ABSTRACT
Objectives: Although low-dose computed tomography has been confirmed to have meaningful diagnostic utility, lung cancer is still the leading cause of cancer-related deaths for both genders worldwide. Thus, a novel panel with a stronger diagnostic performance for lung cancer is needed. This study aimed to investigate the efficacy of a new panel in lung cancer diagnosis.
Materials and Methods: The serum levels of carcinoembryonic antigen (CEA), cancer antigen 125 (CA125) and seven autoantibodies were measured and statistically analyzed in samples from healthy controls and patients with lung cancer. The 316 candidates enrolled in this study were randomly assigned into two groups for the training and validation of a diagnostic panel.
Results: An optimal panel with four biomarkers (CEA, CA125, Annexin A1-Ab, and Alpha enolase-Ab) was established, with an area under the receiver operator characteristic (ROC) curve (AUC) of 0.897, a sensitivity of 86.5%, a specificity of 82.3%, a positive predictive value (PPV) of 88.3%, a negative predictive value (NPV) of 79.7%, and a diagnostic accuracy of 84.8% for the training group. The panel was validated, with an AUC of 0.856 and a sensitivity of 87.5% for the validation group. Furthermore, the new panel performed significantly better in lung cancer screening than did CEA and CA125 in all of the cohorts (p< .05).
Conclusion: The diagnostic performance of CEA and CA125 was significantly enhanced through their combination with two autoantibodies (Annexin A1-Ab, and Alpha enolase-Ab). Optimization of the measured autoantibodies is critical for generating a panel to detect lung cancer in patients.
KEYWORDS: Lung cancer, Diagnostic panel, Autoantibody, Biomarkers, Tumor associated antigens
Introduction
Due to its high incidence rate and poor prognosis, lung cancer, as the leading cause of cancer-related mortality worldwide,1 has become a serious and growing disease burden throughout the world. The National Lung Screening Trial (NLST), which enrolled ‘at-risk’ individuals, demonstrated that low-dose computed tomography (LDCT) could reduce relative lung cancer mortality by 16% to 20%;2 however, the dismal fact is that the proportion of lung cancers in advanced stages remains higher than that in early-stages.1,3 Furthermore, the LDCT screening project had some limitations, including health effects due to high false-positive results, radiation-associated risksand significant economic costs.4,5 Therefore, scientific research aimed to develop a more reliable diagnostic modality to identify early-stage lung cancer is an urgent priority.
Compared with LDCT, blood biomarkers have the advantages of repetitive operation and serial monitoring in lung cancer detection. The clinical value of common biomarkers, carcinoembryonic antigen (CEA) and cancer antigen 125 (CA125), carrying indicative information of lung cancers has been established;6–8 however, studies found that these individual markers have low sensitivity or increasing sensitivity at the cost of specificity.9–11 Correspondingly, effective diagnostic biomarkers that complement these traditional blood tests for lung cancer detection are essential.
Recently, a humoral immune system response against tumor-associated antigens (TAAs) has been documented with increasing evidence. In particular, a cancer-specific humoral response was evident in the sera of individuals, permitting the discovery of disease earlier than that possible with radiographic detection.12 Autoantibodies (AAbs) can be detected earlier than TAAs, and they are also more stable in serum samples, clearly indicating their advantages in the diagnosis of lung cancer.13 For instance, Autoantibody to Annexin A1 was reported significantly higher in lung cancer patients than healthy controls;14,15 Alpha enolase, expressed on the surface of cancer cells, was closely correlated with cell glycolysis, growth, migration, and invasion in non-small cell lung cancer (NSCLC) through FAK-mediated PI3K/AKT pathway,16 and the correlation between Alpha enolase-Ab and tumor progression of lung and breast cancer patients has suggested that Alpha enolase-Ab may be a potentially useful marker for monitoring the staging of cancer patients.17 PGP 9.5, a key regulator to promote tumor cell invasion,18 was highly expressed in primary lung cancers instead of normal lung issue and strongly associated with tumor cancer pathological stages19,20 making it a tumor-associated antigen for lung cancer screening and diagnosis.21 Because of the strong immunogenicity of Cancer/testis antigen 1,22 performance of Cancer/testis antigen 1-Ab in lung cancer diagnosis was superior.23,24 Autoantibody reactivity targeted to 14–3-3 protein theta in sera of lung cancer patients was significantly greater compared with controls, and the serum autoantibody could be detected months earlier prior to the diagnosis of lung cancer.25 Fructose-bisphosphate aldolase A as a prognostic marker in lung cancer correlated with poor prognosis and the epithelial–mesenchymal transition (EMT) process highly,26,27 suggesting the detection of Fructose-bisphosphate aldolase A-Ab is of potentiality in the diagnosis of lung cancers. Autoantibody to cellular tumor antigen p53 is of close correlation with TP53 mutations and could appear before the cancer diagnosis.28
Currently, identifying a combination of biomarkers and/or clinical parameters for the diagnosis of lung cancers is of interest;29,30 Based on previous studies,25,31,32 occurrence of autoantibodies to tumor antigens in lung cancer sera has been presented; thus, seven tumor-associated antigens (Annexin A1, Fructose-bisphosphate aldolase A, Alpha-enolase, PGP 9.5, 14–3-3 protein theta, Cancer/testis antigen 1, Cellular tumor antigen p53) that are closely correlated with tumor cell glycolysis, growth, invasion, migration and prognosis14-16,18,26–28 were firstly analyzed in combination with CEA and CA125 to develop and optimize a mixed diagnostic panel in our study.
