ABSTRACT
HER2 overexpression leads to downregulation of MHC class-I. CD4+ Th1 cytokines, IFNγ and TNFα, and monoclonal antibodies, trastuzumab and pertuzumab, restore MHC class-I expression, and enable CD8+ recognition and cytolysis. Restoration of the anti-HER2 CD4+ Th1 immune response in combination with HER2 targeted therapy appear to be critical to successful anti-HER2 CD8+ immunotherapy.
KEYWORDS: HER2, CD8+ T-cells, MHC class-I, CD4+ T-cells, IFNγ, TNFα, monoclonal antibodies, trastuzumab, pertuzumab
Abbreviations
- CD
cluster of differentiation
- CTL
cytotoxic T lymphocyte
- DTH
delayed type hypersensitivity
- E:T
effector cell to target cell ratio
- HER
human epidermal growth factor receptor
- HLA
human leukocyte antigen
- IFN
interferon
- MCF
mean channel fluorescence
- MHC
major histocompatibility complex
- PD-L1
programmed death-ligand-1
- Th1
type 1 T-helper
- TNF
tumor necrosis factor.
T-cell antitumor responses are associated with improved outcomes in HER2 breast cancer. Increased lymphocytic infiltrate and overexpression of lymphocyte-associated genes in HER2pos tumors correlate with prolonged distant metastasis free survival and decreased recurrence rates.1 Furthermore, there is a strong association between immune gene expression and recurrence free survival following treatment with adjuvant trastuzumab, suggesting that the subset of HER2 positive tumors with a high-level of immunologic activity are better situated to benefit from treatment with adjuvant trastuzumab.2
However, attempts to boost cellular immunity have produced disappointing results. For example, HER2369–377 (KIFGSLAFL; E75), the most widely studied HER2 immunogenic peptide, has been identified endogenously in breast and ovarian cancers. Vaccination with HLA-A2-restricted peptide 369–377 generates HER2369–377 reactive CD8+ T-cells; paradoxically, patients with HER2low-expressing tumors mount a more robust immunologic response after vaccination than patients with HER2high-expressing tumors—patients with HER2low-expressing tumors demonstrate a larger DTH response, a more sustained specific CTL response, and a more prolonged disease free survival.3 Additionally, others have shown that immunization results in peptide-specific CTLs that fail to recognize HLA-A2pos HER2pos tumor cells,4 spurring the controversy as to whether this epitope is processed and presented by HER2 expressing tumors.
We further explored this complex interaction between CD8+ T-cells and HER2pos tumor cells. Overexpression of HER2 has been shown to downregulate MHC class-I.5 The resulting decrease of MHC class-I may explain why peptide-specific CTLs generated by HER2369–377 vaccination fail to recognize the HLA-A2pos HER2pos tumors. Additionally, the magnitude of HER2 expression resulting in MHC class-I downregulation may explain the differential response to HER2low and HER2high expressing cells—CD8+ T-cells recognize HER2low tumor cells with sustained MHC class-I expression, but not HER2high tumor cells with deficient MHC class-I expression. In our recent manuscript, we confirmed that MHC class-I expression is maintained on HER2low cell lines, but is severely diminished on HER2high cell lines. Furthermore, we demonstrated that HER2369–377–specific CD8+ T-cells recognize the epitope HER2369–377 on the HER2low/MHC class-I expressing tumor cells, but not the stealth HER2high/MHC class-I deficient tumor cells.6
We then investigated strategies to restore MHC class-I expression using CD4+ Th1 cytokines, IFNγ and TNFα, and HER2 targeted tyrosine kinase inhibitors, trastuzumab and pertuzumab. The CD4+ cytokine, IFNγ, has been shown to induce MHC class-I expression in HER2 overexpressing tumors in murine models.7 Similarly, we found that all HER2 expressing cell lines treated with IFNγ/TNFα significantly increase MHC class-I expression. However, dual treatment with IFNγ/TNFα was only able to significantly increase anti-HER2 CD8+ mediated recognition and cell lysis in HER2intermediate cell lines, but not HER2high cell lines.6
In contrast, while treatment with monoclonal antibodies alone, trastuzumab, pertuzumab, or both trastuzumab and pertuzumab, had little effect on MHC class-I expression or CD8+ mediated cytotoxicity, the combination of a monoclonal antibody and IFNγ/TNFα dramatically increased MHC class-I expression on all HER2 expressing cell lines, and was significantly better than Th1 cytokine treatment (IFNγ/TNFα) in HER2high cell lines. Similarly, only the combination of a monoclonal antibody and IFNγ/TNFα rendered the HER2high cells susceptible to CD8+ mediated recognition and lysis.6 Consistent with the clinical benefit seen following treatment with dual monoclonal antibodies, trastuzumab and pertuzumab, treatment with IFNγ/TNFα and both trastuzumab and pertuzumab increased MHC class-I expression and CD8+ mediated cytotoxicity more than IFNγ/TNFα or IFNγ/TNFα and either trastuzumab or pertuzumab (Fig. 1).
