Abstract
Skeletal muscle myosin has potent procoagulant activity that is based on its ability to enhance thrombin generation due to binding coagulation factors Xa and Va and accelerating prothrombin activation. A well-studied myosin inhibitor that binds to myosin's neck region inhibits myosin-dependent prothrombin activation. Hence, to identify a potential binding site(s) on skeletal muscle myosin for factor Xa, 19 peptides (25–40 residues) representing the neck region, which consists of a regulatory light chain, an essential light chain, and a heavy chain (HC), were screened for inhibition of myosin-supported prothrombin activation. Peptide HC796–835 comprising residues 796–835 of the heavy chain strongly inhibited myosin-enhanced prothrombin activation by factors Xa and Va (50% inhibition at 1.2 μm), but it did not inhibit phospholipid vesicle-enhanced prothrombin activation. Peptide inhibition studies also implicated several myosin light chain sequences located near HC796–835 as potential procoagulant sites. A peptide comprising HC796–835's C-terminal half, but not a peptide comprising its N-terminal half, inhibited myosin-enhanced prothrombin activation (50% inhibition at 1.2 μm). This inhibitory peptide (HC816–837) did not inhibit phospholipid-enhanced prothrombin activation, indicating its specificity for inhibition of myosin-dependent procoagulant mechanisms. Binding studies showed that purified factor Xa was bound to immobilized peptides HC796–835 and HC816–837 with apparent Kd values of 0.78 and 1.3 μm, respectively. In summary, these studies imply that HC residues 816–835 in the neck region of the skeletal muscle myosin directly bind factor Xa and, with contributions from light chain residues in this neck region, contribute to provision of myosin's procoagulant surface.
Keywords: coagulation factor, skeletal muscle, myosin, peptides, thrombin, factor Xa
Introduction
Recently, it was discovered that skeletal muscle myosin has potent procoagulant and prothrombotic activity (1), providing a novel paradigm regarding how blood clotting may occur following events such as an acute trauma where major disruptions of coagulation (coagulopathy) often occur. Infusion of skeletal muscle myosin reduced blood loss in an acquired hemophilia A mouse bleeding model, thereby evidencing myosin's in vivo prohemostatic activity (2). Mechanistic studies showed that myosin's potent prothrombotic activity involved enhancing thrombin generation due to myosin's ability to bind coagulation factors Xa and Va, which accelerates prothrombin activation (1). However, detailed molecular mechanisms for myosin's binding to coagulation factors have not been established. Skeletal muscle myosin is a dimer of heterotrimers, each trimer comprising a regulatory light chain (RLC),3 an essential light chain (ELC), and a heavy chain (HC). An EM structure of the chick smooth muscle myosin has been obtained in its phosphorylated state (PDB code 3J04) (3), and this extended structure may be used as a homologous model for the human skeletal muscle myosin. Similarly, the X-ray structure of rabbit skeletal muscle myosin (PDB code 5H53) may be used as a basis for studies of procoagulant myosin's structure.
As part of an effort to identify the binding sites on myosin for coagulation factors, myosin peptides were synthesized and assayed for their ability to inhibit myosin-enhanced prothrombin activation by factors Va and Xa. Because a well-known myosin inhibitor, trifluoperazine (TFP), inhibited myosin's procoagulant activity (1) and because this inhibitor binds to the ELC in myosin's “neck” region, which connects the HC head region to the HC tail (4, 5), we initially screened three ELC peptides. Based on positive ELC peptide data, we then screened 19 peptides representing additional myosin sequences located in the myosin neck region where myosin's three polypeptides, the HC, RLC, and ELC, are clustered. Data from these screening assays identified a myosin HC peptide sequence that binds factor Xa and inhibits myosin-enhanced prothrombinase activity. Our findings support the hypothesis that myosin's neck region binds blood coagulation factor Xa and is responsible for myosin's ability to enhance thrombin generation.
Results
Identification of anticoagulant myosin ELC peptides
TFP, an allosteric effector for myosin motor activity, inhibits myosin-supported prothrombin activation, as reported previously (1) and as confirmed in dose-dependent studies here (Fig. S1A). However, several other allosteric myosin effectors, namely (−)blebbistatin (up to 65 μm), omecamtiv mecarbil (CK-1827452) (up to 50 μm), and N-benzyl-p-toluene sulfonamide (up to 0.7 mm), did not inhibit myosin's procoagulant activity (data not shown). This led us to hypothesize that myosin's TFP-binding region on the ELC in the neck region (4, 5) directly contributes to myosin's procoagulant activity (Fig. 1). Thus, three overlapping 16-mer to 20-mer peptides with ELC sequences (Table S1) were synthesized and tested for inhibition of myosin-enhanced or phospholipid-enhanced prothrombin activation by purified factors Xa and Va in the presence of Ca2+ ions. The combination of these three prothrombin-activating factors (Xa, Va, and Ca2+ ions) is termed the prothrombinase complex, whereas activation of prothrombin to generate α-thrombin is termed prothrombinase activity. Two peptides, namely ELC129–144 and ELC138–157, inhibited the myosin-supported prothrombin activation (Fig. S1). Peptide ELC138–157 inhibited prothrombin activation even in the absence of myosin, whereas ELC129–144 did not (Fig. S1). This suggests that peptide ELC129–144 specifically inhibited myosin-supported prothrombin activation, rather than inhibiting myosin-independent clotting factor interactions. This encouraged us to extend the screening for potentially inhibitory peptides to 19 additional peptides containing sequences found on the three myosin polypeptides that are clustered in the neck region (Fig. 1).