Results
Random selection and assignment of the study population
To avoid selection bias, 316 candidates were equally divided into two cohorts (158:158) randomly according to the randomized digital table. Although case matching was not performed in our study, however, no significant differences of basic characteristics were found between the two groups (Table 1). On the other hand, the proportion of subjects with a history of smoking was relatively high in both cohorts: 87.3% and 88.6% in the training and validation cohorts, respectively.
Table 1.
Patients’ characteristics.
| Training Group |
Validation Group |
||
|---|---|---|---|
| Variable | (n = 158) | (n = 158) | p value |
| Age | |||
| ≤60 | 64 | 76 | 0.174 |
| >60 | 94 | 82 | |
| Sex | |||
| Male | 63 | 67 | 0.674 |
| Female | 95 | 91 | |
| Smoking History | |||
| Yes | 138 | 140 | 0.729 |
| No | 20 | 18 | |
| Enrolled Candidates | |||
| NSCLC | 96 | 80 | 0.07 |
| Healthy controls | 62 | 78 |
Abbreviations: NSCLC = Non-Small Cell Lung Cancer
Levels of single biomarkers in the training and validation cohorts
Comparisons of single biomarkers in lung cancer patients and healthy controls were conducted in the two cohorts. In the training group, significantly increased levels of seven tested autoantibodies (p< .05) and two protein markers (CEA and CA125; p< .05) were observed in cancer patients compared with healthy controls. Additionally, the levels of PGP 9.5-Ab, Annexin A1-Ab, CEA and CA125 were significantly higher in cancer patients than those of healthy controls in the validation group (p< .05) (Figure 1).
Figure 1.
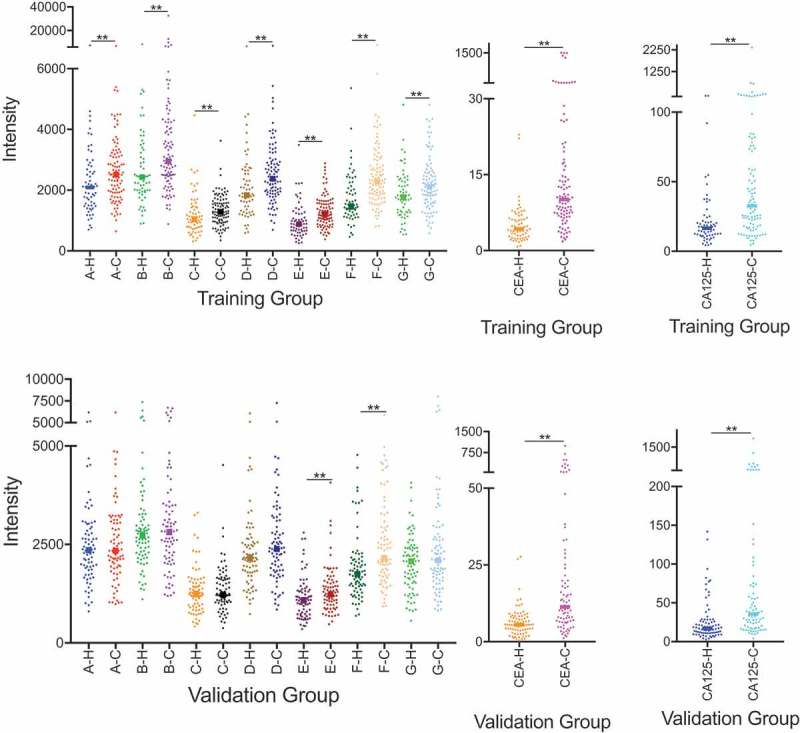
Comparisons of levels of each protein markers and autoantibodies between lung cancers patients and healthy controls in training and validation cohort. CEA = carcinoembryonic antigen, CA125 = cancer antigen 125, A = Fructose-bisphosphate aldolase A; B = Cancer/testis antigen 1; C = Alpha enolase; D = 14–3-3 protein theta; E = Annexin A1; F = PGP 9.5; G = Cellular tumor antigen p53; -H = Healthy controls; -C = Lung cancers patients; ** = p value < .05.
A ROC was generated for each autoantibody and tumor protein using the training group data to demonstrate individual diagnostic performance (Figure 2a). The lowest performance for discriminating lung cancer patients from healthy controls was found for the autoantibody against Alpha enolase, which exhibited an AUC of 0.619 (95%CI: 0.526–0.712); meanwhile, CEA had the highest diagnostic ability with an AUC of 0.835 (95%CI: 0.772–0.898).
Figure 2.
