ABSTRACT
In patients with muscle invasive bladder cancer (MIBC), neoadjuvant chemotherapy (NAC) prior to radical cystectomy has improved survival but there is an urgent unmet need to identify prognostic and predictive biomarkers to stratify patients who will benefit from treatment. This study aimed to examine the composition of tumor-infiltrating immune cells in MIBC, with particular reference to the clinical outcome and the potential modifying effect of NAC. To this end, the expression of CD8+ and FoxP3+ T cells, CD20+ B cells, PD-1+ and PD-L1+ immune cells and PD-L1+ tumor cells was evaluated by immunohistochemistry on tissue microarrays with paired transurethral resection (TURB) specimens, cystectomy specimens and lymph node metastases from 145 patients, 65 of whom had received NAC. Kaplan–Meier and Cox regression analyses were applied to assess the impact of investigated cell subsets on time to recurrence (TTR). In cystectomy specimens, high infiltration of the investigated immune cell populations, but not PD-L1+ tumor cells, were independently associated with a prolonged TTR, whereas in TURB specimens, this association was only seen for CD8+ lymphocytes. An additive beneficial prognostic effect of NAC was seen for the majority of the cell subsets but there was no significant interaction between any immune marker and NAC in relation to TTR. Furthermore, no differences in cell densities prior to NAC treatment were observed between complete and non-complete responders, or pre- and posttreatment in non-complete responders. In conclusion, immune cell infiltration provides important prognostic information in both pre- and postsurgical samples of MIBC, independently of NAC.
Keywords: Bladder cancer, neoadjuvant chemotherapy, tumor microenvironment, immunotherapy, prognosis
Introduction
Bladder cancer (BC) is the ninth most common malignancy worldwide.1 Its high prevalence, in conjunction with high recurrence and progression rates,2 makes BC an important, yet understudied, health issue.3 Despite medical advancements, the prognosis of urothelial muscle invasive BC (MIBC) remains dismal, as nearly half of the patients will develop metastases within few years after radical cystectomy, most of whom will die of their disease.4,5 The high recurrence rate is thought to be predominantly caused by occult micrometastases present at the time of surgery.6 Therefore, cisplatinum-based neoadjuvant chemotherapy (NAC) is recommended to eligible patients with locally advanced but operable MIBC to lower the risk of recurrence.2 Even though clinical studies have consistently reported that NAC improves survival,7 the response to treatment is heterogeneous. Patients in whom NAC elicit a pathological downstaging of the tumor to ≤ pT1 could have a 5-year survival rate of 80–90%, in contrast to 30–40% in non-responders.8 Since the overall response rate to NAC is limited to 30–50% of patients, the overtreatment of non-responders is associated with a risk of chemotherapy-related toxicity and a worse outcome.9 Hence, there is an urgent unmet need to identify prognostic and predictive biomarkers to guide the use of NAC.
Significant research efforts within cancer immunology have been directed towards tumor-infiltrating immune cells (TICs), which have been associated with both disease prognosis as well as response to chemotherapy in various types of cancers.10–13 Cancer surveillance is largely dependent on TICs, and given their key role in the anti-tumor response, infiltration of CD8+ cytotoxic T cells has been identified as a prognostic indicator in MIBC.14,15 Similarly, infiltration of other T cell subsets, including Forkhead box P3+ (FoxP3+) Tregs, has been correlated with an improved survival.16 A reinforced anti-tumor T cell response in lymph nodes after NAC treatment in MIBC has recently been demonstrated by Krantz et al.17 Apart from the T cell response, B cells also play a critical role in anti-tumor immunity and lymphoplasmacytic infiltration has been linked to favorable outcomes in several cancer types, including colorectal cancer, breast, cervical and non-small cell lung cancer.18–20 As of yet, the prognostic significance of tumor-infiltrating cells of the B lineage, including CD20+ B lymphocytes, in MIBC is poorly understood.
In addition to chemotherapy, immune checkpoint inhibitors targeting the programmed-death 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway are approved as first- and second-line treatment options for metastatic BC.21,22 The expression of PD-1 and PD-L1 has been evaluated as possible biomarkers predicting outcome and therapeutic response to both chemotherapy and immunotherapy in MIBC, with conflicting results.23–25 Moreover, discordance between PD-L1 expression in primary tumors and lymph node metastases has been reported.26,27 The observation of spatiotemporal tumoral heterogeneity arises questions whether longitudinal tissue sampling from primary tumors and/or metastatic lesions could add diagnostic information and help guide treatment decisions.21 The expression of CD8+ and FoxP3+ T cells as well as CD20+ B lymphocytes has, to the best of our best knowledge, not been previously compared in matched pre- and postsurgical specimens of primary tumors and lymph node metastases of MIBC.
This study, therefore, aimed to evaluate the densities of tumor-infiltrating T cells, B cells as well as the PD-1/PD-L1 pathway in paired transurethral resection specimens, cystectomy specimens and lymph node metastases of MIBC. Particular emphasis was given to the relationship of TICs with clinical outcome and the potential modifying effect of NAC.
Results
Patient and tumor characteristics in the entire cohort and in strata according to neoadjuvant chemotherapy
The mean follow-up time was 4.3 (range 0.2–7.9) years. At the end of follow-up in August 2018, a total number of 61 (42.1%) patients had died, 50 (34.5%) of whom due to their BC. Recurrent disease was denoted in 51 (35.2%) patients, of whom 6 (4.2%) patients were alive with metastatic spread of BC. The mean time to recurrence was 1.6 (range 0.2–6.2) months. For patients alive, the mean follow-up time was 5.9 (range 4.0–7.9) years.
As demonstrated in Table 1, the patients who received NAC were significantly younger, with a median age of 64.7 years compared to 73.9 years in the untreated population (p <.001). The distribution of sex and preoperative T-stage was well balanced between the two groups. The finding of complete histopathological response (pT0) in cystectomy specimens was more common in NAC treated patients (p < .001) and the presence of concomitant carcinoma in situ (CIS) was significantly lower (p = .022). NAC treated patients had a trend towards lower clinical T-stages. Node-negative disease was more frequent in the NAC treated population (p = .006). Lymphovascular invasion (LVI) was rarely recorded in the pathology report regarding the TURB specimens, with only one case denoted as having LVI. No LVI was found in any of the cystectomy specimens in patients treated with NAC.
Table 1.
Distribution of clinicopathological characteristics in all patients and in strata according to neoadjuvant chemotherapy (NAC).
| All patients | No NAC | NAC | ||
|---|---|---|---|---|
| n (%) | 145 | n = 80 (55.2) | n = 65 (44.8) | p-value |
| Age | ||||
| Mean/median (years) | 68.7/70.6 | 72.7/73.93 | 63.7/64.7 | <0.001 |
| Range (years) | 38.7–83.3 | 38.7–83.3 | 43.1–75.9 | |
| Sex | ||||
| Female | 30 (20.7) | 15 (18.8) | 15 (23.1) | 0.524 |
| Male | 115 (79.3) | 65 (81.3) | 50 (76.9) | |
| Preoperative T-stage | ||||
| T2 | 77 (53.1) | 38 (47.5) | 39 (60.0) | 0.187 |
| T3 | 50 (34.5) | 31 (38.8) | 19 (29.2) | |
| T4 | 18 (12.4) | 11 (13.8) | 7 (10.8) | |
| Postoperative T-stage | ||||
| CIS only | 4 (2.8) | 2 (2.5) | 2 (3.1) | <0.001 |
| pTa | 5 (3.4) | 2 (2.5) | 3 (4.6) | |
| pT0 | 35 (24.1) | 7 (8.8) | 28 (43.1) | |
| pT1 | 13 (9.0) | 7 (8.8) | 6 (9.2) | |
| pT2 | 29 (20.0) | 15 (18.8) | 14 (21.5) | |
| pT3 | 43 (29.7) | 34 (42.5) | 9 (13.8) | |
| pT4 | 16 (11.0) | 13 (16.3) | 3 (4.6) | |
| Grade in TURB specimens | ||||
| Low | 0 (0) | 0 (0) | 0 (0) | - |
| High | 145 (100) | 80 (100) | 65 (100) | |
| Grade in cystectomy specimens | ||||
| Low | 3 (2.9) | 0 (0.0) | 3 (8.6) | <0.001 |
| High | 101 (97.1) | 69 (100) | 32 (91.4) | |
| N/A | 41 | 11 | 30 | |
| N-stage | ||||
| N0 | 103 (71.0) | 50 (62.5) | 53 (81.5) | 0.006 |
| N1 | 16 (11.0) | 9 (11.3) | 7 (10.8) | |
| N2 | 13 (9.0) | 11 (13.8) | 2 (3.1) | |
| N3 | 13 (9.0) | 10 (12.5) | 3 (4.6) | |
| M-stage | ||||
| M0 | 132 (97.8) | 72 (98.6) | 60 (96.8) | 0.468 |
| M1 | 3 (2.2) | 1 (1.4) | 2 (3.2) | |
| Missing data | 10 | 7 | 3 | |
| LVI in cystectomy specimens | ||||
| No | 95 (93.1) | 64 (90.1) | 31 (100) | 0.071 |
| Yes | 7 (6.9) | 7 (9.9) | 0 (0) | |
| Missing data | 43 | 9 | 34 | |
| CIS in TURB specimens | ||||
| Not found | 130 (89.7) | 73 (91.3) | 57 (87.7) | 0.486 |
| Found | 15 (10.3) | 7 (8.8) | 8 (12.3) | |
| CIS in cystectomy specimens | ||||
| Not found | 120 (82.8) | 61 (76.3) | 59 (90.8) | 0.022 |
| Found | 25 (17.2) | 19 (23.8) | 6 (9.2) | |
N/A = No assessable tumour, LVI = Lymphovascular invasion, CIS = Carcinoma in situ.