Figure 1.
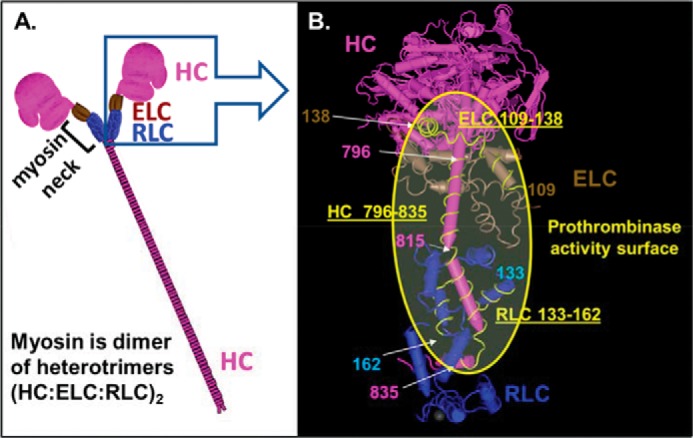
Depiction of the three-dimensional structure of skeletal muscle myosin and location of its neck region where myosin's three polypeptide chains are clustered and where factor Xa is bound to promote prothrombin activation by factors Xa/Va. A, scheme for polypeptide structure of skeletal muscle myosin that is a dimer of heterotrimers, each trimer comprising an RLC, an ELC, and an HC. The neck region depicts where the three polypeptides are clustered and represents the potential region responsible for myosin-enhancement of prothrombin activation by the factor Xa/Va complex. B, expanded view of the neck region of skeletal muscle myosin containing coagulation factor-binding sites. Worm-style structures of rabbit skeletal muscle myosin (PDB code 5H53) are depicted using the NCBI Molecular Modeling Database and Cn3D software (17). Structures shown in pink, brown, and blue are the HC, ELC, and RLC, respectively. Pink and light blue numbers show residue numbers for HC and RLC, respectively. Peptides HC796–835, RLC133–162, and ELC109–138 are shown as yellow lines. Myosin's HC residues 815–835, RLC residues 133–162, and ELC residues 129–138 are suggested to be key for myosin's specific procoagulant activity. The yellow shaded area in the yellow circle shows the potential key region for myosin to exert procoagulant activity that includes a major binding site for factor Xa on HC residues 816–835.
Screening for anticoagulant peptides representing myosin's neck region
Nineteen peptides from skeletal muscle myosin's neck region, four HC peptides (MYH2 sequences), eight ELC peptides (MYL1 sequences), and seven RLC peptides (myosin light chain, phosphorylatable, fast skeletal myosin sequences) (Table 1), were synthesized and tested at three different concentrations for their inhibition of myosin-supported prothrombin activation by purified factor Xa, factor Va, and Ca2+ ions (Fig. 2A). Peptides ELC109–138 and ELC129–159 inhibited myosin-supported prothrombin activation at 100 μm, whereas their partially overlapping neighbor peptides ELC99–122 and ELC149–173 did not. Three HC peptides (peptides HC781–810, HC796–835, and HC815–854) and one RLC peptide (RLC133–162) inhibited myosin-supported prothrombin activation at 100 μm, and each was also inhibitory, to varying degrees, when assayed at 5 μm. Dose-dependence inhibition assays gave IC50 values for the peptides HC781–810, HC796–835, HC815–854, and RLC133–162 of 64, 1.2, 2.3, and 26 μm, respectively (Fig. 3A).
Table 1.
Amino acid sequences of peptides representing human skeletal muscle myosin neck region
Amino acid residue numbers for HC, ELC, and RLC correspond to human MYH2, MYL1, and myosin light chain, phosphorylatable, fast skeletal myosin. Ac denotes acetylation.
