Abstract
Background:
Endometriosis is a widely known benign disease, but 0.5%–1% of cases are associated with malignancy. It has been linked with ovarian neoplasms, particularly endometrioid and clear cell adenocarcinoma histology. Rhabdomyosarcomas are rarely associated with endometriosis.
Case:
A 35-year-old patient underwent surgical management of endometriomas to optimize infertility treatment. She later developed abdominal pain with rapid recurrence of ovarian masses. This prompted additional surgery with biopsies diagnosing ovarian rhabdomyosarcoma. Retroactive review of pathologic specimens from her prior surgery demonstrated the neoplasm originated from her prior endometrioma. Focal areas suggested possible underlying ovarian adenosarcoma with stromal overgrowth.
Discussion:
The incidence of rhabdomyosarcoma arising from endometriosis is exceedingly rare. The accuracy of diagnosing endometriosis and ruling out neoplasm requires coordinated efforts of a multidisciplinary team, involving radiologists, pathologists, oncologists, and gynecologic surgeons.
Keywords: Rhabdomyosarcoma, Endometriosis, Malignant Transformation
INTRODUCTION
Endometriosis is a disease affecting 5%–15% of women and is defined as the presence of endometrial glands and stroma external to the corpus of the uterus.1 It is a process that involves chronic inflammation, excessive estrogen production, and progesterone resistance that can lead to localized or systemic manifestations.2 The exact etiology of endometriosis is unknown but there are a number of proposed theories for its histopathogenesis. These include stem cell theory, retrograde menstruation, coelomic metaplasia, embryonic cell rests, and lymphatic and vascular dissemination.3–5
Endometriosis commonly manifests as pelvic pain, dysmenorrhea, dyspareunia, and infertility. Currently, the diagnosis of endometriosis can only be obtained surgically with procurement of tissue specimens that confirm ectopic endometrial tissue. Defined as a benign disease, endometriosis itself is not fatalistic and causes no disruption in catabolism or metabolism. It however does share many attributes with cancer, including neoangiogenesis, tissue invasion, and decreased apoptosis.6,7 Like cancer metastasis, endometriosis can spread to different organs.8
Endometriosis has been linked with ovarian neoplasms, particularly endometrioid and clear-cell adenocarcinoma histology.1,9–11 There is evidence to support the malignant potential of endometriosis with epidemiologic, histopathologic, and molecular data.9 Molecular alterations have been found in endometriosis-associated neoplasms such as loss of heterozygosity, or mutations in PTEN, ARID1 A, and p53.12,13 Although most endometriosis remains benign, about 0.5%–1% of these cases are complicated by malignancy.14 The most common endometriosis-associated histological types are endometrioid adenocarcinoma and clear cell adenocarcinoma.1 The site of these neoplasms mostly originate in the ovaries and compose 10% of all ovarian adenocarcinomas in developed countries.1
Rhabdomyosarcoma is typically a pediatric malignancy and bodes a favorable prognosis with treatment. However, it is extremely rare among the adult population, comprising only 2%–5% of adult soft-tissue tumors.15 The most prevalent subtypes are embryonal and alveolar types. Approximately 22% of cases affect the genitourinary tract, and these usually originate in the cervix or vagina.16 Developing from undifferentiated mesenchymal cells, rhabdomyosarcoma is an extremely aggressive cancer with a propensity for early progression and dissemination.17 Extrauterine rhabdomyosarcoma of gynecologic origin has been rarely reported, although its relationship with underlying endometriosis is not always discussed. We report a case of an aggressive rhabdomyosarcoma arising in association with extensive endometriosis and postulate that it may have arisen from stromal overgrowth in a subtle adenosarcoma.
CASE DESCRIPTION
A 35-year-old nulligravid woman with no significant medical comorbidities presented to our endometriosis referral center for a surgical consultation. Her only surgical history included a remote laparoscopic right ovarian cystectomy and fulguration of lesions pathologically consistent with endometriosis. She and her partner desired to pursue embryo cryopreservation for future in vitro fertilization. According to her fertility specialists, a 7-cm ovarian mass was discovered on pelvic sonography. The patient was asymptomatic but the presence and location of the mass made potential follicles inaccessible for oocyte retrieval. She was recommended to pursue surgical management to optimize her future fertility.
During her consultation, transvaginal sonography demonstrated honeycomb appearance of both adnexae with adherence to the pelvic sidewalls. No hypervascularity was noted in either adnexa. She underwent an uncomplicated hysteroscopy with endometrial polypectomy and biopsy, laparoscopic lysis of adhesions with extensive enterolysis, ureterolysis, salpingo-ovariolysis, treatment of endometriosis with aspiration of bilateral ovarian endometriomas, and bilateral ovarian cystectomies. (Figure 1, and Figure 2) With some difficulty, the masses were gradually dissected off from adjacent organs and the pelvic sidewalls. They were found to be growing into the retroperitoneum and appeared to be choking the ureters. There were some vegetations noted in the pelvis and adjacent to the masses. The final pathology report confirmed endometriosis of the right cyst with diffuse endometriotic implants. There was no evidence of invasive malignancy.
