ABSTRACT
A significant proportion of human epidermal growth factor receptor 2 (Her2/ErbB2)-positive metastatic breast cancer patients are refractory to Her2-targeted trastuzumab-like therapy. Some of this resistance has been attributed to the upregulation of immune checkpoints such as programmed cell death-1 (PD-1) and its ligand, PD-L1 in Her2-positive breast cancer patients. Therefore, therapies targeting both the PD-1/PD-L1 interaction and oncogenic Her2 signaling are of significant clinical interest. Here, we constructed a mouse bispecific antibody targeting PD-L1 and rat Her2 (referred to as BsPD-L1xrErbB2) aiming to redirect the anti-PD-L1 response toward Her2-expressing tumor cells. BsPD-L1xrErbB2 demonstrated additive binding to interferon (IFN)-γ treated Her2+ TUBO tumor cells, but it did not affect the proliferation of tumor cells in-vitro. BsPD-L1xrErbB2 also blocked the PD-1/PD-L1 interaction. This bispecific antibody was constructed with a mouse IgG2a Fc backbone and interacted with Fcγ receptors and resulted in complement deposition (C3). ADCC and complement action could be potential mechanisms of action of this molecule. BsPD-L1xrErbB2 successfully reduced TUBO tumor growth and increased tumor rejection rate compared to the monovalent anti-PD-L1, monovalent anti-ErbB2 or the combination of anti-PD-L1 and anti-ErbB2 monotherapies. The enhanced anti-tumor effect of BsPD-L1xrErbB2 was dependent on CD8+ T lymphocytes and IFN-γ, as depletion of CD8+ T lymphocytes and neutralization of IFN-γ completely abolished the antitumor activity of the bispecific antibody. Consistently, BsPD-L1xrErbB2 treatment also increased the frequency of intratumor CD8+ T lymphocytes. Taken together, our data support a bispecific antibody approach to enhance the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade in Her2-positive metastatic breast cancers.
KEYWORDS: ErbB2, PD-L1, breast cancer, bispecific antibody
Introduction
Trastuzumab therapy is a standard monoclonal antibody-based therapy targeting oncogenic Her2 (Human epidermal growth factor receptor 2, also called ErbB2) signaling in Her2-positive breast cancers. Apart from its role in blocking tumor promoting oncogenic Her2 signaling, the anti-tumor efficacy of trastuzumab is dependent on antibody-dependent cellular cytotoxicity (ADCC). Its mechanism of action also builds on several innate and adaptive immune factors such as type I and II IFNs, IL-21, CD11b+ and F4/80+ myeloid cells, NK cells, CD4+ and CD8+ T lymphocytes in ErbB2+ mouse models of breast cancers.1–4
Some patients are refractory to trastuzumab treatment.5 In addition to tumor cell-intrinsic factors such as downregulation of cell surface Her2 or mutations in the Her2-mediated PI3-kinase/AKT signaling pathways,5 upregulation of immunosuppressive molecules such as PD-1 ligand, PD-L1 and adenosine generating CD73 are associated with trastuzumab resistance.6,7 Intratumor expression of PD-L1 has been identified in up to 72% Her2+ breast cancers,8,9 and PD-L1 expression along with CD8+ T cell infiltration is associated with favorable prognosis and increased survival.10 Consistently, recent studies have shown that neoadjuvant trastuzumab therapy significantly upregulates immunosuppressive markers such as PD-L1 and indoleamine 2, 3 dioxygenase (IDO) in the tumor-associated macrophages (TAMs) of HER2+ breast cancer patients and this correlates with poor trastuzumab response.11 Therefore, therapies targeting oncogenic Her2 along with these immunosuppressive molecules are of significant clinical interest. Our previous study has also shown that anti-PD-1 monoclonal antibody (mAb) therapy significantly improved anti-ErbB2 therapy4 and we also initiated a clinical trial examining the therapeutic potential of anti-PD-1 mAb in advanced, trastuzumab-resistant, Her2-positive breast cancers.12
Bispecific antibodies carry two antigen specificities in one antibody molecule and so might provide significant opportunities over monospecific antibodies (reviewed in13,14). Firstly, bispecific antibodies targeting both an immune checkpoint and a tumor antigen, may direct specific effectors of the immune system to target tumor cells, anticipating to enhance their cytotoxicity and reducing potential immune-mediated toxicities. Secondly, in contrast to monospecific Abs, bispecific antibodies, when acting in cis, may provide higher binding specificity as they interact with two different surface antigens. Thirdly, it is hypothesized that bispecific antibodies may help to avoid the development of therapy resistance. Disease modulators when present on a single tumor cell are involved in complex signaling pathways and often targeting one pathway may lead to unexpected changes in other pathways that leads to therapy resistance. The use of bispecific antibodies may help to avoid the development of resistance as targeting two disease modulators on a single tumor cell may increase the anticancer effect and thus, avoid the development of resistance.13
Bispecific antibody approaches also have their limitations compared to monotherapies or combinations thereof. They do not allow for sequential therapies and it is not possible to modify the dose of target-binding protein in each arm. Most bispecific antibodies in preclinical and clinical development are engaging T cells, via the CD3 binding arm, for tumor cell killing. Bispecific antibodies targeting Her2 along with CD3 or Her3 have shown enhanced anti-tumor activity in preclinical models of Her2+ breast cancers.15–19
Here, we generated a mouse IgG2a BsPD-L1xrErbB2 bispecific antibody (referred to as BsPD-L1xrErbB2) targeting oncogenic rat ErbB2 and mouse PD-L1 as proof of concept for Her2-directed blockade of the PD-1/PD-L1 interaction. We characterized BsPD-L1xrErbB2 in vitro and successfully demonstrated its enhanced anti-tumor activity in vivo, thereby justifying the rationale that a bispecific antibody approach targeting ErbB2 and blocking the PD-1/PD-L1 interaction might be promising in Her2+ breast cancer patients.
Results
Anti-PD-L1 and anti-ErbB2 monoclonal antibody therapy in ErbB2+ mouse tumors
PD-L1 is expressed in a wide range of cancer types and high PD-L1 expression is associated with improved clinical outcome in triple-negative breast cancers and Her2+ breast cancers likely as an indication of pre-existing immune responses triggering local adaptive resistance.20,21 To understand the therapeutic potential of anti-PD-L1 antibody in Her2+ breast cancers, we tested PD-L1 expression on the rat ErbB2+ mouse TUBO cell line. Untreated TUBO cells did not express any significant level of PD-L1 expression (Figure 1a). IFN-γ is a known regulator of PD-L1 expression and has been shown to contribute toward enhanced immune suppression.22 Upon stimulation with mouse IFN-γ, PD-L1 expression was induced on TUBO cells in a concentration-dependent manner and reached a plateau at around 48 h, while the ErbB2 expression remained constant with around 15,000 antibody binding sites irrespective of the concentration or duration of the IFN-γ exposure (Figure 1a). We confirmed increased PD-L1 expression at the mRNA level after IFN-γ stimulation in several other ErbB2+ tumor cell lines (Supplementary Figure 1A). Next, we evaluated the anti-tumor activity of anti-PD-L1 and anti-ErbB2 mAb in two different mouse models of Her2+ mammary cancers. Consistent with our previous observations,1 H2N100 tumors were completely rejected at higher doses of anti-ErbB2 mAb therapy (Figure 1b, c; 10/10 rejections at 50 μg dose) while TUBO tumors showed significant resistance to anti-ErbB2 mAb therapy at the same dose (Figure 1d, e; 1/10 rejections). In contrast, anti-PD-L1 mAb therapy exhibited slightly better therapeutic activity against TUBO tumors compared to H2N100 tumors. Therefore, in subsequent studies, we used the TUBO tumor model that showed moderate resistance to anti-ErbB2 mAb therapy.