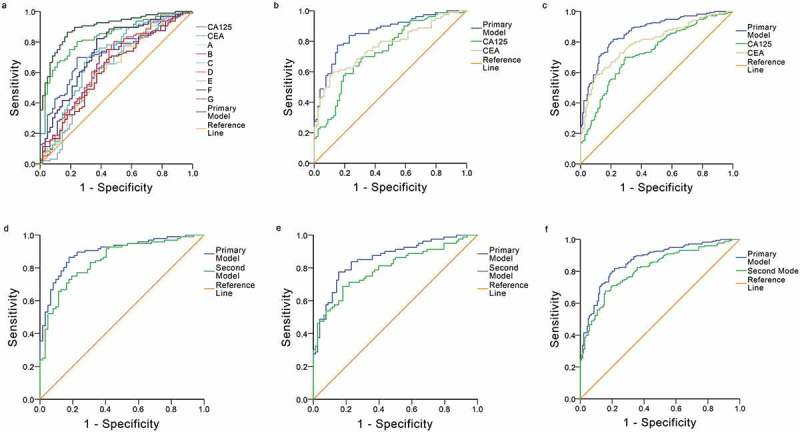
Receiver operator characteristic (ROC) curves for each single tested biomarker and diagnostic models in different cohorts. a: Comparison of the diagnostic ability of the primary model and each biomarker in the training cohort. CEA = carcinoembryonic antigen, CA125 = cancer antigen 125, A = Fructose-bisphosphate aldolase A; B = Cancer/testis antigen 1; C = Alpha enolase; D = 14–3-3 protein theta; E = Annexin A1; F = PGP 9.5; G = Cellular tumor antigen p53. b, c: Comparison of the diagnostic ability of the primary model and protein biomarkers in the validation cohort and the whole cohort. d, e, f: Comparison of the diagnostic ability of the primary model and second model in the training cohort, the validation cohort and the whole cohort, respectively.
Establishing the diagnostic panel
A new combined diagnostic panel was established according to the results of the logistic regression analysis of the training cohort (Appendices Table A1). This analysis revealed that autoantibodies against Alpha enolase and Annexin A1 were significant variables to predict the diagnosis of lung cancers (p< .001). In addition, CEA (p< .001) and CA125 (p= .044) were included in the final model due to their diagnostic value (Table 2). The probability of lung cancer was calculated based on the equation from the logistic regression model (P = EXP/[1+ EXP], EXP = e(-3.046+0.253*CEA+0.015*CA125+0.007*Annexin A1-0.005* Alpha enolase)).
Table 2.
Comparisons of diagnostic models and protein markers in different cohorts.
| Group | Variable | AUC | 95% CI | p value |
|---|---|---|---|---|
| Training Group | CEA | 0.835 | 0.772–0.898 | 0.0081 |
| (n = 158) | CA125 | 0.728 | 0.649–0.807 | <0.0001 |
| Primary Model | 0.897 | 0.848–0.946 | / | |
| Second Model | 0.846 | 0.785–0.907 | 0.0061 | |
| Validation Group | CEA | 0.769 | 0.695–0.842 | 0.0031 |
| (n = 158) | CA125 | 0.736 | 0.659–0.813 | 0.0024 |
| Primary Model | 0.856 | 0.798–0.914 | / | |
| Second Model | 0.797 | 0.727–0.866 | 0.0265 | |
| Whole Group | CEA | 0.797 | 0.749–0.846 | 0.0005 |
| (n = 316) | CA125 | 0.729 | 0.674–0.784 | <0.0001 |
| Primary Model | 0.866 | 0.826–0.905 | / | |
| Second Model | 0.814 | 0.768–0.861 | 0.0025 |
Abbreviations: CEA = carcinoembryonic antigen, CA125 = cancer antigen 125, AUC = area under the curve, 95%CI = 95% Confidence Intervals.
Analysis of diagnostic performance and validation of the new model
To study the diagnostic value of our model, we compared its performance with existing markers (CEA and CA125) in three disparate groups (Table 2).
The comprehensive performance of the diagnostic model in the training group (AUC: 0.897, 95%CI: 0.848–0.946) was significantly better than that of CEA (AUC: 0.835, 95%CI: 0.772–0.898) and CA125 (AUC: 0.728, 95%CI: 0.649–0.807) (p< .05). A YI of 0.688 was used to select a cut-off value of 0.492, and the model had a sensitivity of 86.5%, a specificity of 82.3%, a PPV of 88.3%, an NPV of 79.7%, and a diagnostic accuracy of 84.8%.
In the validation group, ROCs of the panel and two other protein markers were generated and statistically analyzed (Figure 2b). The diagnostic panel was validated (AUC: 0.856, 95%CI: 0.798–0.914), and it performed significantly better than CEA (AUC: 0.769, 95%CI: 0.695–0.842) and CA125 (AUC: 0.736, 95%CI: 0.659–0.813) in the detection of lung cancers (p< .05). With a cut-off value of 0.492, the sensitivity, specificity, PPV, NPV and diagnostic accuracy of the panel were 87.5%, 60.3%, 69.3%, 82.5% and 74.1%, respectively.
