ABSTRACT
Patients with metastatic melanoma were treated with tremelimumab and interferon-α (IFN) in a previously reported clinical trial [NCT00610857]. Responses were assessed by RECIST criteria as complete (CR) or partial (PR), stable disease (SD) or progressive disease (PD). In this study, T-cell receptor (TCR) beta-chain repertoire was immunosequenced in peripheral blood mononuclear cells (PBMC) specimens (N = 33) and tumor samples (N = 18) utilizing the immunoSEQ® Assay to determine repertoire clonality and T cell fractions at pre-treatment (tumor and PBMC), one month (PBMC) and 3 months (PBMC) time points and evaluate its association with clinical outcomes. In the pretreatment tumor microenvironment (TME), T cell clonality was significantly (p = .035) different and greater in patients who achieved disease control (CR, PR, SD) versus those with non-disease control (PD) as best response to treatment. Further, there was significantly (p = .001) increased TCR fraction in tissue of responders (CR, PR) versus non-responders (PD, SD). In examining T cell clonality in the circulation (PBMC), no significant associations were found in the pretreatment samples. However, early on-treatment (4 weeks) there was a significant decrease in T cell clonality that was associated with improved overall survival (p = .01) and progression-free survival (p = .04). In addition, analysis of temporal changes in tumor-infiltrating lymphocytes (TIL) and peripheral TCR repertoire revealed that responders had significantly higher clonal expansion of TIL in the circulation at 4 weeks than non-responders (p = .036). Our study provided interesting mechanistic data related to CTLA-4 Blockade and IFN and potential biomarkers of immunotherapeutic benefit.
KEYWORDS: T-cell receptor (TCR), TCR profiling, TCR repertoire, Immune repertoire, CDR3, CTLA-4, Tremelimumab, Interferon
Introduction
The treatment landscape of metastatic melanoma has improved drastically over the last decade with the identification of small molecule inhibitors of BRAF and MEK and immunotherapeutic antibodies directed at cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell-death protein 1 (PD-1).1,2 However, despite the success with these therapies, more than half of the patients do not experience long-term clinical remission and there is a critical need to investigate novel drug combinations in the salvage setting as well as mechanistic studies and predictive biomarkers of response to extend therapeutic benefits to the majority of patients.
In metastatic melanoma, interferon-α (IFN) has been shown to interrupt tumor immune tolerance, via shifting Th polarity towards Th1 in the tumor microenvironment (TME),3 increasing dendritic cell antigen presentation to immune effectors,4,5 and potentiating natural killer cell-mediated immunity.6 However, tumor tolerance has been shown to limit IFN clinical activity when used as monotherapy. Therefore, adding CTLA-4 blockers plays a critical role in reversing this effect and increasing T cell activation and proliferation.7–13 Based on this hypothesis of potential synergistic antitumor effects of CTLA-blockade and IFN combination treatment, patients with metastatic melanoma were treated with tremelimumab and IFN in a previously reported study.14 The study demonstrated promising durable antitumor activity of the combination with acceptable toxicity.
Early studies have shown that increased T-cell infiltration within the tumor biopsy of primary melanoma is associated with improved clinical outcomes following immunotherapy.15,16 Additionally, increased density of tumor-infiltrating lymphocytes (TILs) is an independent prognostic factor for sentinel lymph node metastatic status and survival.17 Since antitumor effects of anti-CTLA-4 are mediated in part by reprogramming of T-cells, a number of studies have investigated blood and tumor biopsy T-cell subpopulations and correlated immunologic changes with patient outcomes. It has been reported that during treatment with ipilimumab, survival was significantly associated with increased total CD4 and CD8 lymphocyte count.18–21 Other studies have explored the impact of CTLA-4 blockade on the circulating T-cell receptor (TCR) repertoire, as TCR repertoire is proposed to be a mirror of human adaptive immune responses and the presence of diverse T-cell clones may indicate biological effects of treatment. In one study, CTLA-4 blockade using tremelimumab in metastatic melanoma diversified the clonal T cell pool in the blood, without clear correlation with survival.22 In another study, pre-treatment peripheral blood TCR diversity was correlated to clinical outcomes with ipilimumab treatment in a small group of melanoma patients.23
The aim of the present study was to evaluate the effect of combined treatment of tremelimumab and IFN on the clonality of TCR repertoire in tumor and peripheral blood, and correlate it with clinical outcome. Utilizing biospecimens from our previously reported study,14 immunosequencing of the TCR beta chain repertoire was performed in the TME and PBMCs utilizing Adaptive Biotechnologies’ immunoSEQ® Assay to determine repertoire clonality and T cell fraction.
