Abstract
Osteochondral xenografts are potentially inexpensive, widely available alternatives to fresh allografts. However, antigen removal from xenogenic cartilage may damage the extracellular matrix and reduce compressive stiffness. Non-crosslinked xenogenic cartilage may also undergo rapid enzymatic degradation in vivo. We hypothesized that natural crosslinking agents could be used in place of glutaraldehyde to improve the mechanical properties and enzymatic resistance of decellularized cartilage. This study compared the effects of genipin (GNP), proanthocyanidin (PA), and epigallocatechin gallate (EGCG), on the physical and mechanical properties of decellularized porcine cartilage. Glutaraldehyde (GA) served as a positive control. Porcine articular cartilage discs were decellularized in 2% sodium dodecyl sulfate and DNase I followed by fixation in 0.25% GNP, 0.25% PA, 0.25% EGCG, or 2.5% GA. Decellularization decreased DNA by 15% and GAG by 35%. For natural crosslinkers, the average degree of crosslinking ranged from approximately 50% (EGCG) to 78% (GNP), as compared to 83% for the GA control. Among the natural crosslinkers, only GNP significantly affected the disc diameter, and shrinkage was under 2%. GA fixation had no significant effect on disc diameter. Decellularization decreased aggregate modulus; GA and GNP, but not EGCG and PA, were able to restore it to its original level. GNP, PA and GA conferred a similar, almost complete resistance to collagenase degradation. EGCG also conferred substantial resistance but to a lesser degree. Overall, the data support our hypothesis and suggest that natural crosslinkers may be suitable alternatives to glutaraldehyde for stabilization of decellularized cartilage.
Keywords: cartilage, xenograft, crosslinking, genipin, epigallocatechin gallate, proanthocyanidin
Introduction
Cartilage defects are associated with pain, swelling, and functional deficit [1]. According to current clinical guidelines, patients with an ICRS grade III or IV chondral lesion who are under 40 years of age may be good candidates for a restorative procedure, especially those who desire to resume high levels of physical activity [2]. A large review of 25,124 knee arthroscopies performed from 1989 to 2004 showed that isolated cartilage lesions occurred in 18% of patients, usually on the medial femoral condyle or patellar articular surface [3]. In that study patients under 40 years of age with one to three localized grade III and IV cartilage lesions accounted for 7% of all patients. In another series of 1,010 patients who underwent arthroscopic meniscectomy or meniscal repair, 32% of those who were 30–39 years of age displayed articular cartilage changes to at least one compartment of the knee [4]. Thus the orthopaedic surgeon is likely to discover chondral lesions in a sizeable population of relatively young patients who might benefit from hyaline cartilage restoration.
The two most common restorative procedures are autologous chondrocyte implantation (ACI) and osteochondral allograft transplantation (OAT), both of which have drawbacks. ACI necessitates two surgeries, extensive rehabilitation, and a long interval prior to resumption of normal activities [2]. Furthermore, only moderate success rates have been achieved. For example, studies which have examined the outcome of ACI with 4 and 5 years follow-up found 76% and 71% of patients with improved conditions, respectively [5, 6]. Similar success rates are achieved with OAT, and it poses risks of disease transmission, graft rejection, and infection [7, 8]. Perhaps more importantly, graft supply is limited. A new treatment alternative for cartilage lesions, especially one which could be accomplished during the same procedure in which the lesion is discovered would, therefore, be a welcome alternative to current treatment options.
The goal of the current research is to advance development of an “off-the-shelf” treatment for articular cartilage defects discovered during exploratory knee arthroscopy, namely an osteochondral xenograft. If processed appropriately, a xenograft could have the potential advantages of low cost, abundant supply, immediate availability for implantation during exploratory surgery, and restoration of a smooth surface capable of supporting the stress associated with weight bearing. Unlike fresh allografts, a xenograft can be treated with antibacterial and antiviral agents such as deoxycholate and peracetic acid to minimize the risk of disease transmission. In fact, porcine pulmonary artery was demonstrated to be completely free of bacterial, fungal, and viral contamination after decellularization, crosslinking, and sterilization [9]. The pig is a suitable source of human replacement tissue due to similar body size and physiology, as well as rapid growth rate [10].
The purpose of this study was to evaluate selected alternatives to glutaraldehyde for the stabilization of decellularized articular cartilage. Glutaraldehyde is the most frequently used crosslinking agent. However, there are many adverse health effects of glutaraldehyde exposure, including asthmatic symptoms, rhinitis, and skin irritation [11]. Although it has been widely used to stabilize xenograft bioprosthetic heart valves, it has also been implicated in their calcification [12, 13]. Furthermore, glutaraldehyde is cytotoxic, and even commercially available glutaraldehyde-treated bovine pericardium has been shown to elicit inflammatory cytokine release from macrophages [13]. There is evidence to suggest that this inflammatory response can be modulated through the use of alternative crosslinkers. The proportion of anti-inflammatory M2 macrophages infiltrating genipin-treated, decellularized porcine esophagus implanted into rats was observed to increase over time, whereas the proportion of M2 macrphages decreased in response to glutaraldehyde-treated tissue [14]. Thus it is worthwhile to investigate the use of glutaraldehyde alternatives in the course of developing an osteochondral xenograft.
It was hypothesized that natural collagen crosslinking agents could be used to improve the mechanical properties and durability of decellularized cartilage. The crosslinking agents of interest were genipin, grape seed proanthocyanidin, and epigallocatechin gallate (EGCG). Genipin is derived from geniposide, which comes from the fruit of Gardenia jasminoides Ellis. Genipin has been investigated as a means to stabilize cardiovascular xenografts, and has been found to increase tensile stiffness and strength as well as mask residual antigen [15, 16, 17]. Such studies have also shown it to be far less cytotoxic than glutaraldehyde. Genipin has been investigated as a means to stabilize tissue engineered cartilage, and our own previous research suggests that genipin has utility for stabilization of porcine articular cartilage [18, 19]. Proanthocyanidins are condensed tannins within a larger group of polyphenols. They are found in fruits, nuts, seeds, and other plant tissues. Proanthocyanidin is highly concentrated in grape seed extract. When used to crosslink bovine pericardium, grape seed proanthocyanidin was demonstrated to be over 100 times less toxic than glutaraldehyde [20]. It also increased the thermal stability and resistance to collagenase in vitro and slowed the rate of degradation in vivo while promoting cellular infiltration and collagen deposition [20]. EGCG is a polyphenol from green tea. Like proanthocyanidin, EGCG as a crosslinker has been found to increase the ultimate tensile strength and collagenase resistance of dentin collagen matrix [21]. EGCG has specifically been shown to increase the thermal stability and enzymatic resistance of bovine articular cartilage [22]. Intra-articular injection of EGCG was found to have both prophylactic and therapeutic effects in a rat model of arthritis [22]. This study compares the effects of genipin, proanthocyanidin, and EGCG, on the physical characteristics, mechanical properties, and collagenase resistance of decellularized porcine cartilage.
