a role for AUF1 in targeting oxidized mRNA for degradation also identified another 8-oxoG–RNA-specific binding protein PCBP1 (131). In contrast to AUF1, which recognizes RNAs with one 8-oxoG, PCBP1 recognizes heavily oxidized RNAs. The factor does not promote the degradation of its target, but instead induces cell death. Mutations in one of the two RNA-binding KH domains, which abolish 8-oxoG binding, suppress the induction of apoptosis-related reactions, suggesting that its RNA-binding activity is required for the induction of programmed cell death (131). These observations highlight the ability of cells to use damaged mRNA as a signal for stress. Interestingly, this PCBP1-mediated response is only activated in the presence of heavily modified RNAs (131), which in turn only happens if the oxidative stress is excessive. Under these conditions, it would make sense to induce apoptosis. Whether cells have evolved factors that recognize other types of modifications, such as alkylated ones to sense chemical insults and alert surveillance systems, is currently unknown.
RNA repair
Until now our discussion has focused on quality-control pathways that degrade aberrant mRNAs. The transient nature of the molecule makes the utilization of such processes appropriate. However, as the synthesis of mRNAs requires energy, these degradative pathways are costly. Therefore, pathways that could repair mRNAs would save cells energy and be evolutionarily beneficial. It is worth noting that a precedent for this exists for repair of damaged proteins. Like RNA, proteins are transient, and when damaged they are typically degraded through proteasome action or autophagy (132–135). Of the 20 canonical amino acids, cysteine and methionine are most susceptible to ROS. Oxidation of methionine results in methionine sulfoxide (136), whose accumulation may contribute to the progression of neurodegenerative disease (137). The enzymes methionine sulfoxide reductases are able to repair oxidized methionine (138). Therefore, it would not be surprising if cells evolved pathways to repair RNA.
Although similar to DNA, RNA lacks a complementary strand and a homologous partner. As a result, RNA-repair pathways may not use most of the mechanisms used for DNA repair, which take advantage of unaltered information contained within the complementary strand or the homologous chromosome. This leaves at least two direct reversal pathways to potentially repair RNA: 1) methylguanine methyltransferases (MGMT), which repair O6-mG (139), and 2) the oxidative demethylases, which repair m1A and m3C (65, 140). Currently, there are no reports that implicate the MGMT class of proteins in RNA repair, and given the suicide nature of these enzymes it is difficult to imagine why cells would utilize this strategy to repair O6-mG–containing RNA. In contrast, the AlkB family of oxidative demethylases is involved in tRNA modification (141). These natural tRNA modifications, such as m1A and m3C, can also be products of damage, suggesting that these enzymes are capable of repairing damage-induced adducts. This is beside the fact that many of the AlkB enzymes prefer ssRNA (and ssDNA) as substrates in vitro (142). This is especially true for the founding member of the family, the bacterial AlkB, which is capable of reactivating alkylated MS2 RNA phage in vivo (65, 143). The human homologue of AlkB, hAlkB3, displays a similar substrate specificity toward ssRNA (65, 144), suggesting that RNA repair is likely to be conserved. Perhaps the most convincing argument about the existence of RNA–repair pathways and their utility for cellular fitness is the observation that many RNA viruses encode Alkb-like proteins within their RNA genomes (2, 145). Given how compact the RNA genomes of these viruses are, this suggests that the process of RNA repair is very important to maintain the integrity of the polymer.
mRNA damage and disease
In the previous sections, we discussed the susceptibility of RNA to damage and how it impacts the chemical properties and function of the molecule. Naturally, the next question is whether these alterations have any profound effect on organismal fitness. In this section, we discuss the connections between RNA damage and several disease states. Because an mRNA is translated multiple times, and in some cases more than a thousand times, a damaged mRNA has the potential of producing significant amounts of aberrant protein products. As defective protein products are more likely to misfold and aggregate, damaged mRNAs would pose significant challenges to tissues that are more sensitive to protein misfolding. As a result, neurons, with their long lifespan, appear to be the most vulnerable to RNA damage (146). Indeed, more than 2 decades ago, a link between neurodegeneration and RNA oxidation was uncovered (68). Since then, many studies have shown that oxidized RNAs accumulate to very-high levels in neurons during neurodegeneration as well as through aging (147). In one of the initial studies, Nunomura et al. (68) demonstrated that RNA oxidation is a distinct feature of neuronal vulnerability to oxidative stress, as samples from brain tissue of Alzheimer's disease (AD) patients accumulated high levels of 8-oxoG in both cytoplasmic and nuclear RNA (68). A more recent study revealed that neuronal mRNA oxidation in patients with AD can reach as much as 50–70% for some transcripts, whereas the percentage is less than 2% of the total mRNA pool of healthy individuals (147). Aside from AD, the accumulation of 8-oxoG in cellular RNAs has been reported in several neurodegenerative disorders (74, 147–151). For instance, in patients with Parkinson's disease, the presence of 8-oxoG can be observed in cerebrospinal fluid and serum, and an increase in the levels of 8-oxoG was found in substantia nigra compared with other brain regions (149, 152). Highlighting the significance of RNA oxidation for neurodegeneration, the levels of oxidized RNAs have the potential to be used as a diagnostic marker for certain diseases. In particular, oxidized CuZn-superoxide dismutase mRNA can be used as an early pre-clinical feature of amyotrophic lateral sclerosis (74). Given that a hallmark of many neurodegenerative diseases is increased oxidative stress, it should be noted that the accumulation of oxidized RNA under these conditions could be a side effect of the disease pathogenicity. A direct causal relationship between oxidized mRNA and pathogenicity of neurodegenerative disease remains ambiguous (2) and should be the focus of future research. These are important avenues to explore, especially in light of a convincingly-demonstrated causal connection between the inability of cells to resolve stalled ribosomes and neurodegeneration (87).