Results
Patient characteristics, clinical efficacy, and safety
Patient demographics and baseline disease characteristics of the 37 enrolled patients have been summarized in Table 1. Clinical efficacy and toxicity data were reported previously.14
Table 1.
Patient demographics and baseline disease characteristics (N = 37 patients).
| Patient Demographics and Baseline Disease Characteristics (N = 37 patients) | ||
|---|---|---|
| Variable | Number of patients | Percentage |
| Age (years) | ||
| Median | 56 | |
| Range | 28–76 | |
| Cutaneous/unknown primary | 29 | 78 |
| Ocular | 8 | 22 |
| Sex | ||
| Male | 23 | 62 |
| Female | 14 | 38 |
| Performance Status | ||
| 0 | 18 | 49 |
| 1 | 19 | 51 |
| Previous therapy | 22 | 60 |
| No. of previous regimens (range) | 1–5 | |
| Adjuvant IFN | 14 | 38 |
| High-dose IL-2 | 7 | 19 |
| Previous brain metastases | 2 | 5.4 |
| AJCC stage | ||
| M1a | 9 | 24 |
| M1b | 6 | 16 |
| M1c | 22 | 60 |
Association of T-cell receptor clonality in tumor tissue with response
To examine whether pre-treatment TCR clonality was associated with clinical benefit, deep immunosequencing of the T-cell repertoire was done using pre-treatment tumor samples of patients (n = 18) (Figure 1 and supplemental figure 1). It was observed that T cell clonality was significantly (p = .035) different and greater in patients who achieved disease control (CR, PR, SD) versus those with non-disease control (PD) (Figure 1(c)). Further, TCR fraction in tumor tissues was compared in responders and non-responders and it was observed that there was a significant (p = .001) increase in TCR fraction in the tumor tissue in responders (CR, PR) versus non-responders (PD, SD) (Figure 1(b))
Figure 1.
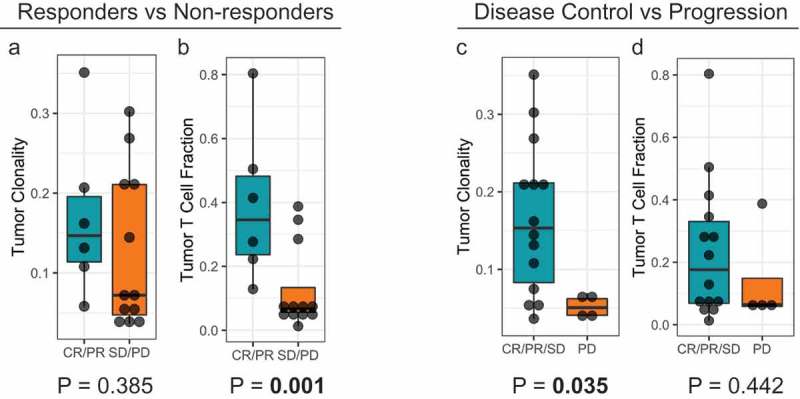
Correlation of T-cell repertoire characteristics of pre-treatment tumor specimens with clinical outcomes. (a) and (b) T-cell clonality and T-cell fraction stratified by responders (CR/PR) and non-responders (SD/PD). (c) and (d) T-cell clonality and T-cell fraction stratified by disease control (CR/PR/SD) and disease progression (PD).
Association of T-cell receptor clonality in tumor tissue with survival
Further, we evaluated if pre-treatment TCR clonality correlated with PFS and OS outcomes. However, we observed no significant correlations. Interestingly, there was a separation of the survival curves where higher TCR clonality at baseline (> 0.12) potentially correlated with better PFS (p = .36) and OS (p = .19) (Figure 2). The lack of significance in terms of survival associations may be a factor of the limited sample size of the study.
Figure 2.
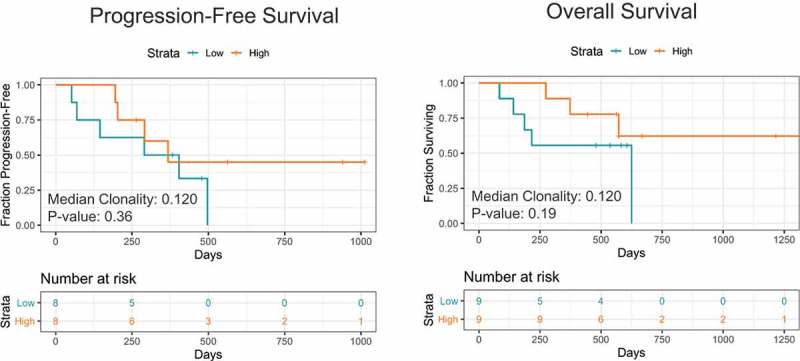
Kaplan-Meier survival plots showing (a) PFS and (b) OS comparing patients with higher (> 0.12) or lower (< 0.12) tumor TCR clonality at baseline.