| Residues | Sequence |
|---|---|
| RLC | |
| 23–57 | Ac-DQTQIQEFKEAFTVIDQNRDGIIDKEDLRDTFAAM-NH2 |
| 36–69 | Ac-VIDQNRDGIIDKEDLRDTFAAMGRLNVKNEELDA-NH2 |
| 63–90 | Ac-KNEELDAMMKEASGPINFTVFLTMFGEK-NH2 |
| 79–105 | Ac-NFTVFLTMFGEKLKGADPEDVITGAFK-NH2 |
| 96–126 | Ac-PEDVITGAFKVLDPEGKGTIKKKFLEELLTT-NH2 |
| 119–143 | Ac-FLEELLTTQCDRFSQEEIKNMWAAF-NH2 |
| 133–162 | Ac-QEEIKNMWAAFPPDVGGNVDYKNICYVITH-NH2 |
| ELC | |
| 49–82 | Ac-KEQQDEFKEAFLLFDRTGDSKITLSQVGDVLRAL-NH2 |
| 74–95 | Ac-QVGDVLRALGTNPTNAEVRKVL-NH2 |
| 87–104 | Ac-TNAEVRKVLGNPSNEELN-NH2 |
| 99–122 | Ac-SNEELNAKKIEFEQFLPMMQAISN-NH2 |
| 109–138 | Ac-EFEQFLPMMQAISNNKDQATYEDFVEGLRV-NH2 |
| 129–159 | Ac-YEDFVEGLRVFDKEGNGTVMGAELRHVLATL-NH2 |
| 149–173 | Ac-GAELRHVLATLGEKMKEEEVEALMA-NH2 |
| 164–193 | Ac-KEEEVEALMAGQEDSNGCINYEAFVKHIMS-NH2 |
| HC | |
| 720–757 | LYADFKQRYKVLNASAIPEGQFIDSKKASEKLLASIDI-NH2 |
| 781–810 | EMRDDKLAQLITRTQARCRGFLARVEYQRM-NH2 |
| 796–835 | ARCRGFLARVEYQRMVERREAIFCIQYNIRSFMNVKHWPW-NH2 |
| 815–854 | EAIFCIQYNIRSFMNVKHWPWMKLFFKIKPLLKSAETEKE-NH2 |
| 795–818 | QARCRGFLARVEYQRMVERREAIF |
| 816–837 | AIFCIQYNIRSFMNVKHWPWMK |
Figure 2.
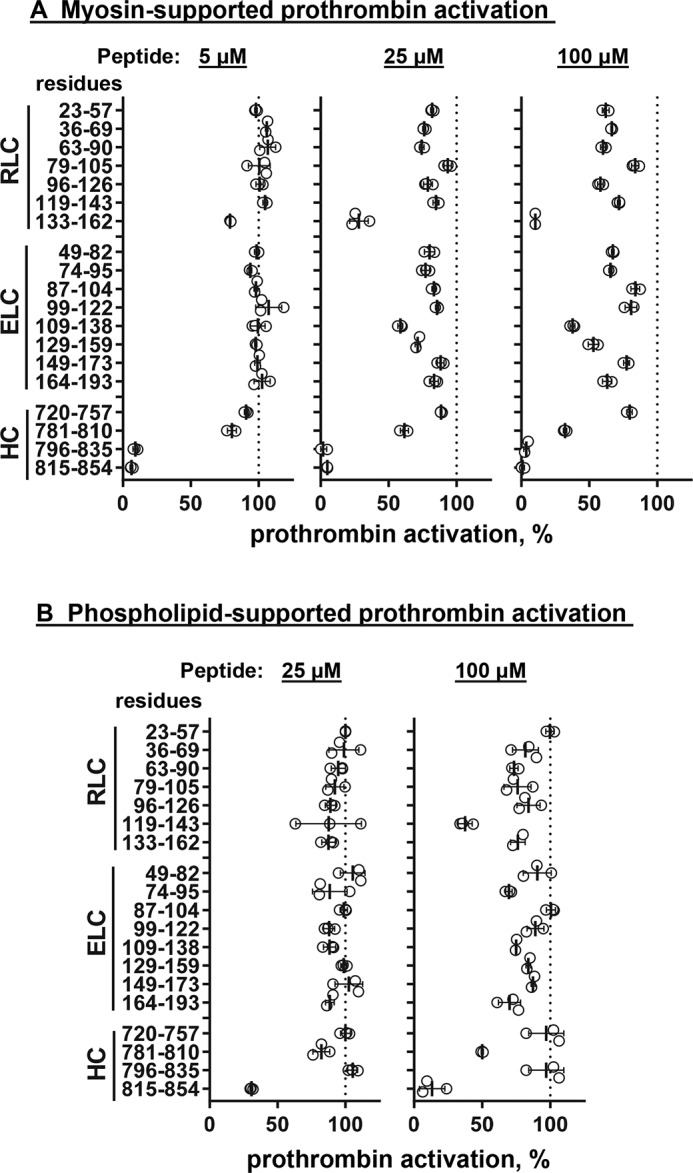
Screening for the inhibitory effects of myosin neck region peptides on myosin-enhanced or phospholipid-enhanced prothrombin activation by factors Xa and Va. Skeletal muscle myosin (2 nm final) (A) or phospholipid vesicles (PC/PS, 80%/20%, w/w) (4 μm final) (B) were incubated with 19 myosin-derived peptides at three different peptide concentrations, 5, 25, and 100 μm, respectively, as indicated, with factor Va (5 nm final) and factor Xa (0.2 nm final) in TBSA plus 5 mm CaCl2 at room temperature. Thrombin generation was determined as described under “Experimental procedures.” 100% was the value for controls in the absence of added peptides. Each value represents the mean ± S.D. (error bars) of triplicate determinations.