Damage to noncoding RNAs and its effect on their functions
So far, the focus of our discussion has been on mRNA damage, but the bulk of the RNA in cells, ∼95%, is noncoding (153, 154). These RNAs carry out important functions in the cell, which include translation, splicing, transcription, RNA-directed modification, and regulation of gene expression (155, 156). For instance, rRNA and tRNA are essential for translation, and given their long-lived nature, modifications that interfere with their function could have drastic consequences on cellular homeostasis. Unlike mRNA, studies addressing the effect of chemical damage to tRNA and rRNA on their function are sparse. However, we note that unlike mRNAs, for which the majority of the nucleotides are seen by the ribosome, changes to the majority of the nucleotides of rRNA would be expected to be tolerated (Fig. 2). Most of the functional sites of the rRNA are buried within the ribosome, making them less accessible to reactive chemicals. Nucleotides on the surface, which are accessible to modification, tend to be less conserved and are often functionally unimportant. Nevertheless, recent studies by Polacek and co-workers (157) showed that the peptidyl-transferase center (PTC) of the ribosome is susceptible to oxidation. Using atomic mutagenesis, the authors were able to show that placing a single 8-oxoA within the PTC inhibits peptide-bond formation, suggesting that oxidation of the ribosome could severely disrupt its function (157). Equivalent studies on the effect of modification to tRNA are lacking. Nevertheless, changes to a tRNA's anticodon that do not affect the aminoacylation reaction but change the base-pairing preference would be expected to cause significant miscoding (Fig. 2). Interestingly, tRNAs harbor modifications that contain sulfur substitutions that are very sensitive to the redox state of cells (158). Many of these reside in the anticodon, and their oxidation is known to affect their base-pairing properties (158).
How cells cope with chemical damage to rRNA and tRNA is largely unknown. It is, however, evident that cells have the ability to recognize defective rRNA and tRNA molecules. In the case of rRNA, nonfunctional rRNA decay (NRD) targets defective ribosomes for rapid turnover (159, 160). NRD has been studied in the context of ribosomes harboring mutations in the PTC of the 25S rRNA and the decoding center of the 18S rRNA. Interestingly, the process by which cells rid themselves of defective small subunits is distinct from that used to degrade defective large subunits. For example, 18S NRD depends on translation, whereas 25S NRD does not. Furthermore, 18S NRD is intimately coupled to mRNA surveillance as it utilizes factors that are used during NGD. In contrast, 25S NRD employs distinct factors and takes place prior to the assembly of the 80S subunit in perinuclear foci (159, 160). Because 18S and 25S NRD recognize defective ribosomes that are unable to carry out protein synthesis, it is tempting to speculate that these processes are induced in response to chemically-damaged ribosomes. Future research should be focused on exploring this potential connection and the utility of these processes in ridding cells of oxidized, alkylated, and cross-linked ribosomes.
Similar to rRNA, defective tRNA molecules are also subject to quality control that ensures that they are rapidly degraded before they can participate in translation. In particular, eukaryotes evolved the process of rapid-tRNA decay (RTD), which targets aberrant tRNAs (161, 162). RTD has been mainly studied in the context of misprocessed tRNAs especially those lacking important post-transcriptional modifications. These tRNAs are subject to rapid decay by Rat1 in the nucleus and Xrn1 in the cytoplasm (161, 162). It is generally accepted that these factors are able to recognize changes to the post-transcriptional modification status of the tRNA through changes to its overall secondary structure. As alkylation and oxidation of the tRNA nucleobases are likely to induce a profound effect on the overall structure of the molecule, it is highly likely that RTD is also responsible for recognizing chemical damage to tRNA molecules.
What about other classes of noncoding RNAs? Almost all of these require base pairing to carry out their function. Many of them are also long lived so that any changes that alter their function would be expected to linger unless cells have pathways to deal with them. For example, snRNAs are used by the spliceosome to identify splice–junction sites through base pairing (163); changes to this class of RNAs is likely to have profound effects on splicing and hence gene expression. Similarly, snoRNAs are used to guide 2′-O-methylation and pseudouridylation on many RNAs (164), and changes to their base-pairing properties could affect the specificity of these reactions and likely be detrimental to the function of the target molecule. As a case in point of the downstream effects of RNA modifications to functional RNAs, Wang et al. (80) showed that oxidation of a microRNA alters its targets and induces apoptosis. How cells cope with chemical damage to these classes of RNAs—be it snRNAs, snoRNAs, and microRNAs—is unclear, and whether they evolved pathways to degrade/repair them should be the subject of future research.
Concluding remarks
As outlined here, we have been aware of the vulnerability of nucleic acids to chemical insults for more than half a century. We have also known how this type of damage would alter their chemical properties and interactions with other molecules. In particular, most modifications affect the base-pairing potential of nucleic acids, which in turn is arguably their most important feature. Interestingly, however, although DNA damage has been systematically studied for decades, RNA damage in contrast has received little attention. Of course, the importance of maintaining genomic integrity supersedes that of RNA, but that cannot fully justify this disparity. RNA plays a central role in many fundamental biological processes, and most of these depend on its ability to base pair correctly. These include intermolecular ones like those between tRNA and mRNA during translation, snRNA and pre-mRNA during splicing, and snoRNA and rRNA during RNA-guided modification; but also the intramolecular ones that are responsible for maintaining the structural integrity of complex molecules like the ribosome. It is not surprising then that RNA damage is detrimental to cellular fitness and has been associated with many disease states.
Recent efforts from our group and others have focused on mRNA damage. These studies have begun to highlight how damage to this molecule affects the efficiency and accuracy of protein synthesis. We are also beginning to appreciate the role of the ribosome in detecting many damage-formed adducts and triggering quality-control processes to degrade the damaged transcript. It is also clear that ribosome-independent mechanisms are at play to degrade or repair damaged mRNA that may escape recognition by the ribosome. This increased interest in how modifications affect mRNA function should hopefully motivate others to look at other species in RNA biology.
Acknowledgments
We are grateful for the careful reading of the manuscript by Carrie Simms. We thank members of the Zaher Laboratory for useful discussion on the manuscript.
This work was supported by National Institutes of Health Grant R01GM11264104 from NIGMS. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ROS
- reactive oxygen species
- 8-oxoG
- 8-oxo-7,8-dihydroguanosine
- 8-oxoA
- 8-oxo-7,8-dihydroadenosine
- SAM
- S-adenosylmethionine
- O6-mG
- O6-methylguanosine
- O4-mU
- O4-methyluridine
- NGD
- no-go decay
- snRNA
- small nuclear RNA
- snoRNA
- small nucleolar RNA
- RQC
- ribosome-quality control
- SMR
- small MutS-related
- MGMT
- methylguanine methyltransferase
- AD
- Alzheimer's disease
- PTC
- peptidyl-transferase center
- NRD
- nonfunctional rRNA decay
- RTD
- rapid-tRNA decay
- ssRNA
- single-stranded RNA.
