ABSTRACT
Dendritic- cells (DCs) have received considerable attention as potential targets for the development of anticancer vaccines. DC-based anticancer vaccination relies on patient-derived DCs pulsed with a source of tumor-associated antigens (TAAs) in the context of standardized maturation-cocktails, followed by their reinfusion. Extensive evidence has confirmed that DC-based vaccines can generate TAA-specific, cytotoxic T cells. Nonetheless, clinical efficacy of DC-based vaccines remains suboptimal, reflecting the widespread immunosuppression within tumors. Thus, clinical interest is being refocused on DC-based vaccines as combinatorial partners for T cell-targeting immunotherapies. Here, we summarize the most recent preclinical/clinical development of anticancer DC vaccination and discuss future perspectives for DC-based vaccines in immuno-oncology.
KEYWORDS: Antigen cross-presentation, DAMPs, immune checkpoint blockers, plasmacytoid dendritic cells, TLR signaling, tumor-infiltrating lymphocytes, clinical trial
Introduction
The presentation of antigenic material to cells of the adaptive immune system, notably CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs), is a crucial function of myeloid cells,1,2 as it stands at the basis of both antigen-specific immune activation and tolerance.3–11 Professional antigen-presenting cells (APCs) are specialized at executing this function, and dendritic cells (DCs) are largely viewed as the most proficient of APCs.7,8,12–18 Indeed, DCs not only present extracellular antigens on MHC Class II molecules to CD4+ T cells,19,20 in thus far resembling macrophages and B cells,21–23 but they also efficiently mediate cross-presentation, i.e., the presentation of extracellular antigens on MHC Class I molecules to CD8+ CTLs, which is key for anticancer immunity.8,24–31 Pioneering research by the team of the Canadian physician Ralph Steinman uncovered the existence of DCs in 1973, an achievement for which he was eventually awarded the 2011 Nobel Prize in Physiology or Medicine.32–35 The name DC reflects the distinguishing morphology of these cells, which can acquire a tree-like shape (from the Greek term “dendron”, translating to “tree”).36–38 Following the discovery of DCs by the Steinman laboratory, considerable efforts have been dedicated to characterize their unique phenotypic and functional features, which firmly position DCs at the interface between innate and adaptive immunity.8,13,39–51
DCs are common throughout the body, although they are relatively more abundant in tissues that are in contact with the external environment, such as epithelial tissues.5,16,52–66 DCs are highly heterogeneous in terms of ontogeny,67–69 function, responsiveness to selected stimuli and preference for specific sources of antigens for presentation.16,70–77 These features have been harnessed to generate ever more refined biological classifications of DCs into different subsets.76,78–81 In particular, both human and mouse DCs can be morphologically classified into 2 major subsets: myeloid DCs, which are also known as conventional or classical DCs (cDCs) and (prior to activation) resemble circulating monocytes,82–84 and plasmacytoid DCs (pDCs), which resemble plasma cells.85–88 Two other DC subsets have been described: CD16+ monocyte-derived (or inflammatory) DCs, and Langerhans cells (LCs), a skin-resident population of DCs with potent immunosuppressive activity.86,89–92 Here, cDCs derive from a common myeloid precursor and can be further classified into two subsets: (1) cDC1, which express CD8α or CD103 in mice, or CD141 in humans; and (2) cDC2, which express CD11b in mice, or CD1c in humans.93 In contrast, pDCs are believed to originate from a poorly defined lymphoid precursor, and are characterized by co-expression of CD45R/B220 and Ly-6C in mice, or CD123 and CD304 in humans.85 An alternative classification for DCs reflects the differential expression of master transcription factors like basic leucine zipper transcription factor ATF-like 3 (BATF3), which is critical for the development of CD8α+ DCs in lymphoid tissues and CD103+CD11b− DCs in peripheral tissues.94–97 High-dimensional analysis techniques (e.g., mass cytometry, single-cell RNA sequencing) have further expanded our understanding on the complexity of DCs, while casting some doubts on the accuracy of the aforementioned classifications.85,86,98
Different DC subsets exhibit functional specialization on a number of immunological levels, including: (1) capturing, processing and presenting antigens (e.g., human CD141+ DCs and mouse CD8α+ DCs are highly proficient at cross-presentation, while CD11b+ DCs efficiently mediate conventional MHC Class II presentation);97,99–105 (2) preferential engagement of adaptive immune cell populations (e.g., CD14+ DCs enriched in the dermis and LCs tend to specifically drive humoral responses and CD8+ T cell effectors, respectively);106,107 and (3) secretion of antiviral and immunomodulatory cytokines such as type I interferon (IFN) (e.g., pDCs mount stronger type I IFN responses as compared to other DC subsets).108–112
In homeostatic conditions, blood-borne as well as tissue-resident DCs exist in an immature functional state, which is key for the preservation of peripheral tolerance to self antigens.113 In particular, immature DCs (iDCs) potently suppress the activity (or even promote the clonal deletion) of self-reactive T cells,45,114 and favor the proliferation of immunosuppressive CD4+CD25+FOXP3+ regulatory T (TREG) cells.27,115–117 The most salient features of iDCs are: (1) prominent potential for the uptake of extracellular material, especially cell corpses, vesicles and other small non-soluble entities, coupled to the secretion of homeostatic cytokines and chemokines; (2) expression of specific chemokine receptors that enable rapid chemotaxis to sites of inflammation; (3) retention of MHC Class II molecules within late endosomes; and (4) minimal surface expression of co-stimulatory molecules like CD40, CD70, CD80, CD83, CD86 and tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4, also known as OX40L).118
In pathological settings, a plethora of microbial cues commonly known as microbe-associated molecular patterns (MAMPs)8,119–121 and endogenous danger signals cumulatively referred to as damage-associated molecular patterns (DAMPs)58,122–128 drive the rapid phenotypic and functional conversion of iDCs into mature DCs (mDCs).129–137 Here, mDCs differ from their immature counterparts as they display: (1) limited phagocytic activity; (2) altered profile of chemokine receptors including high levels of chemokine (C-C motif) receptor 7 (CCR7), which favors DC homing and persistence in lymph nodes; (3) robust exposure of peptide-bound MHC molecules and co-stimulatory ligands on the cell surface; and (4) secretion of pro-inflammatory cytokines such as interleukin 12 (IL12) and T cell-targeting chemokines such as C-X-C motif chemokine ligand 10 (CXCL10).38,116,118,138–149 Thus, mDCs acquire the anatomical location and all the functional properties that are required for optimal T cell priming.150–153
Since the cross-priming of CD8+ CTLs against tumor-associated antigens (TAAs) is critical for the elicitation of robust and durable anticancer immune responses, and this process is often disabled in developing tumors (by a multitude of immunosuppressive strategies deployed by cancer cells),154,155 DCs have attracted considerable attention as potential targets for the development of therapeutic cancer vaccines.156–159 This approach generally involves the differentiation of DCs from autologous, patient-derived monocytes, followed by ex vivo DC exposure to an appropriate source of TAAs in the context of potent maturation cocktails,160 and culminating with the reinfusion of mDCs to the patient. Accumulating clinical evidence demonstrates that this approach often promotes at least some degree of TAA-specific CTLs-driven immunity in patients.8,156,157,161 However, the efficacy of therapeutic DC vaccination is often limited by the robust immunosuppressive circuits that are in place in the microenvironment of most tumors.98,162
Therapeutic DC vaccines developed so far can be broadly classified in different groups based on the approach for TAA-delivery or molecular modifications imposed on DCs before reinfusion.163,164 These groups are: (1) DCs not pulsed with TAAs and employed either in an immature form or used upon maturation with pro-inflammatory cytokines and/or MAMPs or DAMPs;138,165–169 (2) DCs pulsed ex vivo with tumor lysates or tumor-derived mRNA (both of which cover either a broad array of TAAs), specific TAA-based peptides (generally consisting of one or a few selected peptides), or precise TAA-coding mRNAs;59,170–221 (3) DCs provided in vivo with TAAs;222–233 (4) DCs stimulated in situ by immunostimulatory agents applied peritumorally or intratumorally;234 (5) DCs transfected (virally or biochemically) with a genetic vector coding for one or a few TAAs and/or immunostimulatory factors;235 or (6) DC-derived exosomes.236–240 That said, the most common approach to therapeutic cancer vaccination with DCs consists of DCs pulsed with TAAs or tumor lysates and stimulated with standard maturation cocktails.241 Herein, iDC pulsing is usually achieved by: (1) co-incubation with autologous or allogeneic tumor lysates;170–179,242–244 (2) co-incubation with recombinant TAAs or peptides thereof;59,180–186 (3) transfection of TAA-encoding plasmids or mRNAs;187–211,245–248 and, (4) fusion of DCs with inactivated malignant cells, resulting in the generation of so-called “dendritomes”.177–179,212–219,249 The possibility of creating DC vaccines in situ or in vivo by direct TAA delivery has been explored with DC-targeting immunoliposomes,250–252 DC-targeting genetic vectors,253–256 or TAAs fused to monoclonal antibodies or other moieties targeting DC surface receptors.222–228,230,231,233,257,258 The possibility of creating DC vaccines exploiting specific (naturally available) DC subsets has also been explored.259,260 Particularly, specific DC subsets including pDCs, LCs and CD1c+ DCs have been successfully utilized in the clinic for the vaccination of cancer patients.259–261 Notably, sipuleucel-T (commercially sold as Provenge®) is the sole tumor-targeting cellular therapy involving (but not restricted to) DCs that is currently approved by the US Food and Drug Administration (FDA) for use in individuals with asymptomatic or minimally-symptomatic metastatic castration-resistant prostate cancer.262–264
Here, we summarize preclinical and clinical progress in the development of therapeutic cancer vaccines based on DCs. As the cancer immunotherapy landscape is currently dominated by other therapeutic modalities265–269 such as immune checkpoint blockers (ICBs) and adoptive T-cell transfer (ACT),270,271 interest is being refocused on implementing DC-based vaccines as part of multimodal (immuno)therapeutic regimens.15,43,272–278 That said, the number of clinical trials currently open to investigate the safety and therapeutic profile of DC vaccination in cancer patients remains high.
Recent preclinical developments
Several preclinical reports dealing with anticancer DC vaccines were published since our last Trial Watch on this topic (February 2017).279 Of this abundant preclinical literature, we found of particular interest the following selected studies, which are largely representative of the overall trends in the field (not discussed in any specific order). Nimanong et al. (from Medical School Hannover, Hannover, Germany) combined DC vaccination with a co-stimulatory cocktail consisting of a CD40 agonistic antibody,280 an agonist of Toll-like receptor 3 (TLR3) (and different combinations of neoantigen-based or TAA-based peptides) and achieved successful remission of large murine subcutaneous MC38 tumors, accompanied by robust antigen-specific T-cell responses.281 Moreno Ayala et al. (from Universidad de Buenos Aires, Buenos Aires, Paraguay) reported that combining DC vaccines with a FOXP3-targeting synthetic peptide (P60) that inhibits TREG cells results in superior antitumor efficacy against murine LM3 or 4T1 mammary carcinomas.282 Liu et al. (from Chinese PLA General Hospital, Beijing, China) tested the efficacy of DC vaccines involving DCs pulsed with glioma-derived exosomes and α-galactosylceramide (α-GalCer), an agonist for invariant natural killer T (iNKT) cells, in combination with adoptively transferred iNKT cells, in an orthotopic rat glioma model, observing efficient induction of antitumor immunity.283 Similarly, Escriba-Garcia et al. (from Hospital de la Santa Creu i Sant Pau, Barcelona, Spain) found that DC vaccines combined with α-GalCer enabled 100% tumor-free survival in a murine model of B-cell lymphoma, correlating with increased effector T-cell functions and expansion of iNKT cells secreting interferon gamma (IFNG).284 Vo et al. (from Chonnam National University Hwasun Hospital, Jeollanamdo, Republic of Korea) successfully combined TAA-based DC vaccination with the immunomodulatory drug lenalidomide, achieving potent antitumor immunity against murine MC38 colorectal carcinoma tumors, which was accompanied by reduction in myeloid-derived suppressor cells (MDSCs)285 and induction of lymphoid effector functions.286 Dammeijer et al. (from Erasmus MC, Rotterdam, Netherlands) documented that the pharmacological inhibitor of colony stimulating factor 1 receptor (CSF1R), PLX3397 (also known as pexidartinib),287,288 which causes depletion of tumor-associated macrophages (TAMs), synergizes with DC vaccines to achieve eradicating immunity in mouse models of mesothelioma.289 Moreno Ayala et al. (from Universidad de Buenos Aires, Buenos Aires, Paraguay) combined DC vaccines with different TLR7 or TLR9 agonists, observing that whereas DCs activated through TLR9 enabled prolonged tumor- and metastasis-free survival, dual TLR7/TLR9 activation impaired vaccination efficacy, a disparity that could be ameliorated by inhibiting nitric oxide synthase (NOS) and indoleamine-pyrrole-2,3-dioxygenase 1 (IDO1).290,291 Ebrahimi-Nik et al. (from University of Connecticut, Farmington, CT, USA) reported DCs to be superior to macrophages at eliciting neoantigen-targeted eradicating immunity in a DC vaccination setup, but surprisingly found CD11c+MHC-IIlow DCs to be the best APCs in this particular setting.26 Montico et al. (from Centro di Riferimento Oncologico, Aviano, Italy) pulsed human DCs with mantle cell lymphoma (MCL) or diffuse large B-cell lymphoma (DLBCL) cells undergoing immunogenic cell death (ICD)292,293 in response to 9-cis-retinoic acid plus type I IFN, which mediated robust anticancer immunity upon inoculation in immunodeficient tumor-bearing mice reconstituted with human peripheral blood mononuclear cells (PBMCs).42 Antonios et al. (from University of California, Los Angeles, California, USA) treated orthotopic glioma-bearing mice with DC vaccines combined with antibodies against programmed cell death 1 (PDCD1, best known as PD-1) and PLX3397, reporting therapeutic benefits only in the context of vaccination.294 Arab et al. (from Tehran University of Medical Sciences, Tehran, Iran) achieved pronounced inhibition of tumor growth and antitumor immunity upon combining DC vaccination with blockade of adenosine A2A receptor (ADORA2A) and 5ʹ-nucleotidase ecto (NT5E, best known as CD73).295 Komorowski et al. (from Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA) achieved robust therapeutic effects against murine neuroblastomas by combining DC vaccines with an oncolytic vaccinia virus296,297 expressing a C-X-C chemokine receptor type 4 (CXCR4) antagonist.298 Stefanski et al. (from University of Minnesota, Minneapolis, Minnesota, USA) pulsed DCs genetically-engineered to overexpress CCL21 with TAAs, and reported the induction of efficient anticancer immunity in mouse models of leukemia.299 Van Woensel et al. (from KU Leuven, Leuven, Belgium) found that combining DC-based vaccines with the intranasal administration of chitosan nanoparticles loaded with small interfering RNAs targeting galectin 1 (LGALS1) drives robust tumor-targeting immune responses in a murine model of orthotopic glioma, correlating with dramatic changes in the tumor microenvironment in favor of M1-polarized macrophages and effector T cells.300 Huang et al. (from Xinhua Hospital and Shanghai Jiaotong University School of Medicine, Shanghai, China) observed that vaccines obtained by pulsing DCs with exosomes from transforming growth factor beta 1 (TGFB1)-defective cancer cells induce superior anti-leukemia immunity in vivo.301 Lu et al. (from Tianjin Medical University, Tianjin, China) found that DC-derived exosomes from DCs expressing a hepatocellular carcinoma (HCC)-associated antigen are very efficient at eliciting CD8+ T cell-dependent eradicating immunity in 3 different mouse models of the disease.302 Bryson et al. (from University of Southern California, Los Angeles, CA, USA) used a Sindbis virus-based method to deliver breast cancer-associated antigens to tumor-resident DCs, resulting in a potent vaccination effect in situ that amplified CD8+ CTL-dependent immunity against murine breast cancer.303 Liu et al. (from University of North Carolina at Chapel Hill, Chapel Hill, NC, USA) formulated a nanoparticle-based method to deliver mucin 1 (MUC1)-coding mRNA to lymph node-resident DCs in a mannose receptor-dependent fashion, culminating with expansion of MUC1-reactive T cells and regression of MUC1-expressing 4T1 mammary carcinomas, especially in the context of cytotoxic T-lymphocyte associated protein 4 (CTLA4) blockage.304 Oberli et al. (from Harvard Medical School, Boston, Massachusetts, USA) created a cancer vaccine consisting of DCs, macrophages and neutrophils transfected with an mRNA coding for melanoma antigens and delivered with lipid nanoparticles, which effectively eradicated B16 melanomas in a CD8+ CTL-dependent manner.305 Wennhold et al. (from University Hospital of Cologne, Cologne, Germany) compared the efficacy of antigen-pulsed and CD40-activated B cell and DC vaccines against B16 melanoma and E.G7 lymphoma, observing that B cell-based vaccines perform as efficiently as DC vaccines at inducing antitumor immunity.306 Wculek et al. (from Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain) showed that splenic cDC1s pulsed with cancer cells undergoing ICD were able to induce therapeutic immune responses that synergized with PD-1 blockade,307 supporting a mechanistic link between ICD-inducing systemic anticancer therapies and cDC1-dependent antigen cross-presentation.307–320 This finding extends prior observations indicating that human CD1c+ DCs are critical for the presentation of tumor antigens from cancer cells succumbing to ICD.8,103,321–323 Finally, Ventura et al. (Yale University School of Medicine, New Haven, CT, USA) identified a novel approach to generate potent, cytokine-independent DCs, that involves the physical interaction of circulating monocytes with platelets, potentially opening a completely unexplored avenue to DC-based vaccine.324
These are only a few examples of the abundant preclinical literature on therapeutic DC vaccines with oncological applications, demonstrating a persistent interest in this promising but only partially realized therapeutic paradigm.
