Abstract
Heat shock proteins (Hsps) are highly conserved molecular chaperones that are ubiquitously expressed in all species to aid the solubilization of misfolded proteins, protein degradation, and transport. Elevated levels of Hsp70 have been found in the sputum, serum, and bronchoalveolar lavage (BAL) fluid of asthma patients and are known to correlate with disease severity. However, the function of Hsp70 in allergic airway inflammation has remained largely unknown. This study aimed to determine the role of Hsp70 in airway inflammation and remodeling using a mouse model of allergic airway inflammation. WT and Hsp70 double-knockout (Hsp70.1/.3−/−) mice were sensitized and challenged intratracheally with Schistosoma mansoni soluble egg antigens (SEAs) to induce robust Th2 responses and airway inflammation in the lungs. The lack of Hsp70 resulted in a significant reduction in airway inflammation, goblet cell hyperplasia, and Th2 cytokine production, including IL-4, IL-5, and IL-13. An analysis of the BAL fluid suggested that Hsp70 is critically required for eosinophilic infiltration, collagen accumulation, and Th2 cytokine production in allergic airways. Furthermore, our bone marrow (BM) transfer studies show that SEA-induced airway inflammation, goblet cell hyperplasia, and Th2 cytokine production were attenuated in WT mice that were reconstituted with Hsp70-deficient BM, but these effects were not attenuated in Hsp70-deficient mice that were reconstituted with WT BM. Together, these studies identify a pathogenic role for Hsp70 in hematopoietic cells during allergic airway inflammation; this illustrates the potential utility of targeting Hsp70 to alleviate allergen-induced Th2 cytokines, goblet cell hyperplasia, and airway inflammation.
Keywords: heat shock protein (HSP), allergy, inflammation, asthma, cytokine, Th2 immune responses
Introduction
Asthma is a chronic inflammatory disease that affects more than 300 million subjects worldwide and has an increasing prevalence every year in both developing and developed countries (1, 2). Asthma is characterized by a chronic inflammation in the conducting airways and is usually caused by excessive Th22 immune cell activation, which is marked by eosinophilia, goblet cell hyperplasia, mucus secretion, and airway hyperresponsiveness (AHR). Th2 cytokines, including IL-4, IL-5, and IL-13, are implicated in airway inflammation and remodeling in allergic asthma (3). IL-4 is responsible for Th2 cell polarization as well as the class switching of activated B cells to produce IgE. However, IL-13 is implicated in the fibrotic remodeling of airways and AHR. Because both IL-4 and IL-13 share a common receptor, they can induce overlapping inflammatory and airway remodeling effects (4, 5). IL-5 is a primary cytokine that initiates the recruitment, activation, and survival of eosinophils in airway inflammation, whereas IL-13 is critical for AHR through different mechanisms, including the contraction of airway smooth muscle cells, goblet cell hyperplasia, and mucus hypersecretion (6). In support of these findings, clinical trials targeting Th2 cytokines have shown promising outcomes to improve the quality of life and to control asthma exacerbations (7, 8). However, there are endotypes of asthma that are unresponsive to classic corticosteroids and to Th2 cytokine–targeted therapeutic agents; this suggests that multiple Th2 cytokines may concomitantly participate in the development of the disease (9). Additionally, the initiation and development of airway inflammatory responses to allergen exposure involve multiple other pathways that concurrently contribute to the pathophysiology of airway inflammation (10). Therefore, it is important to identify upstream endogenous regulators that might play a pathogenic role by activating multiple Th2 cytokines in allergic airway inflammation.
Hsps represent a family of molecular chaperones that play a critical role in refolding native and immature proteins, protein trafficking, and various cell signaling processes (11–13). Hsps are generally grouped within seven major families according to their molecular weight, including Hsp40, Hsp27, and Hsp70 (14, 15). Most Hsps are constitutively expressed at low levels in different cells, whereas the cellular expression of other Hsps, including Hsp70, is inducible and increased in response to stress conditions such as inflammation, infection, and environmental allergens (12). Hsp70s are highly conserved proteins within species that play a major role in cellular proteostasis and prevent cellular damage in association with other cochaperones such as Hsp27 and Hsp40 (13). Inducible murine Hsp70s include two isoforms, Hsp70.1 and Hsp70.3, which are identical except for one amino acid (16). The role of Hsp70 has been studied in different pathological conditions, including inflammation, malignancy, fibrosis, and autoimmune diseases, and Hsp70 has been reported as either a potential disease marker, a therapeutic target, or a modulator of inflammation (12, 17, 18). In allergic diseases, Hsp70 is elevated in the serum of patients with atopic dermatitis or allergic asthma (19). Previous studies have also reported increased expression of Hsp70 in the nasal and bronchial epithelial cells of patients with asthma or chronic bronchitis (20–22). Furthermore, asthmatic patients have elevated levels of Hsp70 in induced sputum and serum compared with healthy subjects, and this increase correlates with disease severity (23). Those studies have suggested a proinflammatory role for Hsp70 in the development of allergic diseases, whereas other reports have described Hsp70 as an anti-inflammatory mediator (24). The role of Hsp70 in allergic diseases has been associated with divergent or dual conclusions, ranging from pro- to anti-inflammatory effects. However, few studies have examined the relationship among Hsp70, the Th2 immune responses, and allergic airway inflammation.
In this study, we investigated the role of Hsp70 in the regulation of airway inflammatory responses during soluble egg antigen (SEA)-induced allergic lung inflammation in mice. Our data reveal that the loss of Hsp70 is sufficient to attenuate airway inflammation, eosinophilia, goblet cell hyperplasia, and the production of Th2 cytokines in SEA-challenged mice. Furthermore, the bone marrow transfer studies suggest that Hsp70-expressing hematopoietic cells play a pathogenic role by inducing Th2 cytokine production, goblet cell hyperplasia, and airway inflammation.
Results
The loss of Hsp70 attenuates airway inflammation and goblet cell hyperplasia
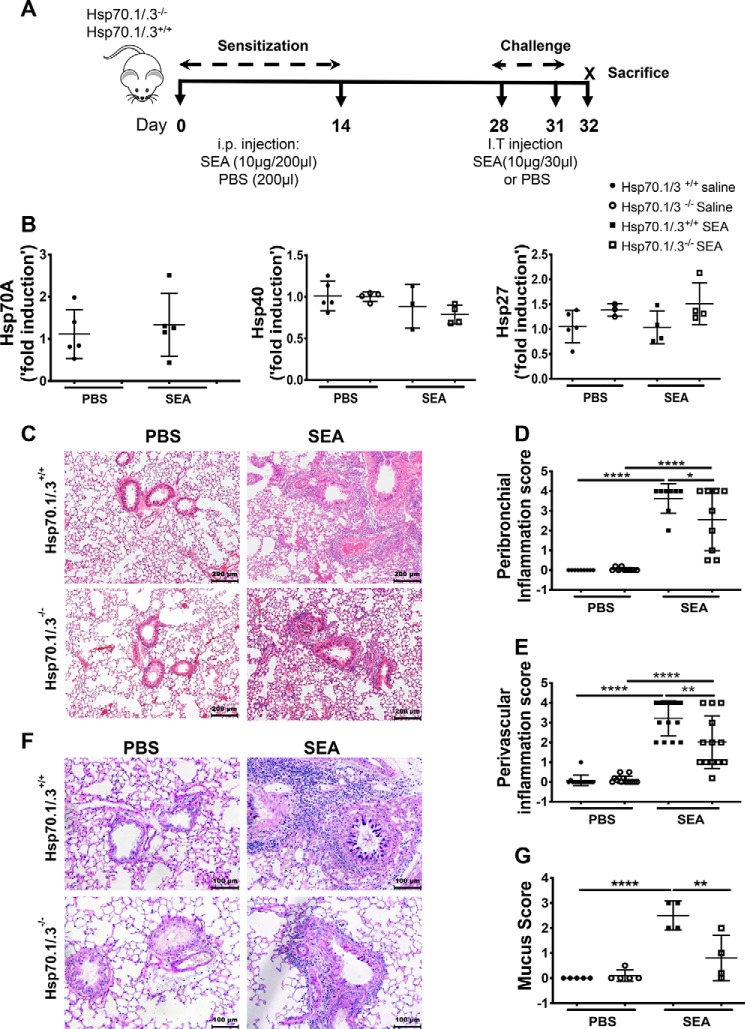
In this study, we used a mouse model of SEA-induced airway inflammation model to study the role of Hsp70 in the development of Th2 responses and inflammation in mouse airways. SEA preparations of Schistosoma mansoni have been used for decades to study Th2-polarized immune responses in chronic lung diseases, including allergic asthma. SEA is well-documented as an inducer of airway inflammation and Th2 immune responses without the use of an adjuvant. Previous studies from our group and others have shown that SEA-induced airway inflammation and Th2 responses share features with other allergens such as house dust mite (5, 25, 26). The WT and Hsp70.1/.3−/− mice were sensitized twice and consecutively challenged with SEA to induce allergic inflammatory responses (Fig. 1A). The absence of Hsp70 gene expression was confirmed by measuring transcripts in the lungs of mice challenged with SEA or saline, and as expected, no expression of Hsp70 was detected in the Hsp70.1/.3−/− mice (Fig. 1B). Additionally, the sensitization and challenge with SEA did not modify the gene expression of Hsp70 in the WT mice. Because Hsp70 interacts with other cochaperones to maintain the protein refolding pathway, we assessed whether its deletion may affect the expression of other chaperone molecules. The loss of Hsp70 did not modify the expression of the cochaperones Hsp27 and Hsp40 nor did the SEA challenge in either the WT or the Hsp70.1/.3−/− mice (Fig. 1B). The lung sections of the WT and Hsp70.1/.3−/− mice that were challenged with SEA or PBS were stained with H&E to measure the airway inflammation (Fig. 1, C–E). As expected, the SEA challenge induced a robust airway inflammation with immune cell infiltration in the WT mice, which was further confirmed by a quantitative measurement of the peribronchial and perivascular inflammation scores. Notably, the Hsp70.1/.3−/− mice that were challenged with SEA developed attenuated airway inflammation with a decrease in both the peribronchial and perivascular inflammation scores compared with the WT mice (Fig. 1, D and E). Alcian blue–periodic acid–Schiff (ABPAS) staining was used to detect mucus-producing goblet cells in lung sections. Hsp70.1/.3−/− mice exhibited attenuated Goblet cell hyperplasia and mucus score compared with WT mice (Fig. 1, F and G). Airway inflammation in allergic asthma is largely due to immune cell infiltration in the airways as well as in the sputum and BAL fluids. We further investigated whether the loss of Hsp70 was also associated with a decrease in bronchial alveolar lavage fluid (BALF) eosinophilia. Concomitant with the decrease in airway inflammation in Hsp70.1/.3−/− mice, the total count of BAL cells was remarkably reduced in Hsp70.1/.3−/− mice compared with that in the WT mice following the SEA challenge. Notably, the loss of Hsp70 induced a significant decrease in airway eosinophilia, whereas the lymphocyte and macrophage counts were increased compared with those of the WT mice (Fig. 2, A and B). Lung sections were further stained with Picrosirius Red to measure collagen deposition in inflamed airways following SEA challenge. Lungs of WT mice challenged with SEA presented an increased deposition of collagen when compared with the saline-treated group. However, Hsp70.1/.3−/− mice presented levels of collagen deposition similar to WT mice with no statistical differences (Fig. S1). These findings suggest that Hsp70 contributes to the development of airway inflammation and mucus production in SEA-induced airway inflammation. The loss of Hsp70 induces partial, rather than complete, suppression of SEA-induced airway inflammation and mucus production.