Materials and Methods
Tissue Collection and Decellularization
Articular cartilage discs of 5 mm diameter were harvested from two stifle joints of skeletally mature pigs using a biopsy punch and scalpel. They were evenly distributed to six 50 ml centrifuge tubes, each of which represented a different experimental group: Control, Decellularized, Glutaraldehyde (GA), Genipin (GNP), epigallocatechin gallate (EGCG), and grape seed proanthocyanidin (PA). Control discs were immediately placed in phosphate-buffered saline (PBS) containing 1 μM PMSF and stored at 4 °C until mechanical testing the following day. The remaining discs were washed with PBS for 30 min at room temperature and then decellularized using sodium dodecyl sulfate (SDS). Each tube was filled with a solution of 2% SDS, 0.5 mg/ml DNase Type I, 0.05 mg/ml RNase, 0.02% EDTA, 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin B. Discs were treated for 6 h at 37 °C with gentle agitation. Following decellularization all discs were washed with PBS for 2 h to remove residual SDS. The wash solution was changed at 30 and 60 min. Discs in the Decellularized group were stored at 4 °C in PBS with 1 μM PMSF until further use.
Histology
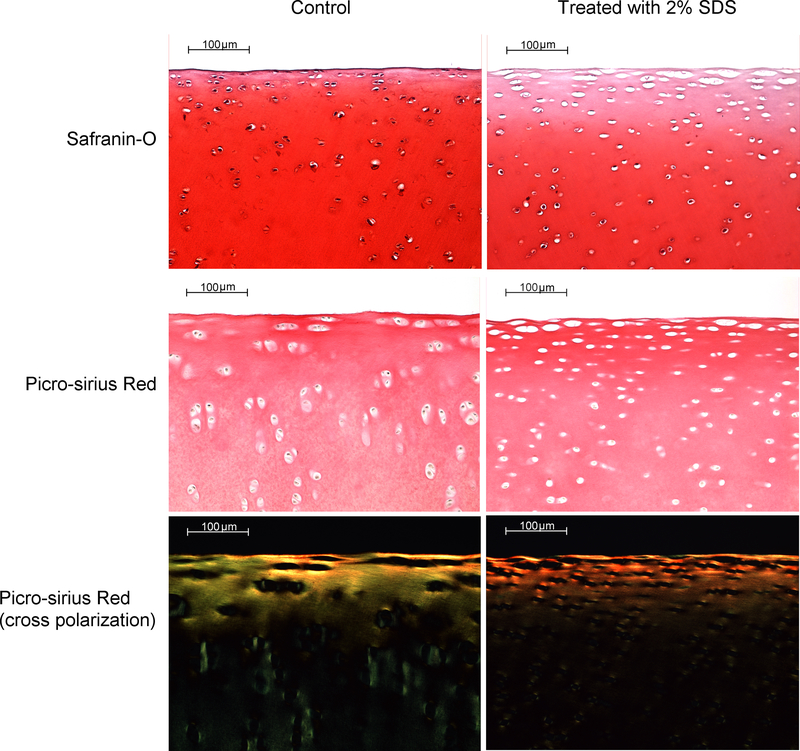
Triplicate discs from the Control and Decellularized groups were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were stained with 0.1% safranin O and 0.1% picro-sirius red to demonstrate GAG and collagen, respectively. Counterstaining with hematoxylin was included to show cell nuclei. Picro-sirius red-stained sections were viewed under typical transmitted light and also with samples positioned between crossed polarizing filters, a technique which reveals collagen birefringence.
Biochemistry
DNA and glycosaminoglycan (GAG) content were measured for discs in the Control and Decellularized groups to characterize the effect of SDS treatment. Each disc was digested overnight at 60 °C in 1 ml of papain digestion buffer (100 mM sodium phosphate buffer, 10 mM Na2EDTA, 10 mM L-cysteine, and 0.125 mg/mL papain, pH 6.5). An aliquot of the digestate was mixed with Hoechst assay buffer containing 10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl, and 0.2 μg/ml bisBenzimide H 33258. Fluorescence (excitation 365 nm, emission 410 – 450 nm) was read using a multimode reader (GloMax®-Multi Jr, Promega, Madison, WI). DNA content was determined by comparison to a standard curve generated from calf thymus DNA. A separate aliquot of the digestate was taken for determination of GAG content using a dimethylmethylene blue assay (Blyscan™ Assay, Biocolor Ltd, Carrickfergus, UK). The assay was performed according to the manufacturer’s recommended protocol.
Crosslinking
Natural collagen crosslinking agents were Genipin (Challenge Bioproducts Co., Ltd., Taiwan), EGCG (Sigma, St. Louis, MO), and Grape seed extract (PureBulk, Inc., Roseburg, OR). Crosslinking solutions were prepared by first dissolving each substance in 100% dimethyl sulfoxide (DMSO) and then diluting with PBS to a final concentration of 0.25 wt/v% in 10% v/v DMSO. Glutaraldehyde solution, 25% in water (Sigma), was diluted 1:10 with PBS to obtain a final concentration of 2.5%. Discs were incubated in their respective crosslinking solutions for 24 h at 37 °C with agitation. Residual crosslinker was removed by washing with PBS for 2 h, changing wash solution after 30 and 60 min. All experiments except for confined compression testing were performed immediately after crosslinking. Discs to be mechanically tested were stored frozen in PBS for no more than 14 days at −20 °C.