P-value <0.05 is considered significant.
Distribution of immunohistochemical expression in TURB specimens, cystectomy specimens and lymph node metastases
Sample immunohistochemical images are shown in Figure 1. Cases with loss of or an insufficient amount of tumor cells in the TMA cores, and cystectomy specimens from cases with complete histopathological response (T0) were excluded from the analyses. The expression of CD8 was assessable in TURB specimens from 140 (96.6%) cases, in cystectomy specimens from 89 (61.4%) cases and in lymph nodes from 27 (18.6%) cases. The corresponding numbers for FoxP3 expression were 143 (98.6%) 93 (64.1%) and 27 (18.6%), for CD20 expression 142 (97.9%), 94 (64.8%) and 27 (18.6%), for PD-1 expression 144 (99.3%), 95 (65.5%) and 27 (18.6%), and for PD-L1 expression 139 (95.9%), 81 (55.9%) and 26 (17.9%).
Figure 1.
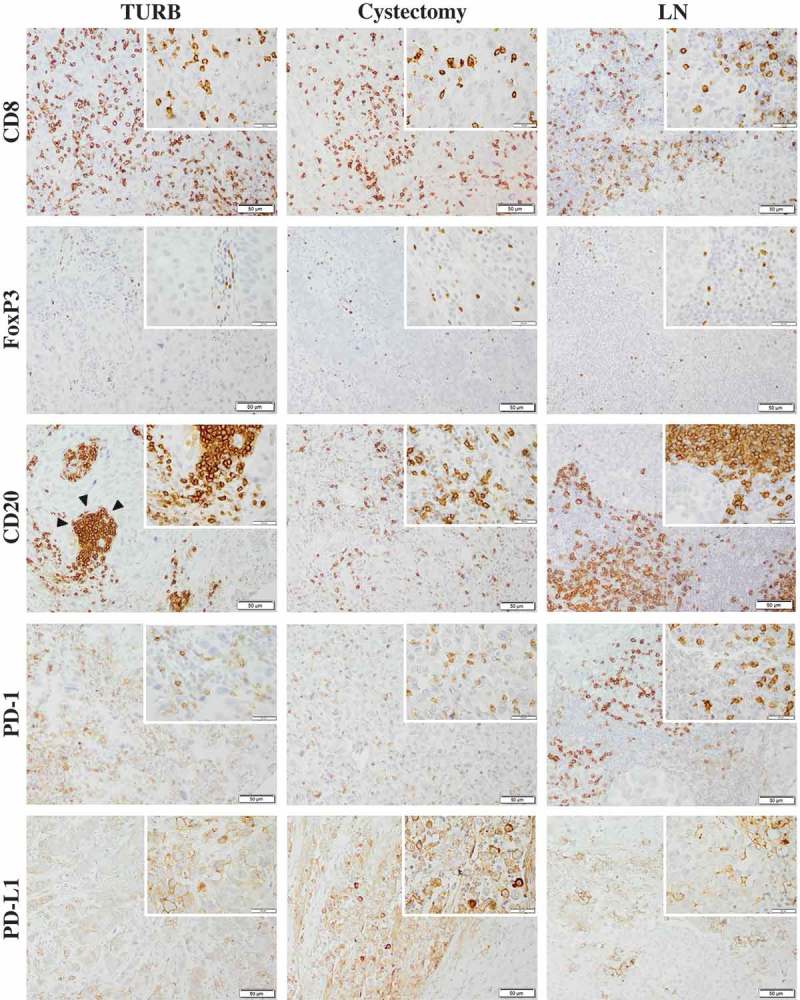
Immunohistochemical staining of CD8, FoxP3, CD20, PD-1 and PD-L1 in muscle invasive bladder cancer. Sample images (10x magnification with 40x insertion) of studied cell subsets in TURB specimens, cystectomy specimens and lymph node metastases (LN). The estimated percentage of stained cells was annotated. Arrow-heads illustrating a lymphoid aggregate of CD20+ B cells. Scale bar = 50 μm (10x) and 20 μm (40x).
The distribution of the densities of investigated cell subsets in paired TURB specimens, cystectomy specimens and lymph node metastases for the entire cohort are demonstrated in Figure 2. Comparison of paired tissue specimens revealed a significantly higher CD8+, CD20+ and PD-1+ immune cell density in cystectomy specimens compared to TURB specimens as well as a significantly higher density of these cell subsets in lymph nodes compared to TURB specimens. The density of CD20+ B cells was also significantly higher in lymph nodes compared to cystectomy specimens. The distribution of the other immune markers did not differ significantly between the different types of specimens.
Figure 2.
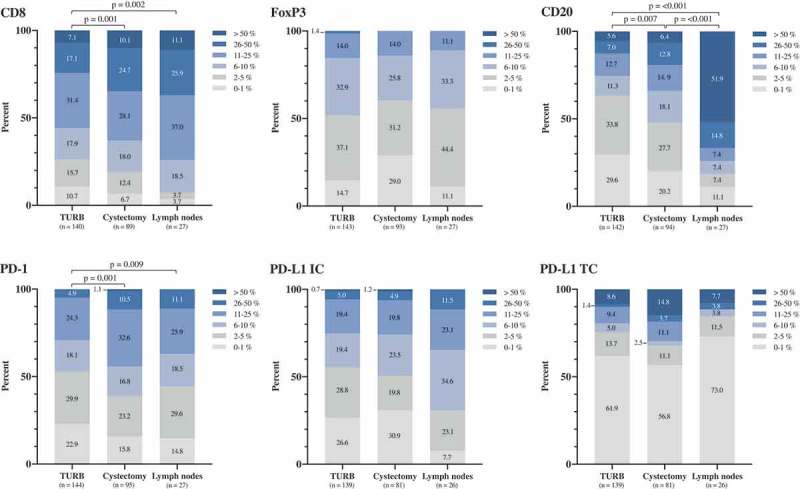
Distribution of immune marker expression in different types of specimens. Bar charts illustrating the distribution of different immune cell subsets and PD-L1TC in A) TURB specimens, B) cystectomy specimens and C) lymph node metastases.
Figure 3 shows the distribution of cell marker expression in strata according to NAC, as well as in relation to the histopathological response. Except for a significantly higher expression of PD-L1TC in TURB specimens from NAC treated patients (p = .037), there were no significant differences in immune cell densities between NAC untreated and treated patients (Figure 3(a)). Comparison of paired tissue specimens revealed significantly higher densities of CD8, CD20 and PD-1 in cystectomy specimens compared to TURB specimens (p = .003, p = .031 and p = .018, respectively) in NAC untreated patients. Furthermore, in NAC untreated patients, the densities of CD8+, CD20+, PD-1+ and PD-L1+ immune cells were higher in lymph nodes compared to TURB specimens (p = .005, 0.001, 0.006 and 0.019, respectively) and there was also an increase in the density of CD20+ B cells in lymph nodes compared to cystectomy specimens (p = .002). In NAC treated patients, a higher density of PD-1+ immune cells in cystectomy specimens compared to TURB specimens was seen (p = .011) as well as a higher density of CD20+ B cells in lymph nodes compared to TURB specimens (p = .028).
Figure 3.