Figure 1.
Deletion of Hsp70.1/.3 attenuates airway inflammatory responses. A, Hsp70.1/.3+/+ (WT) and Hsp70.1/.3−/− (knockout) mice were sensitized and challenged with SEA or PBS at the indicated times to induce allergic airway inflammation. B, the gene expression of the heat shock proteins between the WT (filled circles and squares) and Hsp70.1/.3−/− mice (open circles and squares) in the lung tissues was measured by RT-PCR. The deletion of Hsp70 was confirmed in Hsp70.1/.3−/− mice (left), whereas the expression levels of Hsp40 (middle) and Hsp27 (right) were unaffected. C, 5-μm sections of paraffin-embedded lung tissues from the WT (top) and Hsp70.1/.3−/− (bottom) mice were stained with H&E. Saline- and SEA-treated lung sections are shown on the left and right, respectively; representative images are shown at 10× magnification. The airway inflammation in histological sections was quantified as peribronchial (D) and perivascular (E) inflammation scores. F, the lung sections were also stained with ABPAS for mucus hypersecretion. Representative images are shown as described in C, and images are shown at 20× magnification. G, mucus hypersecretion in ABPAS-stained sections was quantified with the mucus score. The quantitative data are shown as mean ± S.D. (error bars), n = 5–6 per group. Significant p values are presented as *, **, and **** for p < 0.05, p < 0.01, and p < 0.0001, respectively. Data in D and E are shown as pooled results from two repeated experiments with similar results (n = 10–15 mice/group); other figures are representative data of two repeated experiments. i.p., intraperitoneal; I.T., intratracheal.
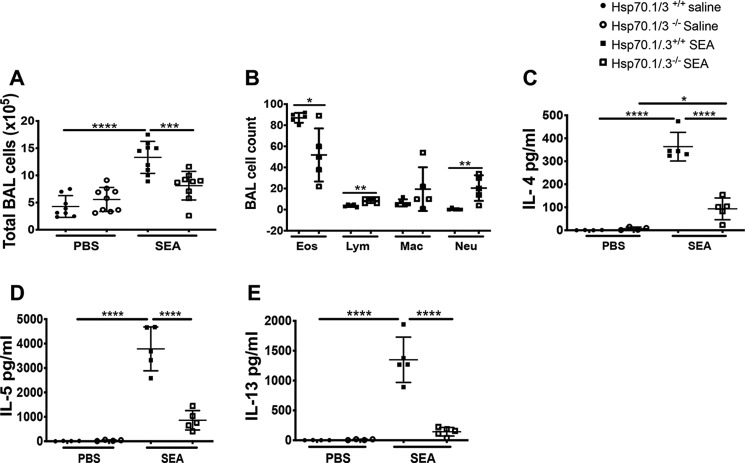
Figure 2.
Hsp70.1/.3−/− mice have decreased numbers of eosinophils and reduced levels of Th2 cytokines in the BAL fluid. BAL fluid was collected from WT (filled circles and squares) and Hsp70.1/.3−/− (open circles and squares) mice challenged with saline or SEA. A, the total BAL cell number was determined. B, the differential cell number in the BAL fluid was determined with Cytospin preparations. C–E, levels of Th2 cytokines IL-4 (C), IL-5 (D), and IL-13 (E) were measured by ELISA. The data are shown as the mean ± S.D. (error bars), n = 5–6 per group. Significant p values are shown as *, **, ***, and **** for p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively. Except for A and B shown as pooled data from two different experiments with similar results (n = 10–12 mice per group), data are shown as a representative of two different experiments with similar results (n = 5–6 mice/group).
The loss of Hsp70 attenuates Th2 immune responses
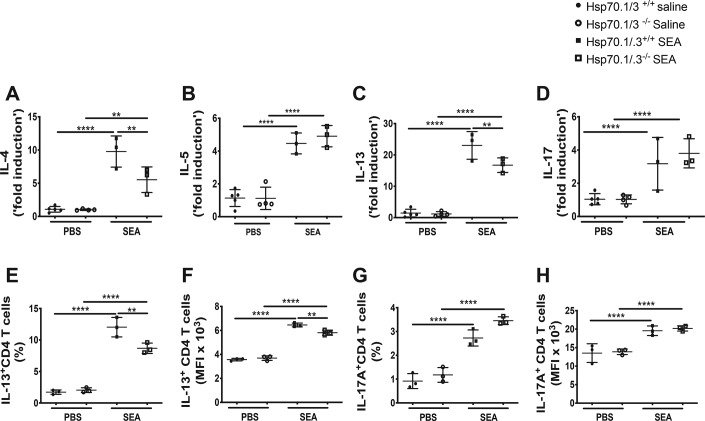
The allergic airway inflammation is characterized by a dominant type 2 immune response with the production of Th2 cytokines such as IL-4, IL-5, and IL-13 (3, 6). Because the loss of Hsp70.1/.3 induced attenuated airway inflammation with decreased airway eosinophilia and mucus production, we further investigated whether Hsp70-mediated airway inflammation was associated with a reduction of Th2 cytokine production. The protein and transcript levels of Th2 cytokines were quantified in the BAL fluid and lung tissues of WT and Hsp70.1/.3−/− mice. The Th2 cytokines IL-4, IL-5, and IL-13 were significantly increased in the BAL fluid of WT mice following the SEA challenge. However, the concentration of these cytokines was decreased in the Hsp70.1/.3−/− mice (Fig. 2, C–E). Similarly, the transcripts of the Th2 cytokines and IL-17 were increased in the lungs of WT and Hsp70.1/.3−/− mice following the SEA challenge (Fig. 3, A–D). Interestingly, the transcript levels of IL-4 and IL-13 were reduced in the Hsp70.1/.3−/− mice compared with the SEA-challenged WT mice, whereas no significant difference in the transcript levels of IL-5 and IL-17 was observed between the WT and Hsp70.1/.3−/− mice that were challenged with SEA (Fig. 3, B and D).
Figure 3.
Hsp70.1/.3 deficiency induces a decrease in gene expression of type 2 cytokines and a reduction of Th2 cytokine–producing T cells in the lungs. A–D, lung gene expression levels of the Th2 cytokines IL-4 (A), IL-5 (B), IL-13 (C), and IL-17 (D) were determined by quantitative RT-PCR. Immune cells were isolated from the lungs of WT and Hsp70.1/.3−/− mice and stimulated with phorbol 12-myristate 13-acetate and ionomycin for intracellular cytokine detection with FACS. The T cell populations were gated as CD3+ and CD4+ cells; the intracellular expression levels of IL-13 (E and F) and IL-17 (G and H) in CD4+ T cells were determined. The percentages (E and G) and MFI (F and H) of the CD4+ T cells expressing IL-13 or IL-17A are shown. The filled circles and squares represent the WT mice, and the open circles and squares represent the Hsp70.1/.3−/− mice. The data are shown as the mean ± S.D. (error bars), with n = 3–6 per group. One-way ANOVA was performed to compare differences between multiple groups. Significant p values are shown as ** and **** for p < 0.01 and p < 0.0001, respectively. Representative data of two different experiments are shown.
Given that T helper cells are a dominant source of type 2 cytokine production in this model (3), we examined whether the Hsp70-dependent reduction of Th2 cytokine production correlates with the decrease in Th2 cytokine–producing T cells. Immune cells that were isolated from the lungs of WT and Hsp70.1/.3−/− mice were analyzed for intracellular cytokine production by flow cytometry. The percentage and median fluorescence intensity (MFI) of IL-13+ CD4 T cells were significantly increased in the lungs of SEA-treated WT mice, whereas Hsp70.1/.3−/− mice presented a significant reduction in the IL-13+ CD4 T cells compared with the WT mice (Fig. 3, E and F). Although the percentage and MFI of the IL-17+ CD4 T cells were increased in both the WT and Hsp70.1/.3−/− mice in response to the SEA challenge, no difference was observed between the groups, suggesting that the production of IL-17 is Hsp70-independent (Fig. 3, G and H). These findings indicate that Hsp70 plays a critical role in type 2 cytokine production and the associated inflammatory responses to these cytokines in allergic airway inflammation.