Degree of Crosslinking
The degree of crosslinking was considered to be the ratio of bound amino groups in the crosslinked samples to the free amino groups in discs from the Decellularized group. It was determined using the ninhydrin assay [23]. Discs were first freeze dried and weighed. Individual samples weighing 15–20 mg were heated to 100 °C with 3 mL ninhydrin solution for 20 min. After cooling to room temperature, each sample was diluted with 4 ml of 50% isopropanol. The amount of free amino groups was then found from the optical absorbance of the solution at 570 nm using glycine as a standard (μQuant, Bio-Tek Instruments, Inc., Winooski, VT). Degree of crosslinking was calculated as follows:
where Mo is the amount of free amino groups in the non-crosslinked native cartilage and Mt is the amount of amino groups remaining in the crosslinked cartilage, both normalized to the tissue dry weight. In this case, Mo was the average amount of free amino groups from 6 control discs.
Dimensional Stability
Individual digital images of 9 discs from each experimental group (including a ruler for scale) were captured, and measurements were made using ImageJ software (NIH). The diameter was measured at 45 degree intervals for a total of four measurements per disc.
Swelling Ratio
Cartilage discs from each group were weighed immediately after freeze drying and again following a 2 h period of rehydration in PBS (n = 12 per group). The swelling ratio percentage was determined by the following equation
where Wd and Ws are the weights of the samples in the dry and swollen states, respectively.
Confined Compression Testing
Mechanical testing was performed using a stepper motor-driven universal testing machine outfitted with 100 N load cell (Mach-1 Mechanical Testing System, Biomomentum, Laval, QC, Canada). Prior to testing, each crosslinked disc was thawed and allowed to equilibrate to room temperature. Disc height was measured under a contact force of 0.25 N in an unconfined configuration between flat, impermeable surfaces. Once its height had been measured, a disc was firmly seated in a flat-bottomed steel chamber of 4.76 mm diameter and the chamber submerged in room-temperature PBS. Compression was applied by a 4.32 mm diameter porous indenter. Stress relaxation testing (n = 12 per group) was carried out in the manner of Soltz and Atheshian [24]. In order to fully seat the indenter against the sample, compression was ramped to 4.5 N and the force monitored until it reached equilibrium (rate of relaxation less than 0.2 g/min). Displacement was then ramped to 10% compressive strain at 5 μm/s and the indenter held at this position until force equilibrium was achieved. Force and displacement data were captured at 20 Hz. A custom Matlab program was used to curve-fit the compressive stress relaxation data to the biphasic theory and obtain the aggregate modulus (HA) and hydraulic permeability (k) [25].
Collagenase Resistance
Type 2 collagenase, 300 U/mg (Worthington Biochemical Corporation, Lakewood, NJ), was dissolved at 1 mg/ml in Dulbecco’s modified Eagle Medium containing 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin B. The solution was passed through a 0.22 μm syringe filter to reduce the risk of microbial contamination and heated to 37 °C. Fresh cartilage discs (n=3), decellularized discs (n=3), and crosslinked discs (n=15 per group) were washed twice in distilled water for 30 minutes and lyophilized for 24 hours. Freeze-dried cartilage discs were weighed to the nearest 0.01 mg. Each disc was placed into a separate microcentrifuge tube and incubated with 1 ml of collagenase solution at 37 °C with agitation. The collagenase solution was replaced every other day. On days 5, 10, and 15, five discs from each crosslinking group were removed from the collagenase, washed twice in distilled water for 30 minutes, and frozen at −20 °C. At the end of the experiment, all discs were lyophilized again for 24 hours and reweighed. Data were expressed as percentage of starting weight.
Statistics
Quantitative data were statistically analyzed using IBM SPSS Statistics 21. Disc diameter, mechanical properties, and swelling ratio were analyzed by one-way ANOVA and the Tukey post hoc test. The collagenase resistance experiment was analyzed using a mixed ANOVA and the Tukey post hoc test. Biochemistry data were examined by independent t-test. The significance level for all tests was α = 0.05.
Results
Histology and Biochemistry
The effect of decellularization on cartilage histology is shown in Figure 1. Treatment with SDS decreased the intensity of safranin-O staining, particularly in the superficial and upper transition zones. This pattern suggests a lower GAG content, and discs were found to contain 35% less GAG than Controls on average (p = 0.030). SDS-treated discs also displayed a loss of cell nuclei in the superficial zone. This decrease is reflected in the DNA content, which was 15% lower in the Decellularized group relative to Control (p = 0.035). There were no apparent differences in the intensity of picro-sirius red staining between Decellularized and Control, nor were there differences in the pattern of collagen birefringence, which reveals alignment of collagen fibers. Thus the picro-sirius red staining suggests that SDS had a negligible effect on the amount and organization of collagen.
Figure 1.
Photomicrographs of porcine stifle joint cartilage which demonstrate the effect of SDS treatment on tissue cellularity, GAG content (safranin-O), collagen content (picro-sirius red), and collagen organization (picro-sirius red under cross polarization). SDS depleted GAG, particularly in the superficial zone, but had no discernable effect on either collagen content or organization.
Degree of Crosslinking, Dimensional Stability, Swelling Ratio
The gross appearance of discs before and after crosslinking is shown in Figure 2. Discs treated with EGCG and proanthocyanidin were stained almost the same color as their respective fixation solutions, whereas the blue color of discs treated with genipin is indicative of the degree of crosslinking (the genipin fixation solution is clear). Degree of crosslinking, diameter, and swelling ratio results are shown in Table 1. The degree of crosslinking after treatment with glutaraldehyde, genipin, and proanthocyanidin was ≥70%. Treatment with EGCG resulted in a substantially lower degree of crosslinking around 50%. Decellularization in SDS reduced disc diameter by 2% relative to Control. The only crosslinking agent which caused a significant amount of further shrinkage was genipin, which reduced the Decellularized disc diameter by an additional 1.6%. Glutaraldehyde and proanthocyanidin lowered the tissue’s swelling ratio by approximately 20% compared to Control, but no crosslinker significantly affected swelling ratio with respect to the Decellularized group.
Figure 2.
Gross appearance of porcine articular cartilage. Control: native tissue; Decellularized: treated with 2% SDS; GA: decellularized and fixed in 2.5% glutaraldehyde; GNP: decellularized and fixed in 0.25% genipin; EGCG: decellularized and fixed in 0.25% epigallocatechin gallate; PA: decellularized and fixed in 0.25% proanthocyanidin (grape seed extract).
Table 1.
