ABSTRACT
In preclinical in vivo models of cancer, the induction of immunogenic cell death (ICD) sensitizes to subsequent immunotherapy. Several clinical trials now confirm that pretreatment with ICD-inducing anthracyclines sensitizes to immune checkpoint blockade targeting PD-1 and PD-L1 interaction. These findings support the translational utility of the concept of ICD.
KEYWORDS: Anthracyclins, immunogenic cell death, PD-1, PD-L1
The durable clinical success of antineoplastic treatment strongly depends on the stimulation of adaptive anticancer immunity and the generation of immunological memory against tumor-associated antigens (TAAs).1 Anticancer immunity and the re-establishment of immunosurveillance can be elicited via the induction of immunogenic cell death (ICD). Thus, exposing cancer cells to ionizing irradiation or antineoplastic agents, such as anthracyclines, oxaliplatin or crizotinib stimulates the liberation of danger associated molecular patterns (DAMPs). Once released by the tumor, DAMPs operate as immunological adjuvants and boost TAA-specific anticancer immune responses.1 Thus, lysosomal secretion of ATP, translocation to, and display on, the cell surface of calreticulin (CALR), a transcriptional type-1 interferon (IFN) response, and exodus of annexin A1 (ANXA1) and nuclear high mobility group box 1 (HMGB1) act together to attract and stimulate dendritic cells (DCs) for TAA uptake and processing. The MHC class I-restricted cross-presentation of TAAs ultimately leads to the priming of T cells and the clonal expansion of cancer-specific cytotoxic T lymphocytes.1,2
We recently observed that the tyrosine kinase inhibitor crizotinib can induce ICD through off-target effects. When combined with non-immunogenic standard of care chemotherapies such as cisplatin or mitomycin c, crizotinib-sensitized models of established orthotopic non-small cell lung cancers (NSCLC) to subsequent immunotherapy with PD-1-based immune checkpoint blockade, reaching a 90% cure rate.3 Similarly, in mice, genetically induced KRAS-positive, TP53-negative NSCLC responded to immunogenic chemotherapy with a combination of two agents, oxaliplatin and cyclophosphamide. This combination treatment strongly enhanced T cell infiltration of the cancers and sensitized them to subsequent checkpoint inhibition targeting both CTLA-4 and PD-1.4
Local immunotherapy such as the intratumoral injection of oncolytic compounds constitutes yet another example of immunogenic anticancer therapies that sensitize to subsequent immune checkpoint blockade. Thus, sequential LTX-401 treatment combined with double checkpoint inhibition of PD-1 and CTLA-4 exhibited strong antineoplastic effects on primary lesions and distant tumors, emphasizing the potency of combining ICD induction with checkpoint blockade.5
Yet another study used HER2-targeting antibody-drug conjugate (ADC) bearing a potent anthracycline derivate (T-PNU) as payload to confirm the induction of ICD in a syngeneic breast cancer model expressing human HER2. Cytotoxic T lymphocytes were identified as drivers of the T-PNU mediated anti-tumor effect. The combination of T-PNU with an antibody targeting PD-1 facilitated tumor eradication and elicited long-lasting immune protection in a murine orthotopic breast cancer model resistant to other HER2-directed therapies.6
Taken together, these results indicate that the induction of ICD sensitizes tumors to subsequent treatment with ICB and that this sensitization strongly relies on the conversion of ‘cold’ into ‘hot’ tumors in which the frequency of tumor-infiltrating leukocytes associated with favorable prognosis was increased. In all preclinical models of cancer outlined here, the combination of ICD inducers with immune checkpoint blockade was superior to monotherapy with either ICD induction or immunotherapy. (Figure 1A)
Figure 1.
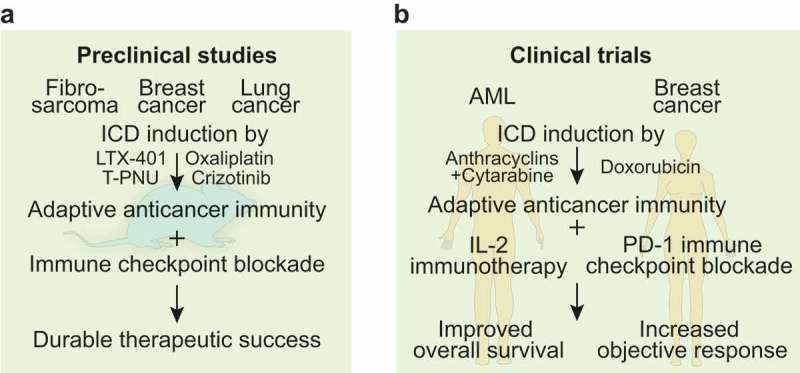
Preclinical and clinical evidence for immunogenic cell death-mediated sensitization to immune checkpoint blockade.