Figure 1.

Anti-ErB2 and anti-PD-L1 mAb therapy in rat ErbB2-positive mouse tumors. (a) Her2 expression and PD-L1 upregulation on TUBO cells upon IFN-γ stimulation. TUBO cells were stimulated with recombinant mouse IFN-γ for 72 h. PD-L1 and Her-2 expression were quantified on the stimulated cells at 24, 48 and 72 h using a QIFI kit and MPDL3280A and 7.16.4 antibodies, respectively. H2N100 (b, c) and TUBO (d, e) tumor cells (5 × 105 cells) were injected subcutaneously into Balb/c wild type mice, and treated with 10 μg or 50 μg of anti-ErbB2 mAb (7.16.4), 200 μg anti-PD-L1 mAb or control Ig (200 μg) injected intraperitoneally on days 17, 21, 24 and 28. Mice were monitored for tumor growth and results are expressed as mean tumor area ± SEM. (c, e) Data is shown for the percentage of mice rejecting tumors/group (gray bars, tumor-bearing mice; black bars, tumor-free mice for at least 60 d). Arrow represents the day treatment was started. Statistical analysis was performed using two-way ANOVA Turkey’s multiple comparisons test at day 30 for B and day 39 for D (***, P < 0.0005; ****, P < 0.0001; ns: not significant).
Characterization of bispecific mmIgG2a-PD-L1 x ErbB2 antibody
Given the reduced tumor growth with anti-PD-L1 therapy in the moderately resistant Her2+ tumors, we next evaluated the combination potential of dual targeting of PD-L1 and ErbB2 in TUBO tumors by generating bispecific antibodies targeting PD-L1 and ErbB2 on mouse IgG2a background (referred as BsPD-L1xrErbB2). Appropriate bispecific controls were also produced as described in detail in Materials and Methods. We first tested the binding affinity of BsPD-L1xrErbB2 on TUBO cells.
In the untreated condition, clear binding of the bivalent anti-rat ErbB2 (clone 7.16.4, expressed as mIgG2a) was shown, as well as binding of the b12xrErbB2 (monovalent control for bispecific) and BsPD-L1xrErbB2 bispecific antibody (Figure 2a). As expected, the potency of the monovalent and bispecific antibodies were lower than the bivalent anti-rat ErbB2 antibody because of loss of avidity to Her2 (Figure 2a). Neither bivalent anti-PD-L1 antibody (MPDL3280A) nor monovalent anti-PD-L1 control antibody (B12xPD-L1), showed any binding to TUBO cells (Figure 2a, b) which might be explained by the minimal amount of PD-L1 expression that is present on these cells in the untreated condition.
Figure 2.
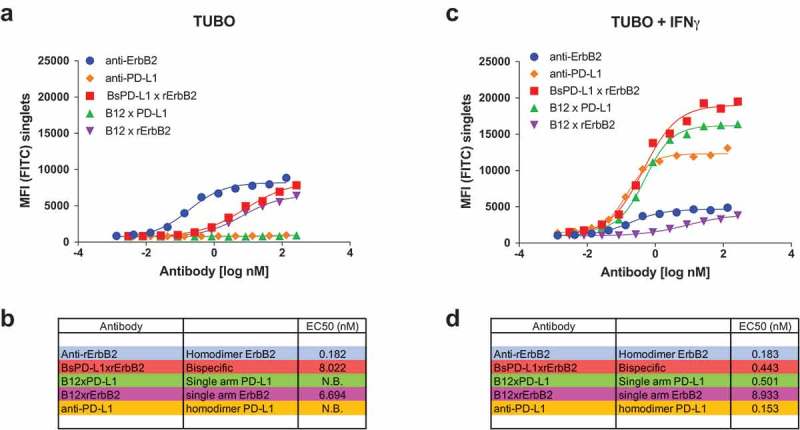
Binding of monospecific and bispecific antibodies to TUBO cells. TUBO tumor were cultured in the absence (a, b) or presence (c, d) of 10ng/ml of mouse IFN-γ for 24 h and incubated with different concentrations of FITC-labeled bivalent (homodimer) anti-rErbB2, anti-PD-L1, monovalent anti-rErbB2 (B12xErbB2) or anti-PD-L1 (B12xPD-L1) or bispecific BsPD-L1xrErbB2 antibodies. On the y-axes, the median fluorescence intensity (MFI) FITC is plotted against the antibody concentration in log nM on the x-axes. EC50 in nM for untreated TUBO (B) and IFN-γ treated TUBO (d) tumor cells is shown.
Treatment of TUBO cells with mouse IFN-γ increased PD-L1 expression which reached even higher levels compared to the Her-2 expression (Figure 1a). Binding of anti-rErbB2 and b12xrErbB2 (monovalent bispecific antibody control for rErbB2) to IFN-γ-treated TUBO cells was comparable to the binding on untreated cells (Figure 2c, d) as IFN-γ treatment did not change ErbB2 expression. Binding of bivalent anti-PD-L1 (MPDL3280A) and monovalent anti-PD-L1 control (B12xPD-L1) on treated cells was more efficacious compared to the anti-rErbB2 bivalent antibody and monovalent antibodies due to the higher PD-L1 expression compared to ErbB2 expression after mouse IFN-γ stimulation. The BsPD-L1xrErbB2 however reached the highest efficacy in binding to IFN-γ-treated TUBO cells with a ~ 20-fold improved EC50 compared to untreated TUBO cells (Figure 2), indicating that this bispecific antibody might be highly potent in binding to ErbB2+ tumors with high PD-L1 expression. These data also suggest that the binding of the BsPD-L1xrErbB2 antibodies to mouse IFN-γ treated TUBO cells was mainly driven by the PD-L1 expression.
To determine the effect of BsPD-L1xrErbB2 on the proliferation of TUBO cells, in the presence or absence of mouse IFN-γ, TUBO cells were incubated with BsPD-L1xrErbB2 and control antibodies for 72 h. Inhibition of proliferation of TUBO cells was observed after treatment with bivalent anti-ErbB2 antibody. This effect was markedly reduced when a monovalent variant of the same antibody B12xErbB2 was used (Figure 3a). BsPD-L1xrErbB2 targeting both PD-L1 and ErbB2 had minimal effect on the TUBO cell proliferation (Figure 3a). Despite the finding that pretreatment with IFN-γ strongly enhanced its binding, the BsPD-L1xrErbB2 bispecific antibody did not affect TUBO cell proliferation (Figure 3b).