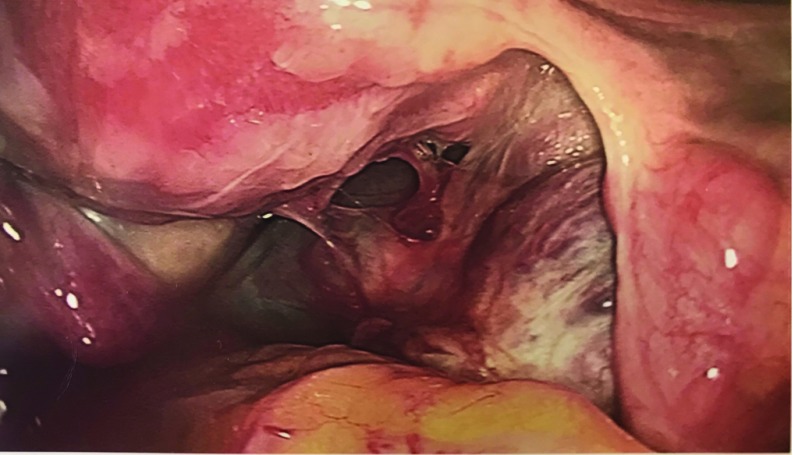
Figure 1.
Extensive adhesions tethering the right adnexa to the posterior aspect of the uterus, the posterior cul-de-sac, and the pelvic sidewall.
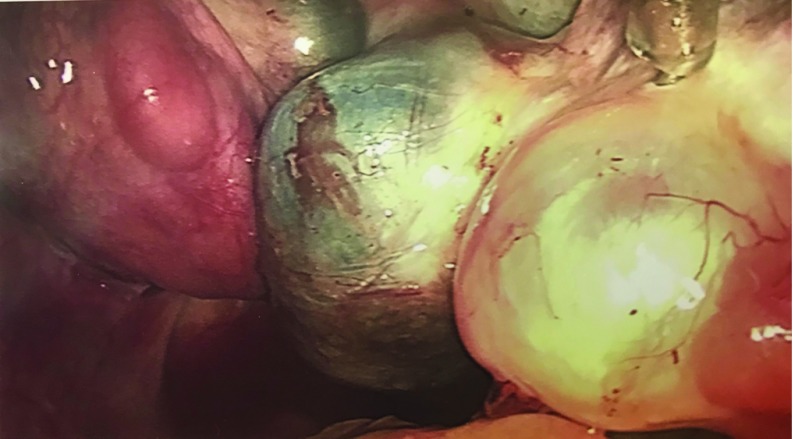
Figure 2.
With the right fallopian tube elevated, the right ovary could be visualized with multilobulated fluid-filled cysts and two endometriomas.
Postoperatively, she was placed on continuous oral combined contraceptive medications to suppress her endometriosis. Three months later, a follow-up transvaginal ultrasound described a retroperitoneal mass and hydrosalpinx. Magnetic resonance imaging of the pelvis demonstrated a normal-sized uterus with likely adenomyosis, bilateral endometriomas with enhancing soft tissue concerning for malignancy and arising from the right endometrioma. There was noted endometriosis involving bilateral ovaries with hydrosalpinx, and endometriotic implants within the pelvic cul-de-sac and bilateral adnexae. The pathology from her prior surgery was rereviewed by multiple gynecologic pathologists given the imaging findings; however, a definitive diagnosis of malignancy could not be rendered on the material.
Despite these imaging findings, the patient did not present in the office for followup until 1 month later. By that time, she was then experiencing severe right lower abdominal pain. She was also referred to a gynecologic oncologist. Given the rapid growth of the adnexal mass over a short duration as well as her acutely severe pain, she immediately underwent a diagnostic laparoscopy. Leakage of her endometriomas was noted with intra-abdominal hemorrhage and dense adhesions found throughout the pelvis. (Figure 3, and Figure 4) Biopsies of only the peritoneum and right ovarian cyst were obtained with frozen pathology sections that were inconclusive.
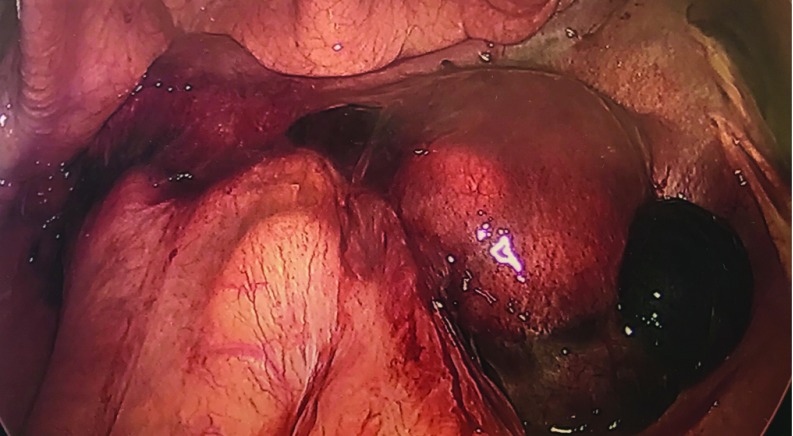
Figure 3.