Effect of Crosslinking on Radial Shrinkage and Swelling Ratio of Porcine Articular Cartilage.
| Group | Degree of crosslinking (%) | Diameter (mm) | Swelling Ratio (%) |
|---|---|---|---|
| Control | NA | 5.04±0.17a | 342±34a |
| Decellularized | 0 | 4.94±0.08b | 311±34a,b |
| GA | 83±5 | 4.95±0.11b | 273±30b |
| GNP | 78±5 | 4.86±0.09c | 308 ±35a,b |
| EGCG | 51±9 | 4.94±0.07b | 324±33a |
| PA | 72±3 | 4.89±0.09b,c | 278 ±26b |
Values are mean±standard deviation.
Letters indicate members of statistically homogeneous groups.
Mechanical Properties
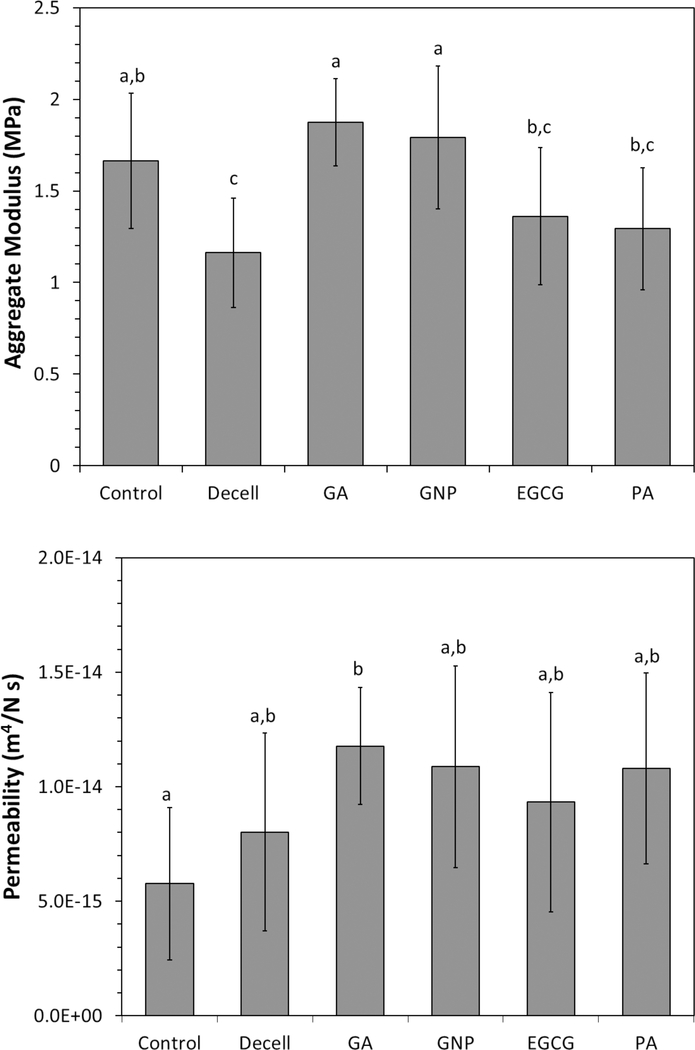
Biphasic properties of the porcine cartilage discs are displayed in Figures 3. SDS decellularization significantly reduced the aggregate modulus of native cartilage by 30%. Treatment of decellularized cartilage with either glutaraldehye or genipin increased the aggregate modulus by over 50% and restored it to the level of native cartilage. Treatment of decellularized cartilage with epigallocatechin gallate and proanthocyanidin also slightly raised the average aggregate modulus, but the improvements were not statistically significant. Decellularization tended to increase permeability of native cartilage, although GA was the only group which was significantly different from Control. None of the crosslinkers had a significant effect on the permeability of decellularized cartilage. These statistically homogeneous groups were therefore pooled together and compared to Control, which revealed a significant increase in permeability associated with decellularization (p = 0.003, two-sample t-test assuming unequal variances).
Figure 3.
Average mechanical properties of porcine articular cartilage determined by confined stress relaxation test and fitting of results to the biphasic model. Decellularization in 2% SDS for 6 h decreased the aggregate modulus, which was restored to its original level by crosslinking in glutaraldehyde or genipin. Crosslinking had little effect on the permeability of decellularized cartilage. Error bars = one standard deviation. Letters above the bars indicate members of statistically homogeneous groups (one-way ANOVA and Tukey HSD).
Collagenase Resistance
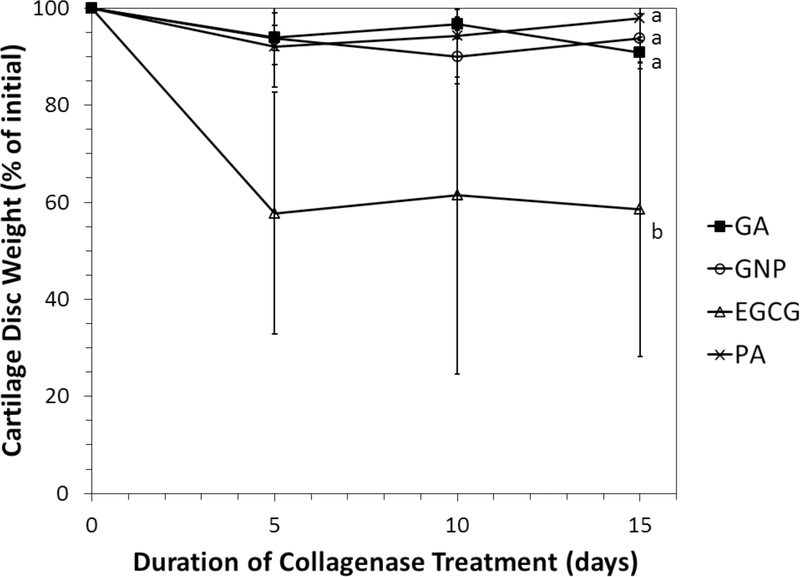
The residual weight of cartilage discs incubated with collagenase is shown in Figure 4. Control and Decellularized discs were completely degraded within 48 h. Crosslinking drastically improved collagenase resistance. Discs crosslinked in GA, GNP, and PA retained overy 90% of their starting weight during the 15-day experimental period. EGCG-treated discs also resisted collagenase degradation, but to a lesser extent. The average percentage of mass retained EGCG discs was approximately 60%. GA, GNP, and PA were statistically equivalent with respect to collagenase resistance, and EGCG was significantly different from the other groups. The re was not a significant effect of collagenase treatment duration. The Control and Decellularized groups were not included in the statistical analysis.