Figure 3.
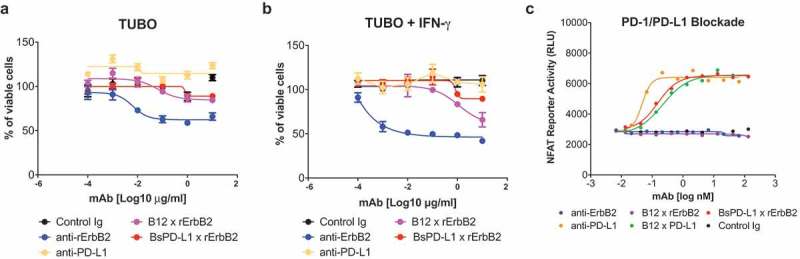
BsPD-L1xrErbB2 blocks PD-1/PD-L1 interaction but does not affect the viability of tumor cells in-vitro. TUBO tumor cells were cultured in the absence (a) or presence (b) of 10ng/ml of mouse IFN-γ overnight and cells were incubated with different concentrations of control Ig, anti-rErbB2, anti-PD-L1, monovalent anti-rErbB2 (B12xErbB2) or BsPD-L1xrErbB2 for 72 h. Viability of TUBO tumor cells is shown as determined by using the CellTiter-Glo system. (c) NFAT Reporter Activity (RLU) as a measure of PD-1/PD-L1 interaction are plotted on the y-axes against the antibody concentrations in log nM on the x-axes.
BsPD-L1xrErbB2 antibody was capable of blocking the PD-1/PD-L1 interaction and might have direct anti-tumor killing efficacy
To validate if the PD-L1 arm in the BsPD-L1xrErbB2 blocked the PD-1/PD-L1 interaction, the bispecific antibody was tested using a PD-1+ Jurkat cell line expressing an NFAT-reporter cocultured with a PD-L1 expressing CHO-K1 cell line. As expected, introduction of the anti-PD-L1 (MPDL3280A) blocked the interaction in a dose-dependent fashion. Surprisingly, the monovalent anti-PD-L1 control antibody (B12xPD-L1) and the BsPD-L1xrErbB2 bispecific antibody had similar potency as the bivalent anti-PD-L1 antibody (Figure 3c). Bivalent anti-ErbB2, monovalent anti-ErbB2 or isotype control (B12) antibody did not block the PD-1/PD-L1 interaction confirming the specificity of the assay (Figure 3c).
Interestingly, although all antibodies used in these experiments were produced with a mouse IgG2a Fc tail, not all demonstrated the same potency in the interaction with FcγRIV. Bivalent anti-PD-L1 antibodies, but not bivalent anti-ErbB2 antibodies, induced activation of the effector cells, and the combination of both bivalent antibodies showed reduced activity. Importantly, the bispecific BsPD-L1xrErbB2 antibody showed activity in FcγRIV reporter assay albeit at a lower level than the bivalent PD-L1 antibody probably due to loss of avidity for PD-L1 (Supplementary Figure 2A). The control bispecific antibody with Fc regions carrying the L234A and L235A mutations that abrogated FcγR binding (‘inert’) showed no activation confirming the specificity of this assay (Supplementary Figure 2A). These results prove that BsPD-L1xrErbB2 has an active FC portion. To further understand the possible effector mechanisms induced by the bispecific antibody, in vitro binding of C3 was determined as a surrogate for putative complement-dependent cytotoxicity (CDC). C3 deposition was observed only on TUBO cells that were treated with anti-ErbB2 antibody. Though TUBO tumor cells were pretreated with IFN-γ to induce PD-L1 expression, no signal was observed in cells treated with anti-PD-L1 antibody. The bispecific BsPD-L1xrErbB2 antibody was less efficacious at low concentrations but demonstrated comparable or even higher C3 deposition at higher concentrations likely due to increased number of bound antibodies per cell (‘single-arm’ binding). As expected, none of the control antibodies with an inert Fc were able to deposit C3 on the TUBO cells. These data suggest that complement activation might be one of the mechanisms of the bispecific and bivalent anti-ErbB2 antibodies (Supplementary Figure 2B).
BsPD-L1xrErbB2 antibody improves therapeutic efficacy of anti-ErbB2 mAb therapy
Next, we evaluated the therapeutic efficacy of the BsPD-L1xrErbB2 in the TUBO mouse tumor model of ErbB2+ mammary cancer. We treated established TUBO tumors (mean tumor size 60–80 mm2) with different doses of anti-ErbB2 (7.16.4), anti-PD-L1 (MPDL3280A), the combination of anti-ErbB2 and anti-PD-L1, BsPD-L1xrErbB2 or control antibodies. Treatment with BsPD-L1xrErbB2 reduced tumor growth and induced more tumor rejections compared to a single agent or the combination therapy at equimolar doses of antibody arms, while the anti-PD-L1 and anti-ErbB2 alone had marginal anti-tumor effects (Figure 4). Although a significant (p > 0.0001) reduction in tumor growth was seen using 200 μg of bispecific antibody, no dramatic changes in tumor rejection rates were identified between 100 μg and 200 μg antibody dose. Importantly, inert anti-PD-L1 antibody (expressed with an inactive Fc tail through the L234A and L235A mutations) showed no anti-tumor effect, further substantiating the importance of Fc-mediated tumor suppression by the anti-PD-L1 antibody (Figure 4a, b). This suggested that anti-tumor efficacy of BsPD-L1xrErbB2 depended on the antibody targeting ErbB2-expressing tumors. To assess the generation of memory anti-tumor response, mice that rejected tumors were re-challenged with a higher dose of TUBO tumor cells in the opposite flank and tumor growth was monitored. BsPD-L1xrErbB2-treated groups provided long-term protection against tumor re-challenge similar to the anti-ErbB2 and anti-PD-L1 treated groups suggesting the successful induction of tumor-specific T cell immunity (Figure 5).
Figure 4.