Association of T-cell receptor clonality in PBMCs with response
We further investigated whether TCR clonality in the PBMC specimens was associated with response to treatment by performing TCR sequencing at baseline and early on-treatment (n = 33 patients) (Figure 3 and supplemental Figure 2 and 3). Unlike the tumor TCR clonality, no significant association was found between pre-treatment PBMC TCR clonality and response. However, there was a trend where patients with disease control tended to have lower clonality at baseline and at 4 weeks.
Figure 3.
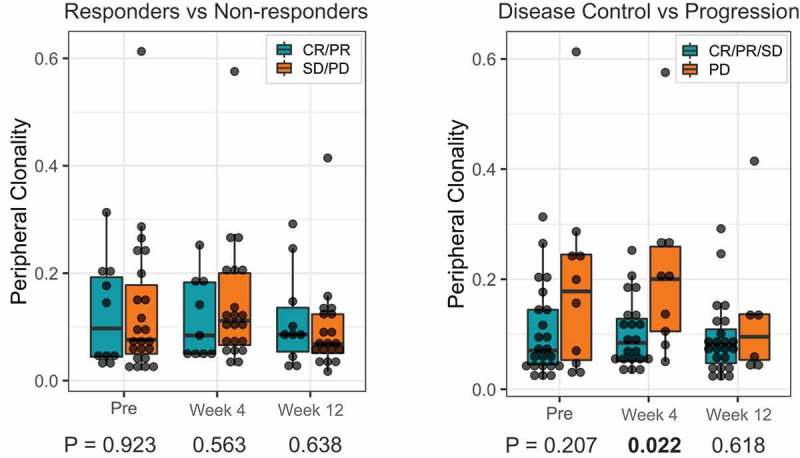
Peripheral T-cell clonality of all patients separated by clinical outcomes and time point. (a) Peripheral T-cell clonality stratified by responders (CR/PR) and non-responders (SD/PD). (b). Peripheral T-cell clonality stratified by disease control (CR/PR/SD) and disease progression (PD).
Association of T-cell receptor clonality in PBMC with survival
Next, we examined if peripheral T-cell repertoire features were associated with survival outcomes (Figure 4). It was found that there was no statistical association of pre-treatment/baseline clonality with PFS and OS. However, early on-treatment (4 weeks), patients with lower peripheral clonality had longer PFS and OS intervals than those with higher peripheral clonality (p = .04 for PFS and p = .01 for OS).
Figure 4.
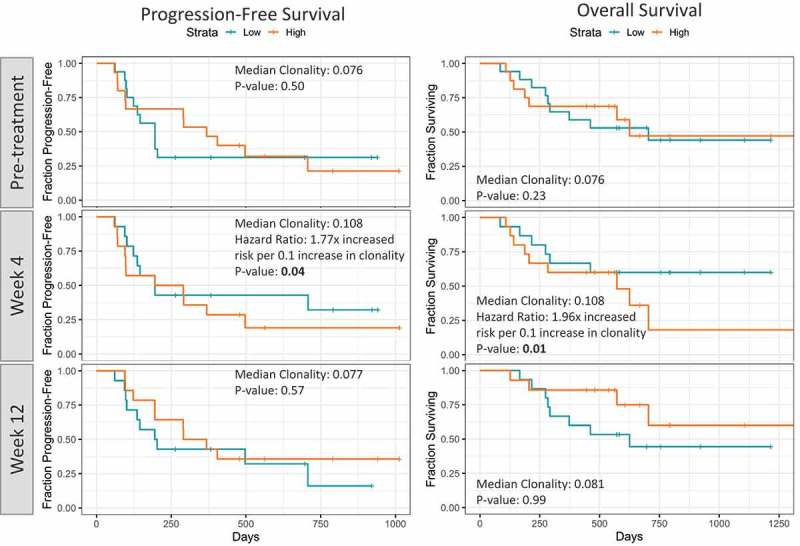
Kaplan-Meier survival plots showing PFS and OS comparing patients with higher or lower median TCR clonality at baseline, week 4 and week 12.