Figure 4.
Effect of crosslinking on the collagenase resistance of decellularized porcine articular cartilage. Improved resistance is indicated by less reduction of starting weight. All of the crosslinking agents tested significantly improved resistance of decellularized tissue to a level which greatly exceeded that of native and decellularized cartilage. Error bars = one standard deviation. Letters beside plots indicate members of statistically homogeneous groups (repeated measures ANOVA and Tukey HSD).
Discussion
Several previous studies demonstrate that osteochondral xenografts have potential to repair articular cartilage defects, but there is room for improvement [26, 27, 28]. For example, Kheir et al. achieved efficient decellularization using freeze/thaw cycles, osmotic shock, detergent and nucleases, but the process degraded the tissue’s mechanical properties [28]. Von Rechenberg and colleagues achieved satisfactory joint resurfacing using non-decellularized, photooxidized osteochondral xenografts in a sheep model, but the transplantation of xenogenic DNA could be problematic [27, 29]. The long-term goal of our research is to develop osteochondral xenografts that perform on par with fresh osteochondral allografts. Such grafts will need to be durable, minimally immunogenic, and capable of supporting host cell invasion and cartilage regeneration.
Natural biomaterials derived from collagen have limitations for use in humans due to short durability and degradation in vivo as a result of inflammatory responses [23]. For any xenogeneic tissue, antigen removal is necessary; however, most of the chemical and physical treatments which are effective for antigen removal also have the potential to disrupt or destroy the ECM. It is particularly difficult to remove antigen from hyaline cartilage and fibrocartilage without depleting GAG [30]. GAG is an important structural component of cartilage, and loss of GAG substantially lowers the aggregate modulus and indentation resistance of cartilage [30, 28]. Decellularization procedures frequently rely on a detergent such as SDS and Triton X-100, plus exogeneous nucleases, to achieve removal of DNA [30, 31, 28, 32]. However, there is a trade-off between DNA removal and maintaining GAG content. Increasing detergent concentration and duration of treatment increases the efficiency of DNA removal, but also increases the amount of GAG which is removed [30, 28, 33].
The antigen removal protocol in this study was relatively mild and reduced DNA content by only 15%. Nonetheless, it removed approximately one-third of the GAG. GAG has a high negative fixed charge density and is the major contributor to the swelling pressure of cartilage. Depletion of GAG has been shown to decrease cartilage’s elastic modulus and increase its permeability [34]. Furthermore, the GAG was depleted mainly from the upper zones of the tissue where most of the matrix consolidation first occurs upon application of a compressive load. The antigen removal process had a negligible impact on the collagen content and organization, thereby providing an opportunity for stabilization of the entire structure through collagen crosslinking. Follow up studies in our laboratory have revealed that extended incubation in the SDS solution can substantially reduce the amount of residual DNA without significantly affecting the tissue’s collagen scaffold.
The collagen molecule has a triple-helical structure resulting from the supercoiling of polypeptide chains around a common axis. Crosslinks within and between the collagen molecules reinforce the structure. The degree of crosslinking determines the rate of resorption of implanted collagenous materials and may also influence the qualitative pattern of tissue regeneration [35]. This study investigates the potential for crosslinking to restore compressive stiffness and increase the enzymatic resistance of cartilage following antigen removal. It focuses on the efficacy of three plant-based crosslinkers with low-cytotoxicity. Genipin is the metabolite of geniposide, which is found in the fruit of Gardenia jasminoides. It forms covalent crosslinks between the free primary amine groups on the lysine and hydroxylysine residues of the collagen molecule [36]. Although aqueous solutions of genipin are clear, the reaction with amino acids and protein forms a blue pigment. The color intensity may be used to gauge the degree of crosslinking. Genipin is estimated to be approximately 10,000 times less cytotoxic than glutaraldehyde [37], and has been shown to depress the inflammatory host response [15]. Epigallocatechin-gallate is the major catechin found in green tea (Camellia sinensis), and has antioxidative, antiangiogenic, and antitumorigenic properties [38]. Although it may induce apoptosis in cancer cells, it has been shown to have little effect on the growth of normal human colon cells and normal mammary gland breast cells in vitro [39]. Several modes of EGCG-mediated collagen stabilization have been proposed, including reaction of EGCG’s hydroxyl groups with collagen’s carboxyl groups to form esteric bonds [40] and stiffening of the collagen backbone via a similar interaction to that which has been postulated for sugar [41]. Proanthocyanidin is a naturally occurring bioflavonoid concentrated in grape seeds. A powerful antioxidant, it is considered to have several health benefits, including the suppression of inflammation and inhibition of tumor growth [42]. The cytotoxic effect of PA on NIH 3T3 cells has been demonstrated to be approximately 120 times lower than that of glutaraldehyde [20]. Hydrogen bonding between the collagen amide carbonyl and the PA hydroxyl is suggested to be the primary mechanism by which PA stabilizes collagen [20].
A first step in the crosslinker screening process was to examine the relative crosslinking potency of each agent. The glutaraldehyde concentration is within the range typically used to fix bioprosthetic heart valves, and it has been shown that complete fixation occurs within a few hours [43]. It has also been demonstrated that the efficiency of genipin crosslinking increases with temperature between 4 °C and 37 °C [44]. To mitigate effects of duration, all crosslinking reactions were carried out for 24 hours at 37 °C, and the relative efficiencies were expected to depend primarily on the agent and concentration. Genipin was the strongest natural crosslinker, and a 0.25% solution of genipin achieved almost the same degree of crosslinking as a 2.5% solution of glutaraldehyde. For genipin, this is a relatively high concentration. For example, an increase of genipin concentration from 0.3% to 0.5% did not increase the degree of crosslinking of type I collagen scaffolds [44]. Proanthocyanidin was less potent than genipin, which is consistent with a previous finding that the degree of PA crosslinking is concentration dependent up to 1% [20]. EGCG was the weakest crosslinker of those studied. The EGCG-mediated degree of crosslinking, as indicated by enzymatic resistance, increases up to at least 0.5% [21], and we have measured a concentration-dependent increase up to 1% via the ninhydrin assay (data not shown).