Hsp70 alters the gene networks involved in allergic airway inflammation
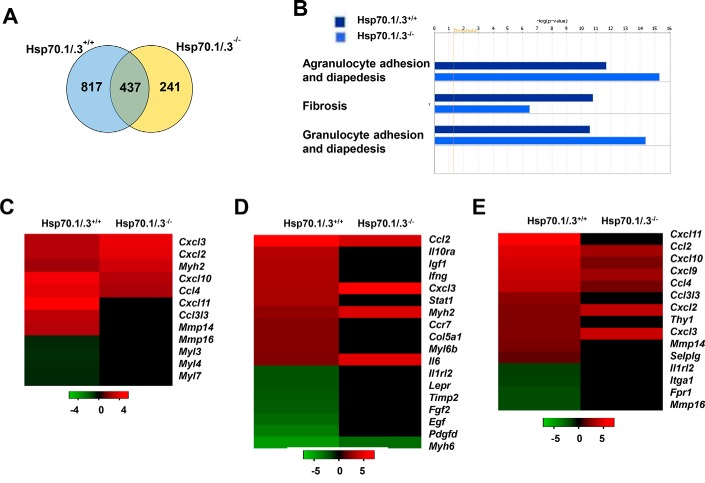
As Hsp70 is a major chaperone that is involved in proteostasis, we aimed to further identify the major cellular pathways that might be regulated by Hsp70 in allergic airway inflammation. The total RNA was extracted from the lung lysates of the WT and Hsp70.1/.3−/− mice that were challenged with SEA or saline, and the RNA was utilized in a comparative microarray assay to identify genes with significant changes in differential expression (1.5-fold up or down) that could be associated with Hsp70.1/.3 deletion as detailed under “Materials and methods.” Differences in gene expression were observed between the WT and Hsp70.1/.3−/− mice. The largest group of genes subject to change following SEA-induced allergic airway inflammation was in the lungs of WT mice (1,254 genes), whereas the loss of the Hsp70 gene resulted in the change of 678 transcripts in the Hsp70.1/.3−/− mice. WT and Hsp70.1/.3−/− mice shared 437 transcripts with differential changes following SEA treatment (Fig. 4A and Tables S1 and S2). Serpin family B genes (Serpinb11), retinoic acid early transcript 1E (Raet1d), mast cell protease 8 (Mcpt8), Acta1, Adra2a, and Calpain 9 (Capn9) were the top up-regulated genes with exclusive differential expression in the Hsp70.1/.3−/− lung transcriptome, whereas Cxcl11, ISG15 ubiquitin-like modifier (Isg15), growth differentiation factor 3 (Gdf3), and interferon regulatory factor 7 (Irf7) were up-regulated in the WT mice (Table S1). Common genes that were up-regulated following the challenge with SEA included Clca1, Rnase2, RetnlB, Retnla, and Ccl8 among the top genes with the highest -fold change (Table S2). We further analyzed the genes that were differentially expressed in the WT and Hsp70.1/.3−/− mice by subjecting them to a functional analysis using Ingenuity Pathway Analysis (IPA). This analysis generated major pathways that are involved in tissue inflammation and remodeling, with the top pathways including the adhesion of agranulocytes and granulocytes and fibrosis (Fig. 4, B–E). The genes involved in cell adhesion and diapedesis were up-regulated, whereas fibrotic pathway–regulated genes were down-regulated in Hsp70.1/.3−/− mice. The genes involved in the cell adhesion and diapedesis pathways were mainly chemokines such as Cxcl9, Cxcl10, and Cxcl11, which are involved in lymphocyte chemoattraction, and Cxcl2 and Cxcl3, which have been shown to regulate neutrophil recruitment (Fig. 4, C and E). These findings mirror the increase in BAL lymphocytes and macrophages of Hsp70.1/.3−/− mice (Fig. 2B). In addition, the genes involved in the T helper cell differentiation pathway were also down-regulated in lung transcriptomes of Hsp70.1/.3−/− mice (data not shown). These findings indicate that Hsp70 plays a significant role in immune cell infiltration and tissue remodeling during SEA-induced allergic airway inflammation.
Figure 4.
Comparative analysis of up- or down-regulated genes in the lungs of WT and Hsp70.1/.3−/− mice during SEA-induced allergic airway inflammation. A, Venn diagram of the ratio of gene expression (SEA/saline) obtained in the lungs isolated from sensitized WT and Hsp70.1/.3−/− mice. Venn diagrams were obtained using raw data and filtering genes that were at least 1.5-fold up- or down-regulated when comparisons were made within each animal group and between the two following conditions: antigen challenge and saline challenge. B, the microarray data were analyzed by Ingenuity Pathway Analysis software, and the top three pathways of differential gene expression between the WT and Hsp70.1/.3−/− mice are shown. C–E, the heat maps present the expression change of genes involved in the agranulocyte adhesion and diapedesis (C), fibrosis (D), and granulocyte adhesion (E) pathways.
Hsp70 in hematopoietic cells alters airway inflammation and remodeling in allergic airway inflammation
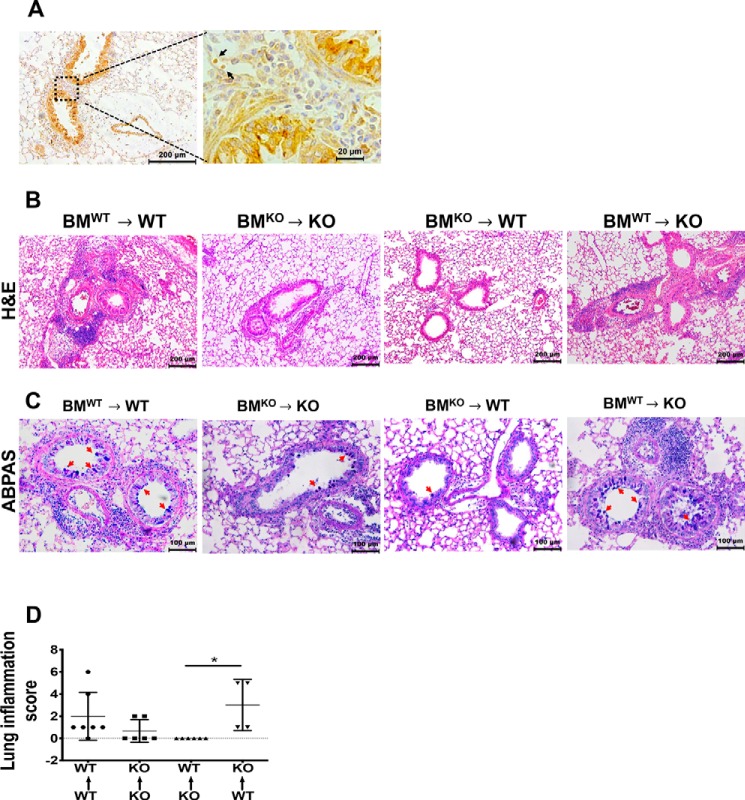
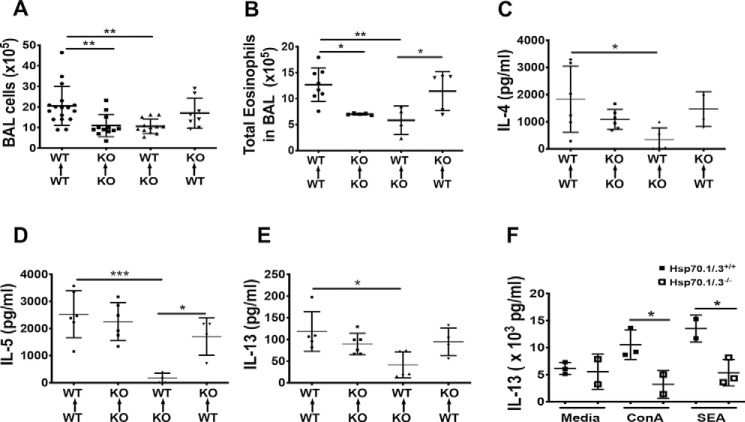
To understand the mechanisms underlying Hsp70-mediated Th2 immune responses and airway inflammation, we assessed the expression of Hsp70 in SEA-challenged mice. The lung sections of WT mice challenged with SEA were immunostained using an anti-Hsp70 antibody. Hsp70 was localized in the bronchial epithelial cells and the infiltrating immune cells in the peribronchial areas of lung sections from SEA-treated mice (Fig. 5A). The observed expression of Hsp70 in both the infiltrating immune cells and the bronchial epithelial cells prompted us to determine which cellular source of Hsp70 might be the key driver of Hsp70-mediated airway inflammation and Th2 immune responses. To address this question, we performed chimeric bone marrow (BM) transfers from WT or Hsp70.1/.3−/− mice into lethally irradiated WT or Hsp70.1/.3−/− mice. Thus, generated chimeric mice were challenged with SEA as described under “Materials and methods.” Chimeric mice that were reconstituted with WT BM displayed airway inflammation with increased goblet hyperplasia compared with chimeric mice with Hsp70.1/.3−/− BM (Fig. 5, B and C). Similarly, the BAL cells and eosinophils were significantly decreased in chimeric mice with Hsp70.1/.3−/− BM, and inversely, the BAL eosinophil number was increased in chimeric mice with WT BM (Fig. 6, A and B). The increased airway inflammatory responses and the BAL eosinophilia in chimeric Hsp70.1/.3−/− mice that received WT BM correlated with the increased lung inflammation score (Fig. 5C). Similar changes were observed with cytokine production in the BAL fluid of chimeric mice challenged with SEA. The Th2 cytokines IL-4, IL-5, and IL-13 were significantly reduced in the BAL fluid of WT mice with Hsp70.1/.3−/− BM. Conversely, these cytokines tended to increase in the BAL fluid of Hsp70.1/.3−/− mice with WT BM, although no significant difference was observed (Fig. 6, C–E). These findings indicate that Hsp70 expression by hematopoietic cells but not by epithelial cells is critical to induce airway inflammation, eosinophilia, and Th2 cytokine production during SEA-induced allergic airway inflammation.