Figure 3.
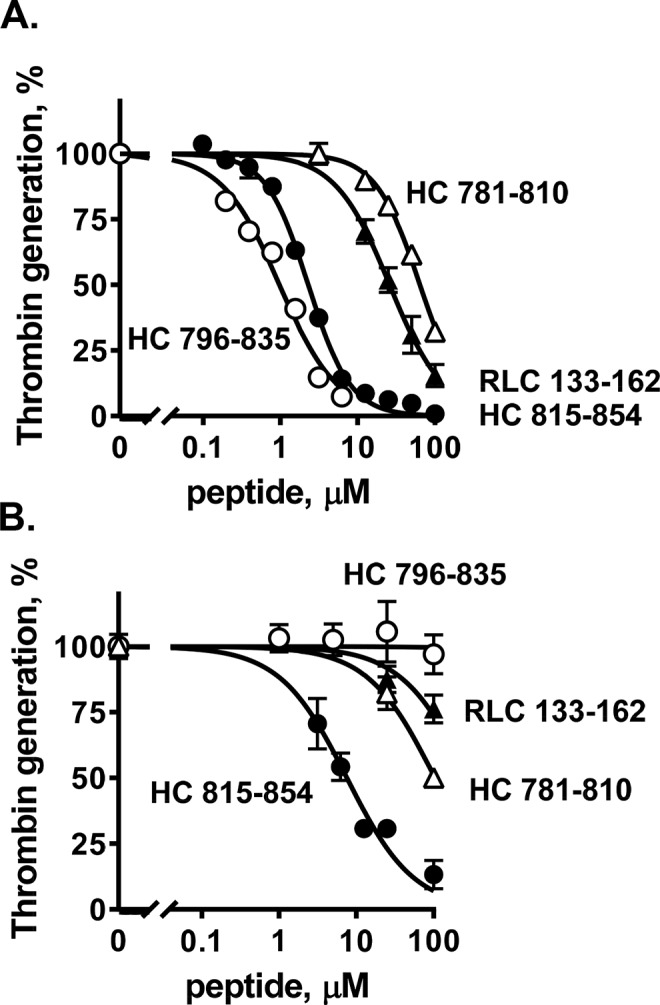
Inhibition of myosin-enhanced prothrombin activation by myosin peptides and binding of peptide HC796–835 to factor Xa. Varying concentrations of peptides HC781–810, HC815–854, HC796–835, and RLC133–162 were incubated with factor Va (5 nm final) and factor Xa (0.2 nm final) in TBSA plus 5 mm CaCl2 at room temperature in the presence of skeletal muscle myosin (2 nm final) (A) or phospholipid vesicles (PC/PS, 80%/20%, w/w) (4 μm final) (B). Thrombin generation was initiated by the addition of prothrombin (0.75 μm) in TBSA containing 5 mm Ca2+. The reaction was quenched by adding EDTA (10 mm final). Thrombin generation was determined as described under “Experimental procedures.” 100% was the value for controls in the absence of added peptides. Each value represents the mean ± S.E. (error bars) of triplicate determinations.
Peptides HC781–810 and HC815–854 inhibited phospholipid vesicles containing 20% l-α-phosphatidylserine (PS)-supported prothrombin activation (IC50 = 7.5 and 104 μm, respectively) (Figs. 2B and 3B). In contrast, the inhibitory effects of peptides HC796–835, RLC133–162, ELC109–138, and ELC129–159 on phospholipid-supported prothrombin activation were not apparent when compared with their strong inhibitory effects on myosin-supported prothrombin activation (Figs. 2 and 3, A and B). This suggests that peptides HC796–835, RLC133–162, ELC109–138, and ELC129–159 specifically inhibited myosin-supported prothrombin activation in contrast to phospholipid-supported prothrombin activation.
Determination of N-terminal and C-terminal regions of peptide HC796–835 to its anticoagulant activity
Two peptides representing the N-terminal and C-terminal sequences of HC796–835, namely HC795–818 and HC816–837, were tested for inhibition of myosin-supported prothrombin activation by purified factor Xa, factor Va, and Ca2+ ions. HC816–837, but not HC795–818, inhibited myosin-supported prothrombin activation (IC50 = 1.2 μm) (Fig. 4A), essentially similar to the inhibition by HC796–835 (Fig. 3A). The inhibitory effect of these two peptides on phospholipid-supported prothrombin activation was not significant (Figs. 3B and 4B).
Figure 4.