References
- 1. Wurtmann E. J., and Wolin S. L. (2009) RNA under attack: cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 44, 34–49 10.1080/10409230802594043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simms C. L., and Zaher H. S. (2016) Quality control of chemically damaged RNA. Cell. Mol. Life Sci. 73, 3639–3653 10.1007/s00018-016-2261-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefani M. (2004) Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim. Biophys. Acta 1739, 5–25 10.1016/j.bbadis.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 4. Chondrogianni N., Petropoulos I., Grimm S., Georgila K., Catalgol B., Friguet B., Grune T., and Gonos E. S. (2014) Protein damage, repair and proteolysis. Mol. Aspects Med. 35, 1–71 10.1016/j.mam.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 5. Mylonas C., and Kouretas D. (1999) Lipid peroxidation and tissue damage. In Vivo 13, 295–309 [PubMed] [Google Scholar]
- 6. Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature. 362, 709–715 10.1038/362709a0 [DOI] [PubMed] [Google Scholar]
- 7. Fu D., Calvo J. A., and Samson L. D. (2012) Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer. 12, 104–120 10.1038/nrc3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miao Z. H., Rao V. A., Agama K., Antony S., Kohn K. W., and Pommier Y. (2006) 4-Nitroquinoline-1-oxide induces the formation of cellular topoisomerase I-DNA cleavage complexes. Cancer Res. 66, 6540–6545 10.1158/0008-5472.CAN-05-4471 [DOI] [PubMed] [Google Scholar]
- 9. Cheung-Ong K., Giaever G., and Nislow C. (2013) DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem. Biol. 20, 648–659 10.1016/j.chembiol.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 10. Deutscher M. P. (2006) Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 34, 659–666 10.1093/nar/gkj472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ross J. (1995) mRNA stability in mammalian cells. Microbiol. Rev. 59, 423–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson L. F., Levis R., Abelson H. T., Green H., and Penman S. (1976) Changes in RNA in relation to growth of the fibroblast: IV. Alterations in the production and processing of mRNA and rRNA in resting and growing cells. J. Cell Biol. 1, 161–165 10.1083/jcb.71.3.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolognani F., and Perrone-Bizzozero N. I. (2008) RNA–protein interactions and control of mRNA stability in neurons. J. Neurosci. Res. 86, 481–489 10.1002/jnr.21473 [DOI] [PubMed] [Google Scholar]
- 14. Simms C. L., Hudson B. H., Mosior J. W., Rangwala A. S., and Zaher H. S. (2014) An active role for the ribosome in determining the fate of oxidized mRNA. Cell Rep. 9, 1256–1264 10.1016/j.celrep.2014.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nunomura A., Moreira P. I., Takeda A., Smith M. A., and Perry G. (2007) Oxidative RNA damage and neurodegeneration. Curr. Med. Chem. 14, 2968–2975 10.2174/092986707782794078 [DOI] [PubMed] [Google Scholar]
- 16. Fimognari C. (2015) Role of oxidative RNA damage in chronic-degenerative diseases. Oxid. Med. Cell. Longev. 2015, 358713 10.1155/2015/358713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohal R. S., and Weindruch R. (1996) Oxidative stress, caloric restriction, and aging. Science 273, 59–63 10.1126/science.273.5271.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schieber M., and Chandel N. S. (2014) ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown G. C., and Borutaite V. (2012) Mitochondrion there is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 12, 1–4 10.1016/j.mito.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 21. Casas-Grajales S., and Muriel P. (2017) in Liver Pathophysiology: Therapies and Antioxidants, pp. 583–604, Elsevier, New York [Google Scholar]
- 22. Lü J. M., Lin P. H., Yao Q., and Chen C. (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell. Mol. Med. 14, 840–860 10.1111/j.1582-4934.2009.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imlay J. A. (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77, 755–776 10.1146/annurev.biochem.77.061606.161055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukai T., and Ushio-Fukai M. (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 15, 1583–1606 10.1089/ars.2011.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zámocký M., and Koller F. (1999) Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 72, 19–66 10.1016/S0079-6107(98)00058-3 [DOI] [PubMed] [Google Scholar]
- 26. Hopkins B. L., and Neumann C. A. (2019) Redox Biology Redoxins as gatekeepers of the transcriptional oxidative stress response. Redox Biol. 21, 101104 10.1016/j.redox.2019.101104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kehrer J. P. (2000) The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50 10.1016/S0300-483X(00)00231-6 [DOI] [PubMed] [Google Scholar]
- 28. Imlay J. A., Chin S. M., and Linn S. (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240, 640–642 10.1126/science.2834821 [DOI] [PubMed] [Google Scholar]
- 29. Neeley W. L., and Essigmann J. M. (2006) Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 19, 491–505 10.1021/tx0600043 [DOI] [PubMed] [Google Scholar]
- 30. Cadet J., and Wagner J. R. (2013) DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 5, a012559 10.1101/cshperspect.a012559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dizdaroglu M., and Jaruga P. (2012) Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 46, 382–419 10.3109/10715762.2011.653969 [DOI] [PubMed] [Google Scholar]
- 32. Gajewski E., Rao G., Nackerdien Z., and Dizdaroglu M. (1990) Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry 29, 7876–7882 10.1021/bi00486a014 [DOI] [PubMed] [Google Scholar]
- 33. Blair I. A. (2008) DNA adducts with lipid peroxidation products. J. Biol. Chem. 283, 15545–15549 10.1074/jbc.R700051200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen Z., Wu W., and Hazen S. L. (2000) Activated leukocytes oxidatively damage DNA, RNA, and the nucleotide pool through halide-dependent formation of hydroxyl radical. Biochemistry 39, 5474–5482 10.1021/bi992809y [DOI] [PubMed] [Google Scholar]