Figure 5.
Hematopoietic BM-derived Hsp70 is responsible for airway inflammation in SEA-induced airway inflammation. A, immunohistochemistry staining of Hsp70 on paraffin-embedded lung sections from the WT mice challenged with SEA. The images are shown at low (10×) and high magnification (63×). The dashed box areas are shown at high magnification to highlight the infiltrating cells (arrows) during airway inflammation. Representative images are shown from five mice per group. The lung sections of chimeric mice were stained with H&E (B) and ABPAS (C), and the chimeric group details are shown above each image column. Representative images are shown at 10× and 20× magnification for H&E and ABPAS, respectively. The red arrows in C highlight the mucus staining in the bronchial epithelial cells. D, airway inflammation was quantified from the histological sections as the lung inflammation score (n = 4–6 mice/group). Data in D are shown as the mean +/− S.D. (error bars). One-way ANOVA was performed to compare differences between chimeric groups: *, p < 0.05. Representative data of two different chimeric assays with similar results are shown. KO, knockout.
Figure 6.
Transfer of WT bone marrow to Hsp70.1/3-deficient mice restored airway inflammation and Th2 cytokine production. BALF was collected from chimeric mice challenged with SEA to induce allergic inflammation of the airways. The total numbers of BAL cells (A) and eosinophils (B) were counted. The Th2 cytokines IL-4 (C), IL-5 (D), and IL-13 (E) were quantified in BAL fluid using ELISA. Chimeric mouse details are shown on the bottom of each related figure. F, lung immune cells were prepared from the WT (filled squares) and Hsp70.1/.3−/− mice (open squares) sensitized and challenged with SEA and then stimulated with ConA or SEA or cultured with media alone. The cytokine levels of IL-13 were determined by ELISA. The data are shown as the mean ± S.D. (error bars). One-way ANOVA with multiple comparison test was used for the chimera assays in A–E, and an unpaired two-tailed Student's t test was used to compare IL-13 cytokine production between the WT and Hsp70.1/.3−/− mice in F. Significant p values are shown as *, **, and *** for p < 0.05, p < 0.01, and p < 0.001, respectively, with n = 4–6 per group. Pooled data from two different experiments are shown in A (n = 8–15); other figures are presented as representative data of two different experiments. KO, knockout.
The loss of Hsp70 attenuates Th2 cytokine production by effector T cells
Because the airway inflammatory responses and Th2 immune responses were dependent on hematopoietic cell–derived Hsp70 (Figs. 5 and 6) and the percentage of IL-13–producing CD4 T cells was decreased in Hsp70.1/.3−/− mice (Fig. 3, E and F), we sought to confirm the effect of an Hsp70 deficiency on T cell activation and type 2 cytokine production following antigen exposure. The lung immune cells were collected from mice challenged with SEA and then stimulated with SEA or concanavalin A (ConA) or with media alone to induce cytokine production in effector T cells. The WT and Hsp70.1/.3−/− mice had similar levels of IL-13 when the immune cells were cultured with media alone. IL-13 was increased in the supernatant of SEA-challenged WT mouse T cells in the presence of ConA or SEA; however, this increase was significantly attenuated in T cells that were deficient for Hsp70 (Fig. 6F). In addition, the level of IL-5 production was similar in both the lung T cells of WT and Hsp70.1/.3−/− mice (data not shown). To address whether Hsp70 participates in the Th2 immune responses through the antigen presentation process, antigen-presenting cells (APCs) were further analyzed for the expression of costimulatory markers. The frequency of APCs in mediastinal lymph nodes was similar between Hsp70.1/.3−/− and WT mice following allergen sensitization and challenge. Moreover, no differences in the expression of APC costimulatory markers were observed between Hsp70.1/.3−/− and WT mice (Fig. S2, A and B). These findings suggest that the loss of Hsp70 reduces allergen-induced Th2 immune responses in the lung via T cell activation rather than acting through structural cells or APCs. Also, we addressed whether Hsp70 has any modulatory effect on IL-13–induced AHR using the PCLS contraction assay (27–29). Lung slices were prepared from naive WT mice and then incubated for 24 h with a recombinant mouse IL-13 to induce AHR with or without YM-08, an Hsp70 small-molecule inhibitor (30). Exposure to methacholine induced a dose-dependent airway contraction in lung slices treated with recombinant IL-13. However, a similar degree of contraction was observed in PCLS treated with IL-13 and YM-08 (Fig. S3). These results suggest that Hsp70 has limited or no effect on IL-13–driven AHR.
Discussion
The level of Hsp70 is increased in multiple diseases, including allergic diseases such as asthma, and it can be detected in the serum, sputum, or BAL fluid of allergy patients (19, 23, 31). However, the function of this major chaperone in the pathophysiology of allergic asthma has remained unknown. Our study demonstrates that Hsp70 is a positive regulator of airway inflammation and Th2 immune responses in allergic airway inflammation. Hsp70 double-deficient mice developed attenuated airway inflammation with decreased airway eosinophilia and goblet cell hyperplasia in response to allergen challenge. Moreover, the Th2 cytokines and the Th2 cytokine–producing T cells were significantly decreased in the Hsp70.1/.3−/− mice compared with those in the WT mice. Bone marrow transfer experiments revealed that the Hsp70-driven airway inflammation and the Th2 immune responses were dependent on Hsp70 expression in hematopoietic cells but not in epithelial cells. Furthermore, when lung immune cells that were isolated from SEA-challenged mice were restimulated with the allergen, the production of IL-13 was significantly reduced in the activated T cells from the Hsp70.1/.3−/− mice compared with that in the activated T cells from the WT mice. These findings indicate that Hsp70 plays a pathogenic role in the amplification of Th2 cell activation and the secretion of type 2 cytokines in allergen-induced airway inflammation. In support of this hypothesis, Yusuf et al. (19) showed that the inhibition of extracellular Hsp70 and Hsp27 with specific antibodies resulted in the reduction of skin contact hypersensitivity in a murine model, supporting the role of Hsp70 in the development of allergic skin hypersensitivity. In addition, Bertorelli et al. (21) have shown that inhaled steroids improved the clinical symptoms of severe asthma and induced a decrease in Hsp70 and HLA-DR expression in those patients. Here, for the first time, we have shown the contribution of Hsp70 in the development of allergic airway inflammation using Hsp70.1/.3-deficient mice, and chimeric BM transfers provide clear evidence that Hsp70-expressing hematopoietic cells contribute to the development of airway inflammation in a murine allergic model of airway inflammation. Our findings show that loss of Hsp70.1/.3 induced a partial protection from allergic airway inflammation (Fig. 1, C–G). This limited protection could be explained by the contribution of different factors, including other isoforms of Hsp70, Hsp40, Hsp27, and Hsp90, which share overlapping function at different levels. In addition to the induction of atopic dermatitis, Hsp27 is known to contribute in the production of proinflammatory cytokines (19, 32, 33). Pezzulo et al. (34) have demonstrated the critical role of Hsp90 in the induction of goblet cell hyperplasia and mucus production.
Min et al. (31) reported an increased level of Hsp70 in the nasal lavage fluid of allergic rhinitis (AR) patients, and interestingly, the stimulation of nasal epithelial cells with Th2 cytokines induced a decreased mRNA expression of cellular Hsp70, whereas the extracellular fraction of this protein was elevated in the supernatant. The authors proposed that Hsp70 may be a mediator of AR and may be a marker of AR, although the pathophysiologic mechanism was not provided (31). During protein homeostasis, proteins containing ubiquitin ligase function interact with Hsp70 to target client proteins for degradation. Conversely, in the absence of proteasome-related proteins, Hsp70 participates in the folding and relocation of mature proteins (35). Wei et al. (36) have explored the role of STIP1 homology and U-box–containing protein 1 (STUB1), a chaperone-dependent ubiquitin ligase, in the regulation of IL-4Rα and airway inflammation. IL-4Rα is a common receptor of the type 2 cytokines IL-4 and IL-13, which are key cytokines in the induction of the Th2 immune response and airway inflammation in allergic asthma. The ubiquitylation function of STUB1 is highly dependent on its interaction with Hsp70 to induce the turnover and degradation of IL-4Rα. When a mutant form of this protein fails to interact with Hsp70, the mice developed spontaneous airway inflammation with elevated expression levels of IL-4Rα; this indirectly suggests the role of Hsp70 in the increased expression of IL-4Rα and the subsequent development of Th2-mediated airway inflammation (36). The study by Wei et al. (36) is in line with our findings, which indicate that Hsp70 induces an increased production of type 2 cytokines by effector T cells in response to antigen stimulation. Furthermore, Hsp70 negatively regulates the expression of the transcription factor Foxp3 in regulatory T cells, thereby promoting inflammatory immune responses (37). Future studies are warranted to determine Hsp70-driven mechanisms underlying T cell activation and cytokine production.
