In this issue, Nguyen et al1 revealed an unexpected role of the protein posttranslational modification (PTM), lysine acetylation, in signal transduction inside cardiac mitochondria. Using a combination of proteomics and biochemical techniques, the authors discovered that the deletion of cyclophilin D (CypD−/−) results in widespread increases in the acetylation of multiple mitochondrial proteins. CypD is a mitochondrial peptidyl prolyl cis-trans isomerase best known for its role in promoting mitochondrial permeability transition (MPT) and the resulting cell death and was previously not associated with PTM signaling. The findings shed light on why CypD−/− mice had decreased fatty acid oxidation capacity in the heart and how CypD−/− loss paradoxically protects against ischemic injury but promotes heart failure.
Many recent works attempted to understand the PTM targets, occupancy, functions, and dynamics of mitochondrial proteins in the heart.2–4 These cellular signaling mechanisms are thought to fine-tune the metabolic and energetic output of the heart. Furthermore, cardioprotective PTM signals converge at cardiac mitochondria to modulate cell death outcome during ischemic injury.5 Therefore, understanding how the signals transduce inside the organelles is an outstanding objective that can lead to better therapies. It has recently emerged that lysine acetylation on nonhistone proteins is a dynamic and reversible PTM that modulates a wide range of cellular processes including metabolism.6 In a previous publication, it was found that CypD loss in mice resulted in altered mitochondrial metabolism. Hypothesizing that this effect may be mediated by lysine acetylation, the authors isolated mitochondrial protein acetylation sites with anti-acetyl lysine antibodies and then used mass spectrometry to identify and quantify them in wild-type and CypD−/− hearts. The hyperacetylated proteins in CypD−/− hearts include the trifunctional protein, a crucial enzyme in fatty acid oxidation, which also showed a concomitant decrease in functions in vitro that can be rescued by the addition of acetylation-promoting acetyl coenzyme A.
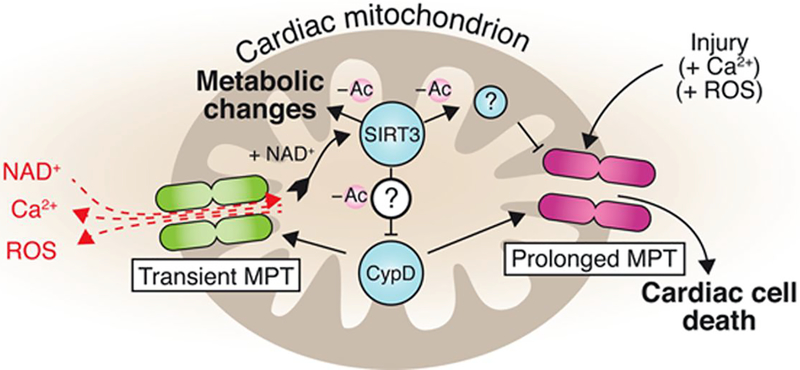
The results carry immediate implications for cardiac research. Foremost, it composed the first comprehensive catalog of protein acetylation sites in cardiac mitochondria. Approximately 200 mitochondrial proteins are now known to be modified by lysine acetylation, making it one of the most pervasive PTM in the heart. The identification of these novel PTM sites is an indispensable first step to elucidating their individual and combinatorial functions and allows future hypothesis testing and functional characterization in different experimental models using targeted PTM-specific identification and quantification techniques.2,7,8 Second, the article provides compelling evidence that lysine acetylation is an important mediator of mitochondrial phenotypes. The acetyl proteins seem to be enriched in respiratory complexes and other metabolic enzymes, suggesting that acetylation may be broadly involved in metabolic regulations (Figure).
Figure. Emerging model of acetylation signaling in mitochondria.
Cyclophilin D (CypD) mediates transient mitochondrial permeability transition (MPT), which equilibrates several metabolites and causes an increase in intramitochondrial NAD+ content. This activates sirtuins such as SIRT3, which deacetylates (−Ac) several mitochondrial protein targets, likely including CypD, to modulate metabolism and possibly cell death. ROS indicates reactive oxygen species.
