ABSTRACT
The development of a single immuno-metabolic adjuvant capable of modulating, in the appropriate direction and intensity, the complex antagonistic and symbiotic interplays between tumor cells, immune cells, and the gut microbiota may appear pharmacologically implausible. Metformin might help solve this conundrum and beneficially impact the state of cancer-immune system interactions.
KEYWORDS: Metformin, immunotherapy, T-cells, immune checkpoints
One of the greatest obstacles to making cancer immunotherapy more broadly effective could be rooted in a basic concept of cell biology, namely metabolism. Immunometabolism, which is a relatively new field in cancer immunotherapy, is gaining momentum through the realization that faulty metabolic remodeling underlies impaired antitumor immune responses, and also that controlling metabolism can enhance antitumor immunity and synergize with existing checkpoint inhibitors.1–5 There is no doubt that harnessing the highly complex, antagonistic and symbiotic metabolite-mediated communication between tumor cells and the range of immune cell compartments residing in the tumor microenvironment (TME) has such potential. The question now is how to resolve the apparent conundrum of simultaneously orchestrating the precise direction and intensity of multiple metabolic checkpoints not only in T-cells, immune suppressor cells (tumor-associated macrophages [TAM], myeloid-derived suppressor cells [MDSC], regulatory T-[Treg]-cells), and cancer cells within the TME, but also in the gut microbiota, and its consequent systemic effects on host metabolism.
Advances in understanding the communication between cancer cells and TME-associated immune cells have highlighted the importance of specific metabolic pathways and nutrient-sensing mechanisms to regulate anti-cancer immune responses and optimize the effectiveness of immunotherapy.6–10 A great deal is known about how the phenotypic characteristics of T-cells for cytotoxicity against tumor cells requires metabolic specialization, and how specific metabolic activities and tumor-driven shifts in the abundance of specific metabolites lead to local immunosuppression and reduce the metabolic fitness of tumor-infiltrating T-cells (TILs). However, while targeting the dynamic interacting and competing metabolic pathways in the TME holds promise for improving immunotherapies, one should acknowledge that the similar metabolic needs between cancer cells and immune cells might abolish the expected synergistic effects of such combinations. Much is expected from tracking the metabolic pathways that are essential to cancer cells and immune cells and, in particular, those that are driven by tumor cells to impose metabolic stress on TILs and result in local immunosuppression. Nevertheless, it might be argued that it is pharmacologically implausible to develop a single drug capable of modulating, in the appropriate direction and intensity, the metabolic checkpoints responsible not only for the antagonistic (tumor cells versus effector/cytotoxic T-cells) and symbiotic (tumor cells, TAM, MDSC, and Treg cells) metabolic interplays of the TME, but also of improving the anticancer profile of gut microbiota to elevate the response rate of cancer immunotherapy.11,12 Although apparently unattainable, the challenge of enhancing cytotoxic T-cell immune surveillance, suppressing the immunosuppressive nature of TME, impeding the expression of immune checkpoints in cancer cells, and shifting the gut microbiota composition towards specific commensal species with a favorable response to cancer immunotherapy, could be achieved with a small metabolic molecule such as the anti-diabetic biguanide metformin (Figure 1(a)). We here present the first comprehensive overview of how metformin might have the capacity to beneficially impact all the cancer-immune system interactions in individual patients (Figure 1(b)).
Figure 1.