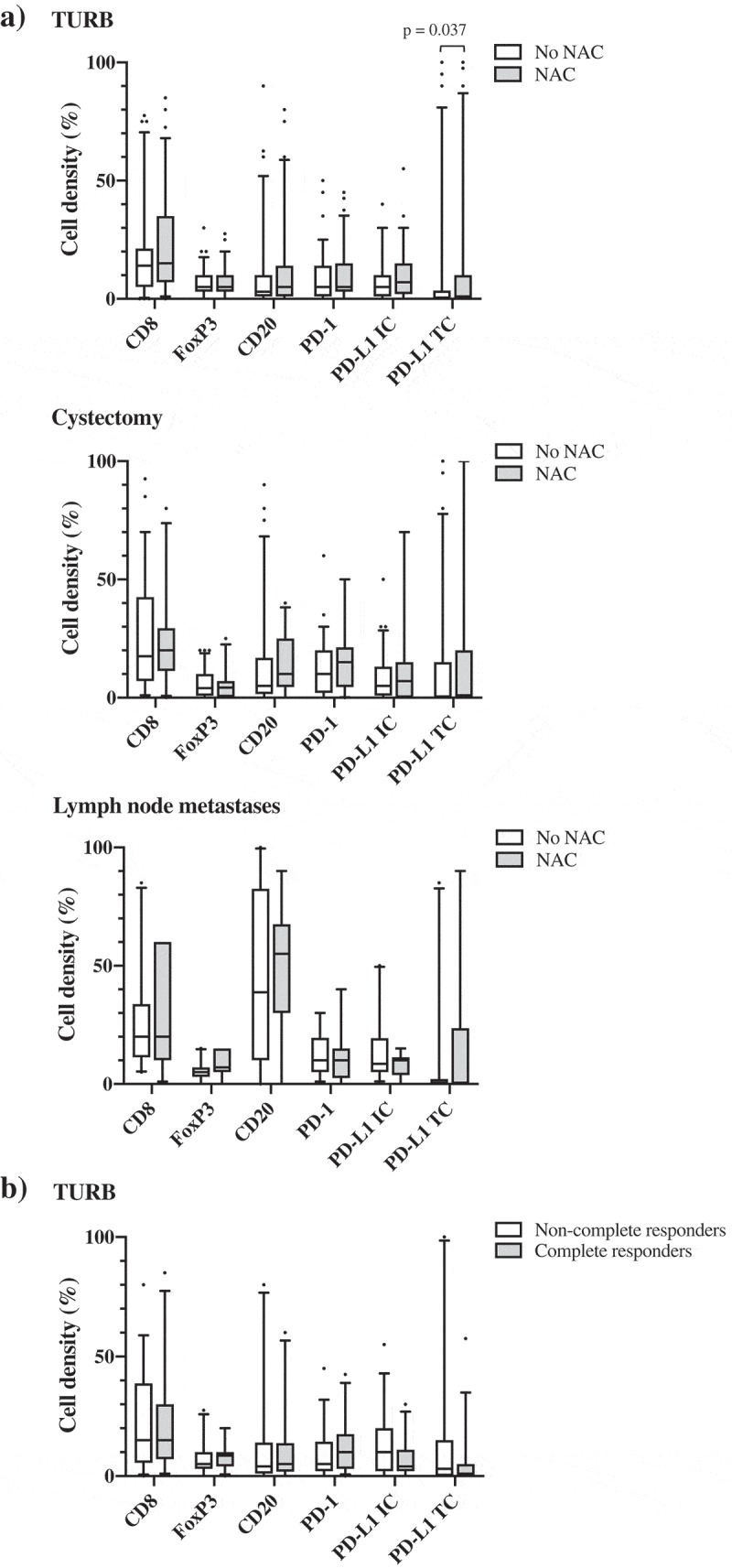
Distribution of immune cell density and PD-L1TC in strata according to neoadjuvant chemotherapy and histopathological response to treatment. A) Densities of different immune cell subsets in tissue specimens from NAC untreated (n = 80) and treated (n = 65) patients. B) Densities of different immune cell subsets prior to NAC treatment in complete (i.e. T-stage 0 or Ta/CIS) and non-complete (i.e. T-stage ≥1) responders. Whiskers represent 5% and 95%.
As shown in Figure 3(b), immune marker expression prior to NAC did not differ significantly between TURB specimens of complete (i.e. T-stage 0 or Ta/CIS only in cystectomy specimens) and non-complete (i.e. T-stage ≥1) responders to treatment.
For all examined immune cell subsets, except CD20+ lymphocytes, the majority of TMA cores from TURB specimens and cystectomy specimens showed immune cell infiltration also in the tumor nest, as presented in Supplementary Figure 1. Lymphoid aggregates of CD20+ B cells were found in TURB specimens in 28 (19.7%) cases and in cystectomy specimens in 12 (12.9%) cases. There were no differences in the spatial distribution of immune cells between stroma and tumor nest nor in the presence of lymphoid aggregates before and after cystectomy (data not shown).
Interrelationship between investigated cell populations and associations with clinicopathological characteristics
The interrelationship between investigated biomarkers in the entire cohort is demonstrated in Supplementary Figure 2. In TURB specimens, Spearman’s rho test revealed significant strong intercorrelations (R = 0.40–0.75) between CD8+, CD20+, PD-1+ and PD-L1+ immune cells as well as PD-L1+ tumor cells, except for a moderate correlation (R = 0.25–0.40) between CD20+ B cells and PD-L1TC. The correlations between FoxP3+ T-cells and the other observed markers were overall moderate. In cystectomy specimens, strong intercorrelations were found between all investigated immune markers, except for a moderate correlation between CD20+ B-cells and PD-L1IC as well as between FoxP3+ cells and PD-L1TC. In paired lymph nodes, significant strong intercorrelations were observed between CD8+, FoxP3+, PD-L1IC and PD-L1TC cell infiltration, as well as between CD20+ and PD-1+ lymphocytes.
The associations between studied cell subsets and clinicopathological characteristics are detailed in Supplementary Table 1–6, and the significant associations are visualized in Figure 4. In TURB specimens, there was a trend towards lower CD8+ T cell density and higher postoperative T-stage. However, in cystectomy specimens, the highest density of CD8+ T cells was observed in T2 tumors, with significant differences to CIS/Ta as well as T3 and T4 tumors, and with a stepwise decrease from T3 to T4 tumors. Similar trends were seen for PD-L1+ tumor cells in cystectomy specimens. Only FoxP3 infiltration was found to differ significantly with age, in that a higher density of FoxP3+ immune cells in TURB specimens was associated with younger age at diagnosis. High density of PD-L1+ tumor cells in TURB specimens as well as high densities of FoxP3+ and PD-L1+ immune cells in cystectomy specimens were associated with a lower prevalence of concomitant carcinoma in situ. High PD-L1TC expression in TURB specimens correlated significantly with lower N-stage. PD-1+ and CD20+ cell infiltration did not correlate significantly with any of the investigated clinicopathological parameters.
Figure 4.
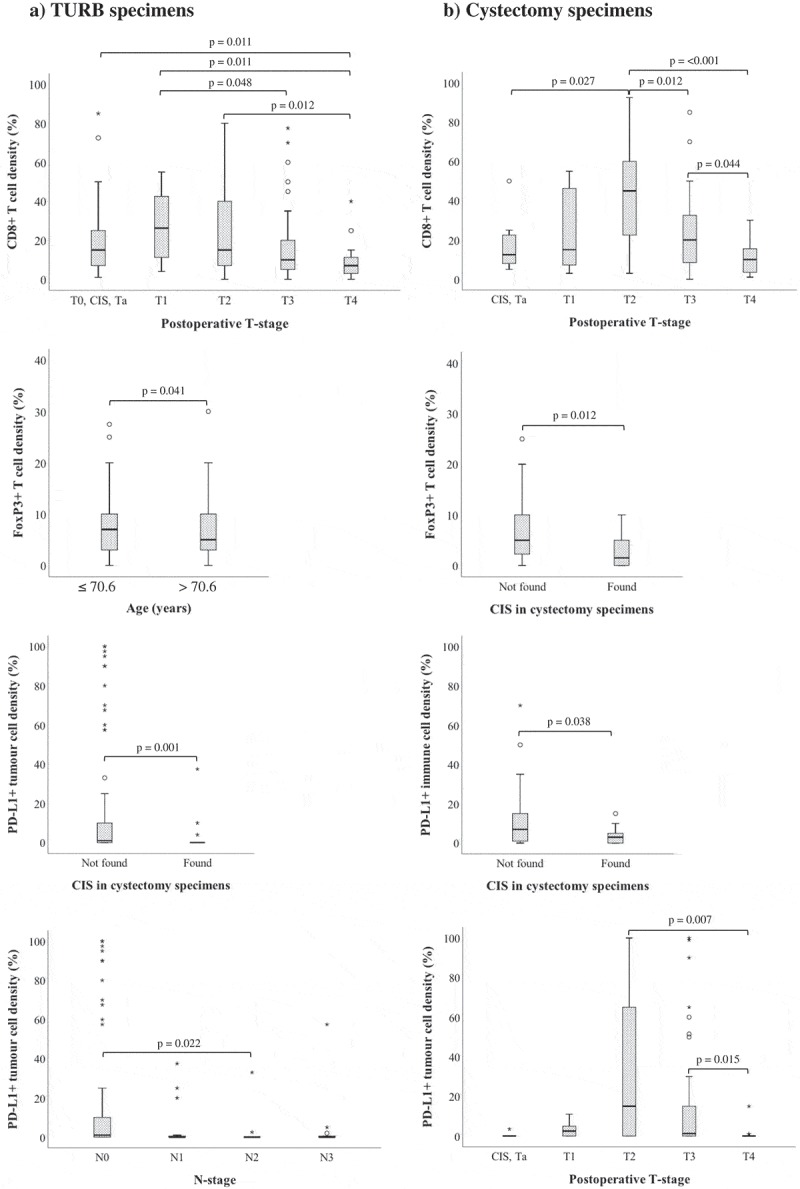
Clinicopathological correlates of different immune cell subsets and PD-L1TC. Box plots visualizing the associations between clinicopathological characteristics and immune marker density in A) TURB specimens and B) cystectomy specimens. Whiskers represent 5% to 95%. P-values are from non-parametric tests, only significant associations (p < .05) are denoted in the panels.