Evolution of T cell repertoire in PBMCs during treatment
To investigate the dynamics of the peripheral T cell repertoire upon CTLA4 blockade and high-dose interferon-alfa (HDI) combination treatment, we compared the peripheral T cell repertoire over different time points (baseline, 4 weeks and 12 weeks). Repertoire turnover, as measured by the Morisita Index, showed a trend towards responders having greater turnover post-treatment than non-responders (Figure 5(a) and supplementary figure 4A). This was evident as non-responders had higher values of Morisita index in comparison to responders, closer to 1, which indicates a more similar repertoire for different time points, while lower values of Morisita index indicate more dis-similarity, consistent with greater repertoire turnover. Similarly, the total number of clones expanding in the peripheral repertoire varied over time within an individual patient (p = .034) with responders showing a greater increase in the number of expanded clones than non-responders, though it was not statistically significant (Figure 5(b) and supplementary figure 4B). When we restricted quantification to only expanded clones that were also found in the tumor repertoire, we observed that responders had significantly more TIL that expanded in the periphery at 4 weeks than non-responders (p = .036) (Figure 5(c) and supplementary figure 4C).
Figure 5.
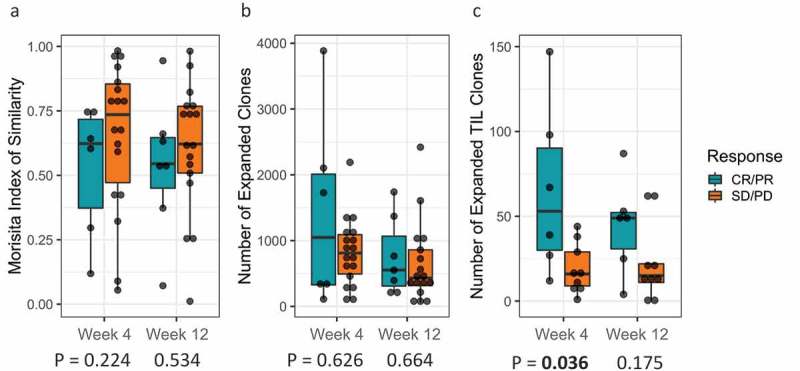
(a) T-cell Repertoire turnover at week 4 and week 12 relative to baseline stratified by clinical response; (b) T-cell clonal expansion at week 4 and week 12 relative to baseline stratified by clinical response; (c) Tumor-infiltrating lymphocytes (TILs) clonal expansion at week 4 and week 12 stratified by clinical response.
Association of T-cell receptor clonality in PBMC with toxicity
We hypothesized that a greater repertoire turnover (as measured by a lower Morisita Index) following immunotherapy treatment may also correlate with the development of immune-related adverse events due to the possible expansion of auto-reactive T cells. We tested PBMC samples from patients who experienced an adverse event and assessed the T-cell repertoire turnover relative to baseline at week 4 and week 12 stratified by presence or absence of adverse event and by adverse event severity. No significant associations were found as tested by linear mixed-effects model in terms of AE incidence (time point p-value = 0.302 and AE p-value = 0.263) (Figure 6(a)), or severity (time point p-value = 0.301 and AE p-value = 0.363) (Figure 6(b)).
Figure 6.
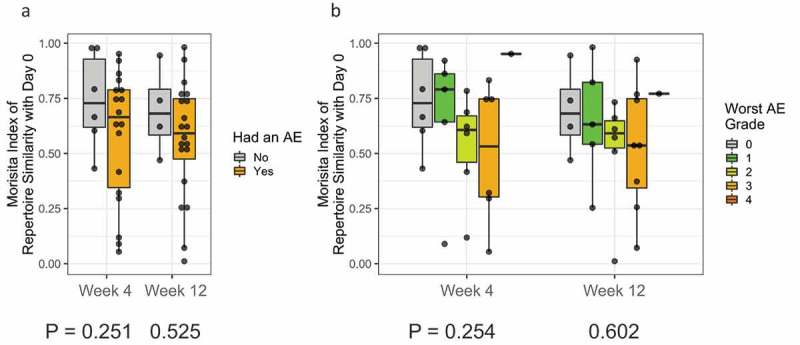
(a) T-cell Repertoire turnover relative to baseline at week 4 and week 12 stratified by presence or absence of adverse event; (b) T-cell Repertoire turnover relative to baseline at week 4 and week 12 stratified by severity of adverse event.