(A). Preclinical work in syngeneic mouse models of fibrosarcoma, breast and lung cancer depicted the potency of immunogenic cell death (ICD) induced by agents such as the oncolytic compound LTX-401, the antibody drug conjugate T-PNU, the tyrosine kinase inhibitor crizotinib and the chemotherapeutic agent oxaliplatin to trigger adaptive anticancer immunity. Sequential combination with immune checkpoint blockade achieved durable therapeutic success. (B). Clinical trials in patients with acute myeloid leukemia (AML) that received consolidation therapy with anthracyclins and cytarabine followed by interleukin-2 (IL-2)-based immunotherapy or women with triple negative breast cancer that received doxorubicin before immune checkpoint inhibition with monoclonal anti-PD-1 antibodies depicted improved overall survival and increased objective response, respectively.
Approximately 450 trials (www.clinicaltrials.gov) are now investigating combination effects between potential ICD inducers and PD-1/PD-L1 checkpoint blockade. Several recent clinical reports now corroborate the hypothesis that pretreatment with ICD-inducing anthracyclines or irradiation sensitizes to immune checkpoint inhibitors.
Patients with acute myeloid leukemia (AML) that underwent consolidation chemotherapy with anthracyclines together with high-dose cytarabine and subsequently received immunotherapy with histamine dihydrochloride and interleukin-2 showed enhanced frequencies of CD8+ effector memory T cells along with improved survival, as compared to individuals that were not treated with anthracylines. Thus, the choice of consolidation therapy prior to AML immunotherapy impacts clinical outcome.7 Non-small cell lung cancer (NSCLC) patients that received chemoradiotherapy (platinum-based, doublet chemotherapy administered with definitive-dose radiotherapy) combined with immune checkpoint blockade targeting PD-L1 had a significantly prolonged overall survival, as compared with the placebo group.8 Thus, the combination of standard of care chemotherapy with immunostimulatory irradiation and immunotherapy was superior to chemoradiotherapy alone. Yet another study compared the efficacy of distinct chemotherapies or irradiation as ‘induction therapy’ (randomized into (i) a negative control group, (ii) 3 × 8 Gy irradiation, (iii) cyclophosphamide, (iv) cisplatin or (v) doxorubicin), followed by PD-1 blockade in metastatic triple-negative breast cancer. The best outcome was obtained in the doxorubicin-treated cohort, yielding an objective response rate of 35% (as compared to a response rate of 20% in the overall cohort). Immunogenic chemotherapy with anthracyclines induced the upregulation of immune-related genes involved in PD-1/PD-L1 and T cell cytotoxicity pathways, suggesting the advent of a more favorable tumor microenvironment that explains the subsequent response to PD-1 blockade9 (Figure 1B).
In summary, these clinical studies support the translational applicability of the concept of ICD. We anticipate that future investigations will lead to the optimization of combination treatment consisting in ‘induction therapies’ with ICD inducers and immunotherapies. It is possible, yet remains to be determined that such ‘induction therapies’ may be used during only one or few cycles and at relatively low doses to avoid the side effects that traditionally have been observed during treatment schedules based on chemotherapy only.
Acknowledgments
GK and LZ are supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Conflict of interest statement
LZ and GK receive research funding by Lytix and are scientific founders of everImmune. OK and GK are scientific founders of Samsara Therapeutics.
References
- 1.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G.. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 3.Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. doi: 10.1038/s41467-019-09415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie W, Mondragón L, Mauseth B, Wang Y, Pol J, Lévesque S, Zhou H, Yamazaki T, Eksteen JJ, Zitvogel L, et al. Tumor lysis with LTX-401 creates anticancer immunity. Oncoimmunology. 2019;8(7):1594555. doi: 10.1080/2162402X.2019.1594555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico L, Menzel U, Prummer M, Müller P, Buchi M, Kashyap A, Haessler U, Yermanos A, Gébleux R, Briendl M, et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J Immunother Cancer. 2019;7(1):16. doi: 10.1186/s40425-018-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aurelius J, Möllgård L, Kiffin R, Ewald Sander F, Nilsson S, Bergh Thorén F, Hellstrand K, Martner A. Anthracycline-based consolidation may determine outcome of post-consolidation immunotherapy in AML. Leuk Lymphoma. 2019;1–8. doi: 10.1080/10428194.2019.1599110. [DOI] [PubMed] [Google Scholar]
- 8.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 9.Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]