- 35. Valavanidis A., Vlachogianni T., and Fiotakis C. (2009) 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 27, 120–139 10.1080/10590500902885684 [DOI] [PubMed] [Google Scholar]
- 36. Sampoli Benítez B., Barbati Z. R., Arora K., Bogdanovic J., and Schlick T. (2013) How DNA polymerase X preferentially accommodates incoming dATP opposite 8-oxoguanine on the template. Biophys. J. 105, 2559–2568 10.1016/j.bpj.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsu G. W., Ober M., Carell T., and Beese L. S. (2004) Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase. Nature 431, 217–221 10.1038/nature02908 [DOI] [PubMed] [Google Scholar]
- 38. Drabløs F., Feyzi E., Aas P. A., Vaagbø C. B., Kavli B., Bratlie M. S., Peña-Diaz J., Otterlei M., Slupphaug G., and Krokan H. E. (2004) Alkylation damage in DNA and RNA–repair mechanisms and medical significance. DNA Repair 3, 1389–1407 10.1016/j.dnarep.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 39. Hoffman D. R., Marion D. W., Cornatzer W. E., and Duerre J. A. (1980) S-Adenosylmethionine and S-adenosylhomocysteine metabolism in isolate rat liver. J. Biol. Chem. 255, 10822–10827 [PubMed] [Google Scholar]
- 40. Rydberg B., and Lindahl T. (1982) Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-l-methionine is a potentially mutagenic reaction. EMBO J. 1, 211–216 10.1002/j.1460-2075.1982.tb01149.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. García-Santos Mdel P., Calle E., and Casado J. (2001) Amino acid nitrosation products as alkylating agents. J. Am. Chem. Soc. 123, 7506–7510 10.1021/ja010348+ [DOI] [PubMed] [Google Scholar]
- 42. Taverna P., and Sedgwick B. (1996) Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J. Bacteriol. 178, 5105–5111 10.1128/jb.178.17.5105-5111.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shuker D. E., and Margison G. P. (1997) Nitrosated glycine derivatives as a potential source of O6-methylguanine in DNA. Cancer Res. 57, 366–369 [PubMed] [Google Scholar]
- 44. Busby W. F. Jr., Shuker D. E., Charnley G., Newberne P. M., Tannenbaum S. R., and Wogan G. N. (1985) Carcinogenicity in rats of the nitrosated bile acid conjugates. Cancer Res. 45, 1367–1371 [PubMed] [Google Scholar]
- 45. Hamilton J. T., McRoberts W. C., Keppler F., Kalin R. M., and Harper D. B. (2003) Chloride methylation by plant pectin: an efficient environmentally significant process. Science 301, 206–209 10.1126/science.1085036 [DOI] [PubMed] [Google Scholar]
- 46. Gribble G. W. (1994) The natural production of chlorinated compounds. Environ. Sci. Technol. 28, 310A–319A 10.1021/es00056a712 [DOI] [PubMed] [Google Scholar]
- 47. McCulloch A., Aucott M. L., Benkovitz C. M., Graedel T. E., Kleiman G., Midgley P. M., and Li Y.-F. (1999) Global emissions of hydrogen chloride and chloromethane from coal combustion, incineration and industrial activities: reactive chlorine emissions inventory. J. Geophys. Res. Atmos. 104, 8391–8403 10.1029/1999JD900025 [DOI] [Google Scholar]
- 48. Ballschmiter K. (2003) Pattern and sources of naturally produced organohalogens in the marine environment: biogenic formation of organohalogens. Chemosphere 52, 313–324 10.1016/S0045-6535(03)00211-X [DOI] [PubMed] [Google Scholar]
- 49. Bolt H. M., and Gansewendt B. (1993) Mechanisms of carcinogenicity of methyl halides. Crit. Rev. Toxicol. 23, 237–253 10.3109/10408449309105011 [DOI] [PubMed] [Google Scholar]
- 50. Hecht S. S. (1999) DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. Mol. Mech. Mutagen. 424, 127–142 10.1016/S0027-5107(99)00014-7 [DOI] [PubMed] [Google Scholar]
- 51. Song P., Wu L., and Guan W. (2015) Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 7, 9872–9895 10.3390/nu7125505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hurley L. H. (2002) DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer 2, 188–200 10.1038/nrc749 [DOI] [PubMed] [Google Scholar]
- 53. Bellacosa A., and Moss E. G. (2003) RNA repair: damage control. Curr. Biol. 13, R482–R484 10.1016/S0960-9822(03)00408-1 [DOI] [PubMed] [Google Scholar]
- 54. Margison G. P., Santibáñez Koref M. F., and Povey A. C. (2002) Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis 17, 483–487 10.1093/mutage/17.6.483 [DOI] [PubMed] [Google Scholar]
- 55. Preston B. D., Singer B., and Loeb L. A. (1986) Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc. Natl. Acad. Sci. 83, 8501–8505 10.1073/pnas.83.22.8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eadie J. S., Conrad M., Toorchen D., and Topal M. D. (1984) Mechanism of mutagenesis by O6-methylguanine. Nature 308, 201–203 10.1038/308201a0 [DOI] [PubMed] [Google Scholar]
- 57. Warren J. J., Forsberg L. J., and Beese L. S. (2006) The structural basis for the mutagenicity of O6-methyl-guanine lesions. Proc. Natl. Acad. Sci. 103, 19701–19706 10.1073/pnas.0609580103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soll J. M., Sobol R. W., and Mosammaparast N. (2017) Regulation of DNA alkylation damage repair: lessons and therapeutic opportunities. Trends Biochem. Sci. 42, 206–218 10.1016/j.tibs.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ezaz-Nikpay K., and Verdine G. L. (1994) The effects of N7-methylguanine on duplex DNA structure. Chem. Biol. 1, 235–240 10.1016/1074-5521(94)90016-7 [DOI] [PubMed] [Google Scholar]
- 60. Boysen G., Pachkowski B. F., Nakamura J., and Swenberg J. A. (2009) The formation and biological significance of N7-guanine adducts. Mutat. Res. Toxicol. Environ. Mutagen. 678, 76–94 10.1016/j.mrgentox.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu K., Henning D., Iwakuma T., Valdez B. C., and Busch H. (1999) Adriamycin inhibits human RH II/Gu RNA helicase activity by binding to its substrate. Biochem. Biophys. Res. Commun. 266, 361–365 10.1006/bbrc.1999.1815 [DOI] [PubMed] [Google Scholar]