The bowel was severely adhered to the uterine fundus and bilateral adnexae. A large hemorrhagic cyst was attached to the right ovary.
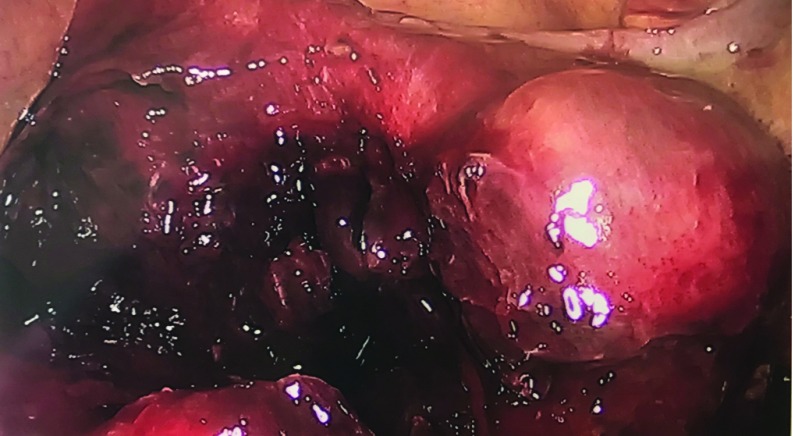
Figure 4.
A frozen pelvis was encountered with significant hemorrhage, blood clots, and inflammatory process noted throughout the cavity.
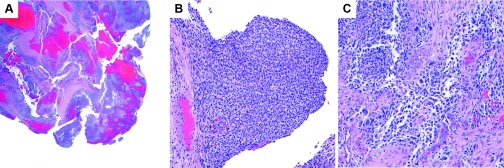
Final pathology of the specimens revealed rhabdomyosarcoma in the right ovarian and peritoneal specimens. Histologic sections of the right ovarian cyst showed fragmented cyst wall with a malignant spindle cell neoplasm with a range of cytologic features. Some areas showed relatively monomorphic spindle cells with hyperchromatic nuclei while others showed significant nuclear pleomorphism with eosinophilic cytoplasm (Figure 5). There were intermixed benign endometrial glands with reactive changes. The malignant spindle-cell proliferation was present in the subepithelial areas but there was no clear phyllodes-like growth. Immunohistochemical stains showed that the spindle cells were positive for myogenin and PAX-7, consistent with rhabdomyoblastic differentiation. (Figure 6) The overall morphologic and immunophenotypic findings were interpreted as malignant sarcoma with rhabdomyoblastic differentiation, consistent with ovarian rhabdomyosarcoma, embryonal type.
Figure 5.
A, Rhabdomyosarcoma in fibrous tissue from the resection (H&E, 2×). B, Area of tumor with more uniform spindle cells (H&E, 20×). C, Area of tumor with pleomorphic tumor cells (H&E, 20×).
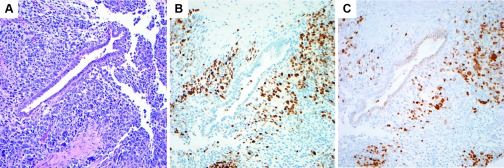
Figure 6.
A, Spindled, pleomorphic cells of the rhabdomyosarcoma surrounding a benign endometrial-type gland (H&E, 20×). B, Positive PAX-7 immunohistochemical staining in the tumor cells (20×). C, Positive myogenin immunohistochemical staining in the tumor cells (20×).