The immunoregulatory effects of Hsp70 have been investigated for decades in different disease models with controversial results on the role of either the intracellular or extracellular forms of Hsp70 (12, 38). The data from our study indicate that Hsp70 positively regulates airway inflammation and Th2 immune responses in SEA-induced allergic airway inflammation. However, other studies have reported that Hsp70 acts as a negative modulator, including in asthma (24, 38). Shevchenko et al. (24) addressed the modulatory effect of extracellular Hsp70 using ovalbumin-induced allergic inflammation of the airways in mouse and showed that an exogenous and autologous oropharyngeal administration of Hsp70 in ovalbumin-challenged mice was sufficient to decrease BAL fluid type 2 cytokine secretion and inflammatory cell infiltration. In our study, we used a complete deletion of Hsp70.1/.3 that suppressed both the intracellular and extracellular fractions of Hsp70 and therefore prevents any compensatory effect of its isoforms. The complete deletion of Hsp70 provided consistent data supporting the positive regulatory function of Hsp70 in allergic airway inflammation. Furthermore, the chimeric transfer of BM has highlighted the cell-specific role of Hsp70, which is critical for airway inflammation. Because the extracellular and intracellular forms of Hsp70 might have different signaling pathways, specifically targeting one form could lead to different conclusions and may explain the divergence observed in previous studies (12, 24, 39). Chimeric transfer of Hsp70.1/.3−/− BM in WT irradiated mice unexpectedly induced a greater decrease in cytokines than in Hsp70.1/.3−/− mice (Fig. 6, D and E). Two possible situations could explain the differences between BM transfers and findings in whole-body deficient mice (Fig. 2, E and F). First, the contribution of Hsp70 or other heat shock proteins that are expressed in nonhematopoietic cells. Second, differences could be due to an activation of alternative stress response pathways in response to irradiation in mice.
Mucus hypersecretion is one of the hallmark features of airway inflammation, and previous studies have demonstrated the contribution of IL-13 in goblet cell hyperplasia and mucus production (5, 6). In this study, goblet cell hyperplasia and mucus hyperproduction were significantly attenuated in Hsp70.1/.3−/− mice, indicating the role of Hsp70 in allergen-induced Th2 immune responses. In support of this hypothesis, Fang et al. (40) demonstrated that the inhibition of Hsp70 in human bronchial epithelial cells with MAL3–101, a small-molecule Hsp70 inhibitor, or knocking down Hsp70 with Hsp70 siRNA induced a significant decrease in mucin production in vitro. These findings further support our findings on the role of Hsp70 in goblet cell hyperplasia, even though our chimeric transfer findings suggested a dispensable role of Hsp70 in the nonhematopoietic compartment.
A nucleotide microarray analysis of the whole-lung transcriptome was used to identify Hsp70-regulated genes in mice challenged with SEA. The genes involved in the fibrotic pathway were down-regulated in the lungs of Hsp70.1/.3−/− mice compared with those in the lungs of WT mice. However, histological analysis of collagen deposition in lungs of Hsp70.1/.3−/− and WT mice following SEA challenge showed a similar level of increased collagen without significant differences between groups (Fig. S1). These findings might suggest that the airway remodeling process, during SEA-induced airway inflammation, is independent of Hsp70 signaling, at least at the protein level. Other studies have also addressed the role of Hsp70 in fibrosis development. González-Ramos et al. (41) demonstrated that extracellular Hsp70 increases the production of extracellular matrix proteins collagen1 and fibronectin in human vascular smooth muscle cells. Another study found increased expression of Hsp70 in fibrotic lesions of idiopathic pulmonary fibrosis (IPF) patients and bleomycin-induced lung fibrosis in mice (42). However, other studies have indicated an antifibrotic function of Hsp70 in various fibrotic disease models, including IPF. A recent study by Sellares et al. (43) showed the antifibrotic effects of inducible intracellular Hsp70 in IPF and bleomycin-induced lung fibrosis in mice. In addition, other studies have reported a connection between drug-induced fibrosis and the decrease in Hsp70 expression; inversely, the antifibrotic drug's effects correlate with the elevated expression of Hsp70 (44, 45). However, the above observed differences can be attributed to the pathological mechanisms underlying these diseases (46–49). Additionally, unlike its reduced expression in the lungs of IPF subjects (43), Hsp70 expression is commonly elevated in lung tissues or biological fluids in cases of allergic diseases (19–23). In a murine model of chronic asthma, Hsp70/CD80 DNA vaccine has been shown to inhibit airway remodeling through regulating the development of Th1/Th2 subsets (50). However, additional investigations are warranted to establish the role of Hsp70 in multiple chronic lung diseases.
The microarray revealed that Hsp70 alters the gene networks involved in allergic airway inflammation (Fig. 4). These new findings indicate a potential role of Hsp70 in regulating transcript expression in allergic airways. Hsp70 interacts with key regulators of many signal transduction pathways through its chaperone function, which subsequently can affect downstream signaling. Studies have shown that Hsp70 blocks the activation of the transcription factor HSF1 to alter gene expression (51, 52), whereas HSF1 activation is critical to induce the expression of heat shock response genes to maintain an endogenous anti-inflammatory system (53). Similarly, Hsp70 has been shown to form a complex with STUB1 to alter Foxp3 degradation and regulatory T (Treg) cell activity (36, 37). These studies indicate that Hsp70 deficiency may influence multiple T cell subsets and facets of allergic asthma. Our study suggests that the production of IL-17 is not affected by the loss of Hsp70 (Fig. 3B, G, and H). In support of this observation, previous studies have shown that, rather than Hsp70, Hsp90 plays an indispensable role in the production of IL-17 (54). In the current study, Hsp70-deficient CD4+ T cells presented a reduced production of Th2 cytokines following antigen stimulation compared with those from WT mice, and similarly, the percentage of IL-13–producing CD4+ T cell was lower, even though slight but significant, in Hsp70.1/.3−/− mice (Figs. 3, E and F, and 6F). These data suggest that, apart from reduced IL-13 production and a decrease in IL-13+ CD4+ T cells, other subsets of T cells or immune cells might also be affected by Hsp70 loss. Previous studies have shown that Hsp70 can influence multiple T cells, including CD4+ and CD8+ T cells, and cytokine production (55). Furthermore, other immune cells, including basophils or mast cells, can produce type 2 cytokines and hence may at some level contribute to differences in Hsp70-driven airway inflammation (56–58). The IgE-mediated activation of basophils is associated with an increased expression of Hsp70, which correlates with basophil degranulation (57). Besides, basophils and mast cells are important mediators of airway inflammation and can secrete IL-4 and IL-13 during the acute phase of allergic diseases. Further studies targeting these cells will elucidate their contribution to Hsp70-driven airway inflammation. AHR is one of the features of allergic asthma. The inhibition of Hsp70 did not alter the IL-13–driven AHR following increasing doses of methacholine challenge (Fig. S3). These findings may suggest that Hsp70 potentially induces allergen-induced Th2 inflammatory responses rather than acting through IL-13–driven AHR. However, future studies are needed to substantiate the role of Hsp70 in AHR using loss-of-function and gain-of-function models. Also, future investigations are required to test the specific contribution of the intracellular versus extracellular fraction of Hsp70 in allergic asthma using mouse models of allergic asthma.
In summary, our findings have identified a critical role for Hsp70 as a positive regulator of airway inflammation and goblet cell hyperplasia during SEA-induced allergic asthma. Current findings of this study suggest that these effects are mediated by hematopoietic cells expressing Hsp70 and via Th2-driven cytokine production. New findings of this study may provide the impetus for future studies to use T cell–specific Hsp70-deficient mice to understand molecular pathways that regulate T cell activation and Th2 cytokine production. Hsp70 can be targeted as a potential candidate for the therapeutic modulation of Th2 cytokine–driven airway inflammation and mucus hypersecretion to provide new insights and alternative means to control asthma.
Materials and methods
Animals
Hsp70.1/.3−/− mice and littermate control Hsp70.1/.3+/+ (WT) mice were used in this study (16). Because inducible Hsp70 has two isoforms, Hsp70.1 and Hsp70.3, which are identical except for one amino acid missing in Hsp70.3, a double deletion of the gene is crucial to control any potential compensatory effects that may occur with a single Hsp70 gene deletion. Male and female mice aged 8–10 weeks were used in different experiments in this study. All mice were housed under specific, pathogen-free conditions at the National Institutes of Health in an American Association for the Accreditation of Laboratory Animal Care–approved facility. The National Institute of Allergy and Infectious Diseases animal care and use committee approved all experimental procedures.
Mouse model of SEA-induced allergic airway inflammation
Allergic airway inflammation in animals was induced using a SEA preparation derived from the helminthic parasite S. mansoni, which is a strong inducer of type 2 inflammation, as described previously (59, 60). Briefly, the mice were sensitized twice on days 0 and 14 by an intraperitoneal injection of 200 μl of PBS containing 10 μg of SEA. On days 28 and 31, the mice were anesthetized and challenged with SEA (10 μg/50 μl) via the intratracheal route, whereas the control mice received saline as described earlier (59). The mice were euthanized 24 h following the final challenge of SEA or saline on day 32.