- 62. Pettersen H. S., Visnes T., Vågbø C. B., Svaasand E. K., Doseth B., Slupphaug G., Kavli B., and Krokan H. E. (2011) UNG-initiated base excision repair is the major repair route for 5-fluorouracil in DNA, but 5-fluorouracil cytotoxicity depends mainly on RNA incorporation. Nucleic Acids Res. 39, 8430–8444 10.1093/nar/gkr563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heminger K. A., Hartson S. D., Rogers J., and Matts R. L. (1997) Cisplatin inhibits protein synthesis in rabbit reticulocyte lysate by causing an arrest in elongation. Arch. Biochem. Biophys. 344, 200–207 10.1006/abbi.1997.0198 [DOI] [PubMed] [Google Scholar]
- 64. Melnikov S. V., Söll D., Steitz T. A., and Polikanov Y. S. (2016) Insights into RNA binding by the anticancer drug cisplatin from the crystal structure of cisplatin-modified ribosome. Nucleic Acids Res. 44, 4978–4987 10.1093/nar/gkw246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aas D. P. Jr., Otterlei M., Falnes P. O., Vågbø C. B., Skorpen F., Akbari M., Sundheim O., Bjørås M., Slupphaug G., Seeberg E., and Krokan H. E. (2003) Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421, 859–863 10.1038/nature01363 [DOI] [PubMed] [Google Scholar]
- 66. Cobley J. N., Fiorello M. L., and Bailey D. M. (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 15, 490–503 10.1016/j.redox.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hofer T., Badouard C., Bajak E., Ravanat J. L., Mattsson A., and Cotgreave I. A. (2005) Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 386, 333–337 10.1515/BC.2005.040 [DOI] [PubMed] [Google Scholar]
- 68. Nunomura A., Perry G., Pappolla M. A., Wade R., Hirai K., Chiba S., and Smith M. A. (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J Neurosci. 19, 1959–1964 10.1523/JNEUROSCI.19-06-01959.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Traut T. W. (1994) Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140, 1–22 10.1007/BF00928361 [DOI] [PubMed] [Google Scholar]
- 70. Fiala E. S., Conaway C. C., and Mathis J. E. (1989) Oxidative DNA and RNA damage in the livers of Sprague-Dawley rats treated with the hepatocarcinogen 2-nitropropane. Cancer Res. 49, 5518–5522 [PubMed] [Google Scholar]
- 71. Hofer T., Seo A. Y., Prudencio M., and Leeuwenburgh C. (2006) A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol. Chem. 387, 103–111 10.1515/BC.2006.014 [DOI] [PubMed] [Google Scholar]
- 72. Wamer W. G., and Wei R. R. (1997) In vitro photooxidation of nucleic acids by ultraviolet A radiation. Photochem. Photobiol. 65, 560–563 10.1111/j.1751-1097.1997.tb08605.x [DOI] [PubMed] [Google Scholar]
- 73. Görg B., Qvartskhava N., Keitel V., Bidmon H. J., Selbach O., Schliess F., and Häussinger D. (2008) Ammonia induces RNA oxidation in cultured astrocytes and brain in vivo. Hepatology 48, 567–579 10.1002/hep.22345 [DOI] [PubMed] [Google Scholar]
- 74. Chang Y., Kong Q., Shan X., Tian G., Ilieva H., Cleveland D. W., Rothstein J. D., Borchelt D. R., Wong P. C., and Lin C. L. (2008) Messenger RNA oxidation occurs early in disease pathogenesis and promotes motor neuron degeneration in ALS. PLoS ONE 3, e2849 10.1371/journal.pone.0002849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shan X., Tashiro H., and Lin C. L. (2003) The identification and characterization of oxidized RNAs in Alzheimer's disease. J. Neurosci. 23, 4913–4921 10.1523/JNEUROSCI.23-12-04913.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McKinlay A., Gerard W., and Fields S. (2012) Global analysis of RNA oxidation in Saccharomyces cerevisiae. BioTechniques 52, 109–111 10.2144/000113801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Núñez M. E., Hall D. B., and Barton J. K. (1999) Long-range oxidative damage to DNA: effects of distance and sequence. Chem. Biol. 6, 85–97 10.1016/S1074-5521(99)80005-2 [DOI] [PubMed] [Google Scholar]
- 78. Zaher H. S., and Green R. (2009) Fidelity at the molecular level: lessons from protein synthesis. Cell 136, 746–762 10.1016/j.cell.2009.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shan X., Chang Y., and Lin C. L. (2007) Messenger RNA oxidation is an early event preceding cell death and causes reduced protein expression. FASEB J. 21, 2753–2764 10.1096/fj.07-8200com [DOI] [PubMed] [Google Scholar]
- 80. Wang J.-X., Gao J., Ding S.-L., Wang K., Jiao J.-Q., Wang Y., Sun T., Zhou L.-Y., Long B., Zhang X.-J., Li Q., Liu J.-P., Feng C., Liu J., Gong Y., Zhou Z., and Li P.-F. (2015) Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol. Cell 59, 50–61 10.1016/j.molcel.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 81. Tanaka M., Chock P. B., and Stadtman E. R. (2007) Oxidized messenger RNA induces translation errors. Proc. Natl. Acad. Sci. U.S.A. 104, 66–71 10.1073/pnas.0609737104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hudson B. H., and Zaher H. S. (2015) O6-Methylguanosine leads to position-dependent effects on ribosome speed and fidelity. RNA 21, 1648–1659 10.1261/rna.052464.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moore M. H., Gulbis J. M., Dodson E. J., Demple B., and Moody P. C. (1994) Crystal structure of a suicidal DNA repair protein: the Ada O6-methylguanine-DNA methyltransferase from E. coli. EMBO J. 13, 1495–1501 10.1002/j.1460-2075.1994.tb06410.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malvezzi S., Farnung L., Aloisi C. M. N., Angelov T., Cramer P., and Sturla S. J. (2017) Mechanism of RNA polymerase II stalling by DNA alkylation. Proc. Natl. Acad. Sci. U.S.A. 114, 12172–12177 10.1073/pnas.1706592114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. You C., Dai X., and Wang Y. (2017) Position-dependent effects of regioisomeric methylated adenine and guanine ribonucleosides on translation. Nucleic Acids Res. 45, 9059–9067 10.1093/nar/gkx515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chu J., Hong N. A., Masuda C. A., Jenkins B. V., Nelms K. A., Goodnow C. C., Glynne R. J., Wu H., Masliah E., Joazeiro C. A., and Kay S. A. (2009) A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 106, 2097–2103 10.1073/pnas.0812819106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ishimura R., Nagy G., Dotu I., Zhou H., Yang X.