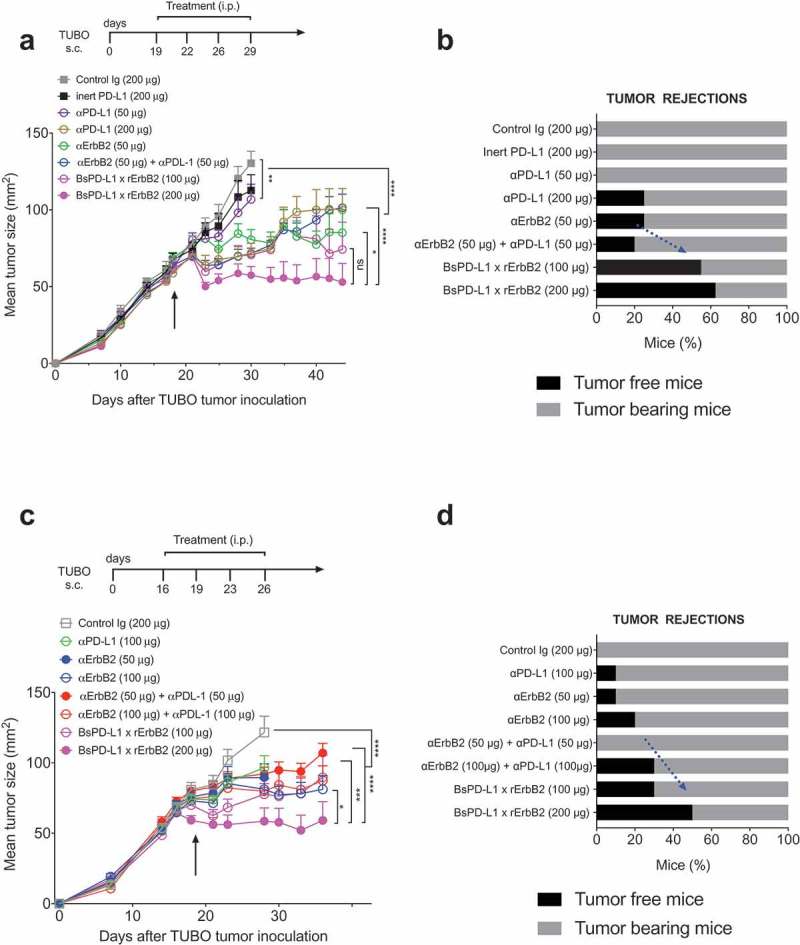
Treatment with BsPD-L1xrErbB2 bispecific antibody reduces tumor growth and increase tumor rejections. TUBO tumor cells (5 × 105 cells) were injected subcutaneously into Balb/c wild type mice, and treated with different concentrations of control Ig, anti-ErbB2 mAb (7.16.4), anti-PD-L1 mAb or BsPD-L1xrErbB2 bispecific antibody injected intraperitoneally on days 19, 22, 26 and 29 (a, b) or on days 16, 19, 23 and 26 (c, d). Mice were monitored for tumor growth and results are expressed as mean tumor area ± SEM. (b, d) Data is shown for the percentage of mice rejecting tumors/group (gray bars, tumor-bearing mice; black bars, tumor-free mice for at least 60 d). Arrow represents the day treatment was started. Statistical analysis was performed using two-way ANOVA Turkey’s multiple comparisons test at day 44 for A and day 28 for C (*, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001; ns: not significant).
Figure 5.
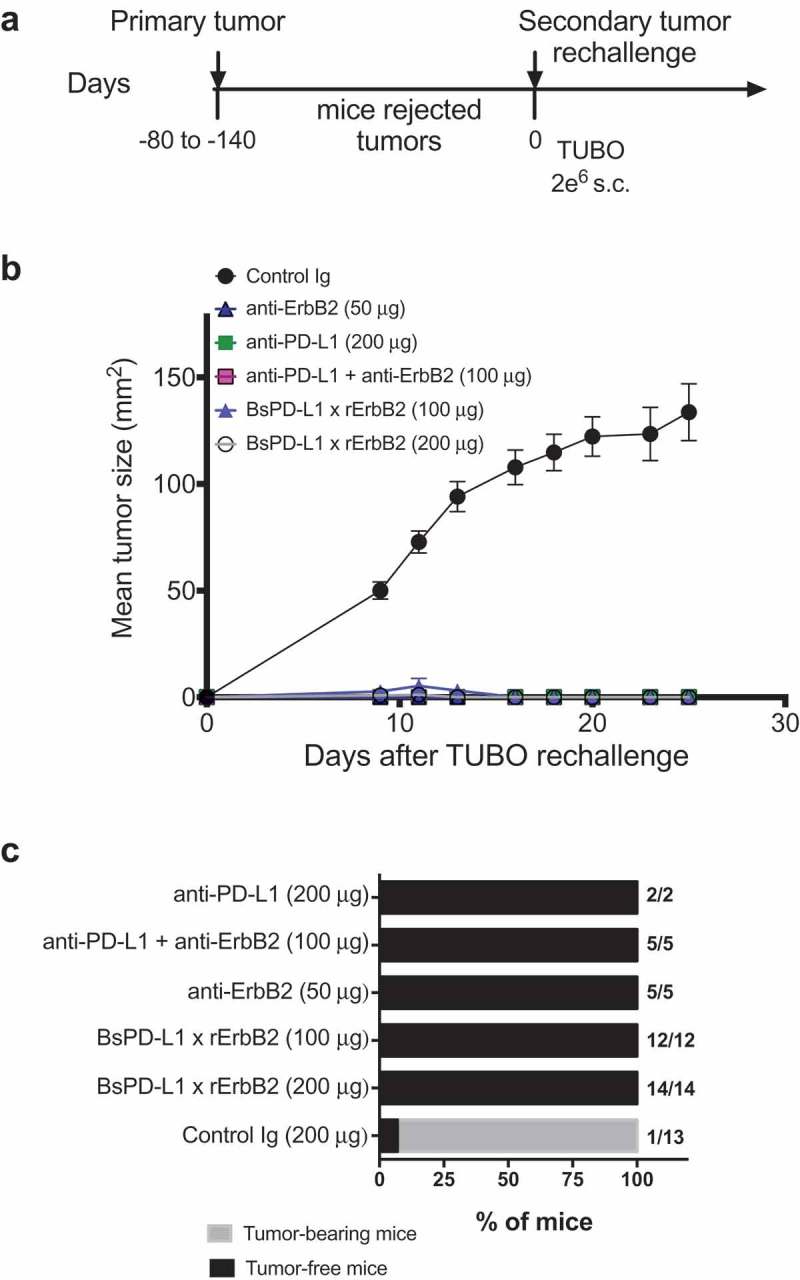
TUBO re-challenge in mice rejected tumors after bispecific antibody therapy. (a, b) Balb/c mice that rejected TUBO tumors after BsPD-L1xrErbB2, anti-ErbB2 alone, anti-PD-L1 alone or the combination therapy from different experiments were collected and 2 × 106 TUBO tumor cells were injected in the opposite flank of mice in each group and tumor growth was monitored. TUBO tumors did not grow in any of the group of mice that had rejected tumors before. (c) Data is shown for the percentage of mice rejecting tumors/group (gray bars, tumor-bearing mice; black bars, tumor-free mice for at least 60 d). Number represents the tumor-free mice/total number of mice in a group.