The multiple-variables model was further analyzed in two cohorts with 316 candidates, yielding an AUC of 0.866 (95%CI: 0.826–0.905) and demonstrating statistically a diagnostic advantage over CEA (AUC: 0.797, 95%CI: 0.749–0.846) and CA125 (AUC: 0.729, 95%CI: 0.674–0.784) (p< .001) (Figure 2c) in the discrimination of lung cancers from healthy controls. In the entire cohort, the diagnostic model had a sensitivity of 85.8%, a specificity of 70.0%, a PPV of 77.8%, an NPV of 79.5% and a diagnostic accuracy of 80.0% (Appendicies Table 2).
Moreover, a second model that included only CEA and CA125 was established based on the same training cohort for pairwise comparison with our primary model using the two groups. In the training group, the AUC of the equation (P = EXP/[1+ EXP], EXP = e(−1.973+0.016*CA125+0.241*CEA)) was 0.846 (95%CI: 0.848–0.946) (Figure 2d), a value significantly smaller than that of our primary model (0.897, 95%CI: 0.848–0.946) (p= .0061). Similarly, in the validation group, the AUC of the second model (0.797, 95%CI: 0.725–0.856) (Figure 2e) was inferior compared to that of our primary model (AUC: 0.856, 95%CI: 0.798–0.914) (p= .0265). The second model also performed unsatisfactorily in the whole group, yielding an AUC of 0.814 (95%CI: 0.768–0.861) (p= .0025) (Figure 2f).
Discussion
Unlike other biomarkers, autoantibodies are produced early in process of oncogenesis, and they are present in serum before tumor-associated antigens can be detected.12,31,33 Therefore, the detection of tumor autoantibodies has great potential in early diagnosis of lung cancers,34 especially for asymptomatic patients. The first diagnostic panel with autoantibodies for the detection of lung cancer, which had an AUC of 0.838, was proposed by Dr. Hanash; however, the study cohort was small, and validation was not performed.25 Since then, the application of tumor-associated antigens in panels for the detection of cancers has been demonstrated by a number of studies. Nevertheless, these publications commonly report high specificity with relatively lower sensitivity and insufficient reproducibility in different cohorts.24,25,31,33,35–38 We speculated that the main reasons for these issues include the heterogenic nature of lung cancer and the screening strategy of using tumor-associated antigens. Although tumor-associated antigens can activate immune responses during the process of cancer immunoediting,39 these responses may not be very strong in patients with indolent lung cancers,12 and they can be affected by treatments.40 The sensitivity of measuring a single autoantibody is also low due to the complexity and heterogeneity of lung cancer as well as tumor-associated antigen expression.33,41 Thus, to enhance the clinical value of a panel for early screening and diagnosis, we focused on optimizing a combination of tumor-associated antigens and autoantibodies instead of simply adding additional antigens for testing.
The tumor protein biomarkers CEA and CA125 have been widely studied as their predictive and prognostic abilities contribute to the detection of lung cancers, early relapse, and progression.6–8,42,43 Both CEA and CA125 are expressed at relatively high levels in advanced-stage cancers compared to early-stage cancers.6,44 Dr. Doseeva studied a mixed panel of three protein markers (CEA, CA125, and CYFRA 21–1) and one autoantibody marker (NY-ESO-1), yielding an AUC of 0.81 in high-risk individuals with smoking history of more than 20 years.44 Although her panel had a sensitivity of 74% and a specificity of 80%, 44 which were lower than our results, the comparable diagnostic performance of a novel multiplexed dual analyte immunoassay was confirmed.
In our study, the levels of two well-investigated biomarkers (CEA and CA125) and seven autoantibodies were separately measured in two randomly divided cohorts to establish a well-optimized model with strong predictive value. Significant differences were observed for each biomarker in the training cohort, but the mean values and standard deviations of single biomarkers were not used for further analysis in our study. Proper statistical analysis is important for data evaluation. Instead of defining positive results based on a single biomarker, a multiple logistic regression analysis was performed to select biomarkers for a mixed model and to generate the ROCs. Furthermore, pairwise comparisons of the ROCs of our primary model and each single protein biomarker were applied to assess significant differences. Unsurprisingly, statistical analysis confirmed that the single biomarkers had relatively low diagnostic performance in the training group, with AUCs ranging from 0.619 to 0.835. While the autoantibody against PGP 9.5 demonstrated the best predictive ability among the seven autoantibodies in both the training and validation groups (AUC: 0.743 (95%CI: 0.662–0.824) and AUC: 0.687 (95%CI: 0.604–0.770), respectively), it was not included in the mixed panel according to the results of the multiple logistic analysis.
Ultimately, the diagnostic model included four markers based on the statistical results, yielding a mixed panel with significant diagnostic performance (AUC: 0.897, 95%CI: 0.848–0.946) compared with that of CEA and CA125 (p< .05). Additionally, our model was validated with the test cohort, showing significantly better performance than that of each included protein marker (p< .05), with an AUC of 0.856 (95%CI: 0.798–0.914). To further analyze the predictive performance of the protein markers and the primary mixed model, a second model including only CEA and CA125 was established based on the same training group. However, our primary model demonstrated significantly better performance than this second model in the training, validation and whole groups (Figure 2d,e,f).