Figure 1.
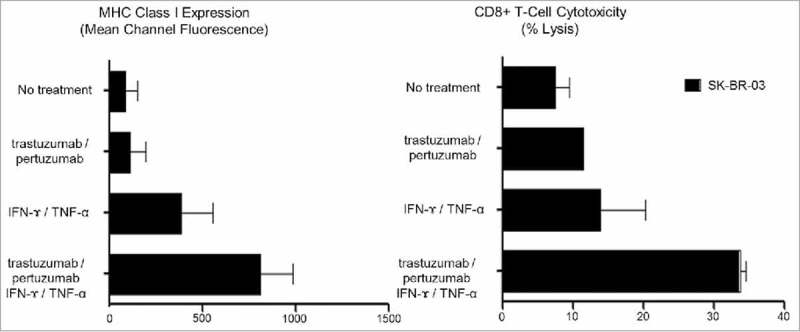
The effect of CD4+ Th1 cytokines (IFNγ/TNFα) and HER2 targeted monoclonal antibodies (Trastuzumab/Pertuzumab) on MHC class-I expression and CD8+ T-cell mediated cytotoxicity in SK-BR-3 cells (E:T = 10:1). Both HLA-A2 MHC class I expression and CD8+ cytotoxicity remained low following treatment with Trastuzumab alone (96.4 ± 70.6 MCF, 11.7%), Pertuzumab alone (103.4 ± 81.6 MCF, 9.7%), and Trastuzumab/Pertuzumab (113.8 ± 83.2 MCF, 11.5%), at levels similar to MHC class I expression without treatment (87.1 ± 64.9 MCF, 9.5%). HLA-A2 MHC class-I expression and CD8+ cytotoxicity both increased following treatment with IFNγ/TNFα (386.6 ± 171.4 MCF, 20.4%), IFNγ/TNFα and Trastuzumab (512.9 ± 95.1 MCF, 28.9%), IFNγ/TNFα and Pertuzumab (486.3 ± 166.7 MCF, 28.6%), with the greatest increase following treatment with IFNγ/TNFα and Trastuzumab/Pertuzumab (813.3 ± 117.8 MCF, 34.6%).