The direct connection between CypD and acetylation is intriguing because CypD exhibits no known deacetylase or acetyltransferase activities. Interestingly, whereas this study shows that CypD and MPT modulate protein acetylation, CypD itself has also been shown to be activated by acetylation to promote MPT.9 An explanation of this mutual regulation is discussed in the article and lies in the distinction between transient and permanent MPT. CypD loss has been shown to disrupt not only death-promoting permanent MPT but also transient MPT, which does not trigger cell death per se but is thought to have a physiological role by providing an escape route for Ca2+ and reactive oxygen species from mitochondria.10,11 The authors propose that increased Ca2+ in CypD−/− mice promotes Krebs cycle flux, which would increase the mitochondrial nicotinamide adenine dinucleotide reduced:oxidized (NADH/NAD+) ratio. Because mitochondrial sirtuins are deacetylases that require NAD+ as cofactors, higher NADH/NAD+ ratio increases acetylation and signals additional metabolic changes. These metabolic changes, including decreased fatty acid oxidation, may account for other observed phenotypes of CypD−/− mice, including their greater tendency for development of heart failure under stress.10 If this model proves to be correct, then the intricacy involved would imply that acetylation is a bona fide regulatory principle of metabolism in the heart. Nguyen et al1 found that CypD−/− mice have increased NADH inside mitochondria as measured by fluorescence microscopy, and a recent article using a complex I deficiency model also showed that NADH/NAD+ ratio can affect overall acetylation level. One unexplored possibility is that CypD may also modulate net NAD+ concentration inside mitochondria. Transient MPT has been proposed to allow the entry of NAD+ through the classically impermeable mitochondrial inner membrane,12 which may then directly affect metabolic flux and acetylation.
Because genetic ablation of CypD protects the heart from ischemic injuries by inhibiting cell death triggered by MPT, this raised the question of whether acetylation is one of the signals for MPT gating. The authors found no difference in MPT probability between wild-type and Sirt3−/− mice, however, concluding that general hyperacetylation per se is not sufficient to instigate protection. The result in Sirt3−/− mice is consistent with some previous observations,9 although other studies have found sirtuin (SIRT)3 to be cardioprotective, with increased acetylation after SIRT3 activity loss associated with MPT opening.13–15 In noncardiac (neuronal) cells, extracellular addition of NAD+ alone is sufficient to inhibit axonal degeneration, a process that shares significant features with MPT (eg, both are triggered by Ca2+ and atractyloside, and both are attenuated by cyclosporine A),16 a result that corroborates that hyperacetylation may regulate cell death among other processes. It is possible that these discrepancies may be attributable to differences in animal models. It should be noted that the present article reported that the hyperacetylation profiles in Sirt3−/− and CypD−/− mice overlapped poorly (<10%). Hence, acetylation is most likely under the regulation of additional enzymes or signaling cues in cardiac mitochondria, and distinguishing the acetylation targets that lead to the observed phenotypes of CypD−/− mice will be a crucial objective.
In summary, the work by Nguyen et al1 is certain to stimulate new studies in cardiac signaling by revealing an extensive acetylation signaling network in the mitochondria, with complexity that approaches that of protein phosphorylation in the cardiac cell. The emerging evidence indicates that hyperacetylation of specific mitochondrial proteins is associated with far-reaching consequences in cell death and metabolic sensing, and future research may elucidate more unsuspected interactions in modulating cardiac phenotypes.
Sources of Funding
This work was supported by the National Institutes of Health awards HL-R37–63901 and HHSN268201000035C, the T.C. Laubisch endowment at University of California, Los Angeles (to Dr Ping), and the American Heart Association fellowships 13POST14700031 (to Dr Lam) and 12PRE11610024 to (E. Lau).
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Nguyen TT, Wong R, Menazza S, Sun J, Chen Y, Wang G, Gucek M, Steenbergen C, Sack MN, Murphy E. Cyclophilin D modulates mitochondrial acetylome. Circ Res. 2013;113:1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam MP, Lau E, Scruggs SB, Wang D, Kim TY, Liem DA, Zhang J, Ryan CM, Faull KF, Ping P. Site-specific quantitative analysis of cardiac mitochondrial protein phosphorylation. J Proteomics. 2013;81: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau E, Wang D, Zhang J, Yu H, Lam MP, Liang X, Zong N, Kim TY, Ping P. Substrate- and isoform-specific proteome stability in normal and stressed cardiac mitochondria. Circ Res. 2012;110:1174–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res. 2013;112:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Z, Scott I, Webster BR, Sack MN. The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ Res. 2009;105:830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zong NC, Li H, Li H, et al. Integration of cardiac proteome biology and medicine by a specialized knowledgebase. Circ Res. 2013;113: 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam MP, Scruggs SB, Kim TY, Zong C, Lau E, Wang D, Ryan CM, Faull KF, Ping P. An MRM-based workflow for quantifying cardiac mitochondrial protein phosphorylation in murine and human tissue. J Proteomics. 2012;75:4602–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY). 2010;2:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. [DOI] [PubMed] [Google Scholar]
- 12.Di Lisa F, Ziegler M. Pathophysiological relevance of mitochondria in NAD(+) metabolism. FEBS Lett. 2001;492:4–8. [DOI] [PubMed] [Google Scholar]
- 13.Shulga N, Pastorino JG. Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3. J Cell Sci. 2010;123:4117–4127. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]