Reasonable statistical methods are important for multiple factors analyses. In our study, Youden’s index was employed to determine the cutoff value for establishing a well-optimized model with good discriminative ability. As the ROC shows, the sensitivity of our model was as high as 86.5% with a specificity of 82.3% in the training group, values higher than those of the previously published studies mentioned above. Although 31 healthy controls were misclassified by our model, resulting in a specificity of 60.0%, its sensitivity and NPV were as high as 87.5% and 82.5%, respectively, in the validation group, and significantly better diagnostic performance was demonstrated by the statistical analysis compared to that of the protein markers. Furthermore, a good combined diagnostic panel should cover broad clinical phenotypes to reflect the presence of lung cancers of different types as well as those in different stages; thus, studying the specific distribution of misclassifications and analyzing the diagnostic performance of subgroups may be of subtle importance. However, the proportion of lung cancers with ground-glass opacity (GGO) areas identified by high-resolution computed tomography (HRCT) is seriously increasing; consequently, a combined panel of blood biomarkers and with scanning for specific abnormalities may be of greater value than application of CT alone in the diagnosis of some specific types of lung cancers. Moreover, as surgical technology improves and the resection regions of small-sized lung cancers without lymph node metastasis have been innovated,45 the underlying correlations between serum levels of autoantibodies and lymph node staging is worth being studied. As the enrollment of our candidates was completely retrospective, a relatively higher proportion of patients with smoking history resulted; thus, our primary model may have more stable predictive performance in cohorts of high-risk smokers, and more samples from non-smokers will be collected for further validation in the future. Although the diagnostic accuracy of our model was 84.8% in the training group, 74.1% in the validation group and 80.0% in the whole group, no marker can provide 100% diagnostic specificity and accuracy. Individuals in the control group with positive results via our mixed model should be intensively followed up by imaging tests to exclude any occult cancers.
Some limitations should be addressed. First, our study was retrospectively designed. Second, additional samples from non-lung cancer diseases such as benign lung nodules or chronic obstructive pulmonary disease (COPD) were not enrolled, which may limit the clinical utility of our model.
Conclusions
Collectively, a newly optimized panel that measures protein markers and serum autoantibodies against tumor-associated antigens was established by statistical analysis. Furthermore, we demonstrated that the model could significantly help detect lung cancer, which showed much higher efficacy than using protein markers alone. In the future, serial samples could be collected to further validate our model in determining lung cancer prognosis.
Material and methods
Blood samples and patient details
In the specimen bank of the Cancer Hospital at the Chinese Academy of Medical Sciences, serum samples of lung cancer patients and healthy controls between August 2017 and October 2018 were collected and stored. Totally, 180 blood samples of lung cancers were collected; however, 176 eligible samples were finally enrolled in our study. Meanwhile, there were 140 blood samples of healthy controls. Therefore, 316 blood samples of lung cancer patients and healthy controls were enrolled. To minimize the selection bias, each enrolled candidate was randomly assigned to the training group or validation group. This study was approved by the Chinese Academy of Medical Sciences Institutional Review Board.
Blood samples of patients with lung cancers and healthy controls were obtained before any anticancer treatment was given. Patients with lung cancers who met the following criteria were included: (a) no history of other malignant tumors and (b) surgical treatment was performed within one month after blood sampling. Healthy individuals in the control group were selected from Physical Examination Centers; these individuals were confirmed to have no lung nodules by chest X-ray or thin-sliced computed tomography as well as no history of malignant tumors. Following informed consent, all sera samples collected were stored in a serum repository at −80°C.
Immunoassay for serum autoantibodies
Before the experiment, we coupled MagPlex microspheres (Luminex Corp.) to seven proteins (10 µg of protein/million beads), individually. The microspheres were activated by N-hydroxysulfosuccinimide (sulfo-NHS) and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) solutions and were then incubated with proteins in the dark for 2 h at room temperature. Concurrently, the serum samples were diluted (1:150) with phosphate buffer.
Multiplexed serum immunoassays were performed using a Luminex MagPix Instrument (Luminex Corp.). Working microsphere mixtures were prepared by diluting the coupled microsphere stock for each protein to a final concentration of 10 beads/µL (50 µL/well, 500 beads/well) in assay buffer. Fifty-microliter aliquots of each prepared working microsphere solution were added to wells in duplicate, and 50 µL of assay buffer were used as blank controls. Subsequently, 50 µL of diluted serum from each patient was added, followed by incubation for 60 min at room temperature on a plate shaker. After two washes with PBS, the immobilized autoantibody-protein complexes were incubated with detection antibodies (anti-Human IgG + IgA + IgM) for 30 min at room temperature on a plate shaker. The plates were then washed twice, and 50 µL of streptavidin phycoerythrin (SAPE, 4 µg/mL; Life Technologies) was added to each well, followed by incubation for 30 min at room temperature on a plate shaker. Finally, each plate was washed again, and 100 µL of drive fluid (Luminex Corp.) was added to each well prior to analysis. In addition, the CEA and CA125 levels were detected using a customized coupled microbead kit (Millipore).