Prognostic role of studied biomarkers overall and in relation to neoadjuvant chemotherapy
For survival analyses, dichotomization of cell densities into high and low expression was based on cut off points from median values and CRT-analyses, respectively. The cut off points based on median values for TURB specimens were as follows (% cases with high expression): CD8 15% (39.3%), FoxP3 5% (48.3%), CD20 3% (45.8%), PD-1 5% (47.2%), PD-L1IC 5% (44.6%) and PD-L1TC 0% (46.0%). The corresponding numbers for median-derived cut off points for cystectomy specimens were: CD8 20% (43.8%), FoxP3 4% (49.5%), CD20 6.75% (50.0%), PD-1 10% (44.2%), PD-L1IC 5% (49.4%) and PD-L1TC 0% (48.1%). CRT-analysis established optimal prognostic cut off points for TURB specimens as follows: CD8 31.25% (22.1%), FoxP3 1.75% (83.9%), CD20 57.50% (4.2%), PD-1 21.25% (11.1%), PD-L1IC 6.75% (42.4%) and PD-L1TC 63.75% (6.5%). The corresponding numbers for CRT-derived cut off points for cystectomy specimens were: CD8 17.0% (52.8%), FoxP3 2.75 (67.7%), CD20 0.50% (84.0%), PD-1 18.75% (32.6%), PD-L1IC 13.75% (24.7%) and PD-L1TC 55.75% (13.6%). Due to the comparatively small number of cases with lymph nodes metastases, survival analyses were not performed.
Unadjusted and adjusted hazard ratios for TTR in relation to high and low infiltration of the investigated cells subsets are shown in Table 2. Using the median values as cut off, in TURB specimens, high CD8 and PD-1 expression were significantly associated with a prolonged TTR, but this association did not remain significant in a multivariable analysis adjusted for age at diagnosis, postoperative T-stage, N-stage, neoadjuvant and adjuvant chemotherapy. In cystectomy specimens, high infiltration of CD8+, FoxP3+, PD-1+ and PD-L1+ immune cells independently signified a prolonged TTR. Furthermore, when including all investigated immune cell subsets in the multivariable analysis, high densities of CD8 (HR = 0.38; 95% CI 0.15–0.95) and PD-1 (HR = 0.30; 95% CI 0.11–0.79) in cystectomy specimens remained significantly associated with a prolonged TTR.
Table 2.
Cox proportional hazards model for time to recurrence (TTR) in relation to clinicopathological factors and investigated immune cell markers and PD-L1TC.
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| n(events) | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (continuous) | 145(57) | 1.04 (1.00–1.07) | 0.028 | 0.99 (0.95–1.04) | 0.723 |
| Sex | |||||
| Female | 30 (10) | 1.00 | 1.00 | ||
| Male | 115 (47) | 1.22 (0.62–2.42) | 0.564 | 2.42 (0.85–6.84) | 0.097 |
| Postoperative T-stage | |||||
| T0, Ta, CIS only | 44 (12) | 1.00 | 1.00 | ||
| T1 | 13 (3) | 0.81 (0.23–2.86) | 0.741 | 1.57 (0.14–17.57) | 0.713 |
| T2 | 29 (10) | 1.27 (0.55–2.93) | 0.583 | 2.74 (0.33–22.51) | 0.347 |
| T3 | 43 (21) | 2.25 (1.11–4.58) | 0.025 | 2.83 (0.36–22.19) | 0.321 |
| T4 | 16 (11) | 4.09 (1.80–9.31) | 0.001 | 3.46 (0.39–31.07) | 0.267 |
| N-stage | |||||
| N0 | 103 (30) | 1.00 | 1.00 | ||
| N1 | 16 (8) | 1.87 (0.86–4.08) | 0.117 | 3.88 (1.44–10.50) | 0.007 |
| N2 | 13 (9) | 4.56 (2.15–9.66) | <0.001 | 4.54 (1.64–12.58) | 0.004 |
| N3 | 13 (10) | 6.08 (2.93–12.60) | <0.001 | 12.11 (3.68–39.80) | <0.001 |
| M-stage | |||||
| M0 | 132 (49) | 1.00 | 1.00 | ||
| M1 | 3 (1) | 0.71 (0.10–5.13) | 0.733 | 1.20 (0.14–10.15) | 0.867 |
| LVI in cystectomy specimens | |||||
| No | 95 (40) | 1.00 | 1.00 | ||
| Yes | 7 (5) | 2.14 (0.84–5.42) | 0.110 | 1.87 (0.56–6.22) | 0.306 |
| Neoadjuvant chemotherapy | |||||
| No | 80 (40) | 1.00 | 1.00 | ||
| Yes | 65 (17) | 0.42 (0.24–0.74) | 0.003 | 0.32 (0.10–0.99) | 0.047 |
| Adjuvant chemotherapy | |||||
| No | 133 (51) | 1.00 | 1.00 | ||
| Yes | 12 (6) | 1.36 (0.58–3.16) | 0.481 | 0.25 (0.08–0.80) | 0.020 |
| TURB specimens (median cut off) | |||||
| CD8 | |||||
| Low | 85 (41) | 1.00 | 1.00 | ||
| High | 55 (15) | 0.48 (0.26–0.86) | 0.014 | 0.56 (0.30–1.07)* | 0.080 |
| FoxP3 | |||||
| Low | 74 (30) | 1.00 | 1.00 | ||
| High | 69 (26) | 0.88 (0.52–1.49) | 0.631 | 1.03 (0.60–1.78)* | 0.907 |
| CD20 | |||||
| Low | 77 (35) | 1.00 | 1.00 | ||
| High | 65 (21) | 0.65 (0.38–1.11) | 0.112 | 0.76 (0.43–1.37)* | 0.364 |
| PD-1 | |||||
| Low | 76 (36) | 1.00 | 1.00 | ||
| High | 68 (20) | 0.58 (0.33–1.00) | 0.049 | 0.62 (0.35–1.11)* | 0.105 |
| PD-L1IC | |||||
| Low | 77 (37) | 1.00 | 1.00 | ||
| High | 62 (19) | 0.58 (0.34–1.01) | 0.055 | 0.71 (0.39–1.27)* | 0.244 |
| PD-L1TC | |||||
| Low | 75 (35) | 1.00 | 1.00 | ||
| High | 64 (21) | 0.64 (0.37–1.10) | 0.108 | 1.21 (0.65–2.23)* | 0.548 |
| Cystectomy specimens (median cut off) | |||||
| CD8 | |||||
| Low | 50 (30) | 1.00 | 1.00 | ||
| High | 39 (12) | 0.39 (0.20–0.77) | 0.006 | 0.28 (0.13–0.60)* | 0.001 |
| FoxP3 | |||||
| Low | 47 (25) | 1.00 | 1.00 | ||
| High | 46 (19) | 0.63 (0.35–1.15) | 0.133 | 0.43 (0.22–0.84)* | 0.013 |
| CD20 | |||||
| Low | 47 (27) | 1.00 | 1.00 | ||
| High | 47 (16) | 0.52 (0.28–0.96) | 0.036 | 0.69 (0.34–1.37)* | 0.286 |
| PD-1 | |||||
| Low | 53 (33) | 1.00 | 1.00 | ||
| High | 42 (12) | 0.35 (0.18–0.68) | 0.002 | 0.34 (0.17–0.70)* | 0.003 |
| PD-L1IC | |||||
| Low | 41 (26) | 1.00 | 1.00 | ||
| High | 40 (15) | 0.52 (0.27–0.98) | 0.042 | 0.47 (0.23–0.95)* | 0.036 |
| PD-L1TC | |||||
| Low | 42 (26) | 1.00 | 1.00 | ||
| High | 39 (15) | 0.50 (0.27–0.95) | 0.034 | 0.59 (0.30–1.17)* | 0.130 |
| TURB specimens (CRT-derived cut off) | |||||
| CD8 | |||||
| Low | 109 (52) | 1.00 | 1.00 | ||
| High | 31 (4) | 0.21 (0.08–0.58) | 0.003 | 0.27 (0.09–0.76)* | 0.014 |
| FoxP3 | |||||
| Low | 23 (14) | 1.00 | 1.00 | ||
| High | 120 (42) | 0.50 (0.27–0.91) | 0.023 | 0.77 (0.39–1.52)* | 0.448 |
| CD20 | |||||
| Low | 136 (56) | 1.00 | - | ||
| High | 6 (0) | 0.05 (0.00–8.60) | 0.248 | - | - |
| PD-1 | |||||
| Low | 128 (56) | 1.00 | - | ||
| High | 16 (0) | 0.04 (0.00–1.10) | 0.057 | - | - |
| PD-L1IC | |||||
| Low | 80 (40) | 1.00 | 1.00 | ||
| High | 59 (16) | 0.48 (0.27–0.86) | 0.013 | 0.57 (0.31–1.06)* | 0.075 |
| PD-L1TC | |||||
| Low | 130 (56) | 1.00 | - | ||
| High | 9 (0) | 0.04 (0.00–2.68) | 0.136 | - | - |
| Cystectomy specimens (CRT-derived cut off) | |||||
| CD8 | |||||
| Low | 42 (29) | 1.00 | 1.00 | ||
| High | 47 (13) | 0.27 (0.14–0.52) | <0.001 | 0.17 (0.08–0.35)* | <0.001 |
| FoxP3 | |||||
| Low | 30 (20) | 1.00 | 1.00 | ||
| High | 63 (24) | 0.41 (0.22–0.74) | 0.003 | 0.35 (0.18–0.69)* | 0.002 |
| CD20 | |||||
| Low | 15 (12) | 1.00 | 1.00 | ||
| High | 79 (31) | 0.26 (0.13–0.50) | <0.001 | 0.25 (0.12–0.55)* | 0.001 |
| PD-1 | |||||
| Low | 64 (38) | 1.00 | 1.00 | ||
| High | 31 (7) | 0.29 (0.13–0.65) | 0.003 | 0.23 (0.10–0.54)* | 0.001 |
| PD-L1IC | |||||
| Low | 61 (36) | 1.00 | 1.00 | ||
| High | 20 (5) | 0.35 (0.14–0.89) | 0.027 | 0.30 (0.11–0.82)* | 0.018 |
| PD-L1TC | |||||
| Low | 70 (40) | 1.00 | 1.00 | ||
| High | 11 (1) | 0.11 (0.02–0.80) | 0.029 | 0.15 (0.02–1.15)* | 0.068 |
LVI = Lymphovascular invasion. * Hazard ratios from multivariable analysis adjusted for age (continuous), postoperative T-stage, N-stage, neoadjuvant and adjuvant chemotherapy. Bold text indicates significant hazard ratios (p < 0.05). ”-” = statistical analyses could not be performed, due to no denoted recurrent disease after radical cystectomy in patients with high density (based on CRT-derived cut off values) of CD20+ and PD-1+ immune cells and PD-L1+ tumor cells in TURB specimens.