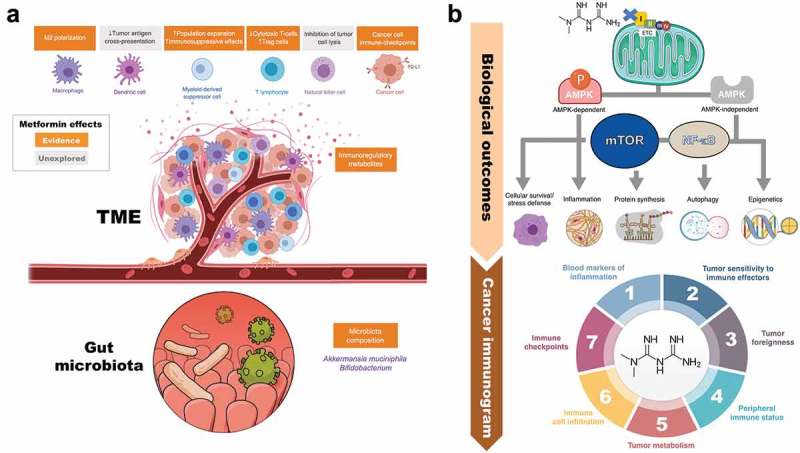
Metformin: A multi-faceted immuno-metabolic adjuvant for cancer immunotherapy. (a). Evidences. The anti-diabetic agent metformin might serve as an archetype immuno-metabolic adjuvant capable of simultaneously regulating, in the appropriate direction and intensity, antitumor immunity-related metabolic checkpoints not only in T-cells, cancer cells and associated immune suppressor cells of the TME, but also in the gut microbiota and its systemic effects on host metabolism. The capacity to improve the metabolic competence of T-cell immune surveillance, suppress the metabolic traits of immunosuppressive cell subsets in the TME, prevent both the constitutive and the inflammation (IFNγ)-inducible expression of immune checkpoint receptors in cancer cells, and shift the gut microbiota composition towards specific commensal microbes might optimize the effectiveness of cancer immunotherapy. Further studies are needed to determine the effects of metformin on tumor antigen cross-presentation by dendritic cells and tumor cell lysis by natural killer cells. (b). Mechanisms. As a consequence of the metformin-mediated inhibition of mitochondrial electron transfer, metformin is able to activate a variety of AMPK-dependent and -independent signaling pathways through which it facilitates the inhibition of mTOR, inhibits the inflammatory pathway, and lastly disturbs inflammation, cellular survival, stress defense, protein synthesis, autophagy, and epigenetic reprogramming .13–20 Downstream of these major biological outcomes, metformin might have the capacity to impact all the cancer-immune system interactions constituting the so-called “cancer immunogram”.21 Metformin might lead to systemically decreased levels of pro-inflammatory soluble inhibitors (e.g., serum levels of C-reactive protein and IL-622–24), which are known to drive tumor-associated inflammation, impair T cell-mediated tumor control, and associate with poor outcomes in response to ICIs (1). Metformin might increase tumor sensitivity to immune effectors by augmenting the levels of major histocompatibility complex (MHC) class I antigens25 (2), which might impact also tumor foreignness by helping T-cells to recognize neoantigens (3). Metformin might alter the general performance of immune system via modification of the microbiome26 (4), specifically by changing microbial folate and serine/methionine metabolism.27–29 Metformin might reverse an inhibitory tumor metabolism by remodeling the hypoxic TME via reduction of intratumoral hypoxia (5), a key driver of poor outcomes upon ICIs. Metformin might sustain or restore the infiltration of tumor-reactive T-cells into the tumor (6) by preventing the occurrence of dysfunctional states characterized by impaired activity and proliferative activity, increase apoptotic rate, and reduced production of effector cytokines (i.e., T-cell exhaustion). Metformin might alter the expression profile of immune checkpoints (7) such as PD-L1 in the tumor compartment [37, Figure 2], thus suggesting that a combination of metformin-CTLA-4 blockade might have the potential to increase the efficacy of cancer immunotherapy.
Metformin enhances the anti-tumor functionality of T-cells
Ten years ago, metformin was shown to target the metabolic switch driving the expansion of CD8+ memory T-cells.30 Metformin appeared to operate in a rapamycin-like manner to facilitate the shift from a glucose-dependent anabolic state (effector T-cell) to a catabolic state of metabolism (memory T-cell) by blocking mTOR signaling downstream of AMPK and restoring mitochondrial fatty acid oxidation.31,32 This ability to directly enhance the number and functionality of memory T-cells proved an effective strategy for improving the functional qualities of vaccine- or infection-induced T-cells, and further protected mice from challenge by tumor cells expressing ovalbumin.30 However, because the cancer-protective effect took place after metformin withdrawal, it should be viewed as a vaccination outcome, which is different from TIL-mediated regression of established solid tumors.