Statistical analysis of the data
Shapiro Wilk`s test was used to assess normal distribution, and differences in the autoantibody levels between the cancer patients and healthy controls in the two cohorts were separately analyzed using nonparametric and Mann–Whitney U tests. In the training group, binary logistic regression was performed to screen for variables to comprise a panel with the best diagnostic performance. The area under the receiver operator characteristic (ROC) curve (AUC) was estimated with 95% confidence intervals (CIs) using predicted probability values to assess the discriminatory capacity of the multiple-variable model. Using the training group, Youden’s index (YI) was calculated to establish the predicted probability cut-off for discriminating lung cancer patients from normal controls. In addition, ROCs for single markers and different subgroups were constructed and compared. Standard descriptive statistics such as frequency, mean value, median value, positive predictive value (PPV), negative predictive value (NPV), and standard deviation (SD) were calculated to describe the study population.
Statistical analysis was performed using SPSS 24.0, GraphPad Prism 5.0, MedCalc (Version 11.4.2.0) and Microsoft Excel. p values are two-tailed, and a p value less than 0.05 was considered to be statistically significant.
Appendices
Table A1.
Multivariable logistic regression
| Variable | Beta Coefficient | S.E. | Wald | df | Sig | Exp(B) |
|---|---|---|---|---|---|---|
| CA125 | 0.015 | 0.007 | 4.04 | 1 | 0.044 | 1.015 |
| CEA | 0.253 | 0.065 | 15.034 | 1 | 0 | 1.287 |
| Alpha enolase | −0.005 | 0.002 | 12.167 | 1 | 0 | 0.995 |
| Annexin A1 | 0.007 | 0.002 | 14.084 | 1 | 0 | 1.007 |
| Constant | −3.046 | 0.702 | 18.827 | 1 | 0 | 0.048 |
Abbreviations: CEA = carcinoembryonic antigen, CA125 = cancer antigen 125, S.E. = Standard Error, df = degrees of freedom, Sig = Significance, Exp(B) = Exponent of B.
Table A2.
Summary of results of the primary model in different cohorts
| Group | N | AUC (95%CI) | Se (%) | Sp (%) | PPV (%) | NPV(%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Training cohort | 158 | 0.897 (0.848–0.946) | 86.5 | 82.3 | 88.3 | 79.7 | 84.8 |
| Validation cohort | 158 | 0.856 (0.798–0.914) | 87.5 | 60.3 | 69.3 | 82.5 | 74.1 |
| Whole cohort | 316 | 0.866 (0.826–0.905) | 85.8 | 70.0 | 77.8 | 79.5 | 80.0 |
Abbreviations : CEA = carcinoembryonic antigen, CA125 = cancer antigen 125, N = Number, AUC = are under the curve, 95%CI = 95% Confidence Intervals, Se = Sensitivity, Sp = Specificity, PPV = Positive Predictive value, NPV = Negative Predictive Value, % = percentage.
Funding Statement
This work was supported by the National Key Research and Development Program of China (grant numbers 2016YFC1303201, 2016YFC0901400), the National Natural Science Foundation of China, CAMS Innovation Fund for Medical Sciences (2016-I2M-1-001, 2017-I2M-1-005), the National Natural Science Foundation of China (81802299, 81502514), the Fundamental Research Funds for the Central Universities (3332018070), and the National Key Basic Research Development Plan (2018YFC1312105).
Abbreviations
| AAbs | Autoantibodies |
| AUC | area under the curve |
| CA125 | cancer antigen 125 |
| CEA | carcinoembryonic antigen |
| CI | 95% confidence interval |
| COPD | chronic obstructive pulmonary disease |
| EMT | epithelial–mesenchymal transition |
| GGO | ground-glass opacity |
| HRCT | high-resolution computed tomography |
| LDCT | low-dose computed tomography |
| NLST | National Lung Screening Trial |
| NPV | negative predictive value |
| PPV | positive predictive value |
| ROC | receiver operator characteristic |
| SD | standard deviation |
| TAAs | tumor-associated antigens |
| YI | Youden’s index. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Disclaimers
The view expressed in the submitted article is not an official position of the institution or funder.