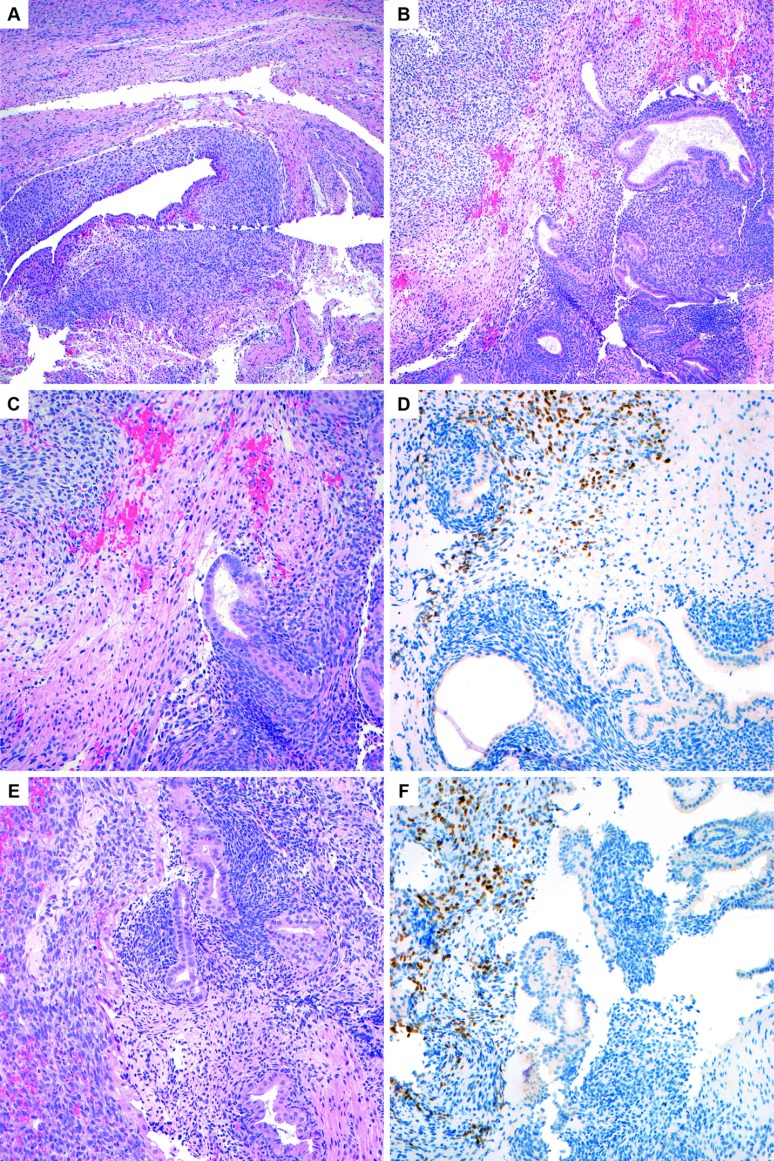
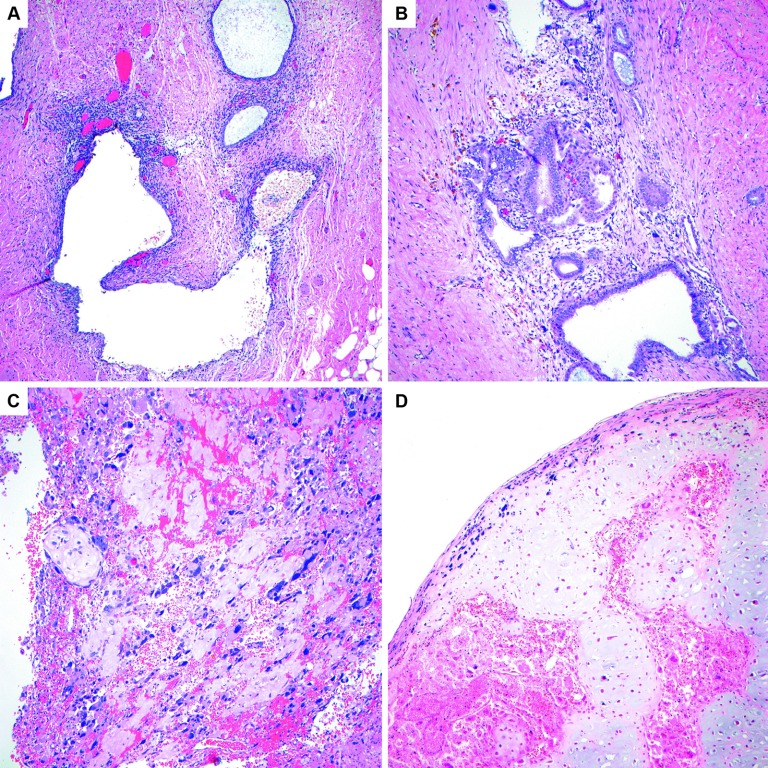
In light of this new histologic information, the specimens from the patient's prior surgery were rereviewed a second time. Immunohistochemical stains were retroactively performed on her prior slides that also demonstrated positive myogenin and PAX-7 in a few strips of paler and mildly atypical stroma adjacent to conventional endometriosis (Figure 7). These foci in retrospect were consistent with focal rhabdomyosarcomatous differentiation. Rare cystic glandular spaces with cuffing/condensation of bland-appearing endometrial-type stroma was noted in the same slide, suggestive of possible adenosarcoma (Figure 8). The cumulative findings were favored to represent extensive stromal overgrowth with rhabdomyosarcomatous differentiation in an adenosarcoma, arising from a background endometrioma. The pathology slides were also sent to two additional large academic institutions for review, which concurred with these diagnostic findings.
Figure 7.
A, Most of the material in the resection consisted of conventional appearing endometriosis, as pictured here in the ovary (H&E, 10×). B, The right aspect of the image shows benign endometrial glands and stroma of endometriosis, and the left upper corner shows stroma with paler cytoplasm (H&E, 10×). C, Higher-power image of the same area to contrast the benign endometrial-type stroma on the bottom right with the paler stroma in the upper left (H&E, 20×). D, Positive PAX-7 immunohistochemical staining in the paler stroma performed retrospectively confirms the presence of focal rhabdomyosarcoma (20×). E, Another area on the same slide to contrast the morphology of the benign endometrial-type stroma in the endometriosis on the right with the paler rhabdomyosarcomatous stroma on the left. In this image, there is slight nuclear enlargement and apoptotic debris noted in the sarcomatous area (H&E, 20×). F, Positive staining for PAX-7 in the stroma on the left confirms the diagnosis of rhabdomyosarcoma (20×).