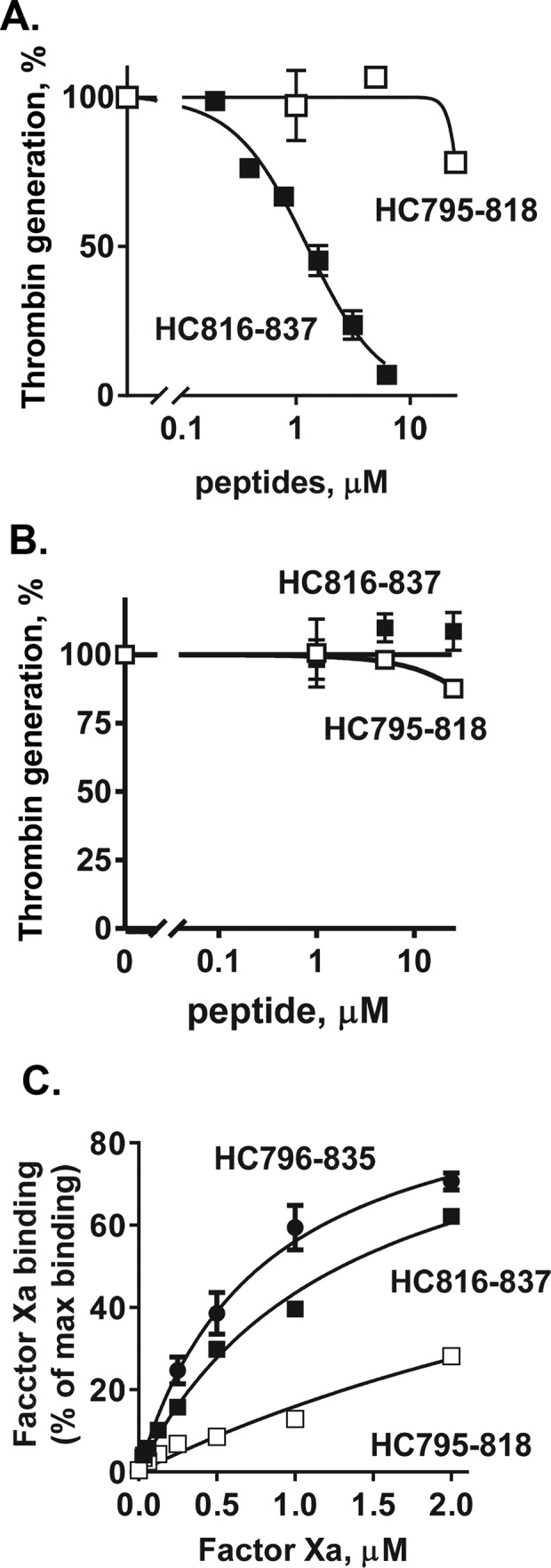
Inhibition of myosin-enhanced prothrombin activation by myosin HC peptides and their binding of factor Xa. Varying concentrations of peptides HC795–818 and HC816–837 were incubated with factor Va (5 nm final) and factor Xa (0.2 nm final) in TBSA plus 5 mm CaCl2 at room temperature in the presence of skeletal muscle myosin (2 nm final) (A) or phospholipid vesicles (PC/PS, 80%/20%, w/w) (4 μm final) (B). Thrombin generation was then initiated by the addition of prothrombin (0.75 μm) in TBSA containing 5 mm Ca2+. The reaction was quenched by adding EDTA (10 mm final), and thrombin generation was determined as described under “Experimental procedures.” 100% was the value for controls in the absence of added peptides. C, peptides HC796–835, HC795–818, and HC816–837 were immobilized in microtiter plate wells, and the binding of factor Xa to HC796–835, HC795–818, and HC816–837 was determined as described under “Experimental procedures.” Each value represents the mean ± S.E. (error bars) of triplicate determinations.
Anticoagulant effects of myosin peptides on thrombin generation in plasma
The 19 synthetic peptides (Table 1) representing the myosin neck region were screened at 100 μm (final concentration) for their inhibition of Ca2+-induced thrombin generation in human plasma, which contains circulating levels of endogenous human skeletal muscle myosin (6–10). Among the 19 peptides, HC796–835 and HC815–854 significantly inhibited thrombin generation (Fig. S2A). When the ability of HC796–835 to inhibit thrombin generation in plasma assays was assayed, it was anticoagulant with IC50 of ∼5.5 μm (Fig. S2B). Because plasma contains circulating levels of myosin whose procoagulant activity is inhibited by anti-myosin antibodies (1), this observation implies that HC796–835 inhibits myosin's procoagulant activity in the plasma milieu.
Factor Xa binding to myosin HC peptides
To test the hypothesis that the inhibitory myosin peptides bind factor Xa, various concentrations of factor Xa were incubated in microtiter plate wells that had been coated with various peptides for binding studies. Immobilized peptide HC796–835 bound factor Xa, and analysis of dose-dependent binding assays were fit with a single binding curve, indicating that factor Xa was bound with an apparent Kd of 0.78 μm (Fig. 4C). Binding assays for peptides representing the N-terminal and C-terminal sequences of HC796–835 showed that peptide HC816–837 bound factor Xa with an apparent Kd of 1.3 μm, whereas HC795–818 bound factor Xa with a weaker apparent Kd of 5.3 μm (Fig. 4C).