Discussion
Over the past few years, application of immunosequencing has gained traction among cancer researchers as it provides a snapshot of the repertoire of immune cells at any given time and assists in tracking adaptive changes as a result of various immunotherapeutic perturbations. Several groups have attempted to study immune repertoire characteristics using immunosequencing in various solid tumors, in an effort to gain insights into the underlying mechanisms and identify novel biomarkers of response and toxicity.24–27 In this study, we have employed immunosequencing techniques to more deeply characterize both intratumoral and peripheral T-cell responses following treatment with combination therapy of tremelimumab and IFN. Our data showed that an increased TCR fraction and T cell clonality in the pre-treatment TME was associated with favorable clinical outcomes. In PBMCs, at 4 weeks post-treatment, a statistically significant association was noted between decrease in T cell clonality and improvement in PFS and OS.
Our findings in terms of the association of high T cell clonality and TCR fraction with immunotherapeutic response are consistent with previous studies that demonstrated increased baseline density and clonality of TIL to be associated with improved survival in solid tumors following treatment with immunotherapy.24,28,29 In metastatic melanoma patients, Tumeh et al. showed that pre-treatment samples from responding patients treated with Pembrolizumab had higher CD8 T cell at the invasive tumor margin and inside tumors and were associated with a more diverse TIL repertoire.24 However, another study involving sequential combination treatment with ipilimumab and nivolumab in melanoma patients did not show significant differences between increased baseline CD8 + T cell density, and clonality in responders vs non-responders.30
TCR portion of T cell plays a critical role in antigen presentation, therefore an increased T cell fraction has been associated with increased antigenicity and tumor antigen presentation by dendritic cells.31,32 Further, increased T cell infiltration in tumor has been associated with a more robust inflammatory response, with marked increase in cytokines and interferon-γ secretion and regulatory ligands inhibitors expression, mitigating checkpoints inhibitors targets.33 On the other hand, increased T cell clonality in responders may reflect a high tumor mutation rate, increased immunogenic neoantigen production, and easier immune cell recognition of the tumor as “foreign”.34,35 Thus, tumors with a high number of clonal neoantigens may be more likely to elicit effective immune responses following immunotherapy.35 Therefore, defining the tumor repertoire at baseline provides a useful tool to determine general immunocompetence and possible response to immunotherapy. It was interesting to observe in our study, that patients with SD had a unique combination of high clonality (a feature associated with favorable response) and low TIL fraction (a feature associated with poor prognosis) (supplementary Figure 1). This is interesting when considering that patients with SD generally do not achieve durable clinical benefits and usually fall in the middle between responders and progressors in terms of clinical benefits. More work is needed to better understand this phenomenon immunologically.
In examining T cell clonality within the circulation, no significant associations were found in the pretreatment samples and baseline TCR diversity was not associated with clinical outcomes. Similar to our findings, a study by Robert et al. found no association between baseline peripheral TCR repertoire diversity and response to CTLA-4 blockade with tremelimumab.22 However, a pilot study by Postow and colleagues reported that higher response rate was observed in metastatic melanoma patients treated with ipilimumab, who presented with a more diverse peripheral TCR repertoire at baseline.23 Similarly, in patients with pancreatic ductal adenocarcinoma receiving ipilimumab, a diverse baseline TCR repertoire was associated with longer median OS.36
In our analysis of PBMC samples obtained early on-treatment (4 weeks), there was a significant association between a decrease in T cell clonality and OS and PFS. In our study, decrease in T cell clonality early on-treatment (4 weeks) and the improvement in OS and PFS can be a restoration of interferon-mediated effect on tumor-killing after using CTLA-4 blockers, increasing T cell activation and broadening the peripheral T cell repertoire compared with baseline.11–13 The addition of IFN interrupts immune tolerance, increases dendritic cell activation and survival4,37 and increases Th1-mediated pro-inflammatory cytokines in peripheral circulation.38
To address clone level changes in the peripheral TCR repertoire following treatment, we compared the peripheral T cell repertoire over 3 time points. In our analysis, no significant association was found between clonal expansion and clinical outcomes, though there was a trend towards greater clonal expansion in responding patients at 4 weeks and 12 weeks. In a previous study by Hopkins et al. long-term survivors of pancreatic cancer treated with ipilimumab had significantly more expanded clones in comparison to short-term survivors (p < .05).36 Further, as individual T cell clones present in tumors can also be tracked in the peripheral blood during treatment, we restricted our clonal analysis to measure only expanded clones that are also found in the tumor repertoire. Interestingly, we observed that responders had significantly more TIL expanded in the periphery at 4 weeks than non-responders, consistent with clonal expansion of tumor antigen-specific T cells. Similar to our findings, Snyder et al. demonstrated that bladder cancer patients with substantial expansion of tumor-associated TCR clones in the peripheral blood 3 weeks after starting treatment with atezolizumab had more favorable outcomes.39 The expansion of tumor-associated TCRs in the peripheral blood of responding patients may suggest that T cells shuttle back and forth between the tumor and the peripheral compartment where circulating immune cells somewhat reflect or overlap with the tumor’s TIL repertoire. Therefore, a greater number of tumor-specific TCR clones could limit the magnitude of tumor escape due to the presence of more specific T-cells in the circulation. With respect to biomarker development, our finding reinforces the potential value of pursuing noninvasive metrics such as peripheral TCR clonal expansion early on-treatment as a potential predictive biomarker for therapeutic decision-making.