A chronic, repeated T-cell receptor presentation from CD8+ TILs specific for tumor antigens to cancer cells leads to a gradual loss in their ability to secrete multiple cytokines (e.g., IL-2, TNFα, IFNγ), and they ultimately undergo apoptotic elimination in a process known as immune exhaustion.33 This worsening of immune function is accompanied by phenotypic changes in CD8+ T-cells, including the expression of exhaustion markers such as the immune checkpoint molecule PD-1. Therapeutic management of functional T-cell exhaustion within tumor tissues is largely based on the administration of blocking antibodies against PD-1 (pembrolizumab and nivolumab) or its ligand PD-L1 (atezolizumab, durvalamab, and avelumab);34–36 however, the possibility exists of metabolically counteracting apoptosis induction and diminished cytokine production in CD8+ TILs to block immune exhaustion within tumor tissues. Interestingly, metformin has been shown to protect PD1+ CD8+ TILs from apoptosis while restoring the production of multiple cytokines via their conversion from a central memory (TCM) to an effector, memory T-cell (TEM) phenotype fully active against tumors.37 This direct effect of metformin on CD8+ T-cells, which occurs even at physiologically relevant low concentrations and markedly alters their multifunctionality following migration into the tumor, appears to be different to that expected from direct mTOR inhibitors. Accordingly, whereas rapamycin has been shown to promote the generation of memory T-cells by increasing the TCM population, which is known to migrate between lymphoid organs, metformin preferentially increases the TEM population, which circulates principally in the blood, spleen, and peripheral tissues.37,38
The ability of metformin to promote anti-tumor effects by rescuing exhausted CD8+ TILs in the TME of highly immunogenic tumors, including leukemia, melanoma, renal cell carcinoma, non–small-cell lung carcinoma, intestinal carcinoma, and breast cancer,37 has been confirmed and extended by the observation that it significantly augments the ability of CD8+ effector memory T-cells to mediate anti-metastatic activity in melanoma models.39 Such a promotion of a strong cancer-protective immune response was accompanied by the additional induction of local and systemic cytokine responses including production of IL-10 by metformin-expanded CD4+ regulatory T-cells, a key mechanism to enhance effector and memory CD8+ T-cell functions.40,41 The supra-additive capacity of metformin to prevent melanoma metastases to the lung when used with other clinically relevant anti-metabolic drugs, such as rapamycin and the dipeptidyl peptidase 4 inhibitor sitagliptin,39 further bolsters the clinical value of metformin against different facets of T-cell immunometabolism.
Metformin neutralizes immune-inhibitory cell populations residing in the tumor microenvironment
Management of the inevitable T-cell exhaustion within the TME should be accompanied by efforts to neutralize the immune-inhibitory cell populations residing in the TME, such as M2-polarized TAMs, MDCSs, and Treg cells, for achieving efficient cancer immunotherapy.