Completed clinical studies
We identified 34 peer-reviewed papers published since the release of our latest Trial Watch on therapeutic DC-based vaccines for oncological indications (February 2017)279 reporting safety and efficacy data from clinical trials assessing this therapeutic paradigm in patients with cancer.
The majority of these studies (Figure 1) tested autologous DCs pulsed with: TAAs or peptides thereof,325–342 autologous cancer cell lysates,343–348 or TAA-coding RNAs.276,349–352 The predominant use of these TAA sources for the generation of DC-based vaccines is in line with previous trends, as documented in our previous Trial Watch on this subject,279 de facto reflecting a broad consensus in the field. Additional TAA sources explored in recent clinical studies include (Figure 1): lysates of autologous (tumor) stem cells, lysates of allogeneic cancer cell lines, and TAA-encoding viral vectors.353–355 Of note, one of these clinical studies involved DC vaccines based on personalized antigenic peptides.335 Moreover, a Phase I clinical study investigated an acute myeloid leukemia (AML)-derived cell line as allogeneic DC vaccine (DCP-001), owing to its DC-like behavior and expression of AML-relevant antigens.356 Finally, autologous DCs have been investigated in combination with cytokine-induced killer (CIK) cells357 for the therapy of advanced pancreatic carcinoma.358
Figure 1.
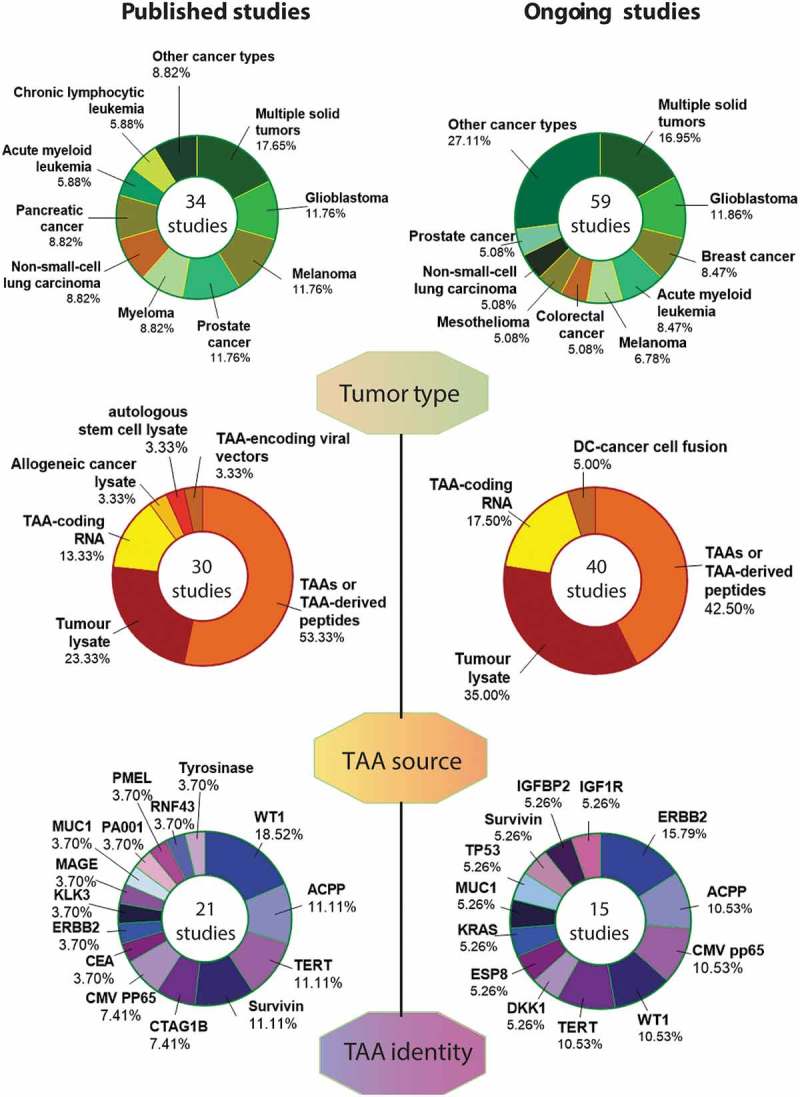
Overview of current strategies of dendritic cell vaccination for cancer therapy. ACPP, acid phosphatase, prostate; AML, acute myeloid leukemia; CEA, carcinoembryonic antigen; CLL, chronic lymphocytic leukemia; CRC, colorectal cancer; DC, dendritic cell; DKK1, dickkopf WNT signaling pathway inhibitor 1; EPS8, epidermal growth factor receptor pathway substrate 8; HCC, hepatocellular carcinoma; KRAS, KRAS proto-oncogene, GTPase; MUC1, mucin 1, cell surface associated; NSCLC, non-small cell lung cancer; NY-ESO-1 (official name: CTAG1B), cancer/testis antigen 1B; CMV pp65, cytomegalovirus 65 kDa phosphoprotein; PSA (official name: KLK3), kallikrein related peptidase 3; RCC, renal cell carcinoma; RNF43, ring finger protein 43; TAA, tumor-associated antigen; TERT, telomerase reverse transcriptase; WT1, WT1 transcription factor.
Most of the aforementioned clinical studies based on specific TAAs focused on WT1 transcription factor (WT1),327,328,337,342,349 acid phosphatase, prostate (ACPP),276,325,329 telomerase reverse transcriptase (TERT)276,333,351 or baculoviral IAP repeating containing 5 (BIRC5, best known as survivin)276,333,340 (Figure 1). This configuration partly deviates from the trend identified in our latest Trial Watch dealing with DC-based vaccines.279 Indeed, while WT1 remains amongst the most commonly targeted TAAs, interest in melanoma-associated differentiation antigens has decreased,337 irrespective of melanoma remaining among the most common indications for experimental DC vaccination (Figure 1).
While most of these clinical studies were basket trials enrolling patients with multiple solid tumors (Figure 1),326,331,336,348 studies focusing on single indications most commonly enrolled patients with melanoma,332,337,339,343 prostate cancer,276,325,329,353 or glioblastoma (GBM).341,346,350,352 This was followed by pancreatic cancer,327,333,358 non-small cell lung carcinoma (NSCLC),338,340,354 and myeloma330,334,345 (Figure 1). This trend was very similar to the one we described in 2017,279 with the single exception of studies enrolling subjects with renal cell carcinoma (RCC), which declined over the past 2 years.
In most clinical studies, DC vaccines were administered in combination with standard-of-care348 or off-label chemotherapeutics (e.g., gemcitabine, cyclophosphamide, S-1, temozolomide, carboplatin, paclitaxel or docetaxel),276,326–328,337,338,341,344,346,350,358 radiotherapy,335,341,346 targeted anticancer agents (e.g., tyrosine kinase inhibitors),328,344 or specific immunotherapeutic regimens, including recombinant colony stimulating factor 2 (CSF2, best known as GM-CSF), recombinant IL2, ACT, and TL3 agonists.326,333,344,352,353
Importantly, the vast majority of these clinical studies confirmed that DC-based vaccines are safe for cancer patients,359 causing mild-to-moderate side effects including fever, erythema, flu-like symptoms, rash and/or fatigue, in a small proportion of patients.328,333,335,340,341,348 Signs of ongoing TAA- or tumor-targeting effector responses upon vaccination, including (but not limited to) increased antigen-specific T- or B-cell activity and tumor infiltration by CD8+ CTLs, were consistently documented.327,332,355 Moreover, multiple clinical studies reported promising clinical responses to vaccination, including disease stabilization326,328,333,342,344,354 as well as a few partial and complete responses.330,335,345,347–349 Encouragingly, a few cases of robust extension in patient survival were also documented.327,351,356,358 For example, DCs pulsed with TAAs from GBM stem cells provided significant progression-free and overall survival advantage (as compared to placebo) to a cohort of 43 patients with GBM enrolled in a Phase II clinical trial.341 Similarly, AML patients at very high risk of relapse receiving DCs transfected with WT1 mRNA exhibited a higher 5-year overall survival rate as compared to historical controls.349 Altogether, these publications capture the promise offered by DC vaccination in specific oncological settings.
Ongoing clinical trials
For the period between February 2017 to February 2019, official sources (http://www.clinicaltrials.gov/) list no less than 59 clinical trials evaluating the safety and therapeutic profile of DC-based vaccines in cancer patients. The specifics of these clinical trials are summarized in Table 1.
Table 1.
Overview of clinical trials currently testing dendritic cell-based immunotherapy in cancer patients.
| Strategy | Indication | Phase | Status | TAA(s) | Combinatorial treatment | Reference |
|---|---|---|---|---|---|---|
| Autologous DCs | Breast cancer | I/II | Recruiting | n.a. | Neoadjuvant chemotherapy | NCT03450044 |
| Colorectal carcinoma | I/II | Recruiting | n.a. | Avelumab | NCT03152565 | |
| Hepatocellular carcinoma | I/II | Recruiting | n.a. | Trans-arterialchemoembolization | NCT03086564 | |
| NSCLC | I | Recruiting | n.a. | Pembrolizumab | NCT03546361 | |
| Prostate cancer | I/II | Recruiting | n.a. | GNRH1 agonist and central memory T cells | NCT03085966 | |
| Solid tumors | I | Not yet recruiting | n.a. | Single agent | NCT03638765 | |
| I | Recruiting | n.a. | Avelumab, ipilimumab and nivolumab | NCT03707808 | ||
| I | Not yet recruiting | n.a. | Anti-PD1 antibody | NCT03815084 | ||
| Autologous DCs loaded with tumor cell lysate | Breast cancer | I | Unknown | Tumor lysate | Single agent | NCT03113019 |
| Colorectal carcinoma | I | Recruiting | Tumor lysate | Single agent | NCT03214939 | |
| Glioblastoma | II | Recruiting | Tumor lysate | Standard therapy | NCT03395587 | |
| I | Not yet recruiting | Tumor lysate | Single agent | NCT03360708 | ||
| Gastric cancer | II | Recruiting | Tumor lysate | Single agent | NCT03410732 | |
| Melanoma | I | Not yet recruiting | Tumor lysate | Anti-PD-1 antibody | NCT03743298 | |
| I/II | Recruiting | Tumor lysate | Pembrolizumab | NCT03325101 | ||
| I | Not yet recruiting | Tumor lysate | Single agent | NCT03803397 | ||
| Mesothelioma | II/III | Recruiting | Tumor lysate. | Best supportive care | NCT03610360 | |
| I | Not yet recruiting | Tumor lysate | Pembrolizumab and IL2 | NCT03546426 | ||
| Ovarian cancer | I/II | Not yet recruiting | Tumor lysate | Autologous NK cell-like CTLs | NCT03735589 | |
| II | Active, not recruiting | n.a. | Standard of care chemotherapy | NCT03657966 | ||
| Renal cell carcinoma | II | Withdrawn | Tumor lysate | Boost radiotherapy and high-dose IL2 | NCT03226236 | |
| Solid tumors | I | Recruiting | Tumor lysate | Single agent | NCT03671720 | |
| Autologous DCs transfected or pulsed with TAA-coding RNA(s) | Acute myeloid leukemia | I/II | Recruiting | WT1 | Single agent | NCT03083054 |
| Glioblastoma | I | Recruiting | Tumor-derived mRNA | TTRNA-xALT, temozolomide, and autologous hematopoietic stem cells | NCT03334305 | |
| I | Recruiting | Tumor-derived mRNA | TTRNA-xALT, temozolomide, lymphodepletive conditioning and autologous hematopoietic stem cells | NCT03396575 | ||
| II/III | Recruiting | Survivin and TERT | Temozolomide | NCT03548571 | ||
| I | Active, not recruiting | CMV pp65 | Tetanus toxoid | NCT03615404 | ||
| II | Not yet recruiting | CMV pp65 | Temozolomide, varlilumab, and tetanus-diphtheria vaccine | NCT03688178 | ||
| NSCLC | I | Recruiting | TERT | Single agent | NCT03371485 | |
| Autologous DCs loaded with recombinant TAAs or TAA-derived peptide(s) | Acute myeloid leukemia | I | Recruiting | WT1 or EPS8 | CAR-expressing T cells | NCT03291444 |
| Breast cancer | I | Recruiting | ERBB2 | Neoadjuvant chemotherapy | NCT03387553 | |
| II | Recruiting | IGFBP2, ERBB2, and IGF1R | DNA-based vaccine | NCT03384914 | ||
| II | Recruiting | ERBB2 | Single agent | NCT03630809 | ||
| Colorectal carcinoma | I | Recruiting | Mutated peptides | Single agent | NCT03730948 | |
| Gastric cancer | I | Not yet recruiting | Tumor peptide pool | Anti-PD-1 antibody | NCT03393416 | |
| Glioblastoma | II | Recruiting | Autologous TAAs | Single agent | NCT03400917 | |
| Hepatocellular carcinoma | I | Recruiting | Personalized neoantigen | Microwave ablation | NCT03674073 | |
| Lung Cancer | II | Recruiting | TP53 | Nivolumab and Ipilimumab | NCT03406715 | |
| Melanoma | I | Recruiting | Melanoma tumor-specific peptides | Cyclophosphamide and pembrolizumab | NCT03092453 | |
| Myeloma | I | Not yet recruiting | DKK1-derived peptide | Single agent | NCT03591614 | |
| Nasopharyngeal cancer | I | Recruiting | EBV proteins | Single agent | NCT03282617 | |
| Pancreatic cancer | I | Recruiting | Mutant KRAS peptides | Single agent | NCT03592888 | |
| Prostate cancer | I/II | Active, not recruiting | MUC1- and WT1-derived peptides | Standard therapy | NCT03114631 | |
| III | Recruiting | ACPP | Single agent | NCT03686683 | ||
| I/II | Recruiting | Pdcd1-/- T Cells | NCT03525652 | |||
| Solid tumors | n.a. | Not yet recruiting | TAAs | Cyclophosphamide | NCT03185429 | |
| II | Recruiting | Immunogenic neoantigens | Single agent | NCT03300843 | ||
| Autologous DC-CIK combinations | Lung cancer | I/II | Recruiting | n.a. | Anti-PD-1 antibody | NCT03360630 |
| Mesothelioma | I/II | Recruiting | n.a. | Anti-PD-1 antibody and hyperthermia | NCT03393858 | |
| Neoplasms | I/II | Recruiting | n.a. | Anti-PD-1 antibody | NCT03190811 | |
| NSCLC | I | Not yet recruiting | Dribbles | Single agent or with imiquimod/GM-CSF | NCT03057340 | |
| Renal cell carcinoma | II | Recruiting | n.a. | Axitinib and anti-PD-1 antibody | NCT03736330 | |
| Solid tumors | I/II | Recruiting | n.a. | Chemotherapy | NCT03047525 | |
| I | Not yet recruiting | n.a. | Anti-PD-1 antibody | NCT03815630 | ||
| Allogenic DCs | Acute myeloid leukemia | II | Recruiting | n.a. | Single agent | NCT03697707 |
| Solid tumors | I/II | Recruiting | n.a. | Pembrolizumab | NCT03735290 | |
| DC-cancer cell fusions | Acute myeloid leukemia | II | Recruiting | n.a. | Single agent | NCT03059485 |
| I | Recruiting | n.a. | Decitabine | NCT03679650 | ||
| Multiple myeloma | II | Not yet recruiting | n.a. | Nivolumab | NCT03782064 |
Abbreviations: ACPP, acid phosphatase, prostate; CAR, chimeric antigen receptor; CIK, cytokine-induced killer; CMV pp65, cytomegalovirus 65 kDa phosphoprotein; CTL, cytotoxic T lymphocyte; DC, dendritic cell; DKK1, dickkopf WNT signaling pathway inhibitor 1; EBV, Epstein-Barr virus; EPS8, epidermal growth factor receptor kinase substrate 8; ERBB2, erb-b2 receptor tyrosine kinase 2; GNRH1, gonadotropin releasing hormone 1; IGF1R, insulin like growth factor 1 receptor; IGFBP2, insulin-like growth factor binding protein 2; IL, interleukin; KRAS, KRAS proto-oncogene, GTPase; MUC1, mucin 1, cell surface associated; n.a., not applicable; NK, natural killer; NSCLC, non-small cell lung cancer; PD-1 (official name PDCD1), programmed cell death 1; TAA, tumor-associated antigen; TERT, telomerase reverse transcriptase; TTRNA-xALT, total tumor RNA-autologous lymphocyte transfer; WT1, WT1 transcription factor.