Just as HER2 cancer cells lack expression of MHC class-I, they also lack expression of programmed death-ligand-1 (PD-L-1). In addition to inducing MHC class-I expression, the CD4+ cytokine, IFNγ, is also known to induce PD-L-1 expression on breast cancer cells with low endogenous PD-L-1 expression.8 We showed that IFNγ upregulated PD-L1 expression in all HER2 expressing cell lines, and that combined IFNγ/TNFα treatment further enhanced PD-L1 expression. Upregulation of PD-L-1 would facilitate the use of PD-1/PD-L-1 inhibition in the treatment of HER2 breast cancer.6
CD4+ T-cells have long been recognized as a critical aide to the survival and function of CD8+ T-cells; our work has shown that CD4+ T-cells further facilitate CD8+ cytotoxicity by modifying the tumor environment. Unfortunately, we have previously shown that there is an early and progressive loss of anti-HER2 CD4+ Th1 response in breast tumorigenesis—healthy patients have a strong anti-HER2 CD4+ Th1 immune response that is decreased in patients with ductal carcinoma in situ and nearly absent in patients with invasive breast cancer.9 We also showed that the magnitude of the immune response correlates with clinical outcomes—a depressed anti-HER2 CD4+ Th1 immune response correlates with an increased risk of recurrence, and a robust response correlates with an increased rate of pathologic complete response following neoadjuvant chemotherapy. Although this deficit does not appear to be corrected by surgery, radiation, chemotherapy, or HER2 targeted monoclonal antibodies, we showed that it can be corrected by HER2 peptide pulsed DC1 vaccination resulting in long term maintenance of anti-HER2 immune response.9,10
These results support the multivalent targeting of HER2. Anti-HER2 Th1 cells together with HER2 directed antibodies may enhance the tumoricidal effects of anti-HER2 CD8+ T-cells and facilitate the potential use of checkpoint inhibitors in the treatment of HER2pos breast cancer. Consideration should be given to restoring the anti-HER2 Th1 in the design of HER2 directed therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, Harris L, Barnard N, Martel M, Levine AJ et al.. High expression of lymphocyte-associated genes in node-negative HER2pos breast cancers correlates with lower recurrence rates. Cancer Res 2007; 67:10669-76; https://doi.org/ 10.1158/0008-5472.CAN-07-0539 [DOI] [PubMed] [Google Scholar]
- 2.Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR, Eckel-Passow JE, Dueck AC, Tenner KS, Jen J et al.. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol 2015; 33:701-8; PMID:25605861; https://doi.org/ 10.1200/JCO.2014.57.6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, Gates JD, Sears AK, Stojadinovic A, Ponniah S et al.. Clinical trial results of the HER2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer 2012; 118:2594-602; PMID:21989902; https://doi.org/ 10.1002/cncr.26574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369-377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res 1998; 58:4902-8; PMID:9809997 [PubMed] [Google Scholar]
- 5.Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N et al.. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer 2011; 128:390-401; PMID:20715101; https://doi.org/ 10.1002/ijc.25613 [DOI] [PubMed] [Google Scholar]
- 6.Datta J, Xu S, Rosemblit C, Smith JB, Cintolo JA, Powell DJ Jr., Czerniecki BJ. CD4+ T-Helper Type 1 Cytokines and Trastuzumab Facilitate CD8+ T-cell Targeting of HER2/neu-Expressing Cancers. Cancer Immunol Res 2015; 3:455-63; PMID:25791067; https://doi.org/ 10.1158/2326-6066.CIR-14-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lollini PL, Nicoletti G, Landuzzi L, De Giovanni C, Rossi I, Di Carlo E, Musiani P, Muller WJ, Nanni P. Down regulation of major histocompatibility complex class I expression in mammary carcinoma of HER-2/neu transgenic mice. Int J Cancer 1998; 77:937-41; PMID:9714068; https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 8.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One 2014; 9:e88557; PMID:24551119; https://doi.org/ 10.1371/journal.pone.0088557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta J, Berk E, Rosemblit C, Showalter S, Mick R, Lee KP, Brod AM, Yang RL, Kelz RR, Fitzpatrick E et al.. Progressive Loss of Anti-HER2 CD4+ T-helper Type 1 Response during Breast Tumorigenesis and the Potential for Immune Restoration. OncoImmunology 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta J, Berk E, Xu S, Fitzpatrick E, Rosemblit C, Lowenfeld L, Goodman N, Lewis DA, Zhang PJ, Fisher C et al.. Anti-HER2 CD4 T-helper type 1 response is a novel immune correlate to pathologic response following neoadjuvant therapy in HER2-positive breast cancer. Breast Cancer Res 2015; 17:71; PMID:25997452; https://doi.org/ 10.1186/s13058-015-0584-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