The glucose-deprived, lactic acid-enriched TME not only impairs T-cell functionality but also polarizes TAMs to an alternatively activated M2 (anti-inflammatory) phenotype, which enhances tumor-associated angiogenesis, promotes tumor migration and invasion, and suppresses anti-tumor immune responses. Metformin has been shown to prevent cancer metastasis by inhibiting the pro-inflammatory polarization of tolerogenic M2-TAMs via AMPK activation.42 The ability of metformin to directly suppress the M2-TAM-driven catabolism of tryptophan to kynurenine – a characteristic immunosuppressive metabolite of the TME that impedes T-cell activation and promotes the development of Treg cells – has not been explored. However, successful metformin treatment of insulin resistance leads to a normalization of the tryptophan-to-kynurenine conversion,43,44 making it mechanistically plausible that metformin decreases the contribution of the kynurenine metabolic pathway in M2-TAMs. Moreover, the immunological ability of metformin to suppress the growth of some tumors such as osteosarcoma is accompanied by a shift from an M2- to M1-like (inflammatory) phenotype of TAMs involving changes in lipid metabolism.45
Metformin can decrease the number of neutrophils and polymorphonuclear MDSCs (PMN-MDCSs) both in the spleen and in tumors.39,45 However, its ability to metabolically reprogram MDCSs to curtail oxidative phosphorylation, decrease glucose uptake, and reduce lipid incorporation is restricted to those cells residing in the TME, in turn pushing it to a metabolic state capable of driving tumor growth inhibition independently of metformin’s affects on T-cells.45 The ability of metformin to generate sustained antitumor immunity in the TME might involve also an attenuation of tumor-infiltrating CD4+CD25+ Treg cells.46 The negative impact of metformin on Treg cells involved the down-regulation of the immune checkpoint molecule CTLA-4, which not only acts on conventional T-cells but also represents a major mechanism of Treg cell function directed by the Treg transcription factor Foxp3.47,48 Accordingly, metformin appears to impede the differentiation of naïve CD4+ T-cells to inducible Treg cells by reducing the expression of Foxp3 protein caused by mTOR activation.46 Beyond impeding Treg cell generation, metformin drives the metabolic reprogramming of Treg cells involving enhanced glycolysis, as evidenced by the increased expression of Glut1 and a decrease in mitochondrial-potential and ROS production.46
Metformin down-regulates PD-L1 in cancer cells
Metabolic changes in cancer cells are closely intertwined with aberrations in oncogenic and tumor-suppressive pathways (e.g., PI3K/PTEN/AKT, MYC, STAT3) that contribute to PD-L1 expression.49–51 Dysregulated activation of immune checkpoints might therefore represent a general mechanism of metabolism-driven tumor immune-tolerance. Accordingly, oncogenic activation of the archetypal PI3K-AKT-mTOR metabolic pathway, which coordinates the uptake and utilization of multiple nutrients including glucose, glutamine, nucleotides, and lipids, promotes immune escape by driving PD-L1 overexpression in tumor cells.52 Not surprisingly, treatment of cancer cells with metformin was shown to inhibit constitutive PD-L1 expression and protein accumulation.53 The AMPK-sensed metabolic crisis imposed by metformin reduced the stability and membrane localization of constitutively expressed PD-L1 by inducing its endoplasmic reticulum (ER)-associated protein degradation (ERAD) in cancer cells.54 In response to metformin, the activated form of AMPK directly phosphorylates PD-L1 in a manner promoting its abnormal glycosylation, resulting in ER accumulation and ERAD, which contributes to an enhanced cytotoxic T-cell activity against cancer cells54 (Figure 2(a)). Concomitantly with AMPK activation, metformin-treated breast cancer tumor tissues exhibit reduced PD-L1 levels. In our hands, stimulating PD-L1 membrane sorting to ERAD via indirect or direct activation of AMPK with metformin or 5-aminoimidazole-4-carboxamide, respectively, sufficed to significantly increase the cytolytic activity of T-cells against highly aggressive basal-like breast carcinoma cells (Figure 2(b)).
Figure 2.