-L., Schimmel P., Senju S., Nishimura Y., Chuang J. H., and Ackerman S. L. (2014) Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 345, 455–459 10.1126/science.1249749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Simms C. L., Thomas E. N., and Zaher H. S. (2017) Ribosome-based quality control of mRNA and nascent peptides. Wiley Interdiscip. Rev. RNA 8, e1366 10.1002/wrna.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brandman O., and Hegde R. S. (2016) Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 23, 7–15 10.1038/nsmb.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shoemaker C. J., and Green R. (2012) Translation drives mRNA quality control. Nat. Struct. Mol. Biol. 19, 594–601 10.1038/nsmb.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Doma M. K., and Parker R. (2006) Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 10.1038/nature04530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsuboi T., Kuroha K., Kudo K., Makino S., Inoue E., Kashima I., and Inada T. (2012) Dom34:Hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol. Cell 46, 518–529 10.1016/j.molcel.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 93. Bengtson M. H., and Joazeiro C. A. (2010) Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 467, 470–473 10.1038/nature09371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brandman O., Stewart-Ornstein J., Wong D., Larson A., Williams C. C., Li G.-W., Zhou S., King D., Shen P. S., Weibezahn J., Dunn J. G., Rouskin S., Inada T., Frost A., and Weissman J. S. (2012) A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151, 1042–1054 10.1016/j.cell.2012.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Defenouillère Q., Yao Y., Mouaikel J., Namane A., Galopier A., Decourty L., Doyen A., Malabat C., Saveanu C., Jacquier A., and Fromont-Racine M. (2013) Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. Proc. Natl. Acad. Sci. U.S.A. 110, 5046–5051 10.1073/pnas.1221724110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pisareva V. P., Skabkin M. A., Hellen C. U., Pestova T. V., and Pisarev A. V. (2011) Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J. 30, 1804–1817 10.1038/emboj.2011.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shoemaker C. J., and Green R. (2011) Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl. Acad. Sci. U.S.A. 108, E1392–E1398 10.1073/pnas.1113956108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shoemaker C. J., Eyler D. E., and Green R. (2010) Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science 330, 369–372 10.1126/science.1192430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Letzring D. P., Wolf A. S., Brule C. E., and Grayhack E. J. (2013) Translation of CGA codon repeats in yeast involves quality control components and ribosomal protein L1. RNA 19, 1208–1217 10.1261/rna.039446.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Saito K., Horikawa W., and Ito K. (2015) Inhibiting K63 polyubiquitination abolishes no-go type stalled translation surveillance in Saccharomyces cerevisiae. PLoS Genet. 11, e1005197 10.1371/journal.pgen.1005197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sitron C. S., Park J. H., and Brandman O. (2017) Asc1, Hel2, and Slh1 couple translation arrest to nascent chain degradation. RNA 23, 798–810 10.1261/rna.060897.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Juszkiewicz S., and Hegde R. S. (2017) Initiation of quality control during poly(A) translation requires site-specific ribosome ubiquitination. Mol. Cell 65, 743–750.e4 10.1016/j.molcel.2016.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sundaramoorthy E., Leonard M., Mak R., Liao J., Fulzele A., and Bennett E. J. (2017) ZNF598 and RACK1 regulate mammalian ribosome-associated quality control function by mediating regulatory 40S ribosomal ubiquitylation. Mol. Cell 65, 751–760.e4 10.1016/j.molcel.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matsuo Y., Ikeuchi K., Saeki Y., Iwasaki S., Schmidt C., Udagawa T., Sato F., Tsuchiya H., Becker T., Tanaka K., Ingolia N. T., Beckmann R., and Inada T. (2017) Ubiquitination of stalled ribosome triggers ribosome-associated quality control. Nat. Commun. 8, 159 10.1038/s41467-017-00188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Simms C. L., Yan L. L., and Zaher H. S. (2017) Ribosome collision is critical for quality control during no-go decay. Mol. Cell 68, 361–373.e5 10.1016/j.molcel.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Juszkiewicz S., Chandrasekaran V., Lin Z., Kraatz S., Ramakrishnan V., and Hegde R. S. (2018) ZNF598 is a quality control sensor of collided ribosomes. Mol. Cell 72, 469–481.e7 10.1016/j.molcel.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ikeuchi K., Tesina P., Matsuo Y., Sugiyama T., Cheng J., Saeki Y., Tanaka K., Becker T., Beckmann R., and Inada T. (2019) Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. EMBO J. 38, e100276 10.15252/embj.2018100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. D'Orazio K. N., Wu C. C., Sinha N., Loll-Krippleber R., Brown G. W., and Green R. (2019) The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during no go decay. Elife 8, e49117 10.7554/eLife.49117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Becker T., Armache J. P., Jarasch A., Anger A. M., Villa E., Sieber H., Motaal B. A., Mielke T., Berninghausen O., and Beckmann R. (2011) Structure of the no-go mRNA decay complex Dom34-Hbs1 bound to a stalled 80S ribosome. Nat. Struct. Mol. Biol. 18, 715–720 10.1038/nsmb.2057 [DOI] [PubMed] [Google Scholar]