In these studies, the most common approaches consist of autologous DCs pulsed with TAAs or peptides thereof, lysates from autologous tumor cells, or TAA-coding RNA (Figure 1 and Table 1). The clinical trials targeting specific TAAs focus on WT1, erb-b2 receptor tyrosine kinase 2 (ERBB2), ACPP, and the human cytomegalovirus protein 65 kDa phosphoprotein (pp65) (Figure 1 and Table 1). As an alternative, multiple ongoing clinical trials favor the use of TAA or TAA-derived peptide mixtures, including blends of mutated peptides or neoantigens (pre-defined or personalized), or melanoma-specific TAA-derived peptides (Table 1).
As for indication, most of ongoing clinical studies focusing on DC vaccines are basket trials and enroll patients with multiple solid tumors (Figure 1 and Table 1). Restricted studies most commonly accrue patients with breast carcinoma and GBM, followed by patients with colorectal carcinoma, melanoma, mesothelioma and prostate cancer (Figure 1 and Table 1). Notably, there are at least three advanced-phase clinical trials testing therapeutic DC-based vaccines in cancer patients: a Phase II/III study enrolling GBM patients (NCT03548571), a Phase II/III trial enrolling patients with mesothelioma (NCT03610360), and a Phase III study open to individuals with prostate cancer (NCT03686683) (Table 1). This indicates that at least some DC-based vaccines have reached advanced clinical development, and may soon be evaluated for approval by regulatory authorities.158,278
Interestingly, the majority of the ongoing clinical trials administer DC-based vaccines in combination with ICBs targeting PD-1, such as pembrolizumab or nivolumab, CD274 (best known as PD-L1), such as avelumab, or CTLA4, like ipilimumab, alone or in different combinations (Table 1). Alternatively, DC vaccination is performed in combination with other immunotherapeutic modalities, including adoptively transferred chimeric antigen receptor (CAR)-expressing T cells, CIK cells or autologous NK cells,360 recombinant cytokines,361,362 immunostimulatory antibodies (e.g., varlilumab),363,364 or MAMP-based immunostimulants (e.g. tetanus toxoid or tetanus-diphtheria vaccine).250,365,366 Additional combinatorial partners for DC-based vaccines in clinical development include: (1) standard-of-care neoadjuvant chemotherapy based on ICD inducers like cyclophosphamide or non-ICD inducers like temozolomide and decitabine;291,367 (2) radiotherapy;368 or (3) targeted therapies (e.g. the tyrosine kinase inhibitor axitinib).289,369,370 Finally, some clinical studies are testing innovative strategies that may support immunity, such as hyperthermia.371,372
In summary, as compared to the clinical trials we discussed in the previous Trial Watch dealing with DC-based vaccination,279 the tendency for currently described studies is to combine DC-based vaccines with other forms of immunotherapy (rather than traditional treatments), which largely reflects the progress of immuno-oncology for the past 2 years.270,271
Status update on clinical trials
These following clinical trials have changed status since the publication of our latest Trial Watch on DC-based vaccination for cancer therapy (February 2017):279 NCT02432846, NCT02366728, NCT02405338, NCT02529072, NCT02692976 and NCT02728102 were previously listed as “Recruiting” but are now listed as “Active, not recruiting”; NCT02993315 shifted from “Active” to “Recruiting”; NCT02709993 and NCT02775292 are now “Recruiting”; and NCT02248402 was previously listed as “Completed” but is now “Unknown”. Moreover, NCT02615574, NCT02745756 and NCT02705703 have been “Withdrawn”, the latter following the decision of the study sponsor to discontinue development, whereas NCT02548169 was “Terminated” due to loss of funding and NCT02851056 is “Suspended”, pending interim analyses.
Concluding remarks
The number of published and ongoing clinical trials testing DC vaccination as a therapeutic approach to malignant disorders suggest that there is a persisting interest in identifying indications for which this immunotherapeutic regimen would offer a good alternative to (or improve the efficacy of) ICBs and ACT. Current efforts in this sense appear to focus on GBM, which is known to be particularly susceptible to DC vaccination.158,373,374
In a scenario in which several new immunotherapies are constantly entering clinical development, such as the current one,270,375–383 it is evident that only clinical trials demonstrating a clear survival advantage may drive the approval of DC-based vaccines by regulatory authorities. It is therefore encouraging to note that there are at least three advanced (Phase II/III-III) clinical trials currently testing DC-based vaccines in patients with cancer. The results of these studies are eagerly awaited, especially since data from another highly anticipated Phase III trial testing DC-based vaccines in subjects with GBM were not as promising as expected.384
The clinical development of DC-based vaccines for oncological indications has been negatively impacted by the commercial failure of Sipuleucel-T as well as by disappointing efficacy in multiple (sometimes poorly designed) clinical studies completed so far.385–387 Indeed, although DC-based vaccines have demonstrated at least some degree of clinical activity in some studies,158 their long-term efficacy depends on a number of parameters that are often underestimated, encompassing the immunosuppressive circuitries that are in place in the microenvironment of most solid tumors,130,271,363,388 the evolution of antigen-loss variants,,389 and the overall immunological competence of the patient.156,157 Furthermore, manufacturing DC-based vaccines involves elevated production costs and robust regulatory scrutiny, which constitute additional obstacles to development.157
Finally, the lack of robust predictive biomarkers of response to DC-based vaccines limit enthusiasm, in particular when production costs are considered.342,390–394 At odds with ICBs,267 DC-based vaccines are indeed associated with mild side effects,156,157 implying that current efforts to identify predictive biomarkers are mainly aimed at increasing signal-to-noise ratio and support the design of clinical trials focused on patient subsets that are most likely to benefit from treatment. Our hope is that such efforts will soon be successful and drive the development of safe and effective DC-based vaccines for oncological indications.
Acknowledgements
ADG and PA are supported by Research Foundation Flanders’ (FWO) Excellence of Science (EOS) grant (30837538) for the ‘DECODE’ consortium. ADG is supported by KU Leuven via POR award funds (POR/16/040) and FWO Postdoctoral Fellowship (2016–2019). PA is supported by the FWO fundamental research grants (G0584.12N, K202313N, GA01111N) and KU Leuven (C16/15/073). AC is supported by the Kom Op Tegen Kanker (Stand up to Cancer), the Flemish cancer society (2016/10728/2603), the Olivia Fund (2017/LUF/00135) and the KU Leuven (C24/18/064). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085 and GDW20181100051), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). LG is supported by a Breakthrough Level 2 grant from the US DoD, Breast Cancer Research Program (BCRP) [#BC180476P1], by a startup grant from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, US), and by donations from Phosplatin (New York, US), the Luke Heller TECPR2 Foundation (Boston, US) and Sotio a.s. (Prague, Czech Republic).
References
- 1.Inglesfield S, Cosway EJ, Jenkinson WE, Anderson G.. Rethinking thymic tolerance: lessons from mice. Trends Immunol. 2019;40:279–291. doi: 10.1016/j.it.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Schuijs MJ, Hammad H, Lambrecht BN.. Professional and ‘amateur’ antigen-presenting cells in type 2 immunity. Trends Immunol. 2019;40:22–34. doi: 10.1016/j.it.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson JS, Abolhalaj M, Lundberg K, Lindstedt M, Greiff L. Dendritic cell subpopulations in nasopharyngeal cancer. Oncol Lett. 2019;17:2557–2561. doi: 10.3892/ol.2018.9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 6.Grees M, Sharbi-Yunger A, Evangelou C, Baumann D, Cafri G, Tzehoval E, Hoadley KA, Print C, Knowlton N, Black MA, et al. Optimized dendritic cell vaccination induces potent CD8 T cell responses and anti-tumor effects in transgenic mouse melanoma models. Oncoimmunology. 2018;7:e1445457. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berraondo P, Labiano S, Minute L, Etxeberria I, Vasquez M, Sanchez-Arraez A, Teijeira A, Melero I. Cellular immunotherapies for cancer. Oncoimmunology. 2017;6:e1306619. doi: 10.1080/2162402X.2017.1306619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, Teijeira A, Kandalaft LE, Romero P, Coukos G, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7:109. doi: 10.1186/s40425-019-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019. doi: 10.1038/s41573-019-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weller M, Roth P, Preusser M, Wick W, Reardon DA, Platten M, Sampson JH. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13:363–374. doi: 10.1038/nrneurol.2017.64. [DOI] [PubMed] [Google Scholar]
- 11.Nagaoka K, Hosoi A, Iino T, Morishita Y, Matsushita H, Kakimi K. Dendritic cell vaccine induces antigen-specific CD8(+) T cells that are metabolically distinct from those of peptide vaccine and is well-combined with PD-1 checkpoint blockade. Oncoimmunology. 2018;7:e1395124. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang S, Agostinis P, Salven P, Garg AD. Decoding cancer cell death-driven immune cell recruitment: an in vivo method for site-of-vaccination analyses. Methods Enzymol. 2019. doi: 10.1016/bs.mie.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Chrisikos TT, Zhou Y, Slone N, Babcock R, Watowich SS, Li HS. Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer. Mol Immunol. 2019;110:24–39. doi: 10.1016/j.molimm.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avigan D, Rosenblatt J. Vaccine therapy in hematologic malignancies. Blood. 2018;131:2640–2650. doi: 10.1182/blood-2017-11-785873. [DOI] [PubMed] [Google Scholar]
- 15.Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 16.Qian C, Cao X. Dendritic cells in the regulation of immunity and inflammation. Semin Immunol. 2018;35:3–11. doi: 10.1016/j.smim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Park HJ, Park JH, Hong EJ, Jang GY, Jung ID, Hoadley KA, Print C, Knowlton N, Black MA, et al. A novel function of API5 (apoptosis inhibitor 5), TLR4-dependent activation of antigen presenting cells. Oncoimmunology. 2018;7:e1472187. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Tak WY, Lee Y, Heo MK, Song JS, Kim HY, Park SY, Bae SH, Lee JH, Heo J, et al. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6:e1328335. doi: 10.1080/2162402X.2017.1328335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanaware PP, Jurewicz MM, Leszyk JD, Shaffer SA, Stern LJ. HLA-DO modulates the diversity of the MHC-II self-peptidome. Mol Cell Proteom: MCP. 2019;18:490–503. doi: 10.1074/mcp.RA118.000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axelrod ML, Cook RS, Johnson DB, Balko JM. Biological consequences of MHC-II expression by tumor cells in cancer. Clin Cancer Res. 2019;25:2392–2402. doi: 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly A, Trowsdale J. Genetics of antigen processing and presentation. Immunogenetics. 2019;71:161–170. doi: 10.1007/s00251-018-1082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerriero JL. Macrophages: their untold story in T cell activation and function. Int Rev Cell Mol Biol. 2019;342:73–93. doi: 10.1016/bs.ircmb.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Saez JJ, Lennon-Dumenil AM, Yuseff MI. Studying MHC class II presentation of immobilized antigen by B lymphocytes. Methods Mol Biol. 2019;1988:419–437. doi: 10.1007/978-1-4939-9450-2_29. [DOI] [PubMed] [Google Scholar]
- 24.Allen F, Bobanga ID, Rauhe P, Barkauskas D, Teich N, Tong C, Hoadley KA, Print C, Knowlton N, Black MA, et al. CCL3 augments tumor rejection and enhances CD8(+) T cell infiltration through NK and CD103(+) dendritic cell recruitment via IFNgamma. Oncoimmunology. 2018;7:e1393598. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 26.Ebrahimi-Nik H, Corwin WL, Shcheglova T, Das Mohapatra A, Mandoiu II, Srivastava PK. CD11c(+) MHCII(lo) GM-CSF-bone marrow-derived dendritic cells act as antigen donor cells and as antigen presenting cells in neoepitope-elicited tumor immunity against a mouse fibrosarcoma. Cancer Immunol Immun: CII. 2018;67:1449–1459. doi: 10.1007/s00262-018-2202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 28.Hsu JL, Bryant CE, Papadimitrious MS, Kong B, Gasiorowski RE, Orellana D, Hoadley KA, Print C, Knowlton N, Black MA, et al. A blood dendritic cell vaccine for acute myeloid leukemia expands anti-tumor T cell responses at remission. Oncoimmunology. 2018;7:e1419114. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharbi-Yunger A, Grees M, Cafri G, Bassan D, Eichmuller SB, Tzehoval E, Utikal J, Umansky V, Eisenbach L. A universal anti-cancer vaccine: chimeric invariant chain potentiates the inhibition of melanoma progression and the improvement of survival. Int J Cancer. 2019;144:909–921. doi: 10.1002/ijc.31795. [DOI] [PubMed] [Google Scholar]
- 30.Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 31.Kroemer M, Spehner L, Mercier-Letondal P, Boullerot L, Kim S, Jary M, Hoadley KA, Print C, Knowlton N, Black MA, et al. SALL4 oncogene is an immunogenic antigen presented in various HLA-DR contexts. Oncoimmunology. 2018;7:e1412030. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanzavecchia A, Sallusto F. Ralph M. Steinman 1943-2011. Cell. 2011;147:1216–1217. [DOI] [PubMed] [Google Scholar]
- 33.Nussenzweig MC, Mellman I. Ralph Steinman (1943-2011). Nature. 2011;478:460. doi: 10.1038/478460a. [DOI] [PubMed] [Google Scholar]
- 34.Mellman I, Nussenzweig M. Retrospective. Ralph M. Steinman (1943–2011). Science. 2011;334:466. doi: 10.1126/science.1215136. [DOI] [PubMed] [Google Scholar]
- 35.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 37.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 38.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudek AM, Martin S, Garg AD, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: toward a DC-cancer cells interface that augments anticancer immunity. Front Immunol. 2013;4:438. doi: 10.3389/fimmu.2013.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Hao X, Zhang Y, Zhang J, Carey CD, Falo LD Jr, Storkus WJ, You Z Intratumoral delivery of tumor antigen-loaded DC and tumor-primed CD4(+) T cells combined with agonist alpha-GITR mAb promotes durable CD8(+) T-cell-dependent antitumor immunity. Oncoimmunology. 2017;6:e1315487. doi: 10.1080/2162402X.2017.1315487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominguez-Andres J, Netea MG. Long-term reprogramming of the innate immune system. J Leukoc Biol. 2019;105:329–338. doi: 10.1002/JLB.MR0318-104R. [DOI] [PubMed] [Google Scholar]