Discussion
A systematic screening of 22 peptides derived from skeletal muscle myosin's neck region (Fig. 1) for their ability to inhibit myosin's enhancement of prothrombin activation in purified clotting factor mixtures identified potent myosin-specific anticoagulant peptide sequences. Peptide HC796–835 was very anticoagulant for myosin-enhanced procoagulant activity. This heavy chain sequence consists of two helical structures (amino acid residues 796–811 and 816–831) (Fig. 1B) connected by and followed by short linker peptides (amino acid residues 812–815 and 832–835, respectively) (PDB code 5H53). One HC peptide, HC781–810, corresponding to amino acid residues 781–810 overlaps the first helical structure of HC796–835, and HC781–810 inhibited myosin-supported prothrombin activation, although it was much weaker than HC796–811. This suggested that the first helical structure corresponding to the amino acid residues 796–810 (Fig. 1B) does not contribute much to the myosin-specific procoagulant activity. Peptide HC815–854 that contains HC residues 815–854 and that overlaps one helical structure and one linker within HC796–835 inhibited myosin-supported prothrombin activation. This implies that the second helical structure in HC796–835 involving residues 815–835 (Fig. 1B) is a key region for the myosin-specific procoagulant activity. These discussions rest on the assumption that the synthetic peptides assume the structures seen for them in the X-ray crystallographic structure of myosin.
In contrast to HC796–835, HC815–854 not only inhibits myosin-stimulated thrombin generation but also inhibits phospholipid-supported prothrombin activation. This indicates that myosin residues 836–854, which do not overlap with HC796–835, interfere with coagulation reactions on phospholipid membrane. HC815–854 may interfere indirectly or directly with the phospholipid-dependent interactions between coagulation factors, or possibly it interacts with phospholipids themselves.
Because the region of HC residues 796–835 involves interactions between the HC and either the ELC or the RLC (11, 12), it is possible that these peptides disrupt myosin's procoagulant structure by disrupting the native structure(s) of myosin's neck region. However, the fact that factor Xa binds directly to the HC796–835 and HC816–837 peptides with a binding affinity that is similar to values for IC50 supports the interpretation that the helical region of residues 816–835 provides a functional procoagulant binding site for factor Xa.
Peptide RLC133–162 inhibited myosin-supported thrombin generation but not phospholipid-supported thrombin generation, suggesting the direct involvement of these residues for myosin's procoagulant activity. Inhibition of myosin-supported prothrombin activation by ELC109–138 and ELC129–159, but not by ELC99–122 and ELC149–173, suggests that the overlapping region of ELC109–138 and ELC129–159, namely myosin's ELC residues 129–138, are key for myosin's procoagulant activity.
It is particularly noteworthy that HC residues 816–835 are located adjacent to RLC residues133–162 in the myosin structure (Fig. 1B). Furthermore, ELC residues 129–138 are on the same surface as HC residues 796–835 and RLC residues 133–162. Thus, we hypothesize that this extended myosin neck region surface area is the key area on myosin for its direct interactions with factors Xa and Va that potentiate prothrombin activation (Fig. 1B).
Peptide HC796–835 was the best inhibitor for myosin-specific procoagulant activity among the screened peptides. Because HC796–835 and HC816–837 did not inhibit phospholipid membrane-supported thrombin generation, this discovery sets the stage for future efforts to identify new antithrombotic drugs that selectively target myosin-dependent procoagulant activities. Such agents may have minimized bleeding risk due to lack of inhibition of myosin-independent prohemostatic reactions. Such agents may offer a therapeutic advantage over traditional anticoagulant drugs that target classical coagulation reactions because anti-myosin anticoagulant drugs do not target intrinsic clotting factor activities or phospholipid membrane-supported coagulation as do the direct new oral anticoagulants, which have an increased bleeding risk (13–15). Antithrombotic drugs targeting myosin, which has never been a target for the anticoagulant therapy, would be an entirely novel class of drugs. These agents might ultimately prove clinically useful for thrombotic situations after further appropriate laboratory, preclinical, and clinical studies as well as studies of patients with increased risk for thrombosis.
In summary, we identified human skeletal muscle myosin peptides that specifically block myosin-enhanced thrombin generation but not phospholipid-stimulated prothrombin activation in purified reaction mixtures and that inhibited blood clotting in plasma. The most potent anticoagulant HC peptide binds purified factor Xa. These findings strongly suggest that the neck region of myosin provides a phospholipid-independent procoagulant surface for thrombin generation (Fig. 1B). Depending on the in vivo physiologic context, this surface of myosin may contribute to either hemostasis or thrombosis.
Experimental procedures
Materials
Peptide design and synthesis
Three peptides representing 129–161 amino acid residues of skeletal muscle myosin ELC and containing overlaps of 7–10 amino acids were synthesized (Table S1). Detailed information is provided in supporting materials and methods.