References
- 1.Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME.. The International Epidemiology of Lung Cancer: latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol. 2016;11(10):1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, et al., National Lung Screening Trial Research T . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(Suppl 1):S108–115. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 5.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, Sykes A-M, Aughenbaugh GL, Bungum AO, Allen KL. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235(1):259–265. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 6.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76(2):138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Salgia R, Harpole D, Herndon JE 2nd, Pisick E, Elias A, Skarin AT. Role of serum tumor markers CA 125 and CEA in non-small cell lung cancer. Anticancer Res. 2001;21:1241–1246. [PubMed] [Google Scholar]
- 8.Cedres S, Nunez I, Longo M, Martinez P, Checa E, Torrejón D, Felip E. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2011;12(3):172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Tarro G, Perna A, Esposito C. Early diagnosis of lung cancer by detection of tumor liberated protein. J Cell Physiol. 2005;203(1):1–5. doi: 10.1002/jcp.20195. [DOI] [PubMed] [Google Scholar]
- 10.Gube M, Taeger D, Weber DG, Pesch B, Brand P, Johnen G, Müller-Lux A, Gross IM, Wiethege T, Weber A, et al. Performance of biomarkers SMRP, CA125, and CYFRA 21-1 as potential tumor markers for malignant mesothelioma and lung cancer in a cohort of workers formerly exposed to asbestos. Arch Toxicol. 2011;85(3):185–192. doi: 10.1007/s00204-010-0580-2. [DOI] [PubMed] [Google Scholar]
- 11.Pastor A, Menendez R, Cremades MJ, Pastor V, Llopis R, Aznar J. Diagnostic value of SCC, CEA and CYFRA 21.1 in lung cancer: a Bayesian analysis. Eur Respir J. 1997;10:603–609. [PubMed] [Google Scholar]
- 12.Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1:513–519. [PubMed] [Google Scholar]
- 13.Desmetz C, Mange A, Maudelonde T, Solassol J. Autoantibody signatures: progress and perspectives for early cancer detection. J Cell Mol Med. 2011;15(10):2013–2024. doi: 10.1111/j.1582-4934.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Zhong W, Chen C, Meng Q, Wei J. Circulating Antibodies to Linear Peptide Antigens Derived from ANXA1 and FOXP3 in Lung Cancer. Anticancer Res. 2017;37(6):3151–3155. doi: 10.21873/anticanres.11673. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Guan S, Sun S, Jin Y, Lee K-H, Chen Y, Wei J. Detection of circulating antibodies to linear peptide antigens derived from ANXA1 and DDX53 in lung cancer. Tumour Biol. 2014;35(5):4901–4905. doi: 10.1007/s13277-014-1643-4. [DOI] [PubMed] [Google Scholar]
- 16.Fu QF, Liu Y, Fan Y, Hua S-N, Qu H-Y, Dong S-W, Li R-L, Zhao M-Y, Zhen Y, Yu X-L, et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol. 2015;8:22. doi: 10.1186/s13045-015-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih NY, Lai HL, Chang GC, Lin H-C, Wu Y-C, Liu JM, Liu K-J, Tseng S-W. Anti-alpha-enolase autoantibodies are down-regulated in advanced cancer patients. Jpn J Clin Oncol. 2010;40(7):663–669. doi: 10.1093/jjco/hyq028. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Kim YM, Lim S, Nam YK, Jeong J, Kim H-J, Lee K-J. Ubiquitin C-terminal hydrolase-L1 is a key regulator of tumor cell invasion and metastasis. Oncogene. 2009;28(1):117–127. doi: 10.1038/onc.2008.364. [DOI] [PubMed] [Google Scholar]
- 19.Hibi K, Westra WH, Borges M, Goodman S, Sidransky D, Jen J. PGP9.5 as a candidate tumor marker for non-small-cell lung cancer. Am J Pathol. 1999;155(3):711–715. doi: 10.1016/S0002-9440(10)65169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittencourt Rosas SL, Caballero OL, Dong SM. da Costa Carvalho Mda G, Sidransky D, Jen J. Methylation status in the promoter region of the human PGP9.5 gene in cancer and normal tissues. Cancer Lett. 2001;170:73–79. [DOI] [PubMed] [Google Scholar]
- 21.Brichory F, Beer D, Le Naour F, Giordano T, Hanash S. Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res. 2001;61:7908–7912. [PubMed] [Google Scholar]
- 22.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen Y-T, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Jiao S, Kang J, Li R, Zhang G. Application of serum NY-ESO-1 antibody assay for early SCLC diagnosis. Int J Clin Exp Pathol. 2015;8:14959–14964. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Shivakumar S, Barker K, Tang Y, Wallstrom G, Park JG, Tsay J-CJ, Pass HI, Rom WN, LaBaer J, et al. Comparative Study of Autoantibody Responses between Lung Adenocarcinoma and Benign Pulmonary Nodules. J Thorac Oncol. 2016;11(3):334–345. doi: 10.1016/j.jtho.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Pereira-Faca SR, Kuick R, Puravs E, Zhang Q, Krasnoselsky AL, Phanstiel D, Qiu J, Misek DE, Hinderer R, Tammemagi M, et al. Identification of 14-3-3 theta as an antigen that induces a humoral response in lung cancer. Cancer Res. 2007;67(24):12000–12006. doi: 10.1158/0008-5472.CAN-07-2913. [DOI] [PubMed] [Google Scholar]