- 42.Montico B, Lapenta C, Ravo M, Martorelli D, Muraro E, Zeng B, Comaro E, Spada M, Donati S, Santini SM, et al. Exploiting a new strategy to induce immunogenic cell death to improve dendritic cell-based vaccines for lymphoma immunotherapy. Oncoimmunology. 2017;6:e1356964. doi: 10.1080/2162402X.2017.1356964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Acker HH, Versteven M, Lichtenegger FS, Roex G, Campillo-Davo D, Lion E, Subklewe M, Van Tendeloo VF, Berneman ZN, Anguille S. Dendritic cell-based immunotherapy of acute myeloid leukemia. J Clin Med. 2019;8:579. doi: 10.3390/jcm8050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santegoets S, de Groot AF, Dijkgraaf EM, Simoes AMC, van der Noord VE, van Ham JJ, Hoadley KA, Print C, Knowlton N, Black MA, et al. The blood mMDSC to DC ratio is a sensitive and easy to assess independent predictive factor for epithelial ovarian cancer survival. Oncoimmunology. 2018;7:e1465166. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H, Tian Y, Wang Y, Mineishi S, Zhang Y. Dendritic cell regulation of graft-vs.-host disease: immunostimulation and tolerance. Front Immunol. 2019;10:93. doi: 10.3389/fimmu.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moussion C, Mellman I. The dendritic cell strikes back. Immunity. 2018;49:997–999. doi: 10.1016/j.immuni.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. 2018;322:63–73. doi: 10.1016/j.jneuroim.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2013;2:e25771. doi: 10.4161/onci.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines-recent breakthroughs and encouraging clinical results. Front Immunol. 2019;10:766. doi: 10.3389/fimmu.2019.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payandeh Z, Yarahmadi M, Nariman-Saleh-Fam Z, Tarhriz V, Islami M, Aghdam AM, Eyvazi S. Immune therapy of melanoma: overview of therapeutic vaccines. J Cell Physiol. 2019;234:14612–14621. doi: 10.1002/jcp.v234.9. [DOI] [PubMed] [Google Scholar]
- 51.Bryant CE, Sutherland S, Kong B, Papadimitrious MS, Fromm PD, Hart DNJ. Dendritic cells as cancer therapeutics. Semin Cell Dev Biol. 2019;86:77–88. doi: 10.1016/j.semcdb.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Penafuerte C, Feldhammer M, Mills JR, Vinette V, Pike KA, Hall A, Migon E, Karsenty G, Pelletier J, Zogopoulos G, et al. Downregulation of PTP1B and TC-PTP phosphatases potentiate dendritic cell-based immunotherapy through IL-12/IFNgamma signaling. Oncoimmunology. 2017;6:e1321185. doi: 10.1080/2162402X.2017.1321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikyskova R, Indrova M, Stepanek I, Kanchev I, Bieblova J, Vosahlikova S, Moserova I, Truxova I, Fucikova J, Bartunkova J, et al. Dendritic cells pulsed with tumor cells killed by high hydrostatic pressure inhibit prostate tumor growth in TRAMP mice. Oncoimmunology. 2017;6:e1362528. doi: 10.1080/2162402X.2017.1362528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swain S, Roe MM, Sebrell TA, Sidar B, Dankoff J, VanAusdol R, Smythies LE, Smith PD, Bimczok D. CD103 (alphaE Integrin) undergoes endosomal trafficking in human dendritic cells, but does not mediate epithelial adhesion. Front Immunol. 2018;9:2989. doi: 10.3389/fimmu.2018.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huo CW, Hill P, Chew G, Neeson PJ, Halse H, Williams ED, Henderson MA, Thompson EW, Britt KL. High mammographic density in women is associated with protumor inflammation. Breast Cancer Res: BCR. 2018;20:92. doi: 10.1186/s13058-018-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bashaw AA, Leggatt GR, Chandra J, Tuong ZK, Frazer IH. Modulation of antigen presenting cell functions during chronic HPV infection. Papillomavirus Res (Amsterdam, Netherlands). 2017;4:58–65. doi: 10.1016/j.pvr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren J, Gwin WR, Zhou X, Wang X, Huang H, Jiang N, Zhou L, Agarwal P, Hobeika A, Crosby E, et al. Adaptive T cell responses induced by oncolytic Herpes Simplex Virus-granulocyte macrophage-colony-stimulating factor therapy expanded by dendritic cell and cytokine-induced killer cell adoptive therapy. Oncoimmunology. 2017;6:e1264563. doi: 10.1080/2162402X.2016.1264563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262–275. doi: 10.1038/nri.2017.9. [DOI] [PubMed] [Google Scholar]
- 59.Ossevoort MA, Feltkamp MC, van Veen KJ, Melief CJ, Kast WM. Dendritic cells as carriers for a cytotoxic T-lymphocyte epitope-based peptide vaccine in protection against a human papillomavirus type 16-induced tumor. J Immunother Emphasis Tumor Immunol. 1995;18:86–94. [DOI] [PubMed] [Google Scholar]
- 60.Wu CJ, Tsai YT, Lee IJ, Wu PY, Lu LS, Tsao WS, Hoadley KA, Print C, Knowlton N, Black MA, et al. Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology. 2018;7:e1477459. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raaijmakers TK, Ansems M. Microenvironmental derived factors modulating dendritic cell function and vaccine efficacy: the effect of prostanoid receptor and nuclear receptor ligands. Cancer Immunol Immun: CII. 2018;67:1789–1796. doi: 10.1007/s00262-018-2205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie K, Xu L, Wu H, Liao H, Luo L, Liao M, Hoadley KA, Print C, Knowlton N, Black MA, et al. OX40 expression in hepatocellular carcinoma is associated with a distinct immune microenvironment, specific mutation signature, and poor prognosis. Oncoimmunology. 2018;7:e1404214. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chyuan IT, Tsai HF, Wu CS, Hsu PN. TRAIL suppresses gut inflammation and inhibits colitogeic T-cell activation in experimental colitis via an apoptosis-independent pathway. Mucosal Immunol. 2019;12:980–989. doi: 10.1038/s41385-019-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129:1441–1451. doi: 10.1172/JCI124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Branchett WJ, Lloyd CM. Regulatory cytokine function in the respiratory tract. Mucosal Immunol. 2019;12:589–600. doi: 10.1038/s41385-019-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones AT, Shen X, Walter KL, LaBranche CC, Wyatt LS, Tomaras GD, Montefiori DC, Moss B, Barouch DH, Clements JD, et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat Commun. 2019;10:798. doi: 10.1038/s41467-019-08739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhodes JW, Tong O, Harman AN, Turville SG. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front Immunol. 2019;10:1088. doi: 10.3389/fimmu.2019.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bassler K, Schulte-Schrepping J, Warnat-Herresthal S, Aschenbrenner AC, Schultze JL. The myeloid cell compartment-cell by cell. Annu Rev Immunol. 2019;37:269–293. doi: 10.1146/annurev-immunol-042718-041728. [DOI] [PubMed] [Google Scholar]
- 69.Strobl H, Krump C, Borek I. Micro-environmental signals directing human epidermal Langerhans cell differentiation. Semin Cell Dev Biol. 2019;86:36–43. doi: 10.1016/j.semcdb.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 70.Ohmatsu H, Humme D, Gonzalez J, Gulati N, Mobs M, Sterry W, Krueger JG. IL-32 induces indoleamine 2,3-dioxygenase(+)CD1c(+) dendritic cells and indoleamine 2,3-dioxygenase(+)CD163(+) macrophages: relevance to mycosis fungoides progression. Oncoimmunology. 2017;6:e1181237. doi: 10.1080/2162402X.2016.1181237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pellegatta S, Eoli M, Cuccarini V, Anghileri E, Pollo B, Pessina S, Hoadley KA, Print C, Knowlton N, Black MA, et al. Survival gain in glioblastoma patients treated with dendritic cell immunotherapy is associated with increased NK but not CD8(+) T cell activation in the presence of adjuvant temozolomide. Oncoimmunology. 2018;7:e1412901. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, Belicova L, Praznovec I, Halaska MJ, Brtnicky T, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer. 2018;6:139. doi: 10.1186/s40425-018-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiss M, Van Gassen S, Movahedi K, Saeys Y, Laoui D. Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol. 2018;330:188–201. doi: 10.1016/j.cellimm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Huber A, Dammeijer F, Aerts J, Vroman H. Current state of dendritic cell-based immunotherapy: opportunities for in vitro antigen loading of different DC subsets? Front Immunol. 2018;9:2804. doi: 10.3389/fimmu.2018.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pyfferoen L, Brabants E, Everaert C, De Cabooter N, Heyns K, Deswarte K, Vanheerswynghels M, De Prijck S, Waegemans G, Dullaers M, et al. The transcriptome of lung tumor-infiltrating dendritic cells reveals a tumor-supporting phenotype and a microRNA signature with negative impact on clinical outcome. Oncoimmunology. 2017;6:e1253655. doi: 10.1080/2162402X.2016.1253655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segura E. Review of mouse and human dendritic cell subsets. Methods Mol Biol. 2016;1423:3–15. doi: 10.1007/978-1-4939-3606-9_1. [DOI] [PubMed] [Google Scholar]
- 77.Thordardottir S, Schaap N, Louer E, Kester MG, Falkenburg JH, Jansen J, Radstake TRD, Hobo W, Dolstra H. Hematopoietic stem cell-derived myeloid and plasmacytoid DC-based vaccines are highly potent inducers of tumor-reactive T cell and NK cell responses ex vivo. Oncoimmunology. 2017;6:e1285991. doi: 10.1080/2162402X.2017.1285991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanpouille-Box C, Galluzzi L. CD103(+) cells at the forefront of anticancer immunity. Oncoimmunology. 2017;6:e1356154. doi: 10.1080/2162402X.2017.1356154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murgaski A, Bardet PMR, Arnouk SM, Clappaert EJ, Laoui D. Unleashing tumour-dendritic cells to fight cancer by tackling their three A’s: abundance, activation and antigen-delivery. Cancers. 2019;11:670. doi: 10.3390/cancers11050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019. doi: 10.1002/JLB.4RI0818-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark GJ, Silveira PA, Hogarth PM, Hart DNJ. The cell surface phenotype of human dendritic cells. Semin Cell Dev Biol. 2019;86:3–14. doi: 10.1016/j.semcdb.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Ovcinnikovs V, Ross EM, Petersone L, Edner NM, Heuts F, Ntavli E, Kogimtzis A, Kennedy A, Wang CJ, Bennett CL, et al. CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells. Sci Immunol. 2019;4. doi: 10.1126/sciimmunol.aaw0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MacNabb BW, Kline DE, Albright AR, Chen X, Leventhal DS, Savage PA, Kline J. Negligible role for deletion mediated by cDC1 in CD8(+) T cell tolerance. J Immunol. 2019;202:2628–2635. doi: 10.4049/jimmunol.1801621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci Immunol. 2019;4. doi: 10.1126/sciimmunol.aau8380. [DOI] [PubMed] [Google Scholar]
- 85.See P, Dutertre CA, Chen J, Gunther P, McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356. doi: 10.1126/science.aag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ali S, Mann-Nuttel R, Schulze A, Richter L, Alferink J, Scheu S. Sources of type I interferons in infectious immunity: plasmacytoid dendritic cells not always in the driver’s seat. Front Immunol. 2019;10:778. doi: 10.3389/fimmu.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vangeti S, Gertow J, Yu M, Liu S, Baharom F, Scholz S, Friberg D, Starkhammar M, Ahlberg A, Smed-Sörensen A. Human blood and tonsil plasmacytoid dendritic cells display similar gene expression profiles but exhibit differential type I IFN responses to influenza A virus infection. J Immunol. 2019;202:2069–2081. doi: 10.4049/jimmunol.1801191. [DOI] [PubMed] [Google Scholar]
- 89.Loughland JR, Woodberry T, Boyle MJ, Tipping PE, Piera KA, Amante FH, Kenangalem E, Price RN, Engwerda CR, Anstey NM, et al. Plasmodium falciparum activates CD16+ dendritic cells to produce tumor necrosis factor and interleukin-10 in subpatent malaria. J Infect Dis. 2019;219:660–671. doi: 10.1093/infdis/jiy555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouteau A, Kervevan J, Su Q, Zurawski SM, Contreras V, Dereuddre-Bosquet N, Le Grand R, Zurawski G, Cardinaud S, Levy Y, et al. DC subsets regulate humoral immune responses by supporting the differentiation of distinct Tfh cells. Front Immunol. 2019;10:1134. doi: 10.3389/fimmu.2019.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, Wang W, Chai Q, Zhu M. Langerhans cells control lymphatic vessel function during inflammation via LIGHT-LTbetaR signaling. J Immunol. 2019;202:2999–3007. doi: 10.4049/jimmunol.1801578. [DOI] [PubMed] [Google Scholar]
- 92.Sumpter TL, Balmert SC, Kaplan DH. Cutaneous immune responses mediated by dendritic cells and mast cells. JCI Insight. 2019;4. doi: 10.1172/jci.insight.122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michea P, Noel F, Zakine E, Czerwinska U, Sirven P, Abouzid O, Goudot C, Scholer-Dahirel A, Vincent-Salomon A, Reyal F, et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol. 2018;19:885–897. doi: 10.1038/s41590-018-0145-8. [DOI] [PubMed] [Google Scholar]
- 95.Macri C, Pang ES, Patton T, O’Keeffe M. Dendritic cell subsets. Semin Cell Dev Biol. 2018;84:11–21. doi: 10.1016/j.semcdb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Durai V, Murphy KM. Functions of murine dendritic cells. Immunity. 2016;45:719–736. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 98.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–65 e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh T-P, Baranek T, Storset AK, Marvel J, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen C-J-J, Dunbar PR, Wadley RB, Jeet V, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Blasio S, Wortel IM, van Bladel DA, de Vries LE, Duiveman-de Boer T, Worah K, de Haas N, Buschow SI, de Vries IJM, Figdor CG, et al. Human CD1c(+) DCs are critical cellular mediators of immune responses induced by immunogenic cell death. Oncoimmunology. 2016;5:e1192739. doi: 10.1080/2162402X.2016.1192739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghinnagow R, De Meester J, Cruz LJ, Aspord C, Corgnac S, Macho-Fernandez E, Soulard D, Fontaine J, Chaperot L, Charles J, et al. Co-delivery of the NKT agonist alpha-galactosylceramide and tumor antigens to cross-priming dendritic cells breaks tolerance to self-antigens and promotes antitumor responses. Oncoimmunology. 2017;6:e1339855. doi: 10.1080/2162402X.2017.1339855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 106.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, et al Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 108.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 109.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 110.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O’Garra A, Liu Y-J. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Medler T, Patel JM, Alice A, Baird JR, Hu HM, Gough MJ. Activating the nucleic acid-sensing machinery for anticancer immunity. Int Rev Cell Mol Biol. 2019;344:173–214. doi: 10.1016/bs.ircmb.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sprooten J, Agostinis P, Garg AD. Type I interferons and dendritic cells in cancer immunotherapy. Int Rev Cell Mol Biol. 2019. doi: 10.1016/bs.ircmb.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19:731–745. doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 114.Collignon A, Silvy F, Robert S, Trad M, Germain S, Nigri J, Hoadley KA, Print C, Knowlton N, Black MA, et al. Dendritic cell-based vaccination: powerful resources of immature dendritic cells against pancreatic adenocarcinoma. Oncoimmunology. 2018;7:e1504727. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell Death Differ. 2016;23:938–951. doi: 10.1038/cdd.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJM, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 120.Aznar MA, Labiano S, Diaz-Lagares A, Molina C, Garasa S, Azpilikueta A, Etxeberria I, Sanchez-Paulete AR, Korman AJ, Esteller M, et al. CD137 (4-1BB) costimulation modifies DNA methylation in CD8(+) T cell-relevant genes. Cancer Immunol Res. 2018;6:69–78. doi: 10.1158/2326-6066.CIR-17-0159. [DOI] [PubMed] [Google Scholar]