BsPD-L1xrErbB2 therapy increased the proportion of tumor-associated CD8+ T cells and decreased the proportion of granulocytic MDSC
To understand the mechanism of anti-tumor efficacy of the BsPD-L1xrErbB2 antibody, we performed tumor-infiltrating leukocyte (TILs) analysis on TUBO tumors treated with control Ig, anti-ErbB2, anti-PD-L1, a combination of anti-ErbB2 and anti-PD-L1 or BsPD-L1xrErbB2. As shown by us and others, anti-ErbB2 therapy increased the proportion of CD4+ T cells, regulatory T cells (Tregs) and CD8+ T cells (Figure 6a–f). In a statistical analysis with multiple comparisons, we found a significant increase of CD8+ T cells harvested from BsPD-L1xrErbB2-treated tumors, but not in other treatment groups (Figure 6e). We also observed a significant decrease in the percentage and absolute number of CD11b+Ly6Ghi granulocytic myeloid-derived suppressor cells (gMDSC) and an increase in the proportion of Tregs in BsPD-L1xrErbB2-treated tumors. These changes were similar to that observed post anti-ErbB2 therapy alone suggesting these effects were largely driven by targeting anti-ErbB2 (Figure 6g, h).
Figure 6.
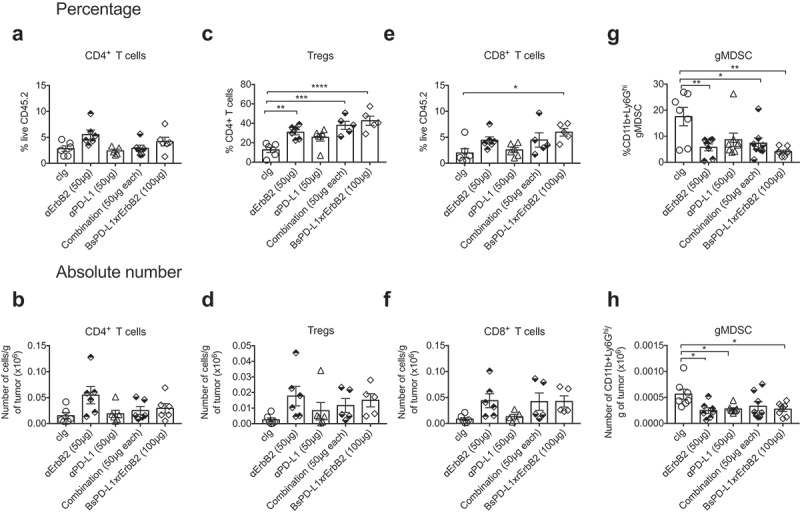
Increased CD8+ T cells in the tumor after BsPD-L1xrErbB2 bispecific antibody therapy. TUBO tumor-bearing mice were treated with two doses of cIg, anti-ErbB2 (7.16.4, 50μg), anti-PD-L1 (MPDL3280A, 50μg), the combination of anti-PD-L1 and anti-ErbB2 (called combination) or the BsPD-L1xrErbB2 at day 15 and 18, and 72 h after the last treatment tumors were resected and analyzed for the presence of TILs via flow cytometry. Percentage (a, c, e, g) and absolute number (b, d, f, h) of CD4+ T cells, CD4+FoxP3+ regulatory T cells (Tregs), CD8+ T cells and CD11b+Ly6Ghi granulocytic MDSC is shown. Data from individual mice are depicted by symbols in bar graphs. Results are expressed in mean ± SEM. Statistical analysis was performed using one-way ANOVA Turkey’s multiple comparisons test; *, P < .05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001 with n = 5–7 mice per group.
CD8+ T cells and IFN-γ were critical for the anti-tumor efficacy of the BsPD-L1xrErbB2 antibody
We and others showed that CD8+ T cells and IFN-γ were required for the anti-ErB2 mAb therapy.1,3,4 In order to test whether CD8+ T cells and IFN-γ are also required for BsPD-L1xrErbB2 therapy, we depleted either CD8+ T cells or CD4+ and CD8+ T cells together, or neutralized mouse IFN-γ in the groups of mice treated with bispecific control Ig (B12), anti-PD-L1 and anti-ErbB2 combination therapy and BsPD-L1xrErbB2 therapy. As shown before, bispecific antibody therapy induced more tumor rejections compared to the anti-PD-L1 and anti-ErbB2 combination therapy group. Both the BsPD-L1xrErbB2 and anti-PD-L1 and anti-ErbB2 combination therapy groups completely lost their anti-tumor activity in the presence of anti-CD8β, anti-CD4/anti-CD8β, or anti-IFN-γ suggesting that CD8 + T cells and IFN-γ were critical for the anti-tumor efficacy of the BsPD-L1xrErbB2 antibody and anti-PD-L1/anti-ErbB2 combination therapy (Figure 7 and Supplementary Figure 3).
Figure 7.
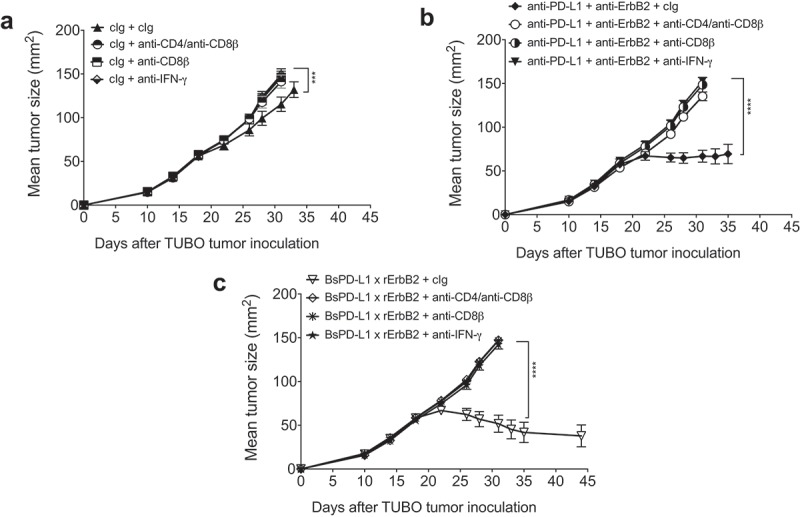
Mechanism of BsPD-L1xrErbB2 antibody therapy is dependent on CD8+ T cells and IFN-γ. TUBO tumor cells (5 × 105 cells) were injected subcutaneously into Balb/c wild type mice, and treated with (a) control Ig (b12, 200μg), (b) anti-PD-L1+ anti-ErbB2 combination (100μg each) or (c) BsPD-L1xrErbB2 bispecific (100μg) injected intraperitoneally on days 19, 22, 26 and 29. Additionally, some groups of mice were treated with control IgG (cIg), CD4- and CD8-depleting anti-CD4/anti-CD8β antibodies or IFN-γ neutralizing anti-IFN-γ antibodies. Mice were monitored for tumor growth and results are expressed as mean tumor area ± SEM. Statistical analysis was performed using two-way ANOVA Turkey’s multiple comparisons test (***, P < 0.0005; ****, P < 0.0001).
Discussion
The potency of an anti-tumor drug is largely dependent on its tumor selectivity. Bispecific antibodies are novel tools in drug armory that can target two different antigens on same or different cell types and can potentially enhance drug potency by dual targeting, or increasing tumor selectivity and reducing immune-mediated systemic toxicities. A few bispecific drugs have been recently been approved and several are under investigation in clinical trials for various malignancies.13 We have previously shown that targeting PD-1/PD-L1 interactions increased the therapeutic activity of targeted anti-Her2 therapy4 and pembrolizumab with trastuzumab displayed durable clinical benefit in patients with PD-L1-positive, trastuzumab-resistant, advanced Her2-positive breast cancers.12 Herein we demonstrate in mice the enhanced anti-tumor activity of BsPD-L1xrErbB2 bispecific antibody targeting oncogenic rat ErbB2 and mouse PD-L1.