- 110. Hilal T., Yamamoto H., Loerke J., Bürger J., Mielke T., and Spahn C. M. (2016) Structural insights into ribosomal rescue by Dom34 and Hbs1 at near-atomic resolution. Nat. Commun. 7, 13521 10.1038/ncomms13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ikeuchi K., and Inada T. (2016) Ribosome-associated Asc1/RACK1 is required for endonucleolytic cleavage induced by stalled ribosome at the 3′ end of nonstop mRNA. Sci. Rep. 6, 28234 10.1038/srep28234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shen P. S., Park J., Qin Y., Li X., Parsawar K., Larson M. H., Cox J., Cheng Y., Lambowitz A. M., Weissman J. S., Brandman O., and Frost A. (2015) Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 347, 75–78 10.1126/science.1259724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Osuna B. A., Howard C. J., Kc S., Frost A., and Weinberg D. E. (2017) In vitro analysis of RQC activities provides insights into the mechanism and function of CAT tailing. Elife 6, e27949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Choe Y.-J., Park S.-H., Hassemer T., Körner R., Vincenz-Donnelly L., Hayer-Hartl M., and Hartl F. U. (2016) Failure of RQC machinery causes protein aggregation and proteotoxic stress. Nature 531, 191–195 10.1038/nature16973 [DOI] [PubMed] [Google Scholar]
- 115. Kostova K. K., Hickey K. L., Osuna B. A., Hussmann J. A., Frost A., Weinberg D. E., and Weissman J. S. (2017) CAT-tailing as a fail-safe mechanism for efficient degradation of stalled nascent polypeptides. Science 357, 414–417 10.1126/science.aam7787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sitron C. S., and Brandman O. (2019) CAT tails drive degradation of stalled polypeptides on and off the ribosome. Nat. Struct. Mol. Biol. 26, 450–459 10.1038/s41594-019-0230-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Verma R., Reichermeier K. M., Burroughs A. M., Oania R. S., Reitsma J. M., Aravind L., and Deshaies R. J. (2018) Vms1 and ANKZF1 peptidyl-tRNA hydrolases release nascent chains from stalled ribosomes. Nature 557, 446–451 10.1038/s41586-018-0022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kuroha K., Zinoviev A., Hellen C. U. T., and Pestova T. V. (2018) Release of ubiquitinated and non-ubiquitinated nascent chains from stalled mammalian ribosomal complexes by ANKZF1 and Ptrh1. Mol. Cell 72, 286–302.e8 10.1016/j.molcel.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Verma R., Oania R. S., Kolawa N. J., and Deshaies R. J. (2013) Cdc48/p97 promotes degradation of aberrant nascent polypeptides bound to the ribosome. Elife 2, e00308 10.7554/eLife.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Patil D. P., Pickering B. F., and Jaffrey S. R. (2018) Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 28, 113–127 10.1016/j.tcb.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., Sorek R., and Rechavi G. (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- 122. Wang X., Lu Z., Gomez A., Hon G. C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G., Ren B., Pan T., and He C. (2014) N6-Methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 10.1038/nature12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Roundtree I. A., Evans M. E., Pan T., and He C. (2017) Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu J., Harada B. T., and He C. (2019) Regulation of gene expression by N-methyladenosine in cancer. Trends Cell Biol. 29, 487–499 10.1016/j.tcb.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yang Y., Hsu P. J., Chen Y.-S., and Yang Y.-G. (2018) Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 28, 616–624 10.1038/s41422-018-0040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu J., Jiang Z., Liu M., Gong X., Wu S., Burns C. M., and Li Z. (2009) Polynucleotide phosphorylase protects Escherichia coli against oxidative stress. Biochemistry 48, 2012–2020 10.1021/bi801752p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hayakawa H., Kuwano M., and Sekiguchi M. (2001) Specific binding of 8-oxoguanine-containing RNA to polynucleotide phosphorylase protein. Biochemistry 40, 9977–9982 10.1021/bi010595q [DOI] [PubMed] [Google Scholar]
- 128. Hayakawa H., Uchiumi T., Fukuda T., Ashizuka M., Kohno K., Kuwano M., and Sekiguchi M. (2002) Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry 41, 12739–12744 10.1021/bi0201872 [DOI] [PubMed] [Google Scholar]
- 129. Hayakawa H., Fujikane A., Ito R., Matsumoto M., Nakayama K. I., and Sekiguchi M. (2010) Human proteins that specifically bind to 8-oxoguanine-containing RNA and their responses to oxidative stress. Biochem. Biophys. Res. Commun. 403, 220–224 10.1016/j.bbrc.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 130. Ishii T., Hayakawa H., Sekiguchi T., Adachi N., and Sekiguchi M. (2015) Role of Auf1 in elimination of oxidatively damaged messenger RNA in human cells. Free Radic. Biol. Med. 79, 109–116 10.1016/j.freeradbiomed.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 131. Ishii T., Hayakawa H., Igawa T., Sekiguchi T., and Sekiguchi M. (2018) Specific binding of PCBP1 to heavily oxidized RNA to induce cell death. Proc. Natl. Acad. Sci. U.S.A. 115, 6715–6720 10.1073/pnas.1806912115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Grune T., Reinheckel T., and Davies K. J. (1997) Degradation of oxidized proteins in mammalian cells. FASEB J. 11, 526–534 10.1096/fasebj.11.7.9212076 [DOI] [PubMed] [Google Scholar]
- 133. Goldberg A. L. (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426, 895–899 10.1038/nature02263 [DOI] [PubMed] [Google Scholar]
- 134. Pickering A. M., and Davies K. J. A. (2012) Degradation of Damaged Proteins: The Main Function of the 20S Proteasome (Tilman G., ed) 1st Ed., Vol. 109, pp. 227–248. Elsevier Inc., New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vilchez D., Saez I., and Dillin A. (2014) The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 5, 5659 10.1038/ncomms6659 [DOI] [PubMed] [Google Scholar]
- 136. Stadtman E. R., Moskovitz J., and Levine R. L. (2003) Oxidation of methionine residues of proteins: biological consequences. Antioxid. Redox Signal. 5, 577–582 10.1089/152308603770310239 [DOI] [PubMed] [Google Scholar]
- 137. Schöneich C. (2005) Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer's disease. Biochim. Biophys. Acta 1703, 111–119 10.1016/j.bbapap.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 138. Boschi-Muller S., Gand A., and Branlant G. (2008) The methionine sulfoxide reductases: catalysis and substrate specificities. Arch. Biochem. Biophys. 474, 266–273 10.1016/j.abb.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 139. Kaina B., Christmann M., Naumann S., and Roos W. P. (2007) MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 6, 1079–1099 10.1016/j.dnarep.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 140. Dinglay S., Gold B., and Sedgwick B. (1998) Repair in Escherichia coli alkB mutants of abasic sites and 3-methyladenine residues in DNA. Mutat. Res. 407, 109–116 10.1016/S0921-8777(97)00065-7 [DOI] [PubMed] [Google Scholar]