- 121.Zafar S, Parviainen S, Siurala M, Hemminki O, Havunen R, Tahtinen S, Bramante S, Vassilev L, Wang H, Lieber A, et al. Intravenously usable fully serotype 3 oncolytic adenovirus coding for CD40L as an enabler of dendritic cell therapy. Oncoimmunology. 2017;6:e1265717. doi: 10.1080/2162402X.2016.1265717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: what, when, and how? Biofactors. 2013;39:355–367. doi: 10.1002/biof.1125. [DOI] [PubMed] [Google Scholar]
- 123.Gonzalez FE, Chernobrovkin A, Pereda C, Garcia-Salum T, Tittarelli A, Lopez MN, Salazar-Onfray F, Zubarev RA. proteomic identification of heat shock-induced danger signals in a melanoma cell lysate used in dendritic cell-based cancer immunotherapy. J Immunol Res. 2018;2018:3982942. doi: 10.1155/2018/3982942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Venkateswaran K, Verma A, Bhatt AN, Shrivastava A, Manda K, Raj HG, Prasad A, Len C, Parmar VS, Dwarakanath BS. Emerging roles of calreticulin in cancer: implications for therapy. Curr Protein Pept Sci. 2018;19:344–357. doi: 10.2174/1389203718666170111123253. [DOI] [PubMed] [Google Scholar]
- 125.Moserova I, Truxova I, Garg AD, Tomala J, Agostinis P, Cartron PF, Vosahlikova S, Kovar M, Spisek R, Fucikova J. Caspase-2 and oxidative stress underlie the immunogenic potential of high hydrostatic pressure-induced cancer cell death. Oncoimmunology. 2017;6:e1258505. doi: 10.1080/2162402X.2016.1258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patidar A, Selvaraj S, Sarode A, Chauhan P, Chattopadhyay D, Saha B. DAMP-TLR-cytokine axis dictates the fate of tumor. Cytokine. 2018;104:114–123. doi: 10.1016/j.cyto.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 127.Carrington EM, Tarlinton DM, Gray DH, Huntington ND, Zhan Y, Lew AM. The life and death of immune cell types: the role of BCL-2 anti-apoptotic molecules. Immunol Cell Biol. 2017;95:870–877. doi: 10.1038/icb.2017.72. [DOI] [PubMed] [Google Scholar]
- 128.Riganti C, Lingua MF, Salaroglio IC, Falcomata C, Righi L, Morena D, Hoadley KA, Print C, Knowlton N, Black MA, et al. Bromodomain inhibition exerts its therapeutic potential in malignant pleural mesothelioma by promoting immunogenic cell death and changing the tumor immune-environment. Oncoimmunology. 2018;7:e1398874. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R, Cirone M, et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Umansky V, Adema GJ, Baran J, Brandau S, Van Ginderachter JA, Hu X, Jablonska J, Mojsilovic S, Papadaki HA, Pico de Coaña Y, et al. Interactions among myeloid regulatory cells in cancer. Cancer Immunol Immun: CII. 2019;68:645–660. doi: 10.1007/s00262-018-2200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zom GG, Willems M, Khan S, van der Sluis TC, Kleinovink JW, Camps MGM, van der Marel GA, Filippov DV, Melief CJM, Ossendorp F. Novel TLR2-binding adjuvant induces enhanced T cell responses and tumor eradication. J Immunother Cancer. 2018;6:146. doi: 10.1186/s40425-018-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. DAMP-induced allograft and tumor rejection: the circle is closing. Am J Transpant. 2016;16:3322–3337. doi: 10.1111/ajt.14012. [DOI] [PubMed] [Google Scholar]
- 133.Santillo BT, Reis DDS, Da Silva LT, Romani NT, Duarte A, Oshiro TM. Phenotypic and functional profile of IFN-alpha-differentiated dendritic cells (IFN-DCs) from HIV-infected individuals. Hum Vaccin Immunother. 2018;1–10. doi: 10.1080/21645515.2018.1547603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krzastek SC, Goliadze E, Zhou S, Petrossian A, Youniss F, Sundaresan G, Wang L, Zweit J, Guruli G. Dendritic cell trafficking in tumor-bearing mice. Cancer Immunol Immun: CII. 2018;67:1939–1947. doi: 10.1007/s00262-018-2187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, Dong W, Nalin AP, Wang Y, Pan P, Xu B, Hoadley KA, Print C, Knowlton N, Black MA, et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology. 2018;7:e1431085. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ho NI, Huis in ‘t Veld LGM, Raaijmakers TK, Adema GJ. Adjuvants enhancing cross-presentation by dendritic cells: the key to more effective vaccines? Front Immunol. 2018;9:2874. doi: 10.3389/fimmu.2018.02874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zafar S, Sorsa S, Siurala M, Hemminki O, Havunen R, Cervera-Carrascon V, Hoadley KA, Print C, Knowlton N, Black MA, et al. CD40L coding oncolytic adenovirus allows long-term survival of humanized mice receiving dendritic cell therapy. Oncoimmunology. 2018;7:e1490856. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, Atianzar K, Kuo BY, Gardner B, Batra RK, et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res. 2004;10:2891–2901. [DOI] [PubMed] [Google Scholar]
- 139.Valentine FT, Golomb FM, Harris M, Roses DF. A novel immunization strategy using cytokine/chemokines induces new effective systemic immune responses, and frequent complete regressions of human metastatic melanoma. Oncoimmunology. 2018;7:e1386827. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mitchell JP, Carmody RJ. NF-kappaB and the transcriptional control of inflammation. Int Rev Cell Mol Biol. 2018;335:41–84. doi: 10.1016/bs.ircmb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 141.Garg AD, Vandenberk L, Fang S, Fasche T, Van Eygen S, Maes J, Van Woensel M, Koks C, Vanthillo N, Graf N, et al. Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. Cell Death Differ. 2017;24:832–843. doi: 10.1038/cdd.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, Luster AD. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity. 2019;50:1498–1512.e5. doi: 10.1016/j.immuni.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, Johnson BD, Dwinell MB. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7:115. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gianello V, Salvi V, Parola C, Moretto N, Facchinetti F, Civelli M, Villetti G, Bosisio D, Sozzani S. The PDE4 inhibitor CHF6001 modulates pro-inflammatory cytokines, chemokines and Th1- and Th17-polarizing cytokines in human dendritic cells. Biochem Pharmacol. 2019;163:371–380. doi: 10.1016/j.bcp.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 145.Lu F, Mosley YC, Carmichael B, Brown DD, HogenEsch H. Formulation of aluminum hydroxide adjuvant with TLR agonists poly(I:C) and CpG enhances the magnitude and avidity of the humoral immune response. Vaccine. 2019;37:1945–1953. doi: 10.1016/j.vaccine.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 146.Oreskovic Z, Nechvatalova K, Krejci J, Kummer V, Faldyna M. Aspects of intradermal immunization with different adjuvants: the role of dendritic cells and Th1/Th2 response. PLoS One. 2019;14:e0211896. doi: 10.1371/journal.pone.0211896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baert T, Garg AD, Vindevogel E, Van Hoylandt A, Verbist G, Agostinis P, Vergote I, Coosemans AN. In vitro generation of murine dendritic cells for cancer immunotherapy: an optimized protocol. Anticancer Res. 2016;36:5793–5801. doi: 10.21873/anticanres.11163. [DOI] [PubMed] [Google Scholar]
- 148.Luty J, Ruckemann-Dziurdzinska K, Witkowski JM, Bryl E. Immunological aspects of autoimmune thyroid disease - complex interplay between cells and cytokines. Cytokine. 2019;116:128–133. doi: 10.1016/j.cyto.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 149.Vieyra-Garcia P, Crouch JD, O’Malley JT, Seger EW, Yang CH, Teague JE, Davis JL, Dong B, Sun C, Page P, et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight. 2019;4. doi: 10.1172/jci.insight.122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 151.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee H-W, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 154.Rao S, Gharib K, Han A. Cancer immunosurveillance by T cells. Int Rev Cell Mol Biol. 2019;342:149–173. doi: 10.1016/bs.ircmb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 155.Chalmin F, Humblin E, Ghiringhelli F, Vegran F. Transcriptional programs underlying Cd4 T cell differentiation and functions. Int Rev Cell Mol Biol. 2018;341:1–61. doi: 10.1016/bs.ircmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 156.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic cell-based immunotherapy: state of the art and beyond. Clin Cancer Res. 2016;22:1897–1906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 158.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–67. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 159.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vanpouille-Box C, Demaria S, Formenti SC, Galluzzi L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell. 2018;34:361–378. doi: 10.1016/j.ccell.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 161.Romero P, Banchereau J, Bhardwaj N, Cockett M, Disis ML, Dranoff G, Helman LJ, Kastan MB, Knapp DW, Levin WJ, et al. The human vaccines project: a roadmap for cancer vaccine development. Sci Transl Med. 2016;8:334ps9. doi: 10.1126/scitranslmed.aaf0746. [DOI] [PubMed] [Google Scholar]
- 162.Truxova I, Hensler M, Skapa P, Halaska MJ, Laco J, Ryska A, Spisek R, Fucikova J. Rationale for the combination of dendritic cell-based vaccination approaches with chemotherapy agents. Int Rev Cell Mol Biol. 2017;330:115–156. doi: 10.1016/bs.ircmb.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 163.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 165.Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, Watkins SC, Todo S, Herberman RB, Lotze MT, et al. Local administration of IL-12-transfected dendritic cells induces antitumor immune responses to colon adenocarcinoma in the liver in mice. J Exp Ther Oncol. 2002;2:337–349. [DOI] [PubMed] [Google Scholar]
- 166.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59:4035–4041. [PubMed] [Google Scholar]
- 167.Hu J, Yuan X, Belladonna ML, Ong JM, Wachsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 168.Endo H, Saito T, Kenjo A, Hoshino M, Terashima M, Sato T, Anazawa T, Kimura T, Tsuchiya T, Irisawa A, et al. Phase I trial of preoperative intratumoral injection of immature dendritic cells and OK-432 for resectable pancreatic cancer patients. J Hepatobiliary Pancreat Sci. 2012;19:465–475. doi: 10.1007/s00534-011-0457-7. [DOI] [PubMed] [Google Scholar]
- 169.Galluzzi L, Vanpouille-Box C, Bakhoum SF, Demaria S. SnapShot: CGAS-STING signaling. Cell. 2018;173:276- e1. doi: 10.1016/j.cell.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 170.Nair SK, Snyder D, Rouse BT, Gilboa E. Regression of tumors in mice vaccinated with professional antigen-presenting cells pulsed with tumor extracts. Int J Cancer. 1997;70:706–715. [DOI] [PubMed] [Google Scholar]
- 171.DeMatos P, Abdel-Wahab Z, Vervaert C, Hester D, Seigler H. Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice. J Surg Oncol. 1998;68:79–91. [DOI] [PubMed] [Google Scholar]
- 172.DeMatos P, Abdel-Wahab Z, Vervaert C, Seigler HF. Vaccination with dendritic cells inhibits the growth of hepatic metastases in B6 mice. Cell Immunol. 1998;185:65–74. doi: 10.1006/cimm.1998.1277. [DOI] [PubMed] [Google Scholar]
- 173.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen Z, Moyana T, Saxena A, Warrington R, Jia Z, Xiang J. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int J Cancer. 2001;93:539–548. [DOI] [PubMed] [Google Scholar]
- 175.Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM. Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells. Cancer Res. 2001;61:2386–2389. [PubMed] [Google Scholar]
- 176.Kokhaei P, Choudhury A, Mahdian R, Lundin J, Moshfegh A, Osterborg A, Mellstedt H. Apoptotic tumor cells are superior to tumor cell lysate, and tumor cell RNA in induction of autologous T cell response in B-CLL. Leukemia. 2004;18:1810–1815. doi: 10.1038/sj.leu.2403517. [DOI] [PubMed] [Google Scholar]
- 177.Kokhaei P, Rezvany MR, Virving L, Choudhury A, Rabbani H, Osterborg A, Mellstedt H. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in B-CLL. Leukemia. 2003;17:894–899. doi: 10.1038/sj.leu.2402913. [DOI] [PubMed] [Google Scholar]
- 178.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 179.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. [DOI] [PubMed] [Google Scholar]
- 181.Mayordomo JI, Loftus DJ, Sakamoto H, De Cesare CM, Appasamy PM, Lotze MT, Storkus WJ, Appella E, DeLeo AB. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med. 1996;183:1357–1365. doi: 10.1084/jem.183.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ Jr.. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]
- 184.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Garcia-Prats MD, DeLeo AB, Lotze MT. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells. 1997;15:94–103. doi: 10.1002/stem.150094. [DOI] [PubMed] [Google Scholar]
- 186.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 187.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
- 190.Irvine AS, Trinder PK, Laughton DL, Ketteringham H, McDermott RH, Reid SC, Haines AM, Amir A, Husain R, Doshi R, et al. Efficient nonviral transfection of dendritic cells and their use for in vivo immunization. Nat Biotechnol. 2000;18:1273–1278. doi: 10.1038/82383. [DOI] [PubMed] [Google Scholar]
- 191.Manickan E, Kanangat S, Rouse RJ, Yu Z, Rouse BT. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukoc Biol. 1997;61:125–132. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- 192.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tuting T, DeLeo AB, Lotze MT, Storkus WJ. Genetically modified bone marrow-derived dendritic cells expressing tumor-associated viral or “self” antigens induce antitumor immunity in vivo. Eur J Immunol. 1997;27:2702–2707. doi: 10.1002/eji.1830271033. [DOI] [PubMed] [Google Scholar]
- 194.Wan Y, Bramson J, Carter R, Graham F, Gauldie J. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther. 1997;8:1355–1363. doi: 10.1089/hum.1997.8.11-1355. [DOI] [PubMed] [Google Scholar]
- 195.McArthur JG, Mulligan RC. Induction of protective anti-tumor immunity by gene-modified dendritic cells. J Immunother. 1998;21:41–47. [DOI] [PubMed] [Google Scholar]
- 196.Ishida T, Chada S, Stipanov M, Nadaf S, Ciernik FI, Gabrilovich DI, Carbone DP. Dendritic cells transduced with wild-type p53 gene elicit potent anti-tumour immune responses. Clin Exp Immunol. 1999;117:244–251. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tuting T, Steitz J, Bruck J, Gambotto A, Steinbrink K, DeLeo AB, Robbins P, Knop J, Enk AH. Dendritic cell-based genetic immunization in mice with a recombinant adenovirus encoding murine TRP2 induces effective anti-melanoma immunity. J Gene Med. 1999;1:400–406. doi:. [DOI] [PubMed] [Google Scholar]