RNA preparation, microarray assay, and real-time PCR
The lung tissues were homogenized using a polytron in TRIzol reagent (Life Technologies), and the total RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA) as described previously (61). The total lung RNA samples were reverse transcribed using Superscript II (Invitrogen). Fluorescent cDNA targets were prepared from a 20-μg experimental RNA sample (SEA-challenged group, dUTP-Cy5 (Amersham Biosciences)) and a 20-μg reference RNA sample (PBS-treated group, dUTP-Cy3 (Amersham Biosciences)). Equal quantities of the above labeled cDNA (experimental and control samples) were mixed, and any free label present in the sample was removed by washing three times using a 10-kDa-cutoff Vivaspin filter (Millipore). The labeled fluorescent cDNA targets were hybridized on the Whole Mouse Genome Oligo Microarray kit (Agilent, Palo Alto, CA) containing more than 41,000 gene probes. Lung transcripts of saline-treated Hsp70.1/.3−/− and WT mice were used as reference samples to subtract baseline differences, respectively. Afterward, gene differential expression was compared between the two experimental groups. The genes that were differentially expressed between the experimental and control samples were selected using a p value of 0.05 and a -fold change of 1.5-fold. The lists of genes that were significantly up- or down-regulated in the WT and Hsp70.1/.3−/− mice were compared to obtain different clusters of Hsp70.1/.3-regulated genes using GeneVenn (62). Functional analyses of gene clusters were performed using Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA), and heat maps were used to illustrate the average -fold change in the comparison of gene expression for WT and Hsp70.1/3−/− mice.
For RT-PCR analysis, the cDNAs were prepared from purified total lung RNA of PBS- and SEA-challenged mice. The relative expression of the genes of interest was quantified using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) as described previously (63). The gene transcripts for each sample were normalized to the expression of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase and then were expressed as the relative -fold induction change compared with that of the saline-treated control mice. The primer sequences used to amplify the cytokines were published previously (5, 59), and the primer sequences for Hsp amplification were as follows: Hsp70a: Fwd, AGGCCTCTGCTGGCTCTC; Rev, TGCAGGACAAACTAAGGAGTGA; Hsp40: Fwd, TTTTCGACCGCTATGGAGAG; Rev, TAGCACCACCACTGCTTCCT; Hsp27: Fwd, AGGAGCTCACAGTGAAGACCA; Rev, CTTTCTTCGTGCTTGCCAGT.
BAL fluid and differential cell count
The preparation of airway-infiltrating cells in the BALF and the differential cell count were performed as described previously with slight modifications (46, 64). Briefly, mice were anesthetized 24 h after the final intratracheal allergen challenge, and the trachea was cannulated followed by two instillation aspirations of 800 μl of PBS. The collected BALF was centrifuged at 1,500 rpm for 5 min at 4 °C, and the supernatant was aliquoted and stored at −80 °C until use. ELISA kits (R&D Systems, Minneapolis, MN) were used to measure the different cytokine levels in BALF following the manufacturer's instructions, whereas the soluble collagen in the BAL fluid was quantified using a Sircol Collagen Assay kit (Biocolor Ltd., Belfast, Northern Ireland, UK). The cell pellets were resuspended in RPMI 1640 culture medium supplemented with 10% fetal calf serum, and the total BAL cell number was enumerated. The Cytospin preparations were stained with a Diff-Quick staining kit (Thermo Fisher Scientific) for the differential counts of BALF cells (59).
Histology
The mice were euthanized 24 h following the last allergen challenge, and the lungs were inflated with 10% neutral formalin and harvested for histological analysis. Paraffin-embedded 5-μm sections of the left lobe were stained with H&E to analyze the airway inflammation. The histological lesions were further quantified and expressed as peribronchial and perivascular inflammation scores (65). Airway inflammation was further quantified with chimera assays through a combination of peribronchial and perivascular inflammation scores and tissue inflammatory cell counts to generate the lung inflammation score. The ABPAS reaction was used to measure the goblet cell hyperplasia and the mucus hypersecretion. Similar to the H&E inflammation score, mucus production was quantified with arbitrary scoring and expressed as a mucus score. All quantified histological data, including the peribronchial and perivascular inflammation scores and the mucus score, were analyzed by an experienced pathologist who was blinded to the experimental protocol and the mouse groups. In addition, for collagen deposition and fibrosis analysis following allergen challenge, lung sections were stained with Picrosirius Red.
Immunohistochemistry
Formalin-fixed lung tissue sections were stained for immunohistochemistry detection of Hsp70 following the challenge with SEA or saline. Paraffin-embedded sections (5 μm) were deparaffinized, extensively washed in PBS, blocked for 2 h with donkey serum, and incubated overnight at 4 °C with a rabbit anti-mouse Hsp70 polyclonal antibody (1:500 dilution; Thermo Fisher, Rockford, IL) in a humidified chamber. The slides were washed and incubated with a horseradish peroxidase–conjugated mouse anti-rabbit antibody (1:200) for 60 min at room temperature as described previously (49). Immunostaining was developed with diaminobenzidine. Gill's hematoxylin was used as the counterstain. Representative images were obtained using a Leica DM2700 bright-field microscope, the low-magnification images were taken with a 10× objective, and the high-magnification images were taken with a 63× objective.
Bone marrow chimeric mice and challenge
The chimeric mice used in this study were generated by transferring BM cells from either the WT or Hsp70.1/.3−/− mice into lethally irradiated recipient mice. The BM cells were prepared as described previously (66). Briefly, the BM was flushed from the femur and tibia of the WT or Hsp70.1/.3−/− mice, and the nucleated BM cells were resuspended in PBS following washing steps and red blood cell lysis. The recipient WT or Hsp70.1/.3−/− mice were irradiated with 11.75 grays (J. L. Shepherd Mark I 68 cesium-137 irradiator), and then the mice were intravenously injected with prepared BM cells from the respective donor mice (1 × 107 cells). Four chimeric mice groups were therefore obtained through the combinatory transfer of WT or Hsp70.1/.3−/− BM cells into either WT or Hsp70.1/.3−/− irradiated recipient mice. Ten weeks after immune reconstitution, the chimeric mice were sensitized and challenged with SEA or PBS as described above and evaluated for airway inflammation.
Intracellular cytokine staining
Lung single-cell suspensions were prepared from the WT and Hsp70.1/.3−/− mice that were challenged with SEA or saline by passing the lung tissues through a 100-μm nylon filter. The red blood cells were lysed with ammonium chloride–potassium lysis buffer, and the immune cells were resuspended in RPMI 1640 culture medium supplemented with 10% fetal bovine serum and antibiotics. For intracellular cytokine staining, the cells were stimulated with phorbol 12-myristate 13-acetate (10 ng/ml) and ionomycin (1 μg/ml) in the presence of brefeldin A for 3 h as described previously (67). The stimulated cells were incubated with anti-CD16/32 Fc blocking antibodies for 30 min and then stained for the T cell surface marker CD4. Following permeabilization (Cytofix/Cytoperm buffer, BD Biosciences), the cells were stained with anti-mouse IL-13 and IL-17 antibodies conjugated with fluorochromes. The stained cells were acquired with a FACSCalibur (BD Immunocytometry), and the data were analyzed with FlowJo (Treestar Inc., Ashland, OR).
T cell stimulation and cytokine production
Lung leukocytes were prepared from the lungs of WT and Hsp70.1/.3−/− mice that were challenged with SEA as described above and restimulated for 72 h in RPMI 1640 medium alone or with SEA (20 μg/ml) or ConA (5 μg/ml) as a positive control in humidified conditions with 5% CO2 at 37 °C. The culture supernatants were collected by centrifugation and stored at −80 °C until use for cytokine production. The cytokine levels of IL-4, IL-5, and IL-13 were determined with DuoSet ELISA kits (R&D Systems) following the manufacturer's instructions.
Statistics
The data were analyzed with Prism (Version 7; GraphPad, San Diego, CA). The quantitative data are presented as scatter plots, including the mean with the standard deviation. The data were considered statistically significant for a p value less than 0.05 that was obtained with a two-tailed, unpaired Student's t test for a comparison of the means between two groups, and Mann–Whitney test was used for data without normal distribution. One-way ANOVA with Tukey's multiple comparison posttest was used to compare the different experimental groups.
Author contributions
D. J. K. Y., M. S. W., T. A. W., and S. K. M. data curation; D. J. K. Y. and S. K. M. software; D. J. K. Y., M. M. M.-K., M. S. W., T. A. W., and S. K. M. formal analysis; D. J. K. Y., M. M. M.-K., M. S. W., T. A. W., and S. K. M. methodology; D. J. K. Y. and S. K. M. writing-original draft; D. J. K. Y. and S. K. M. project administration; D. J. K. Y., M. M. M.-K., M. S. W., T. A. W., and S. K. M. writing-review and editing; M. M. M.-K. visualization; M. S. W., T. A. W., and S. K. M. investigation; T. A. W. and S. K. M. conceptualization; T. A. W. and S. K. M. funding acquisition; S. K. M. supervision.
Supplementary Material
Acknowledgments
We thank the veterinary services and genomics core at the National Institute of Allergy and Infectious Diseases for help with this study.
This work was supported by the United States Department of Defense Grant W81XWH-17-1-0666 (to S. K. M.), National Institutes of Health Grants 1R01 HL134801 and 1R21 AI137309 (to S. K. M.), and the intramural research program of the NIAID, National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We dedicate this study in memory of Allen Cheever who contributed to the analysis of histological sections.
This article contains Tables S1 and S2, Figs. S1–S3, and supporting methods.
- Th
- T helper
- AHR
- airway hyperresponsiveness
- Hsp
- heat shock protein
- BAL
- bronchoalveolar lavage
- SEA
- soluble egg antigen
- BM
- bone marrow
- IL
- interleukin
- H&E
- hematoxylin and eosin
- ABPAS
- Alcian blue–periodic acid–Schiff
- BALF
- bronchial alveolar lavage fluid
- MFI
- median fluorescence intensity
- ConA
- concanavalin A
- APC
- antigen-presenting cell
- PCLS
- precision-cut lung slice
- AR
- allergic rhinitis
- STUB1
- STIP1 homology and U-box–containing protein 1
- IPF
- idiopathic pulmonary fibrosis
- Fwd
- forward
- Rev
- reverse
- ANOVA
- analysis of variance.