Figure 8.
Endometriosis showing stromal condensation around the endometrial-type glands suggestive of underlying adenosarcoma. This area was negative for rhabdomyosarcomatous markers, PAX-7, and myogenin (H&E, 10×).
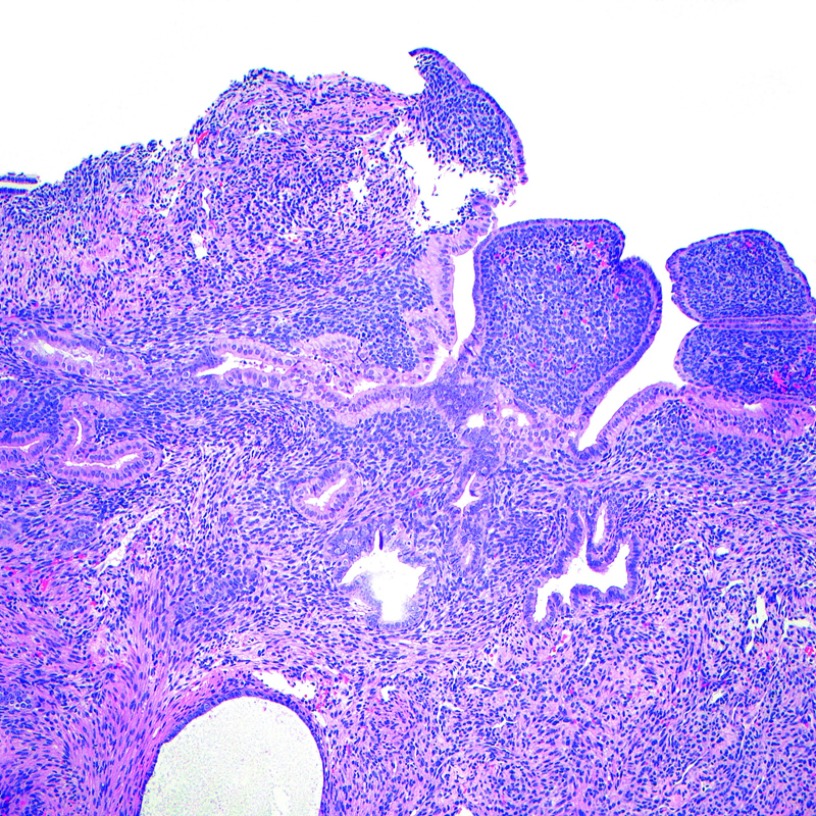
In followup, a magnetic resonance image of the chest, abdomen, and pelvis as well as a positron emission tomography were obtained, which were concerning for metastasis to the chest, hepatic periphery, and lymph nodes in the right inguinal, common iliac, and aortocaval region. After an extensive discussion in a multidisciplinary tumor board and with the patient, she opted to proceed with chemotherapy. She underwent three cycles of neoadjuvant cyclophosphamide, doxorubicin, and vincristine in alternation with ifosfamide and etoposide. Interval imaging showed improvement with decreasing hypermetabolism of her lymph nodes. She then underwent palliative debulking surgery with an exploratory laparotomy, modified radical hysterectomy, bilateral salpingo-oophorectomy, pelvic peritonectomy, resection of the right external iliac and aortic lymph nodes, appendectomy, omentectomy, hepatic flexure mobilization, and optimal cytoreductive surgery to no visible residual disease. Total operative time was 5 hours and 20 minutes with an estimated blood loss of 500 mL. Preoperatively, the patient had required 2 units of packed red blood cells to optimize her to a hemoglobin of 10 g/dL. Postoperatively, her hemoglobin appropriately decreased to 8.5 g/dL. She remained hemodynamically stable and did not require any additional transfusion of blood products. Final pathology demonstrated high-grade sarcoma with rhabdomyoblastic and chondroblastic differentiation in the uterine myoma and serosa, posterior lower uterine segment, bilateral ovaries, and the aortic and right external iliac lymph nodes. (Figure 9) She tolerated the procedure well. Upon her recovery, she completed two more cycles of adjuvant chemotherapy with cyclophosphamide, doxorubicin, and vincristine, and 1 cycle of ifosfamide and etoposide. Additional adjuvant cycles were anticipated but the patient experienced significant cytotoxic symptoms and declined further treatment. She opted for palliative measures. The patient unfortunately succcumbed to her disease as it progressed in her abdomen and caused increasing pain. She decided to pursue end-of-life medications and died in her home surrounded by loved ones 4 months after her last surgery.