- 198.Wan Y, Emtage P, Zhu Q, Foley R, Pilon A, Roberts B, Gauldie J. Enhanced immune response to the melanoma antigen gp100 using recombinant adenovirus-transduced dendritic cells. Cell Immunol. 1999;198:131–138. doi: 10.1006/cimm.1999.1585. [DOI] [PubMed] [Google Scholar]
- 199.Klein C, Bueler H, Mulligan RC. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med. 2000;191:1699–1708. doi: 10.1084/jem.191.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ribas A, Butterfield LH, Hu B, Dissette VB, Chen AY, Koh A, Amarnani SN, Glaspy JA, McBride WH, Economou JS. Generation of T-cell immunity to a murine melanoma using MART-1-engineered dendritic cells. J Immunother. 2000;23:59–66. [DOI] [PubMed] [Google Scholar]
- 201.Okada N, Saito T, Masunaga Y, Tsukada Y, Nakagawa S, Mizuguchi H, Mori K, Okada Y, Fujita T, Hayakawa T, et al. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 2001;61:7913–7919. [PubMed] [Google Scholar]
- 202.Koido S, Kashiwaba M, Chen D, Gendler S, Kufe D, Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol. 2000;165:5713–5719. doi: 10.4049/jimmunol.165.10.5713. [DOI] [PubMed] [Google Scholar]
- 203.Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 204.Yamanaka R, Zullo SA, Tanaka R, Blaese M, Xanthopoulos KG. Enhancement of antitumor immune response in glioma models in mice by genetically modified dendritic cells pulsed with Semliki forest virus-mediated complementary DNA. J Neurosurg. 2001;94:474–481. doi: 10.3171/jns.2001.94.3.0474. [DOI] [PubMed] [Google Scholar]
- 205.Insug O, Ku G, Ertl HC, Blaszczyk-Thurin M. A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res. 2002;22:613–621. [PubMed] [Google Scholar]
- 206.Van Meirvenne S, Straetman L, Heirman C, Dullaers M, De Greef C, Van Tendeloo V, Thielemans K. Efficient genetic modification of murine dendritic cells by electroporation with mRNA. Cancer Gene Ther. 2002;9:787–797. doi: 10.1038/sj.cgt.7700499. [DOI] [PubMed] [Google Scholar]
- 207.Minami T, Nakanishi Y, Izumi M, Harada T, Hara N. Enhancement of antigen-presenting capacity and antitumor immunity of dendritic cells pulsed with autologous tumor-derived RNA in mice. J Immunother. 2003;26:420–431. [DOI] [PubMed] [Google Scholar]
- 208.Schmidt T, Ziske C, Marten A, Endres S, Tiemann K, Schmitz V, Gorschlüter M, Schneider C, Sauerbruch T, Schmidt-Wolf IGH. Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res. 2003;63:8962–8967. [PubMed] [Google Scholar]
- 209.Jung CW, Kwon JH, Seol JG, Park WH, Hyun JM, Kim ES, Kim ST, Lee SJ, Kim BK, Lee YY. Induction of cytotoxic T lymphocytes by dendritic cells pulsed with murine leukemic cell RNA. Am J Hematol. 2004;75:121–127. doi: 10.1002/ajh.10471. [DOI] [PubMed] [Google Scholar]
- 210.Liu BY, Chen XH, Gu QL, Li JF, Yin HR, Zhu ZG, Lin Y-Z. Antitumor effects of vaccine consisting of dendritic cells pulsed with tumor RNA from gastric cancer. World J Gastroenterol. 2004;10:630–633. doi: 10.3748/wjg.v10.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zeis M, Siegel S, Wagner A, Schmitz M, Marget M, Kuhl-Burmeister R, Adamzik I, Kabelitz D, Dreger P, Schmitz N, et al. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol. 2003;170:5391–5397. doi: 10.4049/jimmunol.170.11.5391. [DOI] [PubMed] [Google Scholar]
- 212.Celluzzi CM, Falo LD Jr.. Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J Immunol. 1998;160:3081–3085. [PubMed] [Google Scholar]
- 213.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516–5524. [PubMed] [Google Scholar]
- 214.Tanaka H, Shimizu K, Hayashi T, Shu S. Therapeutic immune response induced by electrofusion of dendritic and tumor cells. Cell Immunol. 2002;220:1–12. [DOI] [PubMed] [Google Scholar]
- 215.Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4–13. doi: 10.1006/cimm.2001.1864. [DOI] [PubMed] [Google Scholar]
- 216.Kjaergaard J, Shimizu K, Shu S. Electrofusion of syngeneic dendritic cells and tumor generates potent therapeutic vaccine. Cell Immunol. 2003;225:65–74. [DOI] [PubMed] [Google Scholar]
- 217.Sukhorukov VL, Reuss R, Endter JM, Fehrmann S, Katsen-Globa A, Gessner P, Steinbach A, Müller KJ, Karpas A, Zimmermann U, et al. A biophysical approach to the optimisation of dendritic-tumour cell electrofusion. Biochem Biophys Res Commun. 2006;346:829–839. doi: 10.1016/j.bbrc.2006.05.193. [DOI] [PubMed] [Google Scholar]
- 218.Cathelin D, Nicolas A, Bouchot A, Fraszczak J, Labbe J, Bonnotte B. Dendritic cell-tumor cell hybrids and immunotherapy: what’s next? Cytotherapy. 2011;13:774–785. doi: 10.3109/14653249.2011.553593. [DOI] [PubMed] [Google Scholar]
- 219.Errington F, Jones J, Merrick A, Bateman A, Harrington K, Gough M, O’Donnell D, Selby P, Vile R, Melcher A. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Ther. 2006;13:138–149. doi: 10.1038/sj.gt.3302609. [DOI] [PubMed] [Google Scholar]
- 220.Chevalier MF, Bobisse S, Costa-Nunes C, Cesson V, Jichlinski P, Speiser DE, Starmann J, Tjwa M, Plate KH, Sültmann H, et al. High-throughput monitoring of human tumor-specific T-cell responses with large peptide pools. OncoImmunology. 2015;4:e1029702. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fromm PD, Papadimitrious MS, Hsu JL, Van Kooten Losio N, Verma ND, Lo TH, Silveira PA, Bryant CE, Turtle CJ, Prue RL, et al. CMRF-56+ blood dendritic cells loaded with mRNA induce effective antigen-specific cytotoxic T-lymphocyte responses. OncoImmunology. 2016;5:e1168555. doi: 10.1080/2162402X.2016.1168555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Adotevi O, Vingert B, Freyburger L, Shrikant P, Lone YC, Quintin-Colonna F, Haicheur N, Amessou M, Herbelin A, Langlade-Demoyen P, et al. B subunit of Shiga toxin-based vaccines synergize with alpha-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J Immunol. 2007;179:3371–3379. doi: 10.4049/jimmunol.179.5.3371. [DOI] [PubMed] [Google Scholar]
- 226.Berraondo P, Nouze C, Preville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–8855. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- 227.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJA, Torensma R, Adema GJ, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 228.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cruz LJ, Tacken PJ, Pots JM, Torensma R, Buschow SI, Figdor CG. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials. 2012;33:4229–4239. doi: 10.1016/j.biomaterials.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 230.Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG, de Vries IJM. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–2292. doi: 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- 231.Tacken PJ, Ginter W, Berod L, Cruz LJ, Joosten B, Sparwasser T, Figdor CG, Cambi A. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood. 2011;118:4111–4119. doi: 10.1182/blood-2011-04-346957. [DOI] [PubMed] [Google Scholar]
- 232.Tacken PJ, Ter Huurne M, Torensma R, Figdor CG. Antibodies and carbohydrate ligands binding to DC-SIGN differentially modulate receptor trafficking. Eur J Immunol. 2012;42:1989–1998. doi: 10.1002/eji.201142258. [DOI] [PubMed] [Google Scholar]
- 233.Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJA, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 234.Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy. 2016;8:315–330. doi: 10.2217/imt.15.120. [DOI] [PubMed] [Google Scholar]
- 235.Fotaki G, Jin C, Kerzeli IK, Ramachandran M, Martikainen MM, Karlsson-Parra A, Hoadley KA, Print C, Knowlton N, Black MA, et al. Cancer vaccine based on a combination of an infection-enhanced adenoviral vector and pro-inflammatory allogeneic DCs leads to sustained antigen-specific immune responses in three melanoma models. Oncoimmunology. 2018;7:e1397250. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. [DOI] [PubMed] [Google Scholar]
- 237.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 239.Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 240.Muller L, Simms P, Hong CS, Nishimura MI, Jackson EK, Watkins SC, Whiteside TL. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6:e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fotaki G, Jin C, Ramachandran M, Kerzeli IK, Karlsson-Parra A, Yu D, Hoadley KA, Print C, Knowlton N, Black MA, et al. Pro-inflammatory allogeneic DCs promote activation of bystander immune cells and thereby license antigen-specific T-cell responses. Oncoimmunology. 2018;7:e1395126. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fucikova J, Rozkova D, Ulcova H, Budinsky V, Sochorova K, Pokorna K, Bartůňková J, Špíšek R. Poly I: C-activated dendritic cells that were generated in CellGro for use in cancer immunotherapy trials. J Transl Med. 2011;9:223. doi: 10.1186/1479-5876-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 244.Bercovici N, Haicheur N, Massicard S, Vernel-Pauillac F, Adotevi O, Landais D, Gorin I, Robert C, Prince HM, Grob -J-J, et al. Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother. 2008;31:101–112. doi: 10.1097/CJI.0b013e318159f5ba. [DOI] [PubMed] [Google Scholar]
- 245.Li M, You S, Ge W, Ma S, Ma N, Zhao C. Induction of T-cell immunity against leukemia by dendritic cells pulsed with total RNA isolated from leukemia cells. Chin Med J (Engl). 2003;116:1655–1661. [PubMed] [Google Scholar]
- 246.Lee J, Boczkowski D, Nair S. Programming human dendritic cells with mRNA. Methods Mol Biol. 2013;969:111–125. doi: 10.1007/978-1-62703-260-5_8. [DOI] [PubMed] [Google Scholar]
- 247.Garg NK, Dwivedi P, Prabha P, Tyagi RK. RNA pulsed dendritic cells: an approach for cancer immunotherapy. Vaccine. 2013;31:1141–1156. doi: 10.1016/j.vaccine.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 248.Sayour EJ, De Leon G, Pham C, Grippin A, Kemeny H, Chua J, Huang J, Sampson JH, Sanchez-Perez L, Flores C, et al. Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. Oncoimmunology. 2017;6:e1256527. doi: 10.1080/2162402X.2016.1256527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.He J, Zheng R, Zhang Z, Tan J, Zhou C, Zhang G, Jiang X, Sun Q, Zhou S, Zheng D, et al. Collagen I enhances the efficiency and anti-tumor activity of dendritic-tumor fusion cells. Oncoimmunology. 2017;6:e1361094. doi: 10.1080/2162402X.2017.1361094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Copland MJ, Baird MA, Rades T, McKenzie JL, Becker B, Reck F, Tyler PC, Davies NM. Liposomal delivery of antigen to human dendritic cells. Vaccine. 2003;21:883–890. [DOI] [PubMed] [Google Scholar]
- 251.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64:4357–4365. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 252.Badiee A, Davies N, McDonald K, Radford K, Michiue H, Hart D, Kato M. Enhanced delivery of immunoliposomes to human dendritic cells by targeting the multilectin receptor DEC-205. Vaccine. 2007;25:4757–4766. doi: 10.1016/j.vaccine.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 253.Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hangalapura BN, Oosterhoff D, de Groot J, Boon L, Tuting T, van Den Eertwegh AJ, Gerritsen WR, van Beusechem VW, Pereboev A, Curiel DT, et al. Potent antitumor immunity generated by a CD40-targeted adenoviral vaccine. Cancer Res. 2011;71:5827–5837. doi: 10.1158/0008-5472.CAN-11-0804. [DOI] [PubMed] [Google Scholar]
- 255.Thacker EE, Nakayama M, Smith BF, Bird RC, Muminova Z, Strong TV, Timares L, Korokhov N, O’Neill AM, de Gruijl TD, et al. A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo. Vaccine. 2009;27:7116–7124. doi: 10.1016/j.vaccine.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Korokhov N, de Gruijl TD, Aldrich WA, Triozzi PL, Banerjee PT, Gillies SD, Curiel TJ, Douglas JT, Scheper RJ, Curiel DT. High efficiency transduction of dendritic cells by adenoviral vectors targeted to DC-SIGN. Cancer Biol Ther. 2005;4:289–294. doi: 10.4161/cbt.4.3.1499. [DOI] [PubMed] [Google Scholar]
- 257.Apostolopoulos V, Thalhammer T, Tzakos AG, Stojanovska L. Targeting antigens to dendritic cell receptors for vaccine development. J Drug Deliv. 2013;2013:869718. doi: 10.1155/2013/869718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cauwels A, Van Lint S, Garcin G, Bultinck J, Paul F, Gerlo S, Hoadley KA, Print C, Knowlton N, Black MA, et al. A safe and highly efficient tumor-targeted type I interferon immunotherapy depends on the tumor microenvironment. Oncoimmunology. 2018;7:e1398876. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJG, van Rossum M, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 260.Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, van de Rakt MWMM, Scharenborg NM, de Boer AJ, Pots JM, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res. 2016;22:2155–2166. doi: 10.1158/1078-0432.CCR-15-2205. [DOI] [PubMed] [Google Scholar]
- 261.Chung DJ, Carvajal RD, Postow MA, Sharma S, Pronschinske KB, Shyer JA, Singh-Kandah S, Dickson MA, D’Angelo SP, Wolchok JD, et al. Langerhans-type dendritic cells electroporated with TRP-2 mRNA stimulate cellular immunity against melanoma: results of a phase I vaccine trial. Oncoimmunology. 2017;7:e1372081. doi: 10.1080/2162402X.2017.1372081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 263.Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 264.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 265.Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology. 2013;2:e24612. doi: 10.4161/onci.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10. doi: 10.1126/scitranslmed.aao4496. [DOI] [PubMed] [Google Scholar]
- 267.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 268.Hendriks D, Choi G, de Bruyn M, Wiersma VR, Bremer E. Antibody-based cancer therapy: successful agents and novel approaches. Int Rev Cell Mol Biol. 2017;331:289–383. doi: 10.1016/bs.ircmb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 269.de la Cruz-Merino L, Chiesa M, Caballero R, Rojo F, Palazon N, Carrasco FH, Sánchez-Margalet V. Breast cancer immunology and immunotherapy: current status and future perspectives. Int Rev Cell Mol Biol. 2017;331:1–53. doi: 10.1016/bs.ircmb.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 270.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 272.Gardner JK, Mamotte CDS, Jackaman C, Nelson DJ. Modulation of dendritic cell and T cell cross-talk during aging: the potential role of checkpoint inhibitory molecules. Ageing Res Rev. 2017;38:40–51. doi: 10.1016/j.arr.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 273.Zhao Y, Qiao G, Wang X, Song Y, Zhou X, Jiang N, Zhou L, Huang H, Zhao J, Morse MA, et al. Combination of DC/CIK adoptive T cell immunotherapy with chemotherapy in advanced non-small-cell lung cancer (NSCLC) patients: a prospective patients’ preference-based study (PPPS). Clin Transl Oncol. 2019;21:721–728. doi: 10.1007/s12094-018-1968-3. [DOI] [PubMed] [Google Scholar]
- 274.Turgeon GA, Weickhardt A, Azad AA, Solomon B, Siva S. Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med J Aust. 2019;210:47–53. doi: 10.5694/mja2.12046. [DOI] [PubMed] [Google Scholar]
- 275.Liu Y, Bewersdorf JP, Stahl M, Zeidan AM. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: the dawn of a new era? Blood Rev. 2019;34:67–83. doi: 10.1016/j.blre.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 276.Kongsted P, Borch TH, Ellebaek E, Iversen TZ, Andersen R, Met O, Hansen M, Lindberg H, Sengeløv L, Svane IM. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy. 2017;19:500–513. doi: 10.1016/j.jcyt.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 277.Hansen M. The role of dendritic cells in cancer. Mediators Inflamm. 2017;39:307–316. [DOI] [PubMed] [Google Scholar]