Cox regression analyses with the CRT-derived cut off points yielded similar results, except for high infiltration of CD8+ T cells in TURB specimens which remained significantly associated with an improved TTR in multivariable analysis. Due to no denoted recurrent disease after radical cystectomy in patients with high densities of CD20+ and PD-1+ immune cells as well as PD-L1+ tumor cells in TURB specimens, Cox regression analysis could not be applied. In cystectomy specimens, high infiltration of CD20+ lymphocytes was shown to be an independent prognostic factor for prolonged TTR. When all investigated immune cell subsets were included in the multivariable analysis, only high expression of CD8 in cystectomy specimens remained significantly associated with a prolonged TTR (HR = 0.28; 95% CI 0.09–0.86).
As for infiltration of immune cells into the tumor nest, the presence of intratumoral CD8+ T cells in TURB specimens was shown to be independently correlated with a prolonged TTR in adjusted analysis (HR = 0.44; 95% CI 0.22–0.89). A similar, however non-significant, trend was also observed in cystectomy specimens. The presence of lymphoid aggregates was not significantly associated with TTR (data not shown).
Finally, the prognostic role of different immune cell subsets and PD-L1TC in relation to NAC was examined. For this purpose, combined variables of high and low expression of studied cell subsets ± NAC were constructed. Kaplan-Meier plots of TTR using median values as cut off are shown in Figures 5 and 6, and the corresponding estimates using CRT-derived cut off values are displayed in Supplementary Figures 3 and 4. In general, the longest TTR was seen for patients having received NAC and with high infiltration of all immune cell subsets and PD-L1TC. However, for FoxP3+ and CD20+ expression in TURB specimens (Figure 5), the longest TTR was observed for patients treated with NAC, regardless of lymphocyte density. Cox proportional hazards analysis showed no significant treatment interaction between NAC and the density of any of the investigated cell subsets in relation to TTR, as shown in Supplementary Table 7.
Figure 5.
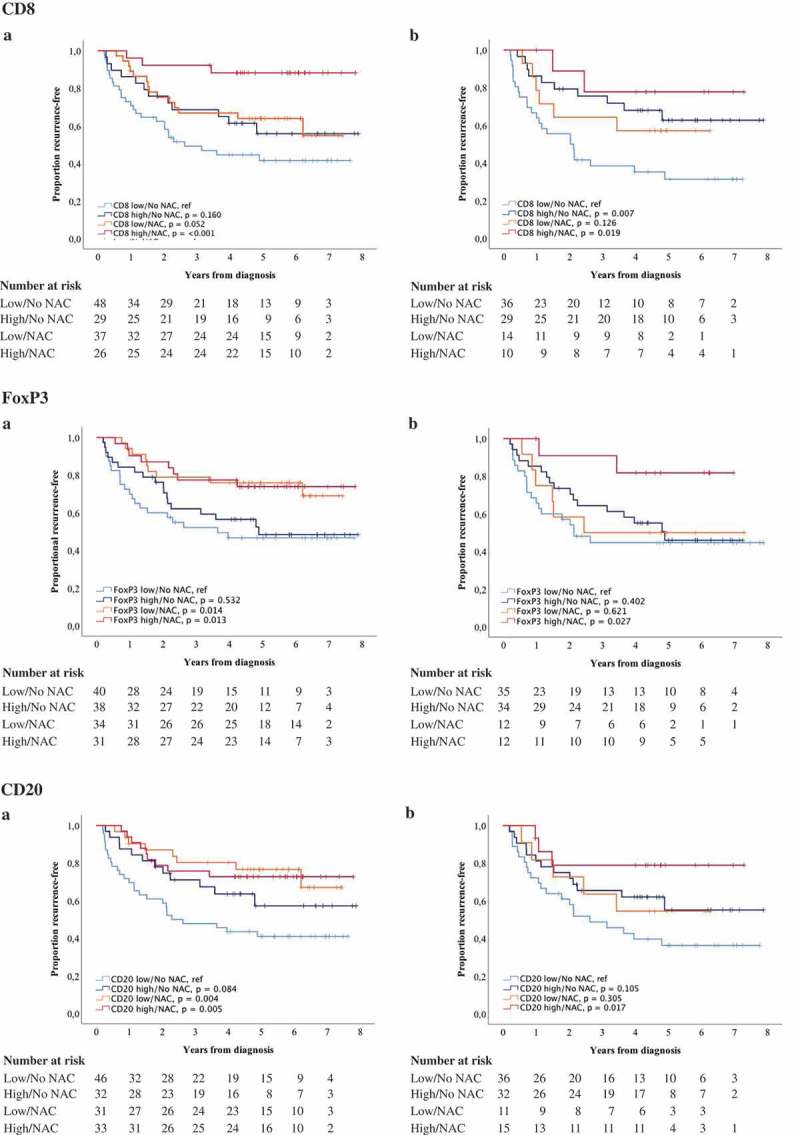
Time to recurrence (TTR) according to the density of tumor-infiltrating immune cells and neoadjuvant chemotherapy. Kaplan-Meier estimates of TTR in combined strata according to high and low expression of CD8, FoxP3 and CD20 and neoadjuvant chemotherapy in A) TURB specimens and B) cystectomy specimens. Dichotomization into high and low expression was based on median values. Number at risk demonstrates the number of patients at risk of recurrence of muscle invasive bladder cancer at given time intervals during follow-up.
Figure 6.
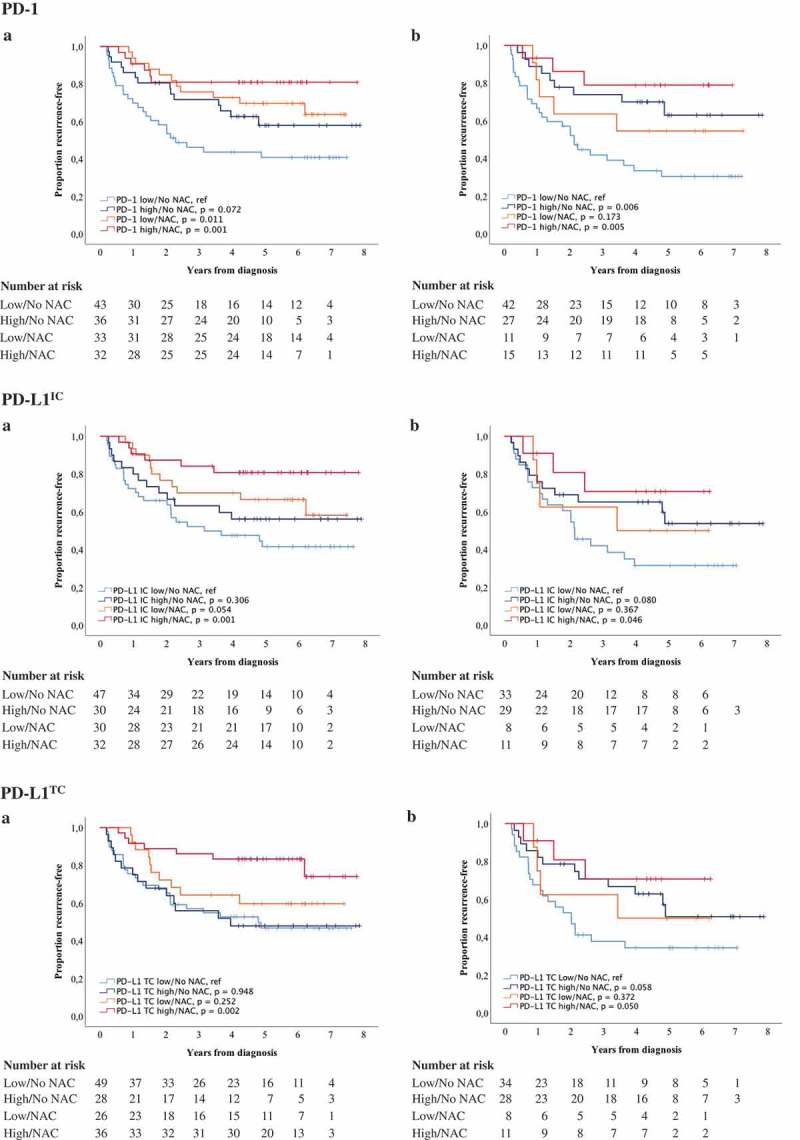
Time to recurrence (TTR) according to PD-1 and PD-L1 expression and neoadjuvant chemotherapy. Kaplan-Meier estimates of TTR in combined strata according to high and low expression of PD-1, PD-L1IC and PD-L1TC and neoadjuvant chemotherapy in A) TURB specimens and B) cystectomy specimens. Dichotomization into high and low expression was based on median values. Number at risk demonstrates the number of patients at risk of recurrence of muscle invasive bladder cancer at given time intervals during follow-up.