Activation of prothrombin by prothrombinase complex
Skeletal muscle myosin (2 nm final) was incubated with factor Va (5 nm final) and factor Xa (0.2 nm final) with 5 mm CaCl2 in TBS (pH 7.4) containing 0.5% albumin (TBSA) in the presence or absence of synthetic peptides for 10 min at room temperature (1). PC/PS (80%/20%) vesicles (4 μm final) were incubated with factor Va (5 nm final) and factor Xa (0.2 nm final) with 5 mm CaCl2 in TBSA in the presence or absence of synthetic peptides for 20, 40, and 60 s at room temperature (1). Thrombin generation was initiated by the addition of prothrombin (0.75 μm final unless noted otherwise). The reaction was quenched by 10 mm EDTA, and the rate of thrombin formation was quantified by measuring thrombin concentration as the rate of thrombin substrate hydrolysis. Prothrombin (0.75 μm final) activation in the absence of factor Va was also determined using factor Xa alone. Prothrombin was mixed with various concentrations of peptides at room temperature and then incubated with factor Xa (1.6 nm final) for 60 min.
Factor Xa peptide binding assays
Peptides HC796–835, HC795–818, and HC816–837 at 10 μg/ml in sodium bicarbonate (pH 9.3) were each separately coated onto the wells of microtiter plates overnight at 4 °C, and then wells were blocked with 5% fatty acid–free BSA in TBS. After washing the plate with TBSA containing 5 mm CaCl2, various concentrations of factor Xa in binding buffer consisting of TBSA containing 5 mm CaCl2 were incubated in plate wells for 1 h at room temperature. Following three rapid washings, bound factor Xa was detected by adding chromogenic substrate for factor Xa (S2222, Chromogenix, Franklin, OH). The absorbance values observed for triplicate noncoated wells lacking peptides served as nonspecific controls for binding and were subtracted from observed values for corresponding duplicate peptide-coated wells. Nonspecific binding of factor Xa ranged from 5 to 20% of maximal total observed binding in various experiments.
Thrombin generation assay in pooled human plasma
The effect of synthetic peptides on thrombin generation in pooled normal human plasma (George King Bio-Medical, Inc., Overland Park, KS) was tested as described (1, 16). Briefly, normal human plasma (30 μl) was mixed with corn trypsin inhibitor followed by the addition of peptide. Then, tissue factor (Innovin) (0.5% pm final) containing 30 mm Ca2+ in TBSA with fluorogenic thrombin substrate solution (I-1140) (Bachem Americas, Inc., Torrance, CA) was added to the plasma to initiate coagulation activation. The first derivative of the time-course data for substrate hydrolysis yielded the thrombin generation curve, allowing measurement of thrombin generation during the initiation, propagation, and termination phases of thrombin generation and the value for peak thrombin generated.
Statistical analysis
S.D. and S.E. values and apparent Kd values using saturation binding curve fit were performed using PrismTM 7.0 software (GraphPad Software Inc., San Diego, CA).
Author contributions
H. D., W. S., and J. H. G. conceptualization; H. D. and Z. G. data curation; H. D. and Z. G. formal analysis; H. D., W. S., and J. H. G. supervision; H. D. validation; H. D., Z. G., M. H., E. P., and S. L. investigation; H. D. and W. S. writing-original draft; E. P., S. L., and W. S. resources; W. S. and J. H. G. writing-review and editing; J. H. G. funding acquisition.
Supplementary Material
This work was supported by National Institutes of Health Grant RO1 HL133728 (to J. H. G.). The Scripps Research Institute has intellectual property rights related to this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supporting materials and methods, Figs. S1 and S2, and Table S1.
- RLC
- regulatory light chain
- TFP
- trifluoperazine
- HC
- heavy chain
- ELC
- essential light chain
- CPI
- carboxypeptidase inhibitor from potato tubers
- TBS
- Tris-buffered saline
- PC
- l-α-phosphatidylcholine
- PS
- l-α-phosphatidylserine
- PDB
- Protein Data Bank.