Figure 9.
A, The postchemotherapy resection showed extensive endometriosis, as shown in this image (H&E, 10×). B, In some areas the benign endometrioid-type glands of the endometriosis were intermixed with residual atypical rhabdomyosarcomatous stroma (H&E, 10×). C, Focal areas showed sheets of pleomorphic tumor cells with abundant eosinophilic cytoplasm, consistent with rhabdomyosarcoma (H&E, 10×). D, The sarcoma in this postchemotherapy resection also demonstrated focal areas with chondrosarcomatous differentiation (H&E, 10×).
REVIEW AND DISCUSSION
An extensive literature review was performed in PubMed with search keywords, “endometriosis,” “rhabdomyosarcoma,” and “malignant transformation.” Most commonly, rhabdomyosarcomas are diagnosed during childhood and typically found in the vagina with pathology consistent with sarcoma botryoides. Ovarian rhabdomyosarcoma was first described by Rudolph Virchow in 1850 and has a uniquely rare occurrence. Cases of ovarian rhabdomyosarcoma have been described as a myriad presentation of malignant mesodermal mixed tumor (carcinosarcoma), mesodermal adenosarcoma, teratoma, or Sertoli-Leydig cell tumors.18–25 There have only been 30 well-documented reports of primary ovarian rhabdomyosarcoma in the literature, with a mean age of 35 at presentation.26 A case series of 13 primary ovarian rhabdomyosarcoma reported 11 embryonal and 2 alveolar subtypes.27
When rhabdomyosarcoma results from the malignant transformation of an underlying ovarian lesion, it is more commonly known to originate from a teratoma. Approximately 0.2%–1.4% of rhabdomyosarcomas arise from mature cystic teratomas. These are tumors with a complexity of heterogeneous elements, including mesenchymal cells. Rhabdomyosarcomas can arise from any site in the body. Of the cases reported, 31% have originated from the genitourinary tract, 25% parameninges, 13% extremities, 9% orbit, 7% head and neck, 7% retroperitoneum, 5% trunk, and 3% from other sites. It is interesting to note 3.5% cases were derived from the female genital and reproductive organs, more notably in the vulva, vagina, uterus, and cervix.28 Ovarian tissue is a site that typically lacks striated muscle, thus making the histologic derivation of rhabdomyosarcoma in the ovary particularly perplexing.26
Malignant transformation from ovarian endometriosis is well documented and was initially described by Sampson in 1925. His criteria for diagnosis of this condition was later supplemented by Scott, and requires 1) the coexistence of carcinoma and endometriosis in the same ovary, 2) the entities share a similar histologic relationship, 3) the exclusion of invasion from other primary sites, and 4) the benign endometriosis must be contiguous with the malignant tissue.29 There is a higher prevalence of ovarian cancer among women with a history of endometriosis compared to the general population.9 In a National Swedish review of 20,686 hospitalized patients with endometriosis, 738 patients developed malignancies and of which 29 were specific to ovarian malignancies. Statistically among their endometriosis cohort, they demonstrated an increased risk of overall malignancy of 1.2, ovarian cancer risk of 1.9, and breast cancer risk of 1.3. Those patients with >10-year history of ovarian endometriosis had a significantly higher relative risk of ovarian cancer with a standardized incidence ratio of 4.2.30 A large pooled analysis of case-control studies demonstrated an association between endometriosis with certain histologic types. Compared to the nonendometriosis control group, women with endometriosis had a higher proclivity for developing invasive clear cell, endometrioid, and low-grade serous subtypes of ovarian cancer, with odds ratios of 3.73, 2.32, and 2.02, respectively. There was no such association for mucinous or high-grade serous subtypes.31 In a review of 76 patients who were diagnosed with stage-I ovarian carcinomas, 40 of these patients had associated findings of ovarian endometriosis, and the majority had endometrioid and clear cell histology.32
Endometriosis can also show malignant transformation into mixed stromal and epithelial or exclusively stromal neoplasms, including endometrial stromal sarcoma,33 clear-cell adenocarcinoma and adenosarcoma with heterologous components of rhabdomyosarcoma34 and ovarian adenosarcoma with rhabdomyosarcoma.35 Thus, only 2 studies reported heterologous components of rhabdomyosarcoma associated with ovarian endometriosis. In our patient, most of the tumor consisted of rhabdomyosarcoma, with only a focal area in the original right ovarian cystectomy with features suggestive of adenosarcoma. We are not certain whether this represents a pure rhabdomyosarcoma arising from extensive endometriosis or whether it is diffuse stromal overgrowth from an inconspicuous adenosarcoma in an endometrioma.
The diagnosis of endometriosis is currently limited to its direct observation during surgery with pathologic confirmation. When surgical intervention is pursued, complete removal of the endometriosis remains the most effective treatment.36 Multiple tissue specimens should be obtained during surgery to maximize the potential for making an accurate histologic diagnosis.