The dimensional change upon fixation was investigated because of the potential for radial cartilage shrinkage to affect the cartilage-bone interface and the formation of a tight junction with the native cartilage upon implantation. Such shrinkage is known to occur with fixation in glutaraldehyde [45]. In the current study it was found that the decellularization process itself caused a radial shrinkage of 2%. Crosslinking with proanthocyanidin and EGCG did not significantly reduce the diameter of decellularized cartilage discs, and genipin reduced it by only 1.6%. Thus cartilage discs which had been decellularized and crosslinked with genipin shrank radially by an average of 3.6%. We have not observed separation between the cartilage and bone subsequent to decellularization and crosslinking of osteochondral plugs, regardless of crosslinker.
The swelling ratio indicates the degree to which absorbed water spontaneously expands the tissue volume. A relatively high swelling ratio is important for maintaining a space into which host cells can migrate and the fluid compartment needed to nourish those cells. However, a higher swelling ratio is indicative of a less stable structure. A reduction in the swelling ratio after decellularization is likely attributable to the decreased GAG content. All crosslinked groups, with exception of EGCG, displayed a significantly lower swelling ratio than native cartilage. The slightly higher swelling ratio of cartilage crosslinked by EGCG could be explained by the lower degree of crosslinking. A swelling ratio of more than 270% was observed in all crosslinked groups, a reduction of less than 20% compared to native cartilage. Therefore, stabilization does not result in a drastic compaction of the extracellular matrix.
Providing resistance to compressive joint loading is a primary function of articular cartilage, and crosslinking must restore the compressive resistance lost during antigen removal. In this study, the compressive resistance was characterized by stress relaxation testing in uniaxial confined compression and fitting results to the biphasic model [25]. Decellularization lowered the aggregate modulus by 30%, most likely because it extracted a significant amount of GAG. Increases in the aggregate modulus of crosslinked cartilage seemed to be correlated with the degree of crosslinking. Treatment with genipin and glutaraldehyde brought the aggregate modulus of decellularized tissue back in line with that of native cartilage, whereas treatment with proanthocyanidin and EGCG produced more modest increases that did not achieve statistical significance. However, we speculate that similar gains can be obtained at higher concentrations. The increases in compressive stiffness associated with fixation in genipin and glutaraldehyde mirror gains in tensile stiffness and strength previously observed following fixation of heart valve leaflets, arteries, and pericardium [15, 17, 16]. The findings are consistent with our previous study indicated that genipin and glutaraldehyde can imbue articular cartilage with supraphysiological compressive stiffness [19]. They are also consistent with the concentration-dependent increase in equilibrium compressive modulus previously reported for fixation of calf articular cartilage in glutaraldehyde up to 0.6% [46]. A previous investigation of the effects of genipin crosslinking on articular cartilage demonstrated an increase in the instantaneous modulus, but not the equilibrium modulus (analogous to aggregate modulus) [47]. The latter phenomenon can be attributed to the short duration of crosslinking (≤6 h) which produced crosslinking of the surface but not through the depth of the cartilage. The instantaneous response of the tissue was not specifically characterized in the current study. Another study involving crosslinking in genipin or glutaraldehyde also showed no increase in aggregate modulus of tissue engineered cartilage [18]. The discrepancy with our finding could be the result of much different genipin treatment durations (3.5 versus 24 h) and possibly of intrinsic differences in native and engineered cartilage. A relatively low concentration of EGCG (0.009%) was found to have no effect on the compressive resistance of bovine articular cartilage, which is consistent with the small and statistically insignificant increase in aggregate modulus after EGCG fixation in this study [22].
The data show that decellularization increased permeability by almost 2-fold on average and that crosslinking produced no further change. Likewise, the permeability of calf articular cartilage has previously been shown to be unaffected by treatment with glutaraldehyde [46], and the permeability of tissue engineered cartilage has been shown to be insensitive to treatment with genipin or glutaraldehyde [18]. Low permeability is crucial to protecting the solid phase of normal cartilage, but its importance in that regard would be diminished in crosslinked tissue in which the solid phase is much stronger. Moreover, increased permeability may support diffusive transport to cells migrating into a graft.
The final issue addressed in this study was enzymatic resistance, which is an indication of the tissue’s in vivo durability. Whereas native and uncrosslinked decellularized cartilage discs were completely digested within 48 hours, crosslinked tissue was highly resistant to degradation by collagenase. Cartilage treated with genipin and proanthocyanidin retained over 90% of its mass after 15 days of exposure to 0.1% collagenase, which was no different from the level of resistance provided by glutaraldehyde. EGCG-treated cartilage was also highly resistant to collagenase, but the level of resistance was lower than that provided by genipin and proanthocyanidin. The lower degree of crosslinking produced by EGCG could possibly account for this difference. These results are generally consistent with previous studies. Cartilage in which had undergone a short treatment with genipin that limited crosslinking to the superficial region displayed increased collagenase resistance for the surface layer but not in the deeper, less crosslinked region [47]. Decellularized porcine aortic heart valves crosslinked with 0.5–2.0% procyanidin displayed approximately the same capacity to resist collagenase degradation as tissue crosslinked with 0.625% glutaraldehyde, similar to the equivalence observed in this study [48]. Bovine articular cartilage fixed in 0.009% EGCG was approximately 25% degraded by collagenase in 4 days [22]. This slightly faster rate of degradation compared to that observed in our study is likely due to the lower concentration of EGCG used for crosslinking. How the rate of degradation in collagenase solution in vitro corresponds to the rate of intra-articular degradation in vivo is unclear. The cartilage portion of decellularized, uncrosslinked osteochondral xenografts displayed little degradation eight weeks after implantation into rabbit joints [49]. And remnants of photo-oxidized bovine cartilage could be found in the stifle joints of sheep up to 12 months after implantation, suggesting that chemically crosslinked cartilage would have similar durability in vivo [50]. In general, crosslinking can affect not only the rate but also the nature of degradation in vivo. Genepin fixation, for example, has been shown to inhibit the overall level of macrophage infiltration as well as lead to recruitment of more immunomodulatory M2 macrophages and fewer pro-inflammatory M1 macrophages [51, 14]. Crosslinking of a decellularized osteochondral xenograft may retard the rate of degradation so as to allow adequate time for invasion of host cells and the gradual replacement of the graft with regenerated tissue. Such host cell repopulation and creeping substitution of a crosslinked bovine osteochondral xenograft has in fact been observed 6 and 12 months after transplantation in a sheep model [27]. Stability of the crosslinks during storage, in addition to the rate of degradation in vivo, may depend on the nature of the crosslinking. Because it involves covalent bonds, genipin-fixed tissue might be expected to degrade at a slower rate than tissue fixed in EGCG or PA, which form hydrogen bonds. However, the optimal rate of osteochondral graft degradation has yet to be determined.