References
- 1. Croisant S. (2014) Epidemiology of asthma: prevalence and burden of disease. Adv Exp. Med. Biol. 795, 17–29 10.1007/978-1-4614-8603-9_2 [DOI] [PubMed] [Google Scholar]
- 2. Loftus P. A., and Wise S. K. (2016) Epidemiology of asthma. Curr. Opin. Otolaryngol. Head Neck Surg. 24, 245–249 10.1097/MOO.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 3. Wynn T. A. (2015) Type 2 cytokines: mechanisms and therapeutic strategies. Nat. Rev. Immunol. 15, 271–282 10.1038/nri3831 [DOI] [PubMed] [Google Scholar]
- 4. Munitz A., Brandt E. B., Mingler M., Finkelman F. D., and Rothenberg M. E. (2008) Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 105, 7240–7245 10.1073/pnas.0802465105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramalingam T. R., Pesce J. T., Sheikh F., Cheever A. W., Mentink-Kane M. M., Wilson M. S., Stevens S., Valenzuela D. M., Murphy A. J., Yancopoulos G. D., Urban J. F. Jr., Donnelly R. P., and Wynn T. A. (2008) Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat. Immunol. 9, 25–33 10.1038/ni1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wills-Karp M. (2004) Interleukin-13 in asthma pathogenesis. Immunol. Rev. 202, 175–190 10.1111/j.0105-2896.2004.00215.x [DOI] [PubMed] [Google Scholar]
- 7. McGregor M. C., Krings J. G., Nair P., and Castro M. (2019) Role of biologics in asthma. Am. J. Respir. Crit. Care Med. 199, 433–445 10.1164/rccm.201810-1944CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flood-Page P., Swenson C., Faiferman I., Matthews J., Williams M., Brannick L., Robinson D., Wenzel S., Busse W., Hansel T. T., Barnes N. C., and International Mepolizumab Study Group (2007) A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am. J. Respir. Crit. Care Med. 176, 1062–1071 10.1164/rccm.200701-085OC [DOI] [PubMed] [Google Scholar]
- 9. Hastie A. T., Steele C., Dunaway C. W., Moore W. C., Rector B. M., Ampleford E., Li H., Denlinger L. C., Jarjour N., Meyers D. A., Bleecker E. R., and NHLBI Severe Asthma Research Program (SARP) (2018) Complex association patterns for inflammatory mediators in induced sputum from subjects with asthma. Clin. Exp. Allergy 48, 787–797 10.1111/cea.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson G. P. (2008) Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 372, 1107–1119 10.1016/S0140-6736(08)61452-X [DOI] [PubMed] [Google Scholar]
- 11. Bukau B., Weissman J., and Horwich A. (2006) Molecular chaperones and protein quality control. Cell 125, 443–451 10.1016/j.cell.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 12. Zininga T., Ramatsui L., and Shonhai A. (2018) Heat shock proteins as immunomodulants. Molecules 23, E2846 10.3390/molecules23112846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malyshev I. (2013) Immunity, Tumors and Aging: the Role of HSP70, pp. 15–29, Springer, Dordrecht, The Netherlands [Google Scholar]
- 14. Kampinga H. H., Hageman J., Vos M. J., Kubota H., Tanguay R. M., Bruford E. A., Cheetham M. E., Chen B., and Hightower L. E. (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14, 105–111 10.1007/s12192-008-0068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rappa F., Farina F., Zummo G., David S., Campanella C., Carini F., Tomasello G., Damiani P., Cappello F., De Macario E. C., and Macario A. J. (2012) HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res. 32, 5139–5150 [PubMed] [Google Scholar]
- 16. Hunt C. R., Dix D. J., Sharma G. G., Pandita R. K., Gupta A., Funk M., and Pandita T. K. (2004) Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol. Cell. Biol. 24, 899–911 10.1128/MCB.24.2.899-911.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim Y. K., Suarez J., Hu Y., McDonough P. M., Boer C., Dix D. J., and Dillmann W. H. (2006) Deletion of the inducible 70-kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation 113, 2589–2597 10.1161/CIRCULATIONAHA.105.598409 [DOI] [PubMed] [Google Scholar]
- 18. Qu B., Jia Y., Liu Y., Wang H., Ren G., and Wang H. (2015) The detection and role of heat shock protein 70 in various nondisease conditions and disease conditions: a literature review. Cell Stress Chaperones 20, 885–892 10.1007/s12192-015-0618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yusuf N., Nasti T. H., Huang C. M., Huber B. S., Jaleel T., Lin H. Y., Xu H., and Elmets C. A. (2009) Heat shock proteins HSP27 and HSP70 are present in the skin and are important mediators of allergic contact hypersensitivity. J. Immunol. 182, 675–683 10.4049/jimmunol.182.1.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baturcam E., Snape N., Yeo T. H., Schagen J., Thomas E., Logan J., Galbraith S., Collinson N., Phipps S., Fantino E., Sly P. D., and Spann K. M. (2017) Human metapneumovirus impairs apoptosis of nasal epithelial cells in asthma via HSP70. J. Innate. Immun. 9, 52–64 10.1159/000449101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertorelli G., Bocchino V., Zhuo X., Chetta A., Del Donno M., Foresi A., Testi R., and Olivieri D. (1998) Heat shock protein 70 upregulation is related to HLA-DR expression in bronchial asthma. Effects of inhaled glucocorticoids. Clin. Exp. Allergy 28, 551–560 10.1046/j.1365-2222.1998.00251.x [DOI] [PubMed] [Google Scholar]
- 22. Vignola A. M., Chanez P., Polla B. S., Vic P., Godard P., and Bousquet J. (1995) Increased expression of heat shock protein 70 on airway cells in asthma and chronic bronchitis. Am. J. Respir. Cell Mol. Biol. 13, 683–691 10.1165/ajrcmb.13.6.7576706 [DOI] [PubMed] [Google Scholar]
- 23. Hou C., Zhao H., Li W., Liang Z., Zhang D., Liu L., Tong W., Cai S. X., and Zou F. (2011) Increased heat shock protein 70 levels in induced sputum and plasma correlate with severity of asthma patients. Cell Stress Chaperones 16, 663–671 10.1007/s12192-011-0271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shevchenko M. A., Troyanova N. I., Servuli E. A., Bolkhovitina E. L., Fedorina A. S., and Sapozhnikov A. M. (2016) Study of immunomodulatory effects of extracellular HSP70 in a mouse model of allergic airway inflammation. Biochemistry 81, 1384–1395 10.1134/S0006297916110158 [DOI] [PubMed] [Google Scholar]
- 25. Lukacs N. W., Strieter R. M., Chensue S. W., and Kunkel S. L. (1994) Interleukin-4-dependent pulmonary eosinophil infiltration in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 10, 526–532 10.1165/ajrcmb.10.5.8179915 [DOI] [PubMed] [Google Scholar]
- 26. Webb L. M., Lundie R. J., Borger J. G., Brown S. L., Connor L. M., Cartwright A. N. R., Dougall A. M., Wilbers R. H., Cook P. C., Jackson-Jones L. H., Phythian-Adams A. T., Johansson C., Davis D. M., Dewals B. G., Ronchese F., et al. (2017) Type I interferon is required for T helper (Th) 2 induction by dendritic cells. EMBO J. 36, 2404–2418 10.15252/embj.201695345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Danov O., Jiménez Delgado S. M., Obernolte H., Seehase S., Dehmel S., Braubach P., Fieguth H. G., Matschiner G., Fitzgerald M., Jonigk D., Knauf S., Pfennig O., Warnecke G., Wichmann J., Braun A., et al. (2018) Human lung tissue provides highly relevant data about efficacy of new anti-asthmatic drugs. PLoS One 13, e0207767 10.1371/journal.pone.0207767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kudo M., Melton A. C., Chen C., Engler M. B., Huang K. E., Ren X., Wang Y., Bernstein X., Li J. T., Atabai K., Huang X., and Sheppard D. (2012) IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 18, 547–554 10.1038/nm.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper P. R., Lamb R., Day N. D., Branigan P. J., Kajekar R., San Mateo L., Hornby P. J., and Panettieri R. A. Jr. (2009) TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L530–L537 10.1152/ajplung.00133.2009 [DOI] [PubMed] [Google Scholar]
- 30. Miyata Y., Li X., Lee H. F., Jinwal U. K., Srinivasan S. R., Seguin S. P., Young Z. T., Brodsky J. L., Dickey C. A., Sun D., and Gestwicki J. E. (2013) Synthesis and initial evaluation of YM-08, a blood-brain barrier permeable derivative of the heat shock protein 70 (Hsp70) inhibitor MKT-077, which reduces tau levels. ACS Chem. Neurosci. 4, 930–939 10.1021/cn300210g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Min H. J., Kim K. S., Yoon J. H., Kim C. H., and Cho H. J. (2017) T-helper 2 cytokine-induced heat shock protein 70 secretion and its potential association with allergic rhinitis. Int. Forum Allergy Rhinol. 7, 530–535 10.1002/alr.21905 [DOI] [PubMed] [Google Scholar]
- 32. Alford K. A., Glennie S., Turrell B. R., Rawlinson L., Saklatvala J., and Dean J. L. (2007) Heat shock protein 27 functions in inflammatory gene expression and transforming growth factor-β-activated kinase-1 (TAK1)-mediated signaling. J. Biol. Chem. 282, 6232–6241 10.1074/jbc.M610987200 [DOI] [PubMed] [Google Scholar]