Discussion
The complex interplay between the tumor and its microenvironment has an indisputable role in the course of cancer development.28 Herein, the densities of tumor-infiltrating T cells, B cells and the PD-1/PD-L1 axis were assessed in paired TURB specimens, cystectomy specimens and lymph node metastases of MIBC, with particular reference to their impact on prognosis as well as the potential modifying effect of NAC. The results demonstrate significant positive intercorrelations between infiltration of the investigated cell subsets within the tumor microenvironment. Furthermore, high densities of immune cells, but not PD-L1TC, signified a prolonged time to recurrence, independently of other established prognostic factors.
The cytotoxic T cell response is driven by cancer-associated antigens29 and bladder cancer ranks among cancers with the highest mutation burden.30 In the present study, high densities of CD8+ T cells in both TURB and cystectomy specimens were found to be independently associated with a prolonged TTR. The prognostic significance of CD8+ T cells is in line with prior findings in several types of cancers, including MIBC, and mirrors their key role in tumor suppression by inducing tumor cell apoptosis.8,14,23 Of note, the spatial location of CD8+ T cells within the tissue landscape appears to be of importance since infiltration into the tumor nest per se was associated with a favorable outcome, suggestive of an active anti-tumor response. It has also been hypothesized that immune cells located at the invasion margin of the tumor might act as a protective barrier against micrometastasis.14
Similarly, infiltration of FoxP3+ Tregs was observed in the majority of cases and a high Treg density in cystectomy specimens was found to be an independent predictor of a prolonged TTR. Tregs have long been viewed as arrant immunosuppressors and are thus expected to be associated with a dismal prognosis.16 Indeed, a meta-analysis by Shang et al. concluded that high Treg infiltration was negatively correlated with survival in most studied solid tumors.31 Nonetheless, a paradoxical positive prognostic impact of Tregs has been reported in studies on colorectal, oesophageal, head and neck as well as bladder cancer.16,31 One proposed explanation for these observations is that infiltration of Tregs may reflect an ongoing CD8+ CTL response.31 Moreover, Winerdal et al. have recently described that the positive prognostic influence of FoxP3+ Tregs on bladder cancer survival could be explained by a Treg mediated restriction of cancer progression, through downregulation of tumor matrix metallo-proteinase 2 (MMP2), a key pro-invasive factor.16 This finding implies that the regulatory T cell population could have other than solely immunosuppressive intrinsic functions, which may in part explain their varying relationship with prognosis, depending on the cancer type.
Most of the literature on cell-mediated anti-tumor responses have been focused on T lymphocytes, while only a few studies have investigated the contribution of the B cell lineage in MIBC. B lymphocytes have been proposed to have dual functions within the tumor microenvironment in that they can either promote or inhibit tumor development.32 Furthermore, persistent tumor antigen exposure may result in the formation of tertiary lymphoid structures of B lymphocytes, which resembles germinal centers.33 The presence of lymphoid aggregates indicates an ongoing clonal B cell expansion and has gained attention due to their prognostic role in some cancers.29,34 Lymphoid aggregates have been found to be more common in high-grade MIBC than in low-grade non-MIBC.29 But their prognostic value has not yet been evaluated. Herein, the denoted lymphoid aggregates were defined as clusters of CD20+ B cells. High density of CD20+ B lymphocytes was shown to be independently associated with a prolonged TTR whereas the presence of lymphoid aggregates of B cells per se was not. Likewise, high CD19+ B cell count has been correlated with prolonged overall survival in patients with MIBC, where these cells were found to serve as antigen-presenting cells to active T lymphocytes.35 However, Ou et al. have demonstrated that infiltrating B cells can enhance bladder cancer invasion and metastasis.36 These contrasting results, suggesting that B cells may either have tumor-inhibiting or tumor-promoting properties depending on the context need to be examined in more detail.
In addition, activated lymphocytes can upregulate the expression of PD-1 on their cell surface and binding to its ligand PD-L1 results in lymphocyte exhaustion. This interaction is one of the major pathways used by some malignancies to evade immune surveillance.12 The prognostic value of PD-1 and PD-L1 expression has been evaluated with inconsistent results, including in MIBC.12,23,37 In the herein investigated cohort, high PD-1+ and PD-L1IC, but not PD-L1TC, expression was found to be independently associated with a prolonged TTR. The previously reported conflicting data on the impact of PD-1/PD-L1 on survival may be due to the fact that a variety of different antibodies, scoring methodologies and prognostic cut off values have been used. A recently published article by Tretiakova et al. compared four commonly used PD-L1 antibodies, including the herein used antibody, in primary and metastatic urothelial carcinomas and found a high concordance between all. PD-L1 was found to be expressed in a substantial proportion of both TICs and tumor cells and to be associated with an improved overall survival.38 It has been speculated that high PD-1/PD-L1 expression could be considered a marker of an active anti-tumor response, explaining the positive correlation between high expression and prognosis.39 In order to validate the prognostic value of PD-L1, standardized criteria for defining PD-L1 positivity should be applied. Furthermore, monoclonal antibodies targeting PD-1/PD-L1 ligation have been approved as treatment options for advanced MIBC, but the value of PD-1/PD-L1 expression in predicting response to checkpoint inhibitors remains an issue of debate.40–42
The finding of a beneficial, in some cases independent, prognostic impact of high immune cell infiltration in the present study, as well as in previous studies, indicates the need to further evaluate the clinical relevance of immune markers in MIBC prognostication. As of today, prognostication as well as choice of treatment strategy for patients with MIBC is usually based on TNM staging and patient characteristics.2 The reliability of these parameters is however limited as there are significant variations in clinical outcome after treatment among patients within the same TNM stage.15 In colorectal cancer, the Immunoscore has been shown to be a stronger predictor of outcome than TNM staging.43 Immunoscore has also shown promise as a potential predictor of clinical outcome in UBC,15 but the value of incorporating this parameter into the current staging classification remains to be determined, as well as which types of TICs that should be included. Also, the optimal prognostic cut off values for different immune cell subsets remain to be established. Herein, the median values as well as the CRT-derived cut off values were used. Both approaches supported that high densities of all investigated immune cell subsets were associated with a favorable outcome.
Given the potentially important prognostic implications of the tumor microenvironment, a better understanding of the effect of drugs on the tumor-stroma interactions is needed. Since NAC is recommended to all patients with locally advanced MIBC, if considered medically fit,2 we investigated its potential effect on the anti-tumor response. Based on the results, an additive beneficial prognostic effect of NAC could be suggested for tumors with high infiltration of the majority of the investigated cell subsets. However, there was no significant interaction between cell density and NAC in relation to prognosis, and the NAC treated patients were also younger and considered fit for chemotherapy. Moreover, an increase in PD-1 expression in cystectomy specimens compared to TURB specimens was seen in both NAC untreated and treated patients. In NAC untreated patients, the quantities of CD8+ and CD20+ cells were higher in cystectomy specimens compared to TURB specimens, which may be due to a general inflammatory response during cystectomy. There was no significant difference in the densities of investigated cell subsets between primary tumors and lymph node metastases in these patients. In non-complete responders to NAC treatment, chemotherapy had no effect on the densities of immune cells or PD-L1TC. Importantly, the similarities in cell densities in paired TURB specimens, cystectomy specimens and lymph node metastases indicate that either type of specimen can be used for prognostication purposes. In line with these findings, Poch et al. have previously reported similarities in the infiltrative patterns of T and B lymphocytes in cystectomy samples from chemonaïve and NAC treated patients44 and Tretiakova et al. have shown a high concordance between PD-L1 expression in matched primary and metastatic lesions.38 But as temporally and spatially discordance of PD-L1 expression between different sites has been observed in MIBC,26,27 the timing of obtaining tissue samples for PD-L1 staining could be of importance.