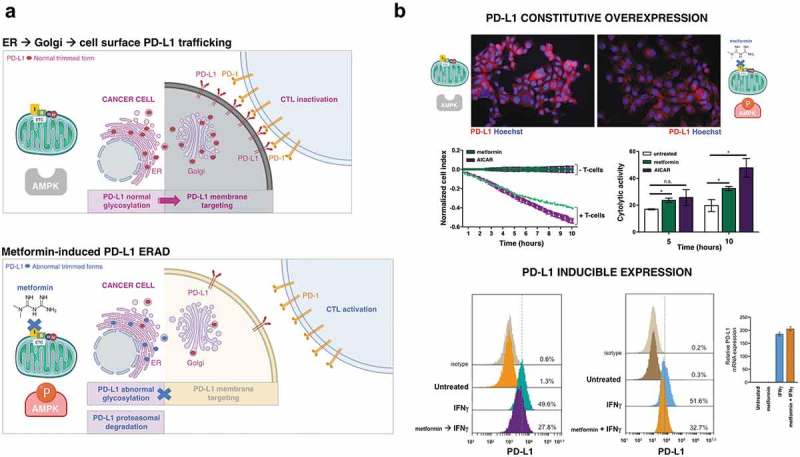
Metformin targets PD-L1 in cancer cells. (a). The AMPK-sensed metabolic crisis imposed by metformin might suffice to promote anti-tumor immunity by reducing the stability and membrane localization of PD-L1. Metformin-induced activation of AMPK promotes PD-L1 phosphorylation and abnormal PD-L1 glycosylation, lastly resulting in ER accumulation and ER-associated PD-L1 protein degradation (ERAD).54,55 (b). Top. Figure exemplifies both the ability of metformin to promote PD-L1 degradation in basal-like (JIMT-1) breast cancer cells exhibiting constitutive overexpression of PD-L156 and how blocking the inhibitory signal by PD-L1 by the AMPK agonistic behavior of metformin (5 mmol/L) enhances cytolytic T cell-mediated tumor cell death as measured by an impedance-based approach (xCELLigence system).57,58 Shown are the mean ± SD, n = 2 in triplicate (* p < .05); [AICAR, 0.5 mmol/L]. Bottom. Figure exemplifies the ability of metformin to prevent the inducible expression of PD-L1 in interferon gamma (IFNγ)-exposed cancer cells. IFNγ plays a pivotal role in PD-L1 expression in cancer cells and the consequent immune escape by the tumor cells. Tumor cells detect the presence of CD8+ T cells via the high concentration of IFNγ secreted from T-cells. IFNγ secreted from CD8+ T cells induced PD-L1 expression on the surface of tumor cells, which become protected from an immune attack by tumor-specific CTLs.59 IFNγ-treated haploid HAP1 cells express high levels of cell surface PD-L1.60 However, cell surface PD-L1 expression is notably reduced in the presence of IFN-γ (100 nmol/L) pre- and co-stimulation following exposure to metformin (5 mmol/L), with no effect on PD-L1 mRNA expression.61 Shown are representative PD-L1 expression histograms analyzed by flow cytometry and PD-L1 mRNA levels analyzed by qRT-PCR (n = 2 in triplicate).
Tumors can express immune checkpoints such as PD-L1 either constitutively, which does not depend on the presence of tumor-infiltrating lymphocytes, or through a more common inducible mechanism in response to inflammatory cytokines, particularly to members of the interferon family. Cytokine-driven expression of PD-L1, which can be detected as patchy pattern of PD-L1 expression in T-cell-enriched tumor areas, is indicative of an ongoing immune response in the TME. We recently took advantage of the observation that human haploid HAP1 cells express high levels of PD-L1 on the cell surface in response to interferon-γ (IFNγ) treatment60 to evaluate the impact of metformin on IFNγ-induced PD-L1 expression. In HAP1 cells, pre-exposure to and concurrent metformin prevented IFNγ-induced PD-L1 expression to a large extent (Figure 2(b)). Because the expression of PD-L1 affects T-cell responsiveness in a quantitative manner, with higher levels of PD-L1 expression leading to an increased impairment of T-cell survival/activity,62,63 the identification of metformin as a new regulator of PD-L1 expression provides a rationale to enhance the effectiveness of currently existing immune checkpoint blocking therapies.