- 278.Garg AD, Coulie PG, Van Den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 279.Garg AD, Vara Perez M, Schaaf M, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: dendritic cell-based anticancer immunotherapy. Oncoimmunology. 2017;6:e1328341. doi: 10.1080/2162402X.2017.1328341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology. 2014;3:e27297. doi: 10.4161/onci.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nimanong S, Ostroumov D, Wingerath J, Knocke S, Woller N, Gurlevik E, Falk CS, Manns MP, Kühnel F, Wirth TC. CD40 signaling drives potent cellular immune responses in heterologous cancer vaccinations. Cancer Res. 2017;77:1918–1926. doi: 10.1158/0008-5472.CAN-16-2089. [DOI] [PubMed] [Google Scholar]
- 282.Moreno Ayala MA, Gottardo MF, Imsen M, Asad AS, Bal de Kier Joffe E, Casares N, Lasarte JJ, Seilicovich A, Candolfi M. Therapeutic blockade of Foxp3 in experimental breast cancer models. Breast Cancer Res Treat. 2017;166:393–405. doi: 10.1007/s10549-017-4414-2. [DOI] [PubMed] [Google Scholar]
- 283.Liu H, Chen L, Liu J, Meng H, Zhang R, Ma L, Wu L, Yu S, Shi F, Li Y, et al. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017;411:182–190. doi: 10.1016/j.canlet.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 284.Escriba-Garcia L, Alvarez-Fernandez C, Tellez-Gabriel M, Sierra J, Briones J. Dendritic cells combined with tumor cells and alpha-galactosylceramide induce a potent, therapeutic and NK-cell dependent antitumor immunity in B cell lymphoma. J Transl Med. 2017;15:115. doi: 10.1186/s12967-017-1219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Heine A, Held SAE, Schulte-Schrepping J, Wolff JFA, Klee K, Ulas T, Schmacke NA, Daecke SN, Riethausen K, Schultze JL, et al. Generation and functional characterization of MDSC-like cells. Oncoimmunology. 2017;6:e1295203. doi: 10.1080/2162402X.2017.1295203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Vo MC, Nguyen-Pham TN, Lee HJ, Jaya Lakshmi T, Yang S, Jung SH, Kim H-J, Lee -J-J. Combination therapy with dendritic cells and lenalidomide is an effective approach to enhance antitumor immunity in a mouse colon cancer model. Oncotarget. 2017;8:27252–27262. doi: 10.18632/oncotarget.15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Merry TL, Brooks AES, Masson SW, Adams SE, Jaiswal JK, Jamieson SMF, Shepherd PR. The CSF1 receptor inhibitor pexidartinib (PLX3397) reduces tissue macrophage levels without affecting glucose homeostasis in mice. Int J Obes. 2019. doi: 10.1038/s41366-019-0355-7. [DOI] [PubMed] [Google Scholar]
- 288.Lee JH, Chen TW, Hsu CH, Yen YH, Yang JC, Cheng AL, Sasaki S-I, Chiu L, Sugihara M, Ishizuka T, et al. A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in Asian patients with advanced solid tumors. Invest New Drugs. 2019. doi: 10.1007/s10637-019-00745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Dammeijer F, Lievense LA, Kaijen-Lambers ME, van Nimwegen M, Bezemer K, Hegmans JP, van Hall T, Hendriks RW, Aerts JG. Depletion of tumor-associated macrophages with a CSF-1R kinase inhibitor enhances antitumor immunity and survival induced by DC immunotherapy. Cancer Immunol Res. 2017;5:535–546. doi: 10.1158/2326-6066.CIR-16-0309. [DOI] [PubMed] [Google Scholar]
- 290.Moreno Ayala MA, Gottardo MF, Gori MS, Nicola Candia AJ, Caruso C, De Laurentiis A, Imsen M, Klein S, Bal de Kier Joffé E, Salamone G, et al. Dual activation of Toll-like receptors 7 and 9 impairs the efficacy of antitumor vaccines in murine models of metastatic breast cancer. J Cancer Res Clin Oncol. 2017;143:1713–1732. doi: 10.1007/s00432-017-2421-7. [DOI] [PubMed] [Google Scholar]
- 291.Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3:e27878. doi: 10.4161/onci.27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Galluzzi L, Kroemer G. Calreticulin and type I interferon: an unsuspected connection. Oncoimmunology. 2017;6:e1288334. doi: 10.1080/2162402X.2017.1288334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6:e1386829. doi: 10.1080/2162402X.2017.1386829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP, Sedighim S, Treger J, Odesa S, Tucker A, et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017;19:796–807. doi: 10.1093/neuonc/now287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Arab S, Kheshtchin N, Ajami M, Ashurpoor M, Safvati A, Namdar A, Mirzaei R, Mousavi Niri N, Jadidi-Niaragh F, Ghahremani MH, et al. Increased efficacy of a dendritic cell-based therapeutic cancer vaccine with adenosine receptor antagonist and CD73 inhibitor. Tumour Biol. 2017;39:1010428317695021. doi: 10.1177/1010428317695021. [DOI] [PubMed] [Google Scholar]
- 296.Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5:e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, Erbs P, Limacher J-M, Preville X, Zitvogel L, et al. Trial watch:: oncolytic viruses for cancer therapy. Oncoimmunology. 2014;3:e28694. doi: 10.4161/onci.28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Komorowski M, Tisonczyk J, Kolakowska A, Drozdz R, Kozbor D. Modulation of the tumor microenvironment by CXCR4 antagonist-armed viral oncotherapy enhances the antitumor efficacy of dendritic cell vaccines against neuroblastoma in syngeneic mice. Viruses. 2018;10:455. doi: 10.3390/v10090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Stefanski HE, Jonart L, Goren E, Mule JJ, Blazar BR. A novel approach to improve immune effector responses post transplant by restoration of CCL21 expression. PLoS One. 2018;13:e0193461. doi: 10.1371/journal.pone.0193461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Van Woensel M, Mathivet T, Wauthoz N, Rosiere R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon L, et al. Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci Rep. 2017;7:1217. doi: 10.1038/s41598-017-01279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Huang F, Wan J, Hao S, Deng X, Chen L, Ma L. TGF-beta1-silenced leukemia cell-derived exosomes target dendritic cells to induce potent anti-leukemic immunity in a mouse model. Cancer Immunol Immun: CII. 2017;66:1321–1331. doi: 10.1007/s00262-017-2028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, Qi H, Guo H, Yin H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739–748. doi: 10.1016/j.jhep.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 303.Bryson PD, Han X, Truong N, Wang P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine. 2017;35:5842–5849. doi: 10.1016/j.vaccine.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, Huang L. Combination Immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26:45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wennhold K, Weber TM, Klein-Gonzalez N, Thelen M, Garcia-Marquez M, Chakupurakal G, Fiedler A, Schlösser HA, Fischer R, Theurich S, et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget. 2017;8:27740–27753. doi: 10.18632/oncotarget.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wculek SK, Amores-Iniesta J, Conde-Garrosa R, Khouili SC, Melero I, Sancho D. Effective cancer immunotherapy by natural mouse conventional type-1 dendritic cells bearing dead tumor antigen. J Immunother Cancer. 2019;7:100. doi: 10.1186/s40425-019-0565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yang M, Li C, Zhu S, Cao L, Kroemer G, Zeh H, Hoadley KA, Print C, Knowlton N, Black MA, et al. TFAM is a novel mediator of immunogenic cancer cell death. Oncoimmunology. 2018;7:e1431086. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Castoldi F, Vacchelli E, Zitvogel L, Maiuri MC, Pietrocola F, Kroemer G. Systemic autophagy in the therapeutic response to anthracycline-based chemotherapy. Oncoimmunology. 2019;8:e1498285. doi: 10.1080/2162402X.2018.1498285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Roselli E, Araya P, Nunez NG, Gatti G, Graziano F, Sedlik C, Benaroch P, Piaggio E, Maccioni M. TLR3 activation of Intratumoral CD103(+) dendritic cells modifies the tumor infiltrate conferring anti-tumor immunity. Front Immunol. 2019;10:503. doi: 10.3389/fimmu.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cancel JC, Crozat K, Dalod M, Mattiuz R. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol. 2019;10:9. doi: 10.3389/fimmu.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Huang FY, Lei J, Sun Y, Yan F, Chen B, Zhang L, Hoadley KA, Print C, Knowlton N, Black MA, et al. Induction of enhanced immunogenic cell death through ultrasound-controlled release of doxorubicin by liposome-microbubble complexes. Oncoimmunology. 2018;7:e1446720. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bottcher JP, Reis ESC. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4:784–792. doi: 10.1016/j.trecan.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hotblack A, Holler A, Piapi A, Ward S, Stauss HJ, Bennett CL. Tumor-resident dendritic cells and macrophages modulate the accumulation of TCR-engineered T cells in melanoma. Mol Ther. 2018;26:1471–1481. doi: 10.1016/j.ymthe.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Meyer MA, Baer JM, Knolhoff BL, Nywening TM, Panni RZ, Su X, Weilbaecher KN, Hawkins WG, Ma C, Fields RC, et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun. 2018;9:1250. doi: 10.1038/s41467-018-03600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Giglio P, Gagliardi M, Tumino N, Antunes F, Smaili S, Cotella D, Hoadley KA, Print C, Knowlton N, Black MA, et al. PKR and GCN2 stress kinases promote an ER stress-independent eIF2alpha phosphorylation responsible for calreticulin exposure in melanoma cells. Oncoimmunology. 2018;7:e1466765. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis E Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–37 e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sanchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Perez-Gracia JL, Sanchez-Arraez A, Sancho D, Melero I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol. 2017;28:xii44–xii55. doi: 10.1093/annonc/mdx237. [DOI] [PubMed] [Google Scholar]
- 319.Santegoets SJ, Stam AG, Lougheed SM, Gall H, Jooss K, Sacks N, Hege K, Lowy I, Scheper RJ, Gerritsen WR, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer. 2014;2:31. doi: 10.1186/s40425-014-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bezu L, Sauvat A, Humeau J, Leduc M, Kepp O, Kroemer G. eIF2alpha phosphorylation: A hallmark of immunogenic cell death. Oncoimmunology. 2018;7:e1431089. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wang L, Fan J, Ye W, Han J, Zhang Y, Zhao L, Duan J, Yin D, Yi Y. The expression of ILT4 in myeloid dendritic cells in patients with hepatocellular carcinoma. Immunol Invest. 2019;1–15. doi: 10.1080/08820139.2019.1571507. [DOI] [PubMed] [Google Scholar]
- 322.Rajendran M, Looney S, Singh N, Elashiry M, Meghil MM, El-Awady AR, Tawfik O, Susin C, Arce RM, Cutler CW. Systemic Antibiotic therapy reduces circulating inflammatory dendritic cells and Treg-Th17 plasticity in periodontitis. J Immunol. 2019;202:2690–2699. doi: 10.4049/jimmunol.1900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Stankovic B, Bjorhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Muller E, Hammarström C, Beraki K, Bækkevold ES, Woldbæk PR, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. 2018;9:3101. doi: 10.3389/fimmu.2018.03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ventura A, Vassall A, Robinson E, Filler R, Hanlon D, Meeth K, Ezaldein H, Girardi M, Sobolev O, Bosenberg MW, et al. Extracorporeal photochemotherapy drives monocyte-to-dendritic cell maturation to induce anticancer immunity. Cancer Res. 2018;78:4045–4058. doi: 10.1158/0008-5472.CAN-18-0171. [DOI] [PubMed] [Google Scholar]
- 325.Wei XX, Perry J, Chang E, Zhang L, Hiatt RA, Ryan CJ, Small EJ, Fong L. Clinical variables associated with overall survival in metastatic castration-resistant prostate cancer patients treated with sipuleucel-T immunotherapy. Clin Genitourin Cancer. 2018;16:184–90 e2. doi: 10.1016/j.clgc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 326.Hijikata Y, Okazaki T, Tanaka Y, Murahashi M, Yamada Y, Yamada K, Takahashi A, Inoue H, Kishimoto J, Nakanishi Y, et al. A phase I clinical trial of RNF43 peptide-related immune cell therapy combined with low-dose cyclophosphamide in patients with advanced solid tumors. PLoS One. 2018;13:e0187878. doi: 10.1371/journal.pone.0187878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yanagisawa R, Koizumi T, Koya T, Sano K, Koido S, Nagai K, Kobayashi M, Okamoto M, Sugiyama H, Shimodaira S. WT1-pulsed Dendritic Cell Vaccine Combined with Chemotherapy for Resected Pancreatic Cancer in a Phase I Study. Anticancer Res. 2018;38:2217–2225. doi: 10.21873/anticanres.12464. [DOI] [PubMed] [Google Scholar]
- 328.Ogasawara M, Miyashita M, Ota S. Vaccination of Urological Cancer Patients With WT1 Peptide-Pulsed Dendritic Cells in Combination With Molecular Targeted Therapy or Conventional Chemotherapy Induces Immunological and Clinical Responses. Ther Apher Dial. 2018;22:266–277. doi: 10.1111/1744-9987.12694. [DOI] [PubMed] [Google Scholar]
- 329.Antonarakis ES, Kibel AS, Yu EY, Karsh LI, Elfiky A, Shore ND, Vogelzang NJ, Corman JM, Millard FE, Maher JC, et al. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin Cancer Res. 2017;23:2451–2459. doi: 10.1158/1078-0432.CCR-16-1780. [DOI] [PubMed] [Google Scholar]
- 330.Oostvogels R, Kneppers E, Minnema MC, Doorn RC, Franssen LE, Aarts T, Emmelot ME, Spierings E, Slaper-Cortenbach I, Westinga K, et al. Efficacy of host-dendritic cell vaccinations with or without minor histocompatibility antigen loading, combined with donor lymphocyte infusion in multiple myeloma patients. Bone Marrow Transplant. 2017;52:228–237. doi: 10.1038/bmt.2016.250. [DOI] [PubMed] [Google Scholar]
- 331.Davis ID, Quirk J, Morris L, Seddon L, Tai TY, Whitty G, Cavicchiolo T, Ebert L, Jackson H, Browning J, et al. A pilot study of peripheral blood BDCA-1 (CD1c) positive dendritic cells pulsed with NY-ESO-1 ISCOMATRIX adjuvant. Immunotherapy. 2017;9:249–259. doi: 10.2217/imt-2016-0132. [DOI] [PubMed] [Google Scholar]
- 332.Wimmers F, de Haas N, Scholzen A, Schreibelt G, Simonetti E, Eleveld MJ, Brouwers HMLM, Beldhuis-Valkis M, Joosten I, de Jonge MI, et al. Monitoring of dynamic changes in Keyhole Limpet Hemocyanin (KLH)-specific B cells in KLH-vaccinated cancer patients. Sci Rep. 2017;7:43486. doi: 10.1038/srep43486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, Scurti G, Salem ML, Nelson MH, Thomas MB, et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017;10:82. doi: 10.1186/s13045-017-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Franssen LE, Roeven MWH, Hobo W, Doorn R, Oostvogels R, Falkenburg JHF, van de Donk NW, Kester MGD, Fredrix H, Westinga K, et al. A phase I/II minor histocompatibility antigen-loaded dendritic cell vaccination trial to safely improve the efficacy of donor lymphocyte infusions in myeloma. Bone Marrow Transplant. 2017;52:1378–1383. doi: 10.1038/bmt.2017.118. [DOI] [PubMed] [Google Scholar]