With this current study design, we were not able to examine the effect of NAC on cell density in cystectomy samples from complete responders, i.e. postoperative T0. Considering the proposed immunostimulatory effects of chemotherapy in MIBC,45,46 further studies monitoring the immune activity during the course of NAC administration in this patient group are highly motivated.
A major issue is the lack of predictors of NAC response that could guide therapeutic decisions. The densities of TICs have been suggested as potential predictive biomarkers of chemotherapy response.8,11 Baras et al. found that a CD8+ CTL to Treg ratio >1 in pretreatment biopsies of MIBC correlated with NAC response, whereas patients with a ratio <1 showed no response.8 Herein, only high CD8+ T cell infiltration in TURB specimens was found to be significantly associated with postoperative T-stage. However, there were no significant differences in the densities of immune cells or PD-L1TC in TURB specimens between complete and non-complete responders to NAC, and the CD8+ T cell to Treg ratio was not found to correlate with therapeutic response.
This study has the known limitations of a retrospective design. But since approximately half of the included patients had received NAC, the comparison of immune marker expression between NAC untreated and treated patients could give some implications of its potential predictive value, despite the retrospective setting. However, the results need further validation, preferably in prospective studies. In this present study, a high concordance of immune marker expression between matched primary tumors and lymph node metastases was seen, but the number of lymph nodes was small and larger comparative studies are needed.
Another limitation is the use of the TMA technique that may not properly reflect the potential heterogeneity of the tumor. In bladder cancer, however, a low frequency of intratumoral heterogeneity has been observed, thus supporting the use of TMA in biomarker studies on this type of cancer.47 Moreover, in an attempt to address the heterogeneity issue, triplicate cores were sampled from representative regions of the tumor and from different donor blocks when possible.
Conclusion
In conclusion, high infiltration of lymphocytes from both the T and B cell lineage within the tumor microenvironment of MIBC independently signified a prolonged time to recurrence. These findings strengthen the potential clinical utility and, hence, need to further evaluate the role of immune markers in MIBC prognostication. The densities of immune cells or PD-L1+ tumor cells in TURB specimens do however not appear to be predictive of response to NAC. Moreover, in patients with residual tumor after NAC treatment, chemotherapy had no effect on the densities of the investigated cell subsets, indicating that assessment of immune cell infiltration in pre- and postsurgical samples provides equally important prognostic information.
Materials and methods Study cohort
Study cohort
The study cohort included a consecutive series of all patients having undergone transurethral resection of the bladder (TURB) and ensuing cystectomy for MIBC at Skåne University Hospital, Malmö, between January 1st 2011 and December 31st 2014. Out of 207 patients, 23 (11.1%) had benign disease or non-urothelial cancers, 22 (10,6%) had CIS only, 3 (1.4%) were misclassified and 14 (6,8%) had missing tumor material from the TURB. This rendered 145 cases eligible for this study, 135 for whom both TURB and cystectomy specimens could be retrieved. Clinical data were obtained from medical records. Follow-up started at MIBC diagnosis and ended at death or August 31st 2018. Clinicopathological characteristics of the study cohort are presented in Table 1. Prior BCG-treatment was denoted in 13 (9.0%) cases. Sixty-five (44.8%) patients had received NAC (MVAC regimen) and 12 (8.3%) patients had been given adjuvant chemotherapy.
Ethical approval
Ethical permission for the study was obtained from the Regional Ethics committee at Lund University, ref no 445–2007. All tissue samples have been handled in accordance with European and national requirements during the conduct of this project; i.e. decision no. 1110/94/EC of the European Parliament and of the Council (OJL126 18,5,94), the Helsinki Declaration on ethical principles for medical research involving human subjects, and the EU Council Convention on human rights and Biomedicine.
Tissue microarray construction and immunohistochemistry
Haematoxylin and eosin-stained slides from all cases were histopathologically re-evaluated by a board-certified pathologist (KJ) and pathological tumor staging was done according to the 7th edition of The American Joint Committee on Cancer TNM classification of malignant tumors. All tumors were T-stage ≥2 at diagnosis. Tissue microarrays (TMA) were constructed from paired TURB specimens, cystectomy specimens and a subset of lymph node metastases (n = 27) using a semi-automated arraying device (TMArrayer, Pathology Devices, Westminster, MD, USA). All tumor samples were represented in triplicate tissue cores of 1 mm in diameter, when possible from different donor paraffin blocks. For immunohistochemical analysis, 4-μm TMA-sections were automatically pretreated using the PT Link system and then stained in an Autostainer Plus (Dako, Glostrup, Denmark) using the following antibodies: anti-CD8-antibody (clone C8/144B, mouse, dilution 1:50, product M7103, Dako, Glostrup, Denmark), anti-FoxP3-antibody (clone 236A/E7, mouse, dilution 1:200, Abcam, Cambridge, UK), anti-CD20-antibody (HPA14341, rabbit, dilution 1:200, Atlas Antibodies AB, Stockholm, Sweden), anti-PD-1-antibody (ab52587, clone NAT105, mouse, dilution 1:50, Abcam, Cambridge, UK) and anti-PD-L1-antibody (clone E1L3N, rabbit, dilution 1:100, Cell Signaling Technology Inc, Danvers, MA 01923, USA).
Evaluation of immunohistochemical staining
The estimated percentage of CD8, FoxP3, CD20, PD-1 and PD-L1 positive immune cells (PD-L1IC) as well as the percentage of tumor cells expressing PD-L1 (PD-L1TC) were manually annotated in each TMA core by two independent observers (SW and KJ) blinded to clinical outcome. Cases of scoring discrepancies were re-evaluated and discussed in order to reach consensus. Staining intensity was not taken into account. A median value of three cores was calculated and used in the subsequent analyses. The spatial location of the stained cell populations was classified as either stromal or in tumor nest (TN, defined as stained immune cells being in juxtaposition to a tumor cell). Lymphoid aggregates of CD20+ cells were denoted as being either present or absent.
Statistical analysis
χ2 and Mann–Whitney U tests were applied to examine differences in the distribution of clinicopathological factors between NAC untreated and treated patients. Non-parametric tests (Mann–Whitney U test, Kruskal–Wallis test) were used for comparison of immune cell density and PD-L1TC with patient and tumor characteristics. Mann–Whitney U test was also used for evaluation of the expression of investigated cell subsets in TURB specimens in relation to chemotherapy response. Wilcoxon signed-rank test was applied to examine differences in immune cell and PD-L1TC densities in paired TURB specimens, cystectomy specimens and lymph nodes. Spearman’s rank correlation test was used to investigate the interrelationship between different immune cell subsets and PD-L1TC categories. For survival analyses, dichotomous variables of high and low density of stained cells were constructed from both median values and prognostic cut off values derived from Classification and regression tree (CRT) analyses. Cox proportional hazards models were used to estimate the impact of immune cell and PD-L1TC density on time to recurrence (TTR) in both univariable and multivariable analyses, adjusted for age at diagnosis, postoperative T-stage, N-stage, neoadjuvant and adjuvant chemotherapy and immune marker expression. TTR was defined as time from TURB to the date of biopsy- or radiology-proven recurrent disease or death from bladder cancer. Kaplan-Meier analyses and log-rank tests were applied to illustrate differences in TTR with respect to biomarker expression and NAC. For evaluation of a potential interaction between neoadjuvant chemotherapy and investigated cell subsets, an interaction variable was constructed of neoadjuvant chemotherapy (±) x lymphocyte density (low/high) and used. All calculations were performed using IBM SPSS Statistics for Mac version 25.0 (IBM, Armonk, NY, USA). All statistical tests were two-sided and p-values <0.05 were considered statistically significant. Graphs were constructed using SPSS or GraphPad Prism version 8 (GraphPad Software, LA Jolla, CA, USA).
Funding Statement
This work was supported by grants from the Swedish Cancer Society (grant number 2016/483), the Swedish Research Council (grant number 2015-03598), the Mrs Berta Kamprad Foundation (grant number 2016-21), Governmental Funding of Clinical Research within the National Health Service (ALF) (grant number F 2014/354), Skåne University Hospital Funds and Donations and Lund University Faculty of Medicine.
Abbreviations
- CRT
Classification and regression tree
- MIBC
Muscle invasive bladder cancer
- NAC
Neoadjuvant chemotherapy
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- PD-L1IC
Immune cells expressing PD-L1
- PD-L1TC
Tumor cells expressing PD-L1
- TICs
Tumor-infiltrating immune cells
- TMA
Tissue microarray
- Tregs
Regulatory T cells
- TTR
Time to recurrence
- TURB
Transurethral resection of the bladder.
Author contribution statement
SW carried out the immunohistochemical analyses, performed the statistical analyses and drafted the manuscript. BN constructed the TMAs and performed the immunohistochemical stainings. KL assisted with the data interpretation. KB collected clinical data and assisted with the statistical analyses. KJ conceived of the study carried out the immunohistochemical analyses and helped draft the manuscript. All authors read and approved the final manuscript.