Further, we also observed that T-cell repertoire turnover was not associated with both the development and severity of toxicities after receiving immunotherapy treatment. Prior studies have reported that increasing T cell repertoire turnover is associated with the development of autoimmune toxicities after receiving tremelimumab22 and ipilimumab.40,41 These observations were explained by the fact that not all T cell activation following immunotherapy can be tumor antigen-specific and development of toxicity may be mechanistically linked to proliferation and mobilization of autoreactive T cell populations.
The major limitation of our study was the small number of patients who were enrolled. Our sample size might have not been adequate to show a statistically significant difference in a number of critical analyses. Nevertheless, our study provides further evidence to support the utility of TCRβ sequencing to elucidate the effects of immunotherapy on TIL and PBMC as well as the assessment of response and immune-related adverse events of immunotherapies.
Conclusion
Higher T cell clonality and TIL fraction in the pretreatment TME were found to be associated with immunotherapeutic response. In the circulation, lower peripheral clonality early on-treatment was significantly associated with improved survival. Further, higher TIL expansion in the peripheral repertoire early on-treatment was associated with response to therapy. These findings warrant further investigation and exploration in relation to other immunotherapeutics.
Patient and methods
Patients
Patients with inoperable metastatic melanoma were enrolled in the study.14 Patients were treated with tremelimumab 15 mg/kg intravenously every 12 weeks. HDI was administered concurrently, including intravenous induction at 20 MU/m2/d for 5 days per week for 4 weeks, followed by maintenance at 10 MU/m2/day subcutaneously three times a week for 8 weeks per cycle of tremelimumab. From cycle 2 onward, HDI was administered subcutaneously. Patients without evidence of disease progression or limiting toxicities were offered additional cycles of therapy up to a maximum of four cycles. The institutional review board at the University of Pittsburgh approved the study and a written informed consent was obtained from all the patients.
Response and toxicity assessment
Response to treatment was evaluated by assessing target lesions with Response Evaluation Criteria in Solid Tumors [RECIST] version 1 using imaging studies, carried out at the end of each cycle or earlier if clinically indicated.42 Patients were classified as having a complete response (CR), partial response (PR), stable disease (SD), or Progressive disease (PD). Descriptions and grading scales found in the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) were used for grading and reporting of adverse events (AEs). In addition, overall survival (OS) and progression-free survival (PFS) data were obtained for all patients.
Analysis of TCRβ repertoire
The TCR repertoire of PBMC specimens (N = 33 patients) and TME (N = 18) was immunosequenced at pre-treatment (TME and PBMC), one month post-treatment (PBMC), and three months post-treatment (PBMC). Immunosequencing of the CDR3 regions of human TCRβ chains was performed using the immunoSEQ® Assay (Adaptive Biotechnologies, Seattle, WA). Extracted genomic DNA was amplified in a bias-controlled multiplex polymerase chain reaction, followed by high-throughput sequencing. Sequences were collapsed and filtered in order to identify and quantitate the absolute abundance of each unique TCRβ CDR3 region for further analysis as previously described.43–45
Clonality and T-cell fraction were calculated for each immunosequenced sample. Clonality is defined as (1 – Peilou’s Evenness) and was calculated on productive rearrangements by Equation 1,
where is the proportional abundance of rearrangement and is the total number of rearrangements in a sample. Clonality values range from 0 to 1 and describe the shape of the frequency distribution: clonality values approaching 0 indicate a very even distribution of frequencies, whereas values approaching 1 indicate an increasingly asymmetric distribution in which a few clones are present at high frequencies.46 T-cell fraction was defined as the proportion of T cells to all nucleated cells in a sample.