Metformin influences the gut microbiota composition
The gut microbiota has been proven to participate in immune surveillance, suppressing malignant transformation,64–66 and specific commensal bacteria synergize with cancer treatments including radiotherapy, chemotherapy, and surgery.67,68 It is now emerging that shits in the gut microbiota/microbiome composition can positively or negatively regulate the efficacy of immune checkpoint inhibitors.11,69–73 For instance, an increased abundance of Akkermansia muciniphila in the gut microbiota of advanced cancer patients improves antitumor immune CD8+ T-cell infiltration and activity, and increases the efficacy of anti-PD1 therapy. Likewise Faecalibacterium, Bifidobacterium, and short-chain fatty acid (SCFA)-producing bacteria, which are associated with anti-inflammatory responses aimed to prevent overactivation of the immune response, positively relate to higher response rates and better clinical outcomes in response to anti-CTLA-4 therapy.
The beneficial effects of metformin on host metabolism are, at least in part, microbially mediated and are associated with inflammatory immune responses.74–80 Metformin treatment of mice on high-fat diet or of patients with diabetes has been shown to shift the microbiota composition to an increased relative abundance of A. muciniphila, a mucolytic bacterium.77–79 Because cancer patients with augmented memory T-cells targeting the gut colonization of A. muciniphila are prone to have a longer clinical benefit from PD-1-based immunotherapy, metformin’s ability to strengthen the intestinal mucosal barrier via enrichment of A. muciniphila and associated improvement in mucin-producing goblet cells might promote a salutatory bacteria-specific synergetic immune response in combination with immune checkpoint inhibitors. Also, modulation of the gut microbiota by metformin results in a higher relative abundance of SCFA (butyrate, propionate, acetate)-producing bacteria including Bifidobacterium, associated with inflammatory immune responses. Because both A. muciniphila and gut microbiota-derived SCFAs such as butyrate and propionate attenuate tissue inflammation by promoting Treg cell differentiation, and augmenting the size of the Treg cell pool by elevating histone H3 acetylation in the Foxp3 promoter region,81,82 it might be argued that metformin-driven anti-inflammatory bacteria and metabolites could induce Treg cell differentiation and proliferation, resulting in higher levels of CTLA-4 and increased sensitivity to CTLA-4 blockade. Further studies are necessary to elucidate whether metformin can promote AMPK/mTOR-related prevention of inducible Treg cells accompanied by elevation of CD4+ TCMs in the tumor bed while simultaneously promoting SCFA-driven suppression of inflammation via augmentation of Treg cells in gut, which is related to colitis incidence and the potent efficacy of CTLA-4 inhibitors.73 Given that changes in host metabolism and microbiota can occur in tandem, the fact that the therapeutic effects of metformin in cancer patients are accompanied by significant elevations in circulating butyrate83 might provide support for the ability of metformin to impact gut microbial diversity and composition to modify the response to immunotherapy.
Clinical efficacy and ongoing trials of metformin combined with immune-checkpoint inhibitors
The ability of metformin to circumvent the tumor-driven metabolic barrier to antitumor immunotherapy by normalizing the hypoxic TME results in a significantly improved intratumoral T-cell function and tumor clearance in pre-clinical models of highly aggressive tumors.84 Such translational potential of metformin to convert immunotherapy-resistant patients into those showing clinical benefit has been supported by the discovery that adjuvant metformin plus anti-PD-1 treatment results in durable antitumor responses by preventing the presentation of PD-1+/CD8+ T-cell infiltrates after drug withdrawal.85 A retrospective cohort study including patients diagnosed with metastatic malignant melanoma and treated with anti-PD-1 only or anti-CTLA4/anti-PD-1 combination therapies, with or without metformin, revealed favorable treatment-related outcomes in terms of objective response rate, disease control rate, overall survival, and progression-free survival in patients who have received metformin in combination with immune-checkpoint inhibitors, albeit without reaching statistical significance likely due to the small sample size.86 An analysis of the immunomodulatory effects of metformin in a clinical trial of head and neck squamous cell carcinoma revealed its ability to increase both CD8+ effector T-cells and FoxP3+ Treg cell infiltrates in the TME.87 A retrospective descriptive analysis carried out in the randomized phase III OAK trial for treatment of advanced or metastatic previously-treated non-small cell lung cancer revealed an encouraging improvement of overall response rate in patients receiving concomitant metformin treatment with the anti-PD-L1 antibody atezolizumab.88 Not surprisingly, large prospective clinical trials are currently underway to study the synergistic effect of metformin in combination with immune-checkpoint inhibitors before its recommendation as routine additive to cancer immunotherapy.