- 335.Shen J, Wang LF, Zou ZY, Kong WW, Yan J, Meng FY, Chen F-J, Du J, Shao J, Xu Q-P, et al. Phase I clinical study of personalized peptide vaccination combined with radiotherapy for advanced hepatocellular carcinoma. World J Gastroenterol. 2017;23:5395–5404. doi: 10.3748/wjg.v23.i29.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lowenfeld L, Mick R, Datta J, Xu S, Fitzpatrick E, Fisher CS, Fox KR, DeMichele A, Zhang PJ, Weinstein SP, et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2(pos) DCIS independent of route: results of randomized selection design trial. Clin Cancer Res. 2017;23:2961–2971. doi: 10.1158/1078-0432.CCR-16-1924. [DOI] [PubMed] [Google Scholar]
- 337.Fukuda K, Funakoshi T, Sakurai T, Nakamura Y, Mori M, Tanese K, Tanikawa A, Taguchi J, Fujita T, Okamoto M, et al. Peptide-pulsed dendritic cell vaccine in combination with carboplatin and paclitaxel chemotherapy for stage IV melanoma. Melanoma Res. 2017;27:326–334. doi: 10.1097/CMR.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 338.Saka H, Kitagawa C, Ichinose Y, Takenoyama M, Ibata H, Kato T, Takami K, Yamashita M, Maeda T, Takeo S, et al. A randomized phase II study to assess the effect of adjuvant immunotherapy using alpha-GalCer-pulsed dendritic cells in the patients with completely resected stage II-IIIA non-small cell lung cancer: study protocol for a randomized controlled trial. Trials. 2017;18:429. doi: 10.1186/s13063-017-2103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Gasser O, Sharples KJ, Barrow C, Williams GM, Bauer E, Wood CE, Mester B, Dzhelali M, Caygill G, Jones J, et al. A phase I vaccination study with dendritic cells loaded with NY-ESO-1 and alpha-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients. Cancer Immunol Immun: CII. 2018;67:285–298. doi: 10.1007/s00262-017-2085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ge C, Li R, Song H, Geng T, Yang J, Tan Q, Song L, Wang Y, Xue Y, Li Z, et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer. 2017;17:884. doi: 10.1186/s12885-017-3859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Yao Y, Luo F, Tang C, Chen D, Qin Z, Hua W, Xu M, Zhong P, Yu S, Chen D, et al. Molecular subgroups and B7-H4 expression levels predict responses to dendritic cell vaccines in glioblastoma: an exploratory randomized phase II clinical trial. Cancer Immunol Immun: CII. 2018;67:1777–1788. doi: 10.1007/s00262-018-2232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhang W, Lu X, Cui P, Piao C, Xiao M, Liu X, Wang Y, Wu X, Liu J, Yang L. Phase I/II clinical trial of a Wilms’ tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol Immun: CII. 2019;68:121–130. doi: 10.1007/s00262-018-2257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Geskin LJ, Damiano JJ, Patrone CC, Butterfield LH, Kirkwood JM, Falo LD. Three antigen-loading methods in dendritic cell vaccines for metastatic melanoma. Melanoma Res. 2018;28:211–221. doi: 10.1097/CMR.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Palma M, Hansson L, Mulder TA, Adamson L, Nasman-Glaser B, Eriksson I, Heimersson K, Ryblom H, Mozaffari F, Svensson A, et al. Lenalidomide as immune adjuvant to a dendritic cell vaccine in chronic lymphocytic leukemia patients. Eur J Haematol. 2018;101:68–77. doi: 10.1111/ejh.13065. [DOI] [PubMed] [Google Scholar]
- 345.Jung SH, Lee HJ, Lee YK, Yang DH, Kim HJ, Rhee JH, Emmrich F, Lee -J-J. A phase I clinical study of autologous dendritic cell therapy in patients with relapsed or refractory multiple myeloma. Oncotarget. 2017;8:41538–41548. doi: 10.18632/oncotarget.14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Inoges S, Tejada S, de Cerio AL, Gallego Perez-Larraya J, Espinos J, Idoate MA, Domínguez PD, de Eulate RG, Aristu J, Bendandi M, et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J Transl Med. 2017;15:104. doi: 10.1186/s12967-017-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Miwa S, Nishida H, Tanzawa Y, Takeuchi A, Hayashi K, Yamamoto N, Mizukoshi E, Nakamoto Y, Kaneko S, Tsuchiya H. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer. 2017;123:1576–1584. doi: 10.1002/cncr.30606. [DOI] [PubMed] [Google Scholar]
- 348.Herbert GS, Vreeland TJ, Clifton GT, Greene JM, Jackson DO, Hardin MO, Hale DF, Berry JS, Nichol P, Yin S, et al. Initial phase I/IIa trial results of an autologous tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine in patients with solid tumors. Vaccine. 2018;36:3247–3253. doi: 10.1016/j.vaccine.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 349.Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N, Nijs G, Stein B, Lion E, Van Driessche A, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon JE, Healy P, et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin Cancer Res. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Khoury HJ, Collins RH Jr., Blum W, Stiff PS, Elias L, Lebkowski JS, Reddy A, Nishimoto KP, Sen D, Wirth ED, et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer. 2017;123:3061–3072. doi: 10.1002/cncr.30696. [DOI] [PubMed] [Google Scholar]
- 352.Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, Norberg PK, Herndon JE, Healy P, Congdon KL, et al. Dendritic Cells Enhance Polyfunctionality Of Adoptively Transferred T Cells that target cytomegalovirus in glioblastoma. Cancer Res. 2018;78:256–264. doi: 10.1158/0008-5472.CAN-17-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sonpavde G, McMannis JD, Bai Y, Seethammagari MR, Bull JMC, Hawkins V, Dancsak TK, Lapteva N, Levitt JM, Moseley A, et al. Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer. Cancer Immunol Immun: CII. 2017;66:1345–1357. doi: 10.1007/s00262-017-2027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, Schaue D, Wang G, Rosen F, Yanagawa J, et al. Phase I trial of intratumoral injection of CCL21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clin Cancer Res. 2017;23:4556–4568. doi: 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Laurell A, Lonnemark M, Brekkan E, Magnusson A, Tolf A, Wallgren AC, Andersson B, Adamson L, Kiessling R, Karlsson-Parra A. Intratumorally injected pro-inflammatory allogeneic dendritic cells as immune enhancers: a first-in-human study in unfavourable risk patients with metastatic renal cell carcinoma. J Immunother Cancer. 2017;5:52. doi: 10.1186/s40425-017-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.van de Loosdrecht AA, van Wetering S, Santegoets S, Singh SK, Eeltink CM, Den Hartog Y, Koppes M, Kaspers J, Ossenkoppele GJ, Kruisbeek AM, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immun: CII. 2018;67:1505–1518. doi: 10.1007/s00262-018-2198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Chen CL, Pan QZ, Weng DS, Xie CM, Zhao JJ, Chen MS, Hoadley KA, Print C, Knowlton N, Black MA, et al. Safety and activity of PD-1 blockade-activated DC-CIK cells in patients with advanced solid tumors. Oncoimmunology. 2018;7:e1417721. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Jiang N, Qiao G, Wang X, Morse MA, Gwin WR, Zhou L, Song Y, Zhao Y, Chen F, Zhou X, et al. Dendritic cell/cytokine-induced killer cell immunotherapy combined with S-1 in patients with advanced pancreatic cancer: a prospective study. Clin Cancer Res. 2017;23:5066–5073. doi: 10.1158/1078-0432.CCR-17-0492. [DOI] [PubMed] [Google Scholar]
- 359.Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int Immunopharmacol. 2018;63:292–298. doi: 10.1016/j.intimp.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 360.Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283. doi: 10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Klener P Jr., Etrych T, Klener P. Biological therapy of hematologic malignancies: toward a chemotherapy- free era. Curr Med Chem. 2019;26:1002–1018. doi: 10.2174/0929867324666171006144725. [DOI] [PubMed] [Google Scholar]
- 362.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vonderheide RH. The immune revolution: a case for priming, not checkpoint. Cancer Cell. 2018;33:563–569. doi: 10.1016/j.ccell.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11:8. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Mangsbo SM, Fletcher EAK, van Maren WWC, Redeker A, Cordfunke RA, Dillmann I, Dinkelaar J, Ouchaou K, Codee JDC, van der Marel GA, et al. Linking T cell epitopes to a common linear B cell epitope: A targeting and adjuvant strategy to improve T cell responses. Mol Immunol. 2018;93:115–124. doi: 10.1016/j.molimm.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 366.Brooks N, Hsu J, Esparon S, Pouniotis D, Pietersz GA. Immunogenicity of a tripartite cell penetrating peptide containing a MUC1 VARIABLE NUMBER OF TANDEM REPeat (VNTR) and A T helper epitope. Molecules. 2018;23:2233. doi: 10.3390/molecules23092233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Starmann J, Tjwa M, Plate KH, Sültmann H, et al. Trial watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15:477–494. doi: 10.1038/s41571-018-0046-7. [DOI] [PubMed] [Google Scholar]
- 369.Taylor J, Pavlick D, Yoshimi A, Marcelus C, Chung SS, Hechtman JF, Benayed R, Cocco E, Durham BH, Bitner L, et al. Oncogenic TRK fusions are amenable to inhibition in hematologic malignancies. J Clin Invest. 2018;128:3819–3825. doi: 10.1172/JCI120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Dummer R, Ramelyte E, Schindler S, Thurigen O, Levesque MP, Koelblinger P. MEK inhibition and immune responses in advanced melanoma. Oncoimmunology. 2017;6:e1335843. doi: 10.1080/2162402X.2017.1335843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Candeias SM, Gaipl US. The immune system in cancer prevention, development and therapy. Anticancer Agents Med Chem. 2016;16:101–107. [DOI] [PubMed] [Google Scholar]
- 372.Lee S, Son B, Park G, Kim H, Kang H, Jeon J, Youn H, Youn B Immunogenic Effect of Hyperthermia on Enhancing Radiotherapeutic Efficacy. Int J Mol Sci. 2018;19:e2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, Helman LJ, Kastan MB, Knapp DW, Levin WJ, et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8:328ra27. doi: 10.1126/scitranslmed.aaf0746. [DOI] [PubMed] [Google Scholar]
- 374.Garg AD, Vandenberk L, Van Woensel M, Belmans J, Schaaf M, Boon L, De Vleeschouwer S, Agostinis P. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers- based clinical predictions in glioblastoma. Oncoimmunology. 2017;6:e1295903. doi: 10.1080/2162402X.2017.1295903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, Bear H, McArthur HL, Frank E, Perlmutter J, et al. current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2018.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Le QT, Colevas AD, O’Sullivan B, Lee AWM, Lee N, Ma B, Siu LL, Waldron J, Lim C-M, Riaz N, et al. Current treatment landscape of nasopharyngeal carcinoma and potential trials evaluating the value of immunotherapy. J Natl Cancer Inst. 2019. doi: 10.1093/jnci/djz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Xiao Y, Ma D, Zhao S, Suo C, Shi J, Xue MZ, Ruan M, Wang H, Zhao J, Li Q, et al. Multi-omics profiling reveals distinct microenvironment characterization and suggests immune escape mechanisms of triple-negative breast cancer. Clin Cancer Res. 2019. doi: 10.1158/1078-0432.CCR-18-3524. [DOI] [PubMed] [Google Scholar]
- 378.Kong BY, Bolton H, Kim JW, Silveira PA, Fromm PD, Clark GJ. On the other side: manipulating the immune checkpoint landscape of dendritic cells to enhance cancer immunotherapy. Front Oncol. 2019;9:50. doi: 10.3389/fonc.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Vitale I, Sistigu A, Manic G, Rudqvist NP, Trajanoski Z, Galluzzi L. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol. 2019;29:396–416. doi: 10.1016/j.tcb.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 380.Jiang N, Schonnesen AA, Ma KY. Ushering in integrated t cell repertoire profiling in cancer. Trends Cancer. 2019;5:85–94. doi: 10.1016/j.trecan.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Pai CS, Huang JT, Lu X, Simons DM, Park C, Chang A, Tamaki W, Liu E, Roybal KT, Seagal J, et al. Clonal deletion of tumor-specific T cells by interferon-gamma confers therapeutic resistance to combination immune checkpoint blockade. Immunity. 2019;50:477–92 e8. doi: 10.1016/j.immuni.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Nadal E, Massuti B, Domine M, Garcia-Campelo R, Cobo M, Felip E. Immunotherapy with checkpoint inhibitors in non-small cell lung cancer: insights from long-term survivors. Cancer Immunol Immun: CII. 2019;68:341–352. doi: 10.1007/s00262-019-02310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16:341–355. doi: 10.1038/s41571-019-0173-9. [DOI] [PubMed] [Google Scholar]
- 384.Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, Heth JA, Salacz M, Taylor S, D’Andre SD, et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16:142. doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gulley JL, Mulders P, Albers P, Banchereau J, Bolla M, Pantel K, Powles T. Perspectives on sipuleucel-T: its role in the prostate cancer treatment paradigm. Oncoimmunology. 2016;5:e1107698. doi: 10.1080/2162402X.2015.1107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Anselmo Da Costa I, Stenzl A, Bedke J. Inmunotherapy in prostate cancer. Arch Esp Urol. 2019;72:211–222. [PubMed] [Google Scholar]
- 387.DeMaria PJ, Bilusic M. Cancer Vaccines. Hematol Oncol Clin North Am. 2019;33:199–214. doi: 10.1016/j.hoc.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 388.Bates JP, Derakhshandeh R, Jones L, Webb TJ, Meunier L, Portelance L, Bernard M, Nelson BH, Bernardini MQ, Bartlett JMS, et al. Mechanisms of immune evasion in breast cancer. BMC Cancer. 2018;18:556. doi: 10.1186/s12885-018-4242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Vitale LA, Thomas LJ, He LZ, O’Neill T, Widger J, Crocker A, Sundarapandiyan K, Storey JR, Forsberg EM, Weidlick J, et al. Development of CDX-1140, an agonist CD40 antibody for cancer immunotherapy. Cancer Immunol Immun: CII. 2019;68:233–245. doi: 10.1007/s00262-018-2267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pan L, Shang N, Shangguan J, Figini M, Xing W, Wang B, Sun C, Yang J, Zhang Y, Hu S, et al. Magnetic resonance imaging monitoring therapeutic response to dendritic cell vaccine in murine orthotopic pancreatic cancer models. Am J Cancer Res. 2019;9:562–573. [PMC free article] [PubMed] [Google Scholar]
- 391.Leeman H, Kaminska E, Green D, Bodman-Smith M, Gravett A, Bodman-Smith K, Copier J, Coulton G, Fusi A, Dalgleish AG. Serum Apolipoprotein E and other inflammatory markers can identify non-responding patients to a dendritic cell vaccine. Transl Oncol. 2019;12:397–403. doi: 10.1016/j.tranon.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ni M, Hoffmann JM, Schmitt M, Schmitt A. Progress of dendritic cell-based cancer vaccines for patients with hematological malignancies. Expert Opin Biol Ther. 2016;16:1113–1123. doi: 10.1080/14712598.2016.1196181. [DOI] [PubMed] [Google Scholar]
- 393.Hsu SC, Lu LC, Chan KY, Huang CH, Cheng SL, Chan YS, YANG Y-S, LAI Y-T, YAO C-L. Large-scale production and directed induction of functional dendritic cells ex vivo from serum-free expanded human hematopoietic stem cells. Cytotherapy. 2019. doi: 10.1016/j.jcyt.2019.04.059. [DOI] [PubMed] [Google Scholar]
- 394.Fennemann FL, de Vries IJM, Figdor CG, Verdoes M. Attacking tumors from all sides: personalized multiplex vaccines to tackle intratumor heterogeneity. Front Immunol. 2019;10:824. doi: 10.3389/fimmu.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]