The Morisita Index is a measure of repertoire similarity, as defined by Equation 2,
where and are the T cell counts of clone in samples A and B, and are the total number of T cells in samples A and B, and is the total number of unique clones in the union of samples A and B. Like clonality, the Morisita Index ranges from 0 to 1, with values closer to 1 indicating high similarity and less repertoire turnover.47 In this study, post-treatment PBMC repertoires were compared to pre-treatment for each patient.
Clonal expansion also compared post-treatment PBMC repertoires to pre-treatment for each patient. As previously described, clones that increased in frequency above our statistical threshold (multiple testing corrected p-value 0.01) in the post-treatment repertoire (relative to pre-treatment) were identified as expanded.48
Statistical methods
Two-tailed, non-parametric statistical tests were used to compare patient response (Wilcoxon Rank Sum or Kruskal-Wallis Tests) and time points (Wilcoxon Signed Rank Test). Multivariate analyses were performed with linear mixed-effects models. Likelihood Ratio Tests were applied to Cox Proportional Hazard models to determine associations between continuous variables and PFS/OS. Unless otherwise specified, p-values 0.05 were considered statistically significant. Statistical analyses were performed in R version 3.5.1. Given the limited sample size, our attempts for adjusting the p-values for multiple testing led to the loss of statistical significance using a p-value of 0.05 (except for the correlation of T-fraction in pre-treatment tumor specimens with an objective response as summarized in Figure 1).
Funding Statement
Pfizer funded the original clinical study. Laboratory services provided at no cost by Adaptive Biotechnologies;Pfizer Foundation.
Disclosure of interest
Arjun Khunger report no conflict of interest
Julie A. Rytlewski, Paul Fields and Erik C. Yusko have equity and employment with Adaptive Biotechnologies.
Ahmad A. Tarhini declared consultant role with Bristol Myers Squibb, Merck, Novartis, Genentech-Roche, Array Biopharma, HUYA, Immunocore, NewLink Genetics.
Disclaimers
immunoSEQ and their associated designs are trademarks of Adaptive Biotechnologies. immunoSEQ Assays are for research use only and not for use in diagnostic procedures.
List of where and when study presented elsewhere: Partly presented at the 2017 and 2019 ASCO annual meeting.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Pasquali S, Hadjinicolaou AV, Sileni VC, Rossi CR, & Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parlato S, Santini SM, Lapenta C, Di Pucchio T, Logozzi M, Spada M, Giammarioli AM, Malorni W, Fais S, Belardelli F.. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98(10):3022–3029. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Richards T, Zarour HM, Sosman J, Ernstoff M, Whiteside TL, Ibrahim J, Blum R, Wieand S, Mascari R.. Immunomodulatory effects of high-dose and low-dose interferon alpha2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer. 2002;95(5):1101–1112. doi: 10.1002/cncr.10775. [DOI] [PubMed] [Google Scholar]
- 5.Wenjun Wang HDE, Uma NM, Rao DM, Jukic SR, Land SF, John M.. Kirkwood, modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNα2b. Clin Cancer Res. 2007;13:1523–1531. [DOI] [PubMed] [Google Scholar]
- 6.Carballido JA, Moltó ML, Manzano L, Olivier C, Salmerón OJ, de Mon MA. Interferon-alpha-2b enhances the natural killer activity of patients with transitional cell carcinoma of the bladder. Cancer. 1993;72(5):1743–1748. [DOI] [PubMed] [Google Scholar]
- 7.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P.. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328(6127):267–270. [DOI] [PubMed] [Google Scholar]
- 8.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–5791. [PubMed] [Google Scholar]
- 9.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH.. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 11.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 13.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarhini AA, Cherian J, Moschos SJ, Tawbi HA, Shuai Y, Gooding WE, Sander C, Kirkwood JM. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30(3):322. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A, Comin-Anduix B, Chmielowski B, Jalil J, de la Rocha P, TA M, Ochoa MT, Seja E, Villanueva A, Oseguera DK, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang RR, Jalil J, JS E, Chmielowski B, RC K, Mok S, Sazegar H, Seja E, Villanueva A, Gomez-Navarro J, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17:4101–4109. doi: 10.1158/1078-0432.CCR-11-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 18.Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63(7):675–683. doi: 10.1007/s00262-014-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for Immunostimulatory Monoclonal Antibodies in Combination Strategies for Melanoma and Other Tumor Types. Clinical Cancer Research. 2013;19(5):1009-1020. [DOI] [PubMed] [Google Scholar]