Based on the pre-clinical capacity of metformin to induce substantial tumor regression and augment the numbers of tumor-infiltrating CD8+ T-cells when combined with the anti-PD-1 antibody nivolumab in mouse models, an investigator-initiated open-label phase-Ib clinical trial has been planned in Japan to investigate the safety, efficacy, and pharmacokinetics of metformin-nivolumab combination treatment.89 Similarly, the anti-tumor efficacy as well as the safety and tolerability profile of metformin-nivolumab combination in patients with non–small-cell lung cancer with and without prior exposure to PD-1/PD-L1 inhibitors is currently being evaluated in the Northwestern University-sponsored NCT03048500 clinical trial (https://clinicaltrials.gov/ct2/show/study/NCT03048500). A pilot phase I trial is investigating the combined effect of metformin and the anti-PD-L1 antibody durvalamab on the TME (i.e., T-cell polarization and TAM M1/M2 ratios) of patients with head and neck squamous cell carcinoma (https://clinicaltrials.gov/ct2/show/NCT03618654). An investigator-initiated phase I clinical trial is evaluating the effectiveness and safety of the combination of the anti-PD-1 antibody pembrolizumab with metformin in advanced-stage melanoma (https://clinicaltrials.gov/ct2/show/NCT03311308). A phase II trial is evaluating the effect of combining metformin with nivolumab on the overall response rate of patients with microsatellite stable stage IV colorectal cancer that has not responded to previous treatment (https://clinicaltrials.gov/ct2/show/NCT03800602). Although data from the FDA adverse event reporting system have suggested a higher risk of inflammatory bowel disease in lung cancer patients during the combined nivolumab-metformin therapy,90 we still lack clinical evidence of the impact of metformin on the risk of immune-related adverse events, which are associated with anti-PD-1/PD-L1 treatment efficacy91 and may include autoimmune diabetes and diabetic ketoacidosis.92–95
Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy: directions and cautions
Metabolic alterations in tumors are emerging as crucial factors affecting the abundance of immune-checkpoints in tumor cells96,97 and, accordingly, the intercrossing of immune evasion and metabolic reprogramming cancer hallmarks might guide the development of new strategies capable of (re)installing immunosurveillance and converting cold tumor cells with primary and acquired resistance to immunotherapy into hot cells, susceptible to immune checkpoint strategies. Here, we delineated the ability of the anti-diabetic biguanide metformin to operate as an archetype immuno-metabolic adjuvant capable of performing several immuno-metabolic tasks simultaneously, given its capacity to improve the metabolic competence of T-cell immune surveillance, suppress the metabolic traits driving TME immunosuppressive cell compartments, prevent both the constitutive and the inflammation-inducible expression of immune checkpoint receptors in cancer cells, and shift the gut microbiota composition to specific commensal microbes associated with a favorable response to cancer immunotherapy.