IFN-γ is a pleiotropic cytokine with opposing effects in tumor microenvironment. IFN-γ from T and NK cells has anti-tumor effects, while alternatively increased IFN-γ levels induce immunosuppressive factors such as IDO and PD-L1, that are likely to provide immune escape mechanisms and contribute to enhanced tumor growth.23 Recently increased PD-L1 expression after trastuzumab therapy was shown to correlate with poor trastuzumab response.11 We did not observe any significant level of PD-L1 expression in rat ErbB2+ mouse tumor cells. However, treatment with mouse IFN-γ increased PD-L1 expression, but not ErbB2 expression, in a time- and concentration-dependent manner. Anti-PD-L1 mAb induced moderate anti-tumor effects in two different rat ErbB2 mouse models suggesting that therapies targeting PD-L1 can enhance anti-Her2 therapeutic effects.
Characterization of a BsPD-L1xrErbB2 antibody revealed that the avidity of BsPD-L1xrErbB2 was similar to the monovalent anti-ErbB2 mAb control. However, its overall binding increased four-fold after treatment of tumor cells with IFN-γ, owing to increased PD-L1 expression on tumor cells, suggesting that bispecific antibody might be highly potent in ErbB2+ tumors with high PD-L1 expression. The BsPD-L1xrErbB2 antibody also blocked PD-1 and PD-L1 interaction and downstream signaling pathway without affecting tumor cell proliferation. When compared to bivalent ErbB2 antibody, the BsPD-L1xrErbB2 showed a strongly reduced effect on tumor cell proliferation. Since PD-L1 is also expressed by immune cells, generally PD-1/PD-L1 blocking antibodies are engineered to have an IgG background limited to no effector function, to avoid elimination of PD-1/PD-L1 expressing immune cells.24,25 The BsPD-L1xrErbB2 antibody with a mouse IgG2a backbone is likely to be capable of eliminating ErbB2+ tumor cells through its active Fc tail that could induce ADCC and/or CDC. Indeed, we demonstrated the enhanced activation of effector T cells toward target ErbB2+ tumor cells upon BsPD-L1xrErbB2 antibody treatment in a Fc-mediated reporter assay, suggesting a potential for ADCC activity. The therapeutic activity of avelumab, a humanized IgG1 mAb against PD-L1 was dependent on NK cell-mediated ADCC.26 Similarly, the anti-tumor effects of anti-ErbB2 mAb therapy were largely attributed to its Fc-mediated ADCC in mouse models of ErbB2+ mammary tumors.3
Importantly, when used in vivo, the BsPD-L1xrErbB2 antibody had improved anti-tumor activity compared with the combination of anti-PD-L1 and anti-ErbB2 mAb therapy. Increased infiltration of CD8+ T cells in the tumor is often associated with improved clinical responses in several malignancies including Her2+ breast cancers,27 while the presence of granulocytic MDSC in the tumor is associated with its pro-tumorigenic immunosuppressive phenotype.28 Consistently, we observed an increased proportion of intratumor CD8+ T cells and decreased proportion of granulocytic MDSC in tumors treated with BsPD-L1xrErbB2 antibody therapy. No statistically significant differences were observed either in intratumor CD8+ T cells or granulocytic MDSCs between BsPD-L1xrErbB2 antibody and the combination of anti-ErbB2 and anti-PDL1 antibodies, suggesting that the observed increased tumor rejection in BsPD-L1xrErbB2 antibody-treated group may involve additional mechanisms including possibility of increased C3 deposition as observed for the BsPD-L1xrErbB2 vs bivalent anti-ErbB2 antibodies. Nevertheless, by depleting either CD8+ T cells or CD4+ and CD8+ T cells together, or neutralizing mouse IFN-γ, we further showed that both CD8+ T cells and IFN-γ were critical for the anti-tumor efficacy of the BsPD-L1xrErbB2 antibody therapy. No macroscopic toxicity was noted and all mice treated with the BsPD-L1xrErbB2 appeared healthy upon treatment.
Collectively our data demonstrated that the BsPD-L1xrErbB2 antibody has several mutually reinforcing anti-cancer properties that are not always available in conventional monoclonal antibodies. In particular, BsPD-L1xrErbB2 antibody was simultaneously able to bind both PD-L1 and rat ErbB2; blocked PD-1/PD-L1 interactions in a Her2-directed manner without affecting tumor cell proliferation in vitro; induced activation of effector cells in FcγRIV reporter assay suggesting ADCC activity; and enhanced the proportion of intratumor CD8+ T cells and improved the therapeutic efficacy of anti-ErbB2 mAb therapy by reducing tumor growth and increasing tumor rejections. Technical progress in the past few years has advanced the development of anti-cancer bispecific antibodies into human clinical trials and our data provide a strong rationale to use this approach to target PD-L1+ ErbB2+ breast cancers to improve Her2+ targeting therapies.
Materials and methods
Mice
Balb/c wild type (WT) mice were purchased from the Walter and Eliza Hall Institute for Medical Research or bred in house. All mice were bred and maintained at the QIMR Berghofer Medical Research Institute and used from the age of 8 weeks. All experiments were approved by the QIMR Berghofer Medical Research Institute Animal Ethics Committee.
Cell culture
Rat ErbB2-positive H2N100 tumor cells were generated from female Balb/c MMTV-ErbB2/neu transgenic mice and cultured as described previously.1,29 TUBO cells were cultured in complete DMEM supplemented with 10% heat-inactivated fetal calf serum (Thermo Scientific), 1X glutamax, 50 U/ml penicillin, 100 μg/ml streptomycin and 10 mM HEPES (Sigma-Aldrich), while other cell lines were cultured in RPMI supplemented with 10% heat-inactivated fetal calf serum, 1X glutamax, 50 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate from Gibco-Life Technologies and 10 mM HEPES, 1% non-essential amino acid solution, and incubated at 37°C in 5% CO2 incubator. The anti-rat ErbB2 mAb (clone 7.16.4) hybridoma was kindly provided by Mark I. Greene (University of Pennsylvania, Philadelphia, PA).
Generation of bispecific antibodies
Bispecific antibodies were made as described previously.30–32 Antibody heavy-chain expression vectors were constructed by de novo synthesis (Geneart) of codon-optimized VH coding regions of antibodies rHer2-7.16.4 and PDL1-MDPL3280A, genetically fused to the heavy-chain constant coding regions of mouse mmIgG2a (V00825) and inserted into expression vector pcDNA3.3 (Invitrogen). Likewise, separate light-chain expression vectors were constructed by inserting the appropriate VL coding regions in frame with the CL coding regions of the mouse (V00807) kappa light chain into expression vector pcDNA3.3. A QuikChange site-directed mutagenesis kit (Stratagene) was used to introduce the L234A, L235A, F405L, K409R, T370K and R411T (EU numbering conventions are used throughout the manuscript) point-mutations. All antibodies were produced under serum-free conditions by co-transfecting relevant heavy and light chain expression vectors in FreeStyleTM 293-F or Expi293FTM cells, using 293fectinTM or ExpiFectamineTM 293, respectively (LifeTechnologies), according to the manufacturer’s instructions.