References
- 1. Deguchi H., Sinha R. K., Marchese P., Ruggeri Z. M., Zilberman-Rudenko J., McCarty O. J. T., Cohen M. J., and Griffin J. H. (2016) Prothrombotic skeletal muscle myosin directly enhances prothrombin activation by binding factors Xa and Va. Blood 128, 1870–1878 10.1182/blood-2016-03-707679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayat M. D., Wyseure T., Mosnier L. O., and Griffin J. H. (2018) Skeletal muscle myosin has pro-hemostatic activity in vivo. Am. J. Hematol. 93, E25 [Google Scholar]
- 3. Baumann B. A., Taylor D. W., Huang Z., Tama F., Fagnant P. M., Trybus K. M., and Taylor K. A. (2012) Phosphorylated smooth muscle heavy meromyosin shows an open conformation linked to activation. J. Mol. Biol. 415, 274–287 10.1016/j.jmb.2011.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang W., Wilson G. J., Brown L. J., Lam H., and Hambly B. D. (1998) EPR and CD spectroscopy of fast myosin light chain conformation during binding of trifluoperazine. Eur. J. Biochem. 257, 457–465 10.1046/j.1432-1327.1998.2570457.x [DOI] [PubMed] [Google Scholar]
- 5. Patel H., Margossian S. S., and Chantler P. D. (2000) Locking regulatory myosin in the off-state with trifluoperazine. J. Biol. Chem. 275, 4880–4888 10.1074/jbc.275.7.4880 [DOI] [PubMed] [Google Scholar]
- 6. Erlacher P., Lercher A., Falkensammer J., Nassonov E. L., Samsonov M. I., Shtutman V. Z., Puschendorf B., and Mair J. (2001) Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin. Chim. Acta 306, 27–33 10.1016/S0009-8981(01)00392-8 [DOI] [PubMed] [Google Scholar]
- 7. Bhatia R., Matsushita K., Yamakuchi M., Morrell C. N., Cao W., and Lowenstein C. J. (2004) Ceramide triggers Weibel-Palade body exocytosis. Circ. Res. 95, 319–324 10.1161/01.RES.0000136519.84279.7a [DOI] [PubMed] [Google Scholar]
- 8. Liu T., Qian W. J., Gritsenko M. A., Xiao W., Moldawer L. L., Kaushal A., Monroe M. E., Varnum S. M., Moore R. J., Purvine S. O., Maier R. V., Davis R. W., Tompkins R. G., Camp D. G. 2nd, Smith R. D., et al. (2006) High dynamic range characterization of the trauma patient plasma proteome. Mol. Cell. Proteomics 5, 1899–1913 10.1074/mcp.M600068-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Löfberg M., Tähtelä R., Härkönen M., and Somer H. (1995) Myosin heavy-chain fragments and cardiac troponins in the serum in rhabdomyolysis. Diagnostic specificity of new biochemical markers. Arch. Neurol. 52, 1210–1214 10.1001/archneur.1995.00540360090020 [DOI] [PubMed] [Google Scholar]
- 10. Guerrero M., Guiu-Comadevall M., Cadefau J. A., Parra J., Balius R., Estruch A., Rodas G., Bedini J. L., and Cusso R. (2008) Fast and slow myosins as markers of muscle injury. Br. J. Sports Med. 42, 581–584 10.1136/bjsm.2007.037945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burke M., Sivaramakrishnam M., and Kamalakannan V. (1983) On the mode of the alkali light chain association to the heavy chain of myosin subfragment 1. Evidence for the involvement of the carboxyl-terminal region of the heavy chain. Biochemistry 22, 3046–3053 10.1021/bi00282a004 [DOI] [PubMed] [Google Scholar]
- 12. Mitchell E. J., Karn J., Brown D. M., Newman A., Jakes R., and Kendrick-Jones J. (1989) Regulatory and essential light-chain-binding sites in myosin heavy chain subfragment-1 mapped by site-directed mutagenesis. J. Mol. Biol. 208, 199–205 10.1016/0022-2836(89)90096-X [DOI] [PubMed] [Google Scholar]
- 13. Kearon C., Akl E. A., Ornelas J., Blaivas A., Jimenez D., Bounameaux H., Huisman M., King C. S., Morris T. A., Sood N., Stevens S. M., Vintch J. R. E., Wells P., Woller S. C., and Moores L. (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149, 315–352 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 14. Li X. S., Deitelzweig S., Keshishian A., Hamilton M., Horblyuk R., Gupta K., Luo X., Mardekian J., Friend K., Nadkarni A., Pan X., and Lip G. Y. H. (2017) Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “real-world” clinical practice. A propensity-matched analysis of 76,940 patients. Thromb. Haemost. 117, 1072–1082 10.1160/TH17-01-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Held C., Hylek E. M., Alexander J. H., Hanna M., Lopes R. D., Wojdyla D. M., Thomas L., Al-Khalidi H., Alings M., Xavier D., Ansell J., Goto S., Ruzyllo W., Rosenqvist M., Verheugt F. W., et al. (2015) Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur. Heart J. 36, 1264–1272 10.1093/eurheartj/ehu463 [DOI] [PubMed] [Google Scholar]
- 16. Deguchi H., Elias D. J., Trauger S., Zhang H. M., Kalisiak E., Siuzdak G., and Griffin J. H. (2014) Warfarin untargeted metabolomics study identifies novel procoagulant ethanolamide plasma lipids. Br. J. Haematol. 165, 409–412 10.1111/bjh.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madej T., Lanczycki C. J., Zhang D., Thiessen P. A., Geer R. C., Marchler-Bauer A., and Bryant S. H. (2014) MMDB and VAST+: tracking structural similarities between macromolecular complexes. Nucleic Acids Res. 42, D297–D303 10.1093/nar/gkt1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