Declaration of Potential Conflicts of Interest
The authors declare that they have no competing interest.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F.. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):1–14. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernandez V, Espinos EL, Dunn J, Rouanne M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic burden of bladder cancer across the European union. Eur Urol. 2016;69(3):438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Raghavan D, Shipley WU, Garnick MB, Russell PJ, Richie JP. Biology and management of bladder cancer. N Engl J Med. 1990;322(16):1129–1138. doi: 10.1056/NEJM199004193221607. [DOI] [PubMed] [Google Scholar]
- 5.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 6.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr., Raghavan D, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 7.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol. 2005;48(2):202–5. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Baras AS, Drake C, Liu JJ, Gandhi N, Kates M, Hoque MO, Meeker A, Hahn N, Taube JM, Schoenberg MP, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016;5(5):e1134412. doi: 10.1080/2162402X.2015.1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee FC, Harris W, Cheng HH, Shenoi J, Zhao S, Wang J, Champion T, Izard J, Gore JL, Porter M, et al. Pathologic response rates of gemcitabine/cisplatin versus methotrexate/vinblastine/adriamycin/cisplatin neoadjuvant chemotherapy for muscle invasive urothelial bladder cancer. Adv Urol. 2013;2013:317190. doi: 10.1155/2013/317190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taube JM, Galon J, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, Baras AS, Patel SS, Anders RA, Rimm DL, et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31(2):214–234. doi: 10.1038/modpathol.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Zhu Y, Xu L, Zhang J, Xie H, Fu H, Zhou Q, Chang Y, Dai B, Xu J. Tumor stroma-infiltrating mast cells predict prognosis and adjuvant chemotherapeutic benefits in patients with muscle invasive bladder cancer. Oncoimmunology. 2018;7(9):e1474317. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pichler R, Heidegger I, Fritz J, Danzl M, Sprung S, Zelger B, Brunner A, Pircher A. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget. 2017;8(40):66849–66864. doi: 10.18632/oncotarget.19913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang HS, Su HY, Li PH, Chiang PH, Huang CH, Chen CH, Hsieh MC. Prognostic impact of tumor infiltrating lymphocytes on patients with metastatic urothelial carcinoma receiving platinum based chemotherapy. Sci Rep. 2018;8(1):7485. doi: 10.1038/s41598-018-25944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104(10):3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu A, Mansure JJ, Solanki S, Siemens DR, Koti M, Dias ABT, Burnier MM, Brimo F, Kassouf W. Presence of lymphocytic infiltrate cytotoxic T lymphocyte CD3+, CD8+, and immunoscore as prognostic marker in patients after radical cystectomy. PLoS One. 2018;13(10):e0205746. doi: 10.1371/journal.pone.0205746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winerdal ME, Krantz D, Hartana CA, Zirakzadeh AA, Linton L, Bergman EA, Rosenblatt R, Vasko J, Alamdari F, Hansson J, et al. Urinary bladder cancer tregs suppress MMP2 and potentially regulate invasiveness. Cancer Immunol Res. 2018;6(5):528–538. doi: 10.1158/2326-6066.CIR-17-0466. [DOI] [PubMed] [Google Scholar]
- 17.Krantz D, Hartana CA, Winerdal ME, Johansson M, Alamdari F, Jakubczyk T, Huge Y, Aljabery F, Palmqvist K, Zirakzadeh AA, et al. Neoadjuvant chemotherapy reinforces antitumour T cell response in urothelial urinary bladder cancer. Eur Urol. 2018;74(6):688–692. doi: 10.1016/j.eururo.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Berntsson J, Nodin B, Eberhard J, Micke P, Jirstrom K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer. 2016;139(5):1129–1139. doi: 10.1002/ijc.30138. [DOI] [PubMed] [Google Scholar]
- 19.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol. 2018;15(2):92–111. doi: 10.1038/nrurol.2017.179. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol. 2018;59(5):285–296. doi: 10.4111/icu.2018.59.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faraj SF, Munari E, Guner G, Taube J, Anders R, Hicks J, Meeker A, Schoenberg M, Bivalacqua T, Drake C, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology. 2015;85(3):703.e1–6. doi: 10.1016/j.urology.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlmeier F, Seitz AK, Hatzichristodoulou G, Stecher L, Retz M, Gschwend JE, Weichert W, Kubler HR, Horn T. The role of PD-L1 expression and intratumoral lymphocytes in response to perioperative chemotherapy for urothelial carcinoma. Bladder Cancer. 2016;2(4):425–432. doi: 10.3233/BLC-160067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherji D, Jabbour MN, Saroufim M, Temraz S, Nasr R, Charafeddine M, Assi R, Shamseddine A, Tawil AN. Programmed death-ligand 1 expression in muscle-invasive bladder cancer cystectomy specimens and lymph node metastasis: a reliable treatment selection biomarker? Clin Genitourin Cancer. 2016;14(2):183–187. doi: 10.1016/j.clgc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Burgess EF, Livasy C, Hartman A, Robinson MM, Symanowski J, Naso C, Doherty S, Guerrieri R, Riggs S, Grigg CM, et al. Discordance of high PD-L1 expression in primary and metastatic urothelial carcinoma lesions. Urol Oncol. 2019. doi: 10.1016/j.urolonc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Pottier C, Wheatherspoon A, Roncarati P, Longuespee R, Herfs M, Duray A, Delvenne P, Quatresooz P. The importance of the tumor microenvironment in the therapeutic management of cancer. Expert Rev Anticancer Ther. 2015;15(8):943–954. doi: 10.1586/14737140.2015.1059279. [DOI] [PubMed] [Google Scholar]
- 29.Koti M, Xu AS, Ren KYM, Visram K, Ren R, Berman DM, Siemens DR. Tertiary lymphoid structures associate with tumour stage in urothelial bladder cancer. Bladder Cancer. 2017;3(4):259–267. doi: 10.3233/BLC-170120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng W, Fu D, Xu F, Zhang Z. Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis. 2018;7(1):2. doi: 10.1038/s41389-017-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen M, Sun Q, Wang J, Pan W, Ren X. Positive and negative functions of B lymphocytes in tumors. Oncotarget. 2016;7(34):55828–55839. doi: 10.18632/oncotarget.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 34.Pimenta EM, Barnes BJ. Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers. 2014;6(2):969–997. doi: 10.3390/cancers6020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Q, Fu Q, Chang Y, Liu Z, Zhang J, Xu L, Zhu Y, Wang Y, Zhang W, Xu J. CD19(+) tumor-infiltrating B-cells prime CD4(+) T-cell immunity and predict platinum-based chemotherapy efficacy in muscle-invasive bladder cancer. Cancer Immunol Immunother. CII. 2019;68(1):45-56. doi: 10.1007/s00262-018-2250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou Z, Wang Y, Liu L, Li L, Yeh S, Qi L, Chang C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget. 2015;6(28):26065–26078. doi: 10.18632/oncotarget.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang X, Yu PC, Long D, Liao XL, Zhang S, You XM, Zhong JH, Li LQ. Prognostic value of PD -L1 expression in patients with primary solid tumors. Oncotarget. 2018;9(4):5058–5072. doi: 10.18632/oncotarget.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tretiakova M, Fulton R, Kocherginsky M, Long T, Ussakli C, Antic T, Gown A. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod Pathol. 2018;31(4):623–632. doi: 10.1038/modpathol.2017.188. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget. 2017;8(38):64066–64082. doi: 10.18632/oncotarget.19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghatalia P, Zibelman M, Geynisman DM, Plimack E. Approved checkpoint inhibitors in bladder cancer: which drug should be used when? Ther Adv Med Oncol. 2018;10:1758835918788310. doi: 10.1177/1758835918788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz H, Wassie E, Alsharedi M. Checkpoint inhibitors: the new treatment paradigm for urothelial bladder cancer. Med Oncol. 2017;34(10):170. doi: 10.1007/s12032-017-1029-8. [DOI] [PubMed] [Google Scholar]
- 42.Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 43.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 44.Poch M, Hall M, Joerger A, Kodumudi K, Beatty M, Innamarato PP, Bunch BL, Fishman MN, Zhang J, Sexton WJ, et al. Expansion of tumor infiltrating lymphocytes (TIL) from bladder cancer. Oncoimmunology. 2018;7(9):e1476816. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherif A, Winerdal M, Winqvist O. Immune responses to neoadjuvant chemotherapy in muscle invasive bladder cancer. Bladder Cancer. 2018;4(1):1–7. doi: 10.3233/BLC-170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zirakzadeh AA, Kinn J, Krantz D, Rosenblatt R, Winerdal ME, Hu J, Hartana CA, Lundgren C, Bergman EA, Johansson M, et al. Doxorubicin enhances the capacity of B cells to activate T cells in urothelial urinary bladder cancer. Clin Immunol. 2017;176:63–70. doi: 10.1016/j.clim.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Jakobsson L, Chebil G, Marzouka NA, Liedberg F, Sjodahl G. Low frequency of intratumor heterogeneity in bladder cancer tissue microarrays. Bladder Cancer. 2018;4(3):327–337. doi: 10.3233/BLC-180176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