In hindsight, a different approach in our case could have been pursued to expedite the appropriate diagnosis. What differed from other endometriosis cases was the presence of vegetations. The growth of the endometrioma into the retroperitoneum and its impingement on the ureters were also hints of aggressive behavior that could have suggested potential malignancy. There were areas of the mass that were extremely difficult to resect. When encountering severe endometriosis, suspicion for malignancy should be considered in the setting of rapid growth rate, invasion into the retroperitoneum, difficult and cumbersome resections, or the presence of vegetations. Earlier immunohistologic testing should also have been performed. However, immunohistochemical stains are not routinely performed in the histopathologic evaluation of endometriosis or mature teratomas. It should be utilized in the setting of suspicious malignancy.
Although malignant transformation is a rare occurrence, vigilance should be practiced when it is suspected. Imaging studies can also be helpful in alluding to potential malignant transformation. Sonography is appropriate for detecting homogenous hypoechogenic features consistent with ovarian endometriomas. However, it is often ineffective in identifying potential malignant processes or small transformative changes. On the other hand, magnetic resonance imaging findings could demonstrate contrast enhanced mural nodules, suggestive of malignant transformation.10,11 Another clue could be an enlarged endometrioma with disappearance of shading on T2-weighted imaging.37
Practitioners should identify endometriosis patients who have a higher propensity for developing ovarian cancer. These include patients with a long-standing history of the disease, endometriosis diagnosis at an early age, history of infertility, or the presence and rapid growth of endometriomas.11 When encountering this susceptible group of patients, a more comprehensive approach should be pursued. Consideration of magnetic resonance imaging with findings of mural formation may be suggestive of malignant transformation from endometriomas. Complete surgical resection of the endometriosis should be performed with all specimens sent for histological analysis. Adjuvant therapy with hormonal suppression can aid in decreasing the recurrence of endometriomas as well as the possible potential for malignant transformation. Close follow-up of these patients with surveillance for recurrence is recommended. Although malignant transformation from endometriosis affects only a small subset of patients, it can be detrimental with a high rate of morbidity and mortality when it does manifest. Given the multifactorial processes of both endometriosis and ovarian cancer, there currently exists no sound screening tests or reliable diagnostic biomarkers for these diseases. Until these advances are made, reliance must be placed on the collaborative efforts across multiple disciplines to diagnose and appropriately treat these patients.
Contributor Information
Camran Nezhat, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Stanford University Medical Center, Palo Alto, California, USA.; University of California at San Francisco School of Medicine, San Francisco, California, USA.
Mailinh Vu, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Stanford University Medical Center, Palo Alto, California, USA.; University of California at San Francisco School of Medicine, San Francisco, California, USA.
Nataliya Vang, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Stanford University Medical Center, Palo Alto, California, USA..
Kristen Ganjoo, Stanford Comprehensive Cancer Center, Stanford University Medical Center, Stanford, California, USA..
Amer Karam, Stanford Women's Cancer Center, Stanford University Medical Center, Stanford, California, USA..
Ann Folkins, Department of Pathology, Stanford University School of Medicine, Stanford, California, USA..
Azadeh Nezhat, Camran Nezhat Institute, Center for Special Minimally Invasive and Robotic Surgery, Stanford University Medical Center, Palo Alto, California, USA.; University of California at San Francisco School of Medicine, San Francisco, California, USA.
Farr Nezhat, Nezhat Surgery for Gynecology/Oncology, Weill Cornell Medical College of Cornell University, New York City, New York, USA.; Stony Brook University School of Medicine, Stony Brook, New York, USA. New York University, Winthrop Hospital, Mineola, New York, USA.