- 141. Fu D., Brophy J. A., Chan C. T., Atmore K. A., Begley U., Paules R. S., Dedon P. C., Begley T. J., and Samson L. D. (2010) Human AlkB homolog ABH8 is a tRNA methyltransferase required for wobble uridine modification and DNA damage survival. Mol. Cell. Biol. 30, 2449–2459 10.1128/MCB.01604-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. A Alemu E., He C., and Klungland A. (2016) ALKBHs-facilitated RNA modifications and de-modifications. DNA Repair 44, 87–91 10.1016/j.dnarep.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ougland R., Zhang C.-M., Liiv A., Johansen R. F., Seeberg E., Hou Y.-M., Remme J., and Falnes P. Ø. (2004) AlkB restores the biological function of mRNA and tRNA inactivated by chemical methylation. Mol. Cell 16, 107–116 10.1016/j.molcel.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 144. Falnes P. Ø., Bjørås M., Aas P. A., Sundheim O., and Seeberg E. (2004) Substrate specificities of bacterial and human AlkB proteins. Nucleic Acids Res. 32, 3456–3461 10.1093/nar/gkh655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. van den Born E., Omelchenko M. V., Bekkelund A., Leihne V., Koonin E. V., Dolja V. V., and Falnes P. Ø. (2008) Viral AlkB proteins repair RNA damage by oxidative demethylation. Nucleic Acids Res. 36, 5451–5461 10.1093/nar/gkn519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gandy S. (2005) The role of cerebral amyloid accumulation in common forms of Alzheimer disease. J. Clin. Invest. 115, 1121–1129 10.1172/JCI25100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shan X., and Lin C. L. (2006) Quantification of oxidized RNAs in Alzheimer's disease. Neurobiol. Aging 27, 657–662 10.1016/j.neurobiolaging.2005.03.022 [DOI] [PubMed] [Google Scholar]
- 148. Nunomura A., Chiba S., Kosaka K., Takeda A., Castellani R. J., Smith M. A., and Perry G. (2002) Neuronal RNA oxidation is a prominent feature of dementia with Lewy bodies. Neuroreport 13, 2035–2039 10.1097/00001756-200211150-00009 [DOI] [PubMed] [Google Scholar]
- 149. Zhang J., Perry G., Smith M. A., Robertson D., Olson S. J., Graham D. G., and Montine T. J. (1999) Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 154, 1423–1429 10.1016/S0002-9440(10)65396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ding Q., Markesbery W. R., Chen Q., Li F., and Keller J. N. (2005) Ribosome dysfunction is an early event in Alzheimer's disease. J. Neurosci. 25, 9171–9175 10.1523/JNEUROSCI.3040-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bradley-Whitman M. A., Timmons M. D., Beckett T. L., Murphy M. P., Lynn B. C., and Lovell M. A. (2014) Nucleic acid oxidation: an early feature of Alzheimer's disease. J. Neurochem. 128, 294–304 10.1111/jnc.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kikuchi A., Takeda A., Onodera H., Kimpara T., Hisanaga K., Sato N., Nunomura A., Castellani R. J., Perry G., Smith M. A., and Itoyama Y. (2002) Systemic increase of oxidative nucleic acid damage in Parkinson's disease and multiple system atrophy. Neurobiol. Dis. 9, 244–248 10.1006/nbdi.2002.0466 [DOI] [PubMed] [Google Scholar]
- 153. Rosenow C., Saxena R. M., Durst M., and Gingeras T. R. (2001) Prokaryotic RNA preparation methods useful for high density array analysis: comparison of two approaches. Nucleic Acids Res. 29, E112 10.1093/nar/29.22.e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Westermann A. J., Gorski S. A., and Vogel J. (2012) Dual RNA-seq of pathogen and host. Nat. Rev. Microbiol. 10, 618–630 10.1038/nrmicro2852 [DOI] [PubMed] [Google Scholar]
- 155. Bushati N., and Cohen S. M. (2007) microRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175–205 10.1146/annurev.cellbio.23.090506.123406 [DOI] [PubMed] [Google Scholar]
- 156. Esteller M. (2011) Non-coding RNAs in human disease. Nat. Rev. Genet. 12, 861–874 10.1038/nrg3074 [DOI] [PubMed] [Google Scholar]
- 157. Willi J., Küpfer P., Evéquoz D., Fernandez G., Katz A., Leumann C., and Polacek N. (2018) Oxidative stress damages rRNA inside the ribosome and differentially affects the catalytic center. Nucleic Acids Res. 46, 1945–1957 10.1093/nar/gkx1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nawrot B., Sochacka E., and Düchler M. (2011) tRNA structural and functional changes induced by oxidative stress. Cell. Mol. Life Sci. 68, 4023–4032 10.1007/s00018-011-0773-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cole S. E., LaRiviere F. J., Merrikh C. N., and Moore M. J. (2009) A convergence of rRNA and mRNA quality control pathways revealed by mechanistic analysis of nonfunctional rRNA decay. Mol. Cell 34, 440–450 10.1016/j.molcel.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. LaRiviere F. J., Cole S. E., Ferullo D. J., and Moore M. J. (2006) A late-acting quality control process for mature eukaryotic rRNAs. Mol. Cell 24, 619–626 10.1016/j.molcel.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 161. Chernyakov I., Whipple J. M., Kotelawala L., Grayhack E. J., and Phizicky E. M. (2008) Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 22, 1369–1380 10.1101/gad.1654308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Whipple J. M., Lane E. A., Chernyakov I., D'Silva S., and Phizicky E. M. (2011) The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 25, 1173–1184 10.1101/gad.2050711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Will C. L., and Lührmann R. (2011) Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 3, a003707 10.1101/cshperspect.a003707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Watkins N. J., and Bohnsack M. T. (2012) The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip. Rev. RNA 3, 397–414 10.1002/wrna.117 [DOI] [PubMed] [Google Scholar]