- 20.Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology. 2016;5(4):e1100788. doi: 10.1080/2162402X.2015.1100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins AM, Rowland A, Kichenadasse G, Wiese MD, Gurney H, McKinnon RA, Karapetis CS, Sorich MJ. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117(7):913. doi: 10.1038/bjc.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B, Koya RC. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clinical Cancer Research 2014;20(9):2424-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, Perez S, Tanneau I, Noel M, Courtier A, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015;3(1):23. doi: 10.1186/s40425-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roh W, Chen P-L, Reuben A, Spencer CN, Prieto PA, Miller JP, Gopalakrishnan V, Wang F, Cooper ZA, Reddy SM, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9(379):eaah3560. doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page DB, Yuan J, Redmond D, Wen YH, Durack JC, Emerson R, Solomon S, Dong Z, Wong P, Comstock C, et al. Deep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapy. Cancer Immunol Res. 2016;4(10):835–844. doi: 10.1158/2326-6066.CIR-16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martín-Algarra S, Mandal R, Sharfman WH, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(4):934–949.e16. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, Liniker E, Kong BY, Cooper AJ, Howle JR, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in Melanoma. Clin Cancer Res. 2017;23(17):5024–5033. doi: 10.1158/1078-0432.CCR-16-0698. [DOI] [PubMed] [Google Scholar]
- 29.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6(8):827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei-Shen Chen MCA, Spencer C, Tawbi HA-H, Lazar A, Tetzlaff MT, Tetzlaff MT, Patel SP, Hwu P, Hwu W-J, Diab A, et al. Molecular and immune predictors of response and toxicity to combined CTLA-4 and PD-1 blockade in metastatic melanoma (MM) patients (pts). J Clin Oncol. 2017;35(15):9579. doi: 10.1200/JCO.2017.35.15_suppl.9579. [DOI] [Google Scholar]
- 31.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Li T, Pignon J-C, Wang B, Wang J, Shukla SA, Dou R, Chen Q, Hodi FS, Choueiri TK, et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet. 2016;48(7):725–732. doi: 10.1038/ng.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack SM, He Q, Yearley JH, Emerson R, Vignali M, Zhang Y, Redman MW, Baker KK, Cooper S, Donahue B, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123(17):3291–3304. doi: 10.1002/cncr.30726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riaz N, Havel JJ, Kendall SM, Makarov V, Walsh LA, Desrichard A, Weinhold N, Chan TA. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat Genet. 2016;48(11):1327–1329. doi: 10.1038/ng.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins AC, Jacobsen KM, Henry CJ, Huey MG, Parker RE, Page LS, Hill AA, Wang X, Frye SV, Earp HS, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight. 2018;3(13). doi: 10.1172/jci.insight.97941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Edington HD, Rao UNM, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNα2b. CCR. 2007;06:1387. [DOI] [PubMed] [Google Scholar]
- 38.Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, Gorelik E, Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13(8):2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 39.Snyder A, Wordsworth S, Fermont JM, Page S, Kaur K, Camps C, Kaisaki P, Gupta A, Talbot D, Middleton M, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med. 2017;14(5):e1002309. doi: 10.1371/journal.pmed.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, Faham M, Fong L. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 2017;77(6):1322–1330. doi: 10.1158/0008-5472.CAN-16-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L, et al Clonal expansion of CD8 T cells in the systemic circ. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 43.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood. 2009;114(19):4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robins H, Desmarais C, Matthis J, Livingston R, Andriesen J, Reijonen H, Carlson C, Nepom G, Yee C, Cerosaletti K. Ultra-sensitive detection of rare T cell clones. J Immunol Methods. 2012;375(1–2):14–19. doi: 10.1016/j.jim.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung M-W, Parsons JM, Steen MS, LaMadrid-Herrmannsfeldt MA, Williamson DW, Livingston RJ, et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun. 2013;4:2680. doi: 10.1038/ncomms3680. [DOI] [PubMed] [Google Scholar]
- 46.Kirsch I, Vignali M, Robins H. T-cell receptor profiling in cancer. Mol Oncol. 2015;9(10):2063–2070. doi: 10.1016/j.molonc.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rempala GA, Seweryn M. Methods for diversity and overlap analysis in T-cell receptor populations. J Math Biol. 2013;67:1339–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeWitt WS, Emerson RO, Lindau P, Vignali M, Snyder TM, Desmarais C, Sanders C, Utsugi H, Warren EH, McElrath J, Makar KW.. Dynamics of the cytotoxic T cell response to a model of acute viral infection. J Virol. 2015;89(8):4517–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