References:
- 1. Matias-Guiu X, Stewart CJR. Endometriosis-associated ovarian neoplasia. Pathology. 2018;50:190–204. [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117–26. [DOI] [PubMed] [Google Scholar]
- 4. Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–69. [DOI] [PubMed] [Google Scholar]
- 5. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110-43. [PMC free article] [PubMed] [Google Scholar]
- 6. Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. [DOI] [PubMed] [Google Scholar]
- 7. Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–75. [DOI] [PubMed] [Google Scholar]
- 8. Siufi Neto J, Kho RM, Siufi DF, Baracat EC, Anderson KS, Abrao MS. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J Minim Invasive Gynecol. 2014;21:55–63. [DOI] [PubMed] [Google Scholar]
- 9. Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C. The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril. 2008;90:1559–70. [DOI] [PubMed] [Google Scholar]
- 10. Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102:342–4. [DOI] [PubMed] [Google Scholar]
- 11. Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24:623–8. [DOI] [PubMed] [Google Scholar]
- 12. Krawczyk N, Banys-Paluchowski M, Schmidt D, Ulrich U, Fehm T. Endometriosis-associated malignancy. Geburtshilfe Frauenheilkd. 2016;76:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nezhat F, Cohen C, Rahaman J, Gretz H, Cole P, Kalir T. Comparative immunohistochemical studies of BCL-2 and p53 proteins in benign and malignant ovarian endometriotic cysts. Cancer. 2002;94:2935–40. [DOI] [PubMed] [Google Scholar]
- 14. Wei JJ, William J, Bulun S. Endometriosis and ovarian cancer: a review of clinical, pathologic, and molecular aspects. Int J Gynecol Pathol. 2011;30:553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogilvie CM, Crawford EA, Slotcavage RL, et al. Treatment of adult rhabdomyosarcoma. Am J Clin Oncol. 2010;33:128–31. [DOI] [PubMed] [Google Scholar]
- 16. Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44. [PubMed] [Google Scholar]
- 17. Esnaola NF, Rubin BP, Baldini EH, et al. Response to chemotherapy and predictors of survival in adult rhabdomyosarcoma. Ann Surg. 2001;234:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramaekers FC, Verheijen RH, Moesker O, Kant A, Vooijs GP, Herman CJ. Mesodermal mixed tumor. Diagnosis by analysis of intermediate filament proteins. Am J Surg Pathol. 1983;7:381–5. [PubMed] [Google Scholar]
- 19. Dehner LP, Norris HJ, Taylor HB. Carcinosarcomas and mixed mesodermal tumors of the ovary. Cancer. 1971;27:207–16. [DOI] [PubMed] [Google Scholar]
- 20. Dictor M. Malignant mixed mesodermal tumor of the ovary: a report of 22 cases. Obstet Gynecol. 1985;65:720–4. [PubMed] [Google Scholar]
- 21. de Brito PA, Silverberg SG, Orenstein JM. Carcinosarcoma (malignant mixed mullerian (mesodermal) tumor) of the female genital tract: immunohistochemical and ultrastructural analysis of 28 cases. Hum Pathol. 1993;24(2):132–142. [DOI] [PubMed] [Google Scholar]
- 22. Kawai M, Kano T, Furuhashi Y, et al. Immature teratoma of the ovary. Gynecol Oncol. 1991;40:133–7. [DOI] [PubMed] [Google Scholar]
- 23. Ornvold K, Detlefsen GU, Horn T, Rorth M. Immature ovarian teratoma in a postmenopausal woman. Acta Obstet Gynecol Scand. 1987;66:473–6. [DOI] [PubMed] [Google Scholar]
- 24. Prat J, Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors with heterologous elements. II. Cartilage and skeletal muscle: a clinicopathologic analysis of twelve cases. Cancer. 1982;50:2465–75. [DOI] [PubMed] [Google Scholar]
- 25. Chan YF, Leung CS, Ma L. Primary embryonal rhabdomyosarcoma of the ovary in a 4-year-old girl. Histopathology. 1989;15:309–11. [PubMed] [Google Scholar]
- 26. Allende DS, Yang B. Primary ovarian rhabdomyosarcoma with heterologous elements: a case report. Int J Gynecol Pathol. 2008;27:402–6. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen GP, Oliva E, Young RH, Rosenberg AE, Prat J, Scully RE. Primary ovarian rhabdomyosarcoma: a report of 13 cases. Int J Gynecol Pathol. 1998;17:113–9. [DOI] [PubMed] [Google Scholar]
- 28. Arndt CA, Donaldson SS, Anderson JR, et al. What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract? Cancer. 2001;91:2454–68. [PubMed] [Google Scholar]
- 29. Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283–9. [PubMed] [Google Scholar]
- 30. Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–9. [DOI] [PubMed] [Google Scholar]
- 31. Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deligdisch L, Penault-Llorca F, Schlosshauer P, Altchek A, Peiretti M, Nezhat F. Stage I ovarian carcinoma: different clinical pathologic patterns. Fertil Steril. 2007;88:906–10. [DOI] [PubMed] [Google Scholar]
- 33. Back JA, Choi MG, Ju UC, Kang WD, Kim SM. A case of advanced-stage endometrial stromal sarcoma of the ovary arising from endometriosis. Obstet Gynecol Sci. 2016;59:323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yasuoka H, Tsujimoto M, Fujita S, et al. Coexistence of a clear cell adenocarcinoma and an adenosarcoma with a heterologous rhabdomyosarcoma in an endometriotic cyst of the ovary: a case study. Int J Gynecol Pathol. 2009;28:362–6. [DOI] [PubMed] [Google Scholar]
- 35. Mikami M, Tanaka K, Onouchi M, Komiyama S, Ishikawa M, Hirose T. A case of ovarian adenosarcoma with a heterologous rhabdomyosarcoma component: a brief case report. Eur J Obstet Gynecol Reprod Biol. 2004;117:112–4. [DOI] [PubMed] [Google Scholar]
- 36. Nezhat C, Vang N, Tanaka P, Nezhat C. Optimal Management of Endometriosis and Pain. Obstet Gynecol. 2019;134(4):834–839. [DOI] [PubMed] [Google Scholar]
- 37. Takeuchi M, Matsuzaki K, Uehara H, Nishitani H. Malignant transformation of pelvic endometriosis: MR imaging findings and pathologic correlation. Radiographics. 2006;26:407–17. [DOI] [PubMed] [Google Scholar]