Antibodies (all mmIgG2a and variants thereof generated for this study) were purified by protein A affinity chromatography (MabSelect SuRe; GE Health Care), dialyzed overnight to PBS, and filter-sterilized over 0.2-µM filters. Alternatively, antibodies were purified by protein G affinity chromatography (GE Health Care). The purity was determined by SDS-PAGE/CE-SDS and the concentration was measured by absorbance at 280 nm (specific extinction coefficients were calculated for each protein). Batches of purified antibody were tested by high-performance size-exclusion chromatography (HP-SEC) to determine the presence of aggregates or degradation products. Purified antibodies were stored at 2–8°C. Endotoxin levels of batches used in vivo were below 0.2 endotoxin units/mg IgG. Batches were visually inspected before use and in case of precipitates the samples were centrifuged and the concentration was determined.
Controlled Fab-arm exchange
Equimolar amounts of mmIgG2a-F405L-R411T and mmIgG2a-T370K-K409R (for effector function-silenced (inert) variants mmIgG2a-L234A-L235A-F405L-R411T and mmIgG2a-L234A-L235A-T370K-K409R) antibodies were mixed and incubated with 2-Mercaptoethylamine (2-MEA; Sigma) at a final concentration of 1 mg/mL per antibody. The final concentration of 2-MEA was 75 mM. The mixtures were typically incubated for 5 h at 31°C. To remove 2-MEA, the mixtures were buffer-exchanged against PBS using PD-10 desalting columns (5 kDa molecular weight cut-off; GE Healthcare) or dialysis using Slide-A-Lyzer cassettes (10 kDa molecular weight cut-off30). Samples were stored overnight at 4°C to allow for the re-oxidation of the disulfide bonds. Quality of the batches were verified by biochemical characterization in the assays shown in supplementary table 1.
In-vitro proliferation assay
Cells were plated in 96 well flat bottom plates at a density of 2500 cells per well in serum containing medium supplemented with 10 ng/ml mouse IFN-γ (R&D Systems) or control medium. The cells were incubated overnight in an incubator at 37°C and 5% CO2. After removal of the medium, fresh serum-free medium containing a titration series of the test antibodies was added to the cells. As positive control, cells were treated with 5 µM staurosporine to induce maximal killing. The cells were incubated for additional 72 h and cell viabilities were determined using the CellTiter-Glo system. After addition of 50 μl of CellTiter-Glo reagent, the cells were incubated for 90 min at 37°C and 5% CO2. The samples were transferred to white optiplates and luminescence was measured using an EnVision®Envision 2104 multilabel counter Microplate reader (PerkinElmer, Inc.). Viability was calculated as follows: Viable cells (% of control) = (signal-background)/(no mAb control-background)*100%), in which the background was defined as the signal measured in the wells treated with staurosporine and cells incubated in medium without antibodies as negative control sample.
NFAT reporter assay
To validate if the PD-L1 arm in the BsPD-L1xrErbB2 antibody blocked the PD-1/PD-L1 interaction, the bispecific antibody was tested in an NFAT reporter assay. PD-1/PD-L1 reporter assay (Promega) use a PD-L1+ aAPC/CHO-K1 cell line and a PD-1+ Jurkat reporter cell line (effector cells). In short, concentrated antibody dilution series were plated and subsequently both the PD-1+ effector cells and PD-L1+ aAPC/CHO-K1, both in thaw-and-use format, were added. After a 6 h incubation period at 37°C and 5% CO2, plates were equilibrated to room temperature and after addition of Bio-Glo Luciferase Assay substrate (Promega), plates were measured for luminescence on the Spectramax iD5 (Molecular Devices).
ADCC reporter assay
The experiment was performed in accordance with the protocol supplied with the Bio-Glo assay kit (Bio-Glo luciferase assay system, Promega). Prior to the experiment, the Bio-Glo luciferase substrate was added to the Bio-Glo Buffer (1 vial in 10 mL buffer). Targets cells (TUBO) were plated in a flat bottom 96 well plate at a density of 12,500 cells/well in the presence of 1 ng/ml IFN-γ. After overnight incubation, the medium was replaced by assay buffer and effector Jurkat T cells genetically engineered with mouse FcγRIV and an NFAT-response element that drives luciferase expression (NFAT-RE-luc2) (75000/well). Antibody dilutions were added and the plates were incubated at 37°C and 5% CO2 for 6 h. Then, Bio-Glo Luciferase assay reagent was added to the wells and after 15-min incubation luminescence was measured using Envision reader. In parallel, the expression of both ErbB2 and PD-L1 on the target cells was confirmed using flow cytometry.
Mouse C3 deposition assay
TUBO cells were trypsinized after O/N treatment with 1 ng/ml IFN-γ and plated in 96-well round bottom plate in serum-free medium at 40 × 103 cells/well. Antibody titrations were added and the cells were incubated for 15 min at room temperature. Mouse serum (1%) was added to stop the reaction and samples were incubated at 37°C and 5% CO2 for 15 min. C3 deposition was determined using flow cytometry with anti-mouse C3 antibody.
Receptor expression on tumor cells
The TUBO cell line expressing rat Erbb2 was cultured up to 80% confluency. The cells were stimulated with a mouse IFN-γ (R&D Systems) at a concentration range 1 to 10 to 100 ng/ml and subsequently, the cells were incubated for 24h-72h for receptor expression analysis. TUBO cells were washed with PBS, harvested using trypsin and stained with antibodies detecting Rat ErbB2 (IgG2amm-rHer2-7164-T370K-K409R) and mPD-L1 (IgG2amm-PDL1-MDPL3280A-F405-R411T). Quantification was performed using a QIFIKIT (DAKO) and the samples were analyzed by flow cytometry.
In vivo treatments
H2N100 (5 × 105) and TUBO (5 × 105) tumor cells were injected subcutaneously into Balb/c WT mice or IFN-γ-deficient Balb/c mice. Mice were treated with four intraperitoneal injections of anti-ErbB2 (7.16.4) anti-PD-L1 (MPDL3280A), bispecific antibodies or control immunoglobulin (cIg) twice a week for 2 weeks after the tumors reached a size of 60–80 mm2. A subset of mice additionally received α-CD4 (GK1.5), α-CD8β (53.5.8) to deplete T cell subsets and α-asialoGM1 to deplete NK cells, and neutralizing anti-IFN-γ (H22) antibody as indicated. Tumor growth was monitored and tumor size was recorded with a digital caliper every 2–3 d as the product of two perpendicular diameters.