Testing the crosslinking agents at a single concentration is a limitation of this study. The main purpose was to perform an initial screening of the crosslinkers, including evaluation of their relative potencies. Follow-up studies are planned to compare them after adjusting the concentrations to achieve equivalent degrees of crosslinking. Another limitation is this study’s focus on the cartilage portion of an osteochondral xenograft. However, bone’s properties are dominated by the mineral phase, and crosslinking is expected to have less of an impact on bone remodeling than it does on cartilage regeneration. This study also does not characterize cellular responses to crosslinked collagen. The general non-cytoxic nature of genipin, proanthocyanidin, and EGCG are well established, and genipin and EGCG have been shown to be compatible with chondrogenesis in vitro [52, 53].
Conclusion
This study indicates crosslinking with plant-derived agents of low cytotoxicity is a feasible approach to stabilization of decellularized osteochondral xenografts. On a mass concentration basis, genipin is the strongest crosslinker among those studied and its effects are comparable to those of a 10-fold higher concentration of glutaraldehyde. Treatment with 0.25% wt/vol genipin was able to fully restore the aggregate modulus of decellularized cartilage and drastically increase its collagenase resistance with minor effects on its dimensions and swelling ratio. These properties and genipin’s proven biocompatibility with mesenchymal stem cells and chondrocytes distinguishes it from the other agents evaluated [54, 52].
Acknowledgement
Research reported in this publication was partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R15AR066926. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was provided by the National Science Foundation, REU Site: Physical Properties of Materials, Award Number 1359437. The authors are grateful for the technical assistance of Patrick Barton and Preston Smith. None of the authors have a conflict of interest to disclose.
Contributor Information
Amand Pinheiro, Department of Biomedical Engineering, The University of Akron, Akron, Ohio, 44325, USA.
Avery Cooley, Department of Pathobiology and Population Medicine, Mississippi State University, Starkville, Mississippi, 39762, USA.
Jun Liao, Department of Agricultural & Biological Engineering, Mississippi State University, Starkville, Mississippi, 39762, USA.
Raj Prabhu, Department of Agricultural & Biological Engineering, Mississippi State University, Starkville, Mississippi, 39762, USA.
Steven Elder, Department of Agricultural & Biological Engineering, Mississippi State University, Starkville, Mississippi, 39762, USA.
References
- 1.Gooding C, Bartlett W, Bentley G, Skinner J, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13(3):230–10. [DOI] [PubMed] [Google Scholar]
- 2.Cole B, Pascual-Garrido C, Grumet R. Surgical managment of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91(7):1778–90. [PubMed] [Google Scholar]
- 3.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–82. [DOI] [PubMed] [Google Scholar]
- 4.Ciccotti M, Kraeutler M, Austin L, et al. The prevalence of articular cartilage changes in the knee joint in patients undergoing arthroscopy for meniscal pathology. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2012;28(10):1437–1444. [DOI] [PubMed] [Google Scholar]
- 5.Zaslav K, Cole B, Brewster R, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37(1):42–55. [DOI] [PubMed] [Google Scholar]
- 6.Browne J, Anderson A, Arciero R, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;436:237–45. [DOI] [PubMed] [Google Scholar]
- 7.Gross A, Shash N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. [DOI] [PubMed] [Google Scholar]
- 8.McCulloch P, Kang R, Sobhy M, Hayden J, Cole B. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411–20. [DOI] [PubMed] [Google Scholar]
- 9.Balasundari R, Gupta R, Sivasubramanian V, et al. Complete microbe free processed porcine xenograft for clinical use. Ind J Thorac Cardiovasc Surg. 2007;23:240–245. [Google Scholar]
- 10.Chen R, Kadner A, Mitchell R, Adams D. Mechanism of delayed rejection in transgenic pig-to-primate cardiac xenotransplantation. J Surg Res. 2000;90:119–125. [DOI] [PubMed] [Google Scholar]
- 11.Takigawa T, Endo Y. Effects of glutaraldehyde exposure on human health. J Occup Health. 2006;48:75–87. [DOI] [PubMed] [Google Scholar]
- 12.Tam H, Zhang W, Feaver K, Parchment N, Sacks M, Vyavahare N. A novel crosslinking method for improved tear resistance and biocompatibility of tissue based biomaterials. Biomaterials. 2015;66:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umashankar P, Mohanan P, Kumari TV. Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol Int. 2012;19(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch H, Graneist C, Emmrich F, et al. Xenogenic esophagus scaffolds fixed with several agents: comparative in vivo study of rejection and inflammation. Journal of Biomedicine and Biotechnology. 2012;2012:948320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somers P, De Somer F, Cornelissen M, et al. Genipin blues: an alternative non-toxic crosslinker for heart valves? J Heart Valve Dis. 2008;17(6):682–8. [PubMed] [Google Scholar]
- 16.Yoo J, Kim Y, Kim S, Choi S. Study on genipin: a new alternative natural crosslinking agent for fixing heterograft tissue. Korean J Thorac Cardiovasc Surg. 2011;44(3):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi-xun Y, Fei L, Yuan-ting X, Chang-xiu W. In vitro study in the endothelial cell compatibility and endothelialization of genipin-crosslinked biological tissues for tissue-engineered vascular scaffolds. J Mater Sci Mater Med. 2010;21(2):777–85. [DOI] [PubMed] [Google Scholar]
- 18.Elder B, Mohan A, Athanasiou K. Beneficial effects of exogenous crosslinking agents on self-assembled tissue engineered cartilage construct biomechanical properties. Journal of Mechanics in Medicine and Biology. 2011;11(2):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King T, Elder S. Effects of genipin on decellularized porcine cartilage. Journal of the Mississippi Academy of Sciences. 2014;59(supplemental):367–370. [Google Scholar]
- 20.Han B, Jaurequi J, Tang BW, Nimni M. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65(1):118–24. [DOI] [PubMed] [Google Scholar]