- 33. Breed E. R., Hilliard C. A., Yoseph B., Mittal R., Liang Z., Chen C. W., Burd E. M., Brewster L. P., Hansen L. M., Gleason R. L. Jr., Pandita T. K., Ford M. L., Hunt C. R., and Coopersmith C. M. (2018) The small heat shock protein HSPB1 protects mice from sepsis. Sci. Rep. 8, 12493 10.1038/s41598-018-30752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pezzulo A. A., Tudas R. A., Stewart C. G., Buonfiglio L. G. V., Lindsay B. D., Taft P. J., Gansemer N. D., and Zabner J. (2019) HSP90 inhibitor geldanamycin reverts IL-13- and IL-17-induced airway goblet cell metaplasia. J. Clin. Investig. 129, 744–758 10.1172/JCI123524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDonough H., and Patterson C. (2003) CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8, 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei Q., Sha Y., Bhattacharya A., Abdel Fattah E., Bonilla D., Jyothula S. S., Pandit L., Khurana Hershey G. K., and Eissa N. T. (2014) Regulation of IL-4 receptor signaling by STUB1 in lung inflammation. Am. J. Respir. Crit. Care Med. 189, 16–29 10.1164/rccm.201305-0874OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Z., Barbi J., Bu S., Yang H. Y., Li Z., Gao Y., Jinasena D., Fu J., Lin F., Chen C., Zhang J., Yu N., Li X., Shan Z., Nie J., et al. (2013) The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 10.1016/j.immuni.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferat-Osorio E., Sanchez-Anaya A., Gutierrez-Mendoza M., Bosco-Garate I., Wong-Baeza I., Pastelin-Palacios R., Pedraza-Alva G., Bonifaz L. C., Cortes-Reynosa P., Perez-Salazar E., Arriaga-Pizano L., Lopez-Macias C., Rosenstein Y., and Isibasi A. (2014) Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J. Inflamm. 11, 19 10.1186/1476-9255-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Eden W., Spiering R., Broere F., and van der Zee R. (2012) A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones 17, 281–292 10.1007/s12192-011-0311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang S., Crews A. L., Chen W., Park J., Yin Q., Ren X. R., and Adler K. B. (2013) MARCKS and HSP70 interactions regulate mucin secretion by human airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L511–L518 10.1152/ajplung.00337.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. González-Ramos M., Calleros L., López-Ongil S., Raoch V., Griera M., Rodríguez-Puyol M., de Frutos S., and Rodríguez-Puyol D. (2013) HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int. J. Biochem. Cell Biol. 45, 232–242 10.1016/j.biocel.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 42. Luzina I. G., Kopach P., Lockatell V., Kang P. H., Nagarsekar A., Burke A. P., Hasday J. D., Todd N. W., and Atamas S. P. (2013) Interleukin-33 potentiates bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 49, 999–1008 10.1165/rcmb.2013-0093OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sellares J., Veraldi K. L., Thiel K. J., Cárdenes N., Alvarez D., Schneider F., Pilewski J. M., Rojas M., and Feghali-Bostwick C. A. (2019) Intracellular heat shock protein 70 deficiency in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 60, 629–636 10.1165/rcmb.2017-0268OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Namba T., Tanaka K., Hoshino T., Azuma A., and Mizushima T. (2011) Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PLoS One 6, e27296 10.1371/journal.pone.0027296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanaka K., Tanaka Y., Namba T., Azuma A., and Mizushima T. (2010) Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem. Pharmacol. 80, 920–931 10.1016/j.bcp.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 46. Wilson M. S., Madala S. K., Ramalingam T. R., Gochuico B. R., Rosas I. O., Cheever A. W., and Wynn T. A. (2010) Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 207, 535–552 10.1084/jem.20092121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gieseck R. L. 3rd, Wilson M. S., and Wynn T. A. (2018) Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 18, 62–76 10.1038/nri.2017.90 [DOI] [PubMed] [Google Scholar]
- 48. Vannella K. M., Ramalingam T. R., Borthwick L. A., Barron L., Hart K. M., Thompson R. W., Kindrachuk K. N., Cheever A. W., White S., Budelsky A. L., Comeau M. R., Smith D. E., and Wynn T. A. (2016) Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci. Transl. Med. 8, 337ra365 10.1126/scitranslmed.aaf1938 [DOI] [PubMed] [Google Scholar]
- 49. Sontake V., Wang Y., Kasam R. K., Sinner D., Reddy G. B., Naren A. P., McCormack F. X., White E. S., Jegga A. G., and Madala S. K. (2017) Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight 2, e91454 10.1172/jci.insight.91454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan L., Xiao-Ling S., Zheng-Yan C., Guo-Ping L., Sen Z., and Zhuang C. (2013) HSP70/CD80 DNA vaccine inhibits airway remodeling by regulating the transcription factors T-bet and GATA-3 in a murine model of chronic asthma. Arch. Med. Sci. 9, 906–915 10.5114/aoms.2013.33180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abravaya K., Myers M. P., Murphy S. P., and Morimoto R. I. (1992) The human heat shock protein Hsp70 interacts with Hsf, the transcription factor that regulates heat shock gene expression. Gene Dev. 6, 1153–1164 10.1101/gad.6.7.1153 [DOI] [PubMed] [Google Scholar]
- 52. Krakowiak J., Zheng X., Patel N., Feder Z. A., Anandhakumar J., Valerius K., Gross D. S., Khalil A. S., and Pincus D. (2018) Hsf1 and Hsp70 constitute a two-component feedback loop that regulates the yeast heat shock response. Elife 7, e31668 10.7554/eLife.31668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ianaro A., Ialenti A., Maffia P., Pisano B., and Di Rosa M. (2001) HSF1/hsp72 pathway as an endogenous anti-inflammatory system. FEBS Lett. 499, 239–244 10.1016/S0014-5793(01)02569-8 [DOI] [PubMed] [Google Scholar]
- 54. Tukaj S., Zillikens D., and Kasperkiewicz M. (2014) Inhibitory effects of heat shock protein 90 blockade on proinflammatory human Th1 and Th17 cell subpopulations. J. Inflamm. 11, 10 10.1186/1476-9255-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Figueiredo C., Wittmann M., Wang D., Dressel R., Seltsam A., Blasczyk R., and Eiz-Vesper B. (2009) Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood 113, 3008–3016 10.1182/blood-2008-06-162727 [DOI] [PubMed] [Google Scholar]
- 56. Mortaz E., Redegeld F. A., Nijkamp F. P., Wong H. R., and Engels F. (2006) Acetylsalicylic acid-induced release of HSP70 from mast cells results in cell activation through TLR pathway. Exp. Hematol. 34, 8–18 10.1016/j.exphem.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 57. Li X., Kanegasaki S., Jin F., Deng Y., Kim J. R., Chang H. W., and Tsuchiya T. (2018) Simultaneous induction of HSP70 expression, and degranulation, in IgE/Ag-stimulated or extracellular HSP70-stimulated mast cells. Allergy 73, 361–368 10.1111/all.13296 [DOI] [PubMed] [Google Scholar]
- 58. Harkins M. S., Moseley P. L., and Iwamoto G. K. (2003) Regulation of CD23 in the chronic inflammatory response in asthma: a role for interferon-γ and heat shock protein 70 in the TH2 environment. Ann. Allergy Asthma Immunol. 91, 567–574 10.1016/S1081-1206(10)61536-0 [DOI] [PubMed] [Google Scholar]
- 59. Wilson M. S., Elnekave E., Mentink-Kane M. M., Hodges M. G., Pesce J. T., Ramalingam T. R., Thompson R. W., Kamanaka M., Flavell R. A., Keane-Myers A., Cheever A. W., and Wynn T. A. (2007) IL-13Rα2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J. Clin. Investig. 117, 2941–2951 10.1172/JCI31546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edukulla R., Singh B., Jegga A. G., Sontake V., Dillon S. R., and Madala S. K. (2015) Th2 cytokines augment IL-31/IL-31RA interactions via STAT6-dependent IL-31RA expression. J. Biol. Chem. 290, 13510–13520 10.1074/jbc.M114.622126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Madala S. K., Pesce J. T., Ramalingam T. R., Wilson M. S., Minnicozzi S., Cheever A. W., Thompson R. W., Mentink-Kane M. M., and Wynn T. A. (2010) Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J. Immunol. 184, 3955–3963 10.4049/jimmunol.0903008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pirooznia M., Nagarajan V., and Deng Y. (2007) GeneVenn—a web application for comparing gene lists using Venn diagrams. Bioinformation 1, 420–422 10.6026/97320630001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sontake V., Kasam R. K., Sinner D., Korfhagen T. R., Reddy G. B., White E. S., Jegga A. G., and Madala S. K. (2018) Wilms' tumor 1 drives fibroproliferation and myofibroblast transformation in severe fibrotic lung disease. JCI Insight 3, 121252 10.1172/jci.insight.121252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilson M. S., Pesce J. T., Ramalingam T. R., Thompson R. W., Cheever A., and Wynn T. A. (2008) Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J. Immunol. 181, 6942–6954 10.4049/jimmunol.181.10.6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alrifai M., Marsh L. M., Dicke T., Kılıç A., Conrad M. L., Renz H., and Garn H. (2014) Compartmental and temporal dynamics of chronic inflammation and airway remodelling in a chronic asthma mouse model. PLoS One 9, e85839 10.1371/journal.pone.0085839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Madala S. K., Edukulla R., Schmidt S., Davidson C., Ikegami M., and Hardie W. D. (2014) Bone marrow-derived stromal cells are invasive and hyperproliferative and alter transforming growth factor-α-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 50, 777–786 10.1165/rcmb.2013-0042OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Choy D. F., Hart K. M., Borthwick L. A., Shikotra A., Nagarkar D. R., Siddiqui S., Jia G., Ohri C. M., Doran E., Vannella K. M., Butler C. A., Hargadon B., Sciurba J. C., Gieseck R. L., Thompson R. W., et al. (2015) TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci. Transl. Med. 7, 301ra129 10.1126/scitranslmed.aab3142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.