Flow cytometry analysis
TUBO tumors were cut into small pieces and digested in medium containing RPMI with collagenase II (1 mg/mL) and DNAse (20 μg/mL) for 45 min. Tumors and spleen samples were filtered through 70 μm filter and washed in PBS. Samples were lysed for red blood cells by ACK lysis buffer, washed with PBS and single-cell suspensions were incubated for 20 min in Fc blocking buffer (2.4G2 antibody). Thereafter, samples were stained with viability stain Zombie Yellow (Biolegend) and fluorescence-conjugated mAbs, all diluted in 2% FCS containing PBS: anti-mouse-CD45.2 (104), TCR-β (H57-597), CD4 (RM4-5), CD8 (53.6.7), CD11b (M1/70) and Ly6G (1A8) for 30 min in ice. Samples were fixed and permeabilized using fixation permeabilization buffer from eBioscience for 15 min in ice and stained with anti-mouse FoxP3 (FJK-16S). For staining of cells in culture, tumors cells were stained with anti-mouse-PD-L1 (MIH5) and anti-ErbB2 (7.16.4) for 30 min in ice. All mAbs were purchased either from Biologend or eBioscience. Samples were acquired on LSR Fortessa IV Flow Cytometer (BD Biosciences) and data were analyzed on FlowJo V10 (Treestar).
Statistical analysis
Statistical analysis was performed using Graphpad Prism Software. Data were compared using a Mann–Whitney U test or one-way or two-way ANOVA as indicated. Data were considered statistically significant where the p values were equal to or less than 0.05.
Funding Statement
MJS is supported by a National Health and Medical Research Council of Australia (NHMRC) Senior Principal Research Fellowship [1078671] and Program Grant [1013667]. Financial support for this work was obtained from Aduro Biotech Europe and Genmab.
Acknowledgments
We thank Renske Goedemans and Laura Van Der Tuijn (Genmab) for their technical support with in vitro experiments and Janine Schuurman (Genmab) for discussion.
Conflict of interest
MJS has research agreements with Bristol Myers Squibb, Aduro Biotech and Tizona Pharmaceuticals. The following authors have a financial interest in Genmab: J.N. have stock and/or warrants. The following authors have a financial interest in Aduro Biotech Inc.: J.K., M.H., H.v.E. and A.v.E. have stocks and/or warrants. All other authors have no conflict of interest to declare.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Mittal D, Caramia F, Michiels S, Joensuu H, Kellokumpu-Lehtinen PL, Sotiriou C, Loi S, Smyth MJ. Improved treatment of breast cancer with anti-HER2 therapy requires Interleukin-21 signaling in CD8+ T cells. Cancer Res. 2016;76:264–274. [DOI] [PubMed] [Google Scholar]
- 2.Mortenson ED, Park S, Jiang Z, Wang S, Fu YX.. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clin Cancer Res. 2013;19:1476–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ.. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. [DOI] [PubMed] [Google Scholar]
- 6.Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, Weiner LM, Fan Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett. 2018;430:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, Jose V, Pommey S, Delisle V, Loi S, Joensuu H, et al. CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 2017;77:5652–5663. [DOI] [PubMed] [Google Scholar]
- 8.Hou Y, Nitta H, Wei L, Banks PM, Parwani AV, Li Z. Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer: the association with response to anti-HER2 neoadjuvant therapy. Clin Breast Cancer. 2018;18:e237–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang JY, Au WL, Lo KY, Ni YB, Hlaing T, Hu J, Chan S-K, Chan K-F, Cheung S-Y, Tse GM, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat. 2017;162:19–30. [DOI] [PubMed] [Google Scholar]
- 10.Hou Y, Nitta H, Wei L, Banks PM, Lustberg M, Wesolowski R, Ramaswamy B, Parwani AV, Li Z. PD-L1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J. 2018;24:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, Chen J, Su F, Liu Q, Song E. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175:442–57 e23. [DOI] [PubMed] [Google Scholar]
- 12.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20:371–382. [DOI] [PubMed] [Google Scholar]
- 13.Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther. 2018;12:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. [DOI] [PubMed] [Google Scholar]
- 15.Brack S, Attinger-Toller I, Schade B, Mourlane F, Klupsch K, Woods R, Hachemi H, von der Bey U, Koenig-Friedrich S, Bertschinger J, et al. A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol Cancer Ther. 2014;13:2030–2039. [DOI] [PubMed] [Google Scholar]
- 16.Geuijen CAW, De Nardis C, Maussang D, Rovers E, Gallenne T, Hendriks LJA, Visser T, Nijhuis R, Logtenberg T, de Kruif J, et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell. 2018;33:922–36 e10. [DOI] [PubMed] [Google Scholar]
- 17.Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res. 2014;74:5561–5571. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Ybarra R, Mak J, Herault A, De Almeida P, Arrazate A, Ziai J, Totpal K, Junttila MR, Walsh KB, et al. IFNgamma-induced chemokines are required for CXCR3-mediated T-cell recruitment and antitumor efficacy of anti-HER2/CD3 bispecific antibody. Clin Cancer Res. 2018;24:6447–6458. [DOI] [PubMed] [Google Scholar]
- 19.Rius Ruiz I, Vicario R, Morancho B, Morales CB, Arenas EJ, Herter S, Freimoser-Grundschober A, Somandin J, Sam J, Ast O, et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci Transl Med. 2018;10.pii: eaat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries MP, Hynes S, Bingham V, Cougot D, James J, Patel-Socha F, Parkes EE, Blayney JK, O’Rorke MA, Irwin GW, et al. Automated tumour recognition and digital pathology scoring unravels new role for PD-L1 in predicting good outcome in ER-/HER2+ breast cancer. J Oncol. 2018;2018:2937012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim A, Lee SJ, Kim YK, Park WY, Park DY, Kim JY, Lee CH, Gong G, Huh GY, Choi KU. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep. 2017;7:11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, Kadel EE, Wistuba I, Chaft J, Rizvi NA, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A. 2018;115:E10119–E10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, Watkins A, Mullins S, Chodorge M, Andrews J, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–1062. [DOI] [PubMed] [Google Scholar]
- 26.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–1543. [DOI] [PubMed] [Google Scholar]
- 28.Zilio S, Serafini P. Neutrophils and Granulocytic MDSC: the Janus God of Cancer Immunotherapy. Vaccines (Basel). 2016;4.pii: E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, Johnstone RW, Smyth MJ. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci U S A. 2008;105:16254–16259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labrijn AF, Meesters JI, Bunce M, Armstrong AA, Somani S, Nesspor TC, Chiu ML, Altintaş I, Verploegen S, Schuurman J, et al. Efficient generation of bispecific murine antibodies for pre-clinical investigations in syngeneic rodent models. Sci Rep. 2017;7:2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labrijn AF, Meesters JI, de Goeij BE, van Den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110:5145–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labrijn AF, Meesters JI, Priem P, de Jong RN, van Den Bremer ET, van Kampen MD, Gerritsen AF, Schuurman J, Parren PWHI. Controlled Fab-arm exchange for the generation of stable bispecific IgG1. Nat Protoc. 2014;9:2450–2463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