- 21.Hiraishi N, Sono R, Sofiqul I, et al. In vitro evaluation of plant-derived agents to preserve dentin collagen. Dental Materials. 2013;29:1048–1054. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan V, Madhan B, Tiku M. Intra-articular injections of polyphenols protect articular cartilage from inflammation-induced degradation: suggesting a potential role in cartilage therapeutics. PLoS One. 2015;10(6):e0127165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui L, Jia J, Guo Y, Lu Y, Zhu P. Preparation and characterization of IPN hydrogels composed on chitosan and gelatin crosslinked by genipin. Carbohydr Polym. 2014;99:31–8. [DOI] [PubMed] [Google Scholar]
- 24.Soltz M, Ateshian G. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. Journal of Biomechanics. 1998;31:927–934. [DOI] [PubMed] [Google Scholar]
- 25.Mow V, Kuei S, Lai W, Armstrong C. Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng. 1980;102(1):73–84. [DOI] [PubMed] [Google Scholar]
- 26.Toolan B, Frenkel S, Pereira D, Alexander H. Development of a novel osteochondral graft for cartilage repair. J Biomed Mater Res. 1998;41:244–250. [DOI] [PubMed] [Google Scholar]
- 27.von Rechenberg B, Akens M, Nadler D, et al. Mosaicplasty with photooxidized, mushroom shaped, bovine, oteochondral xenografts in experimental sheep. Vet Comp Orthop Traumatol. 2006;19:147–56. [PubMed] [Google Scholar]
- 28.Kheir E, Stapleton T, Shaw D, Jin Z, Fisher J, Ingham E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res A. 2011;99A(2):283–294. [DOI] [PubMed] [Google Scholar]
- 29.Zheng M, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–67. [DOI] [PubMed] [Google Scholar]
- 30.Elder B, Eleswarapu S, Athanasiou K. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30:3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavassoli A, Mahdavi-Shahri N, Matin M, Fereidoni M, Shahabipour F. Bovine articular cartilage decellularized matrix as a scaffold for use in cartilage tissue engineering. Iranian Journal of Veterinary Science and Technology. 2012;4(1):1–8. [Google Scholar]
- 32.Yang Q, Peng J, Lu S, et al. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J. 2011;124(23):3930–3938. [PubMed] [Google Scholar]
- 33.Somers P, De Somer F, Cornelissen M, Thierens H, Van Nooten G. Decellularization of heart valve matrices: search for the ideal balance. Artificial Cells, Blood Substitutes, and Biotechnology. 2012;40:151–162. [DOI] [PubMed] [Google Scholar]
- 34.Katta J, Stapleton T, Ingham E, Jin Z, Fisher J. The effect of glycosaminoglycan depletion on the friction and deformation of articular cartilage. Proc Inst Mech Eng H. 2008;222(1):1–11. [DOI] [PubMed] [Google Scholar]
- 35.Liang H, Chang Y, Hsu C, Lee M, Sung H. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials. 2004;25(17):3541–52. [DOI] [PubMed] [Google Scholar]
- 36.Butler M, Ng YF, Pudney P. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. Journal of Polymer Science Part A: Polymer Chemistry. 2003;41(24):3941–3953. [Google Scholar]
- 37.Sung H, Huang RN, Huang L, Tsai C. In vitro evaluation of cytotoxicity of a naturally occurring crosslinking reagent for biological tissue fixation. J Biomater Sci Polym Edn. 1999;10:63–78. [DOI] [PubMed] [Google Scholar]
- 38.Singh B, Shankar S, Srivastava R. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Schell J, Ho CT, Chen K. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Letters. 1998;129(2):173–179. [DOI] [PubMed] [Google Scholar]
- 40.Goo H, Hwang YS, Choi Y, Cho H, Suh H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials. 2003;24:5099–5113. [DOI] [PubMed] [Google Scholar]
- 41.Vidal C, Aguiar T, Phansalkar R, et al. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomaterialia. 2014;10(7):3288–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Iglesia R, Milagro F, Campion J, Boque N, Martinez J. Healthy properties of proanthocyanidins. Biofactors. 2010;36(3):159–68. [DOI] [PubMed] [Google Scholar]
- 43.Duncan A, Boughner D, Vesely I. Viscoelasticity of dynamically fixed bioprosthetic valves. II. Effect of glutaraldehyde concentration. The Journal of Thoracic and Cardiovascular Surgery. 1997;113(2):302–310. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Chen X, Yang T, et al. The effects of different crossing-linking conditions of genipin on type I collagen scaffolds: an in vitro evaluation. Cell Tissue Bank. 2014;15(4):531–41. [DOI] [PubMed] [Google Scholar]
- 45.Hopwood D Some aspects of fixation with glutaraldehyde. J Anat. 1967;101(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Oungoulian S, Hehir K, Zhu K, et al. Effect of glutaraldehyde fixation on the frictional response of immature bovine articular cartilage explants. Journal of Biomechanics. 2014;47:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGann M, Bonitsky C, Jackson M, Ovaert T, Trippel S, Wagner D. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J Orthop Res. 2015;33(11):1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhai W, Chang J, Lin K, Wang J, Zhao Q, Sun X. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials. 2006;27:3684–3690. [DOI] [PubMed] [Google Scholar]
- 49.Mosher M, Butler R, Elder S, et al. A comparison of decellularization methods applied to porcine osteochondral xenografts for articular cartilage repair. Journal of the Mississippi Academy of Sciences. 2014;59(supplemental):363–366. [Google Scholar]
- 50.Akens M, von Rechenberg B, Bittmann P, Nadler D, Zlinszky K, Auer J. Long term in-vivo studies of a photo-oxidized bovine osteochondral transplant in sheep. BMC Musculoskelet Disord. 2001;2(9):Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhrany A, Lien C, Beckstead B, et al. Crosslinking of an oesophagus acellular matrix tissue scaffold. Journal of Tissue Engineering and Regenerative Medicine. 2008;2:365–372. [DOI] [PubMed] [Google Scholar]
- 52.Lima E, Tan A, Tai T, et al. Genipin enhances the mechanical properties of tissue engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009;91(3):692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, Liu Q, Liu L, Wu H, Zheng L. Effect of epigallocatechin-3-gallate on proliferation and phenotype maintenance in rabbit articular chondrocytes in vitro. Exp Ther Med. 2015;9(1):213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng N, Estes B, Young T, Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19(3–4):484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]