- 26.Chang YC, Chan YC, Chang WM, Lin Y-F, Yang C-J, Su C-Y, Huang M-S, Wu ATH, Hsiao M. Feedback regulation of ALDOA activates the HIF-1alpha/MMP9 axis to promote lung cancer progression. Cancer Lett. 2017;403:28–36. doi: 10.1016/j.canlet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Falck Miniotis M, Arunan V, Eykyn TR, Marais R, Workman P, Leach MO, Beloueche-Babari M. MEK1/2 inhibition decreases lactate in BRAF-driven human cancer cells. Cancer Res. 2013;73(13):4039–4049. doi: 10.1158/0008-5472.CAN-12-1969. [DOI] [PubMed] [Google Scholar]
- 28.Trivers GE, De Benedetti VM, Cawley HL, Caron G, Harrington AM, Bennett WP, Jett JR, Colby TV, Tazelaar H, Pairolero P, et al. Anti-p53 antibodies in sera from patients with chronic obstructive pulmonary disease can predate a diagnosis of cancer. Clin Cancer Res. 1996;2:1767–1775. [PubMed] [Google Scholar]
- 29.Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Consortium for Early Detection of Lung Cancer, Guida F, Sun N, Bantis LE, Muller DC, Li P, et al. Assessment of Lung Cancer Risk on The Basis of a Biomarker Panel of Circulating Proteins. JAMA Oncol. 2018;4(10):e182078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D, Zhang X, Powell CA, Ni J, Wang B, Zhang J, Zhang Y, Wang L, Xu Z, Zhang L, et al. Probability of cancer in high-risk patients predicted by the protein-based lung cancer biomarker panel in China: LCBP study. Cancer. 2018;124(2):262–270. doi: 10.1002/cncr.31020. [DOI] [PubMed] [Google Scholar]
- 31.Qiu J, Choi G, Li L, Wang H, Pitteri SJ, Pereira-Faca SR, Krasnoselsky AL, Randolph TW, Omenn GS, Edelstein C, et al. Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol. 2008;26(31):5060–5066. doi: 10.1200/JCO.2008.16.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohue Y, Wada H, Oka M, Nakayama E. Antibody response to cancer/testis (CT) antigens: A prognostic marker in cancer patients. Oncoimmunology. 2014;3(11):e970032. doi: 10.4161/21624011.2014.970032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Türeci O, Wiewrodt R, Barnes AC, Robertson JF. Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax. 2008;63(3):228–233. doi: 10.1136/thx.2007.083592. [DOI] [PubMed] [Google Scholar]
- 34.Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. Febs J. 2009;276(23):6880–6904. doi: 10.1111/j.1742-4658.2009.07396.x. [DOI] [PubMed] [Google Scholar]
- 35.Boyle P, Chapman CJ, Holdenrieder S, Murray A, Robertson C, Wood WC, Maddison P, Healey G, Fairley GH, Barnes AC, et al. Clinical validation of an autoantibody test for lung cancer. Ann Oncol. 2011;22(2):383–389. doi: 10.1093/annonc/mdq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam S, Boyle P, Healey GF, Maddison P, Peek L, Murray A, Chapman CJ, Allen J, Wood WC, Sewell HF, et al. EarlyCDT-Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila). 2011;4(7):1126–1134. doi: 10.1158/1940-6207.CAPR-10-0328. [DOI] [PubMed] [Google Scholar]
- 37.Jett JR, Peek LJ, Fredericks L, Jewell W, Pingleton WW, Robertson JF. Audit of the autoantibody test, EarlyCDT(R)-lung, in 1600 patients: an evaluation of its performance in routine clinical practice. Lung Cancer. 2014;83(1):51–55. doi: 10.1016/j.lungcan.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Shan Q, Lou X, Xiao T, Zhang J, Sun H, Gao Y, Cheng S, Wu L, Xu N, Liu S. A cancer/testis antigen microarray to screen autoantibody biomarkers of non-small cell lung cancer. Cancer Lett. 2013;328(1):160–167. doi: 10.1016/j.canlet.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4(4):1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Chang W, Zhao J, Yu Y, Tan X, Su T, Zhao L, Huang S, Liu S, Cao G. Development of autoantibody signatures as novel diagnostic biomarkers of non-small cell lung cancer. Clin Cancer Res. 2010;16(14):3760–3768. doi: 10.1158/1078-0432.CCR-10-0193. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 42.Sakao Y, Tomimitsu S, Takeda Y, Natsuaki M, Itoh T. Carcinoembryonic antigen as a predictive factor for postoperative tumor relapse in early-stage lung adenocarcinoma. Eur J Cardiothorac Surg. 2004;25(4):520–522. doi: 10.1016/j.ejcts.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Buccheri G, Ferrigno D. Identifying patients at risk of early postoperative recurrence of lung cancer: a new use of the old CEA test. Ann Thorac Surg. 2003;75:973–980. [DOI] [PubMed] [Google Scholar]
- 44.Doseeva V, Colpitts T, Gao G, Woodcock J, Knezevic V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med. 2015;13:55. doi: 10.1186/s12967-015-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veluswamy RR, Ezer N, Mhango G, Goodman E, Bonomi M, Neugut AI, Swanson S, Powell CA, Beasley MB, Wisnivesky JP. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: impact of Histology. J Clin Oncol. 2015;33(30):3447–3453. doi: 10.1200/JCO.2014.60.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]