During the last decade, an ever-growing number of epidemiological and preclinical studies have suggested that metformin may reduce overall cancer risk and mortality.98–102 Accordingly, many randomized clinical studies, ranging from proof-of-principle studies in the prevention setting to phase II/III trials in the adjuvant and metastatic settings, have been planned and/or currently underway (as of June 2019, the clinicaltrials.gov database lists more than 300) to test the causal nature of the suggested correlation between metformin use and clinical benefit in cancer patients. We should acknowledge, however, that recently reported first-generation clinical trials using metformin in combination with systemic therapy have failed to significant improve outcomes in cancer patients.103–105 Therefore, before we can recommend the use of metformin as a bona fide immuno-metabolic adjuvant in a combination with immune checkpoint inhibitors (ICIs) regardless of the metabolic status of the patient, more patient-level data collection (retrospective and especially prospective) are urgently needed. Indeed, the clinical relevance of the immunomodulatory functions of metformin, which might be synergistic and could overcome resistance to single agent anti-PD1-/PD-L1 and anti-CTLA-4 inhibitors,106 should be reweighted when considering the apparently paradoxical association between obesity and increased anti-tumor efficacy and survival after PD-1/PD-L1 blockade.107,108 First, obesity and other metabolic disorders (e.g., diabetes) heighten PD1-driven T-cell dysfunction and tumor progression. Second, these very same immune-tumorigenic loops amplify the clinical benefits that derive from the normalization of T-cell metabolism imposed by ICIs, which impede the so-called immune metabolic anergy that take place upon the interaction of immune checkpoints such as the PD-L1 ligand in tumor cells with its cognate receptor PD-1 on T-cells and involves several metabolic pathways and mitochondrial fitness in these cells.109,110 Third, the anti-cancer effects of metformin might vary with host characteristics such as overweight or obesity with metabolic disturbances.111,112 Therefore, although ongoing clinical trials using metformin in combination with ICIs might begin to validate the role of metformin or novel biguanides as immuno-metabolic adjuvants capable of broadening the spectra of cancer patients and indications that could benefit from immunotherapy, a careful consideration to the metabolic characteristics of the study population should be given as it might significantly modify the capacity of metformin to impact the tumor and host-specific parameters characterizing the cancer-immune interaction and required for successful immunotherapy treatment.21,113 Indeed, enriching future metformin-based clinical trials with the inclusion of “cancer immunograms”114–117 (Figure 1(b)), could help identifying the population of metformin-responders by prospectively capturing those aspects of the cancer-immune interaction that characterize the dynamic process of antitumor immunity in an individual patient, thereby realizing the potential of precision immunotherapy for more cancer patients.
Funding Statement
Work in the Menendez laboratory is supported by the Spanish Ministry of Science and Innovation (Grant SAF2016-80639-P, Plan Nacional de l+D+I, founded by the European Regional Development Fund, Spain) and by an unrestricted research grant from the Fundació Oncolliga Girona (Lliga catalana d’ajuda al malalt de càncer, Girona).
Acknowledgments
The authors would like to thank Dr. Kenneth McCreath for editorial support.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Ho PC, Liu PS.. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer. 2016;4:4. doi: 10.1186/s40425-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison KE, Coomber BL, Bridle BW. Metabolic reprogramming in the tumour microenvironment: a hallmark shared by cancer cells and T lymphocytes. Immunology. 2017;152:175–184. doi: 10.1111/imm.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiura A, Rathmell JC. Metabolic barriers to T cell function in tumors. J Immunol. 2018;200:400–407. doi: 10.4049/jimmunol.1701041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer K, Cheng WC, Kreutz M, Ho PC, Siska PJ. Immunometabolism in cancer at a glance. Dis Model Mech. 2018;11(8). pii: dmm034272. doi: 10.1242/dmm.034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Wenes M, Romero P, Huang SC, Fendt SM, Ho PC. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019. March 26. doi: 10.1038/s41571-019-0203-7. [DOI] [PubMed] [Google Scholar]
- 6.Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol. 2017;8:248. doi: 10.3389/fimmu.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ 3rd, Kopinski PK, Wang L, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol. 2018;19:1319–1329. doi: 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 10.Galluzzi L, Kroemer G. Potent immunosuppressive effects of the oncometabolite R-2-hydroxyglutarate. Oncoimmunology. 2018;7:e1528815. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, Chu Q, Wu K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018;11:47. doi: 10.1186/s13045-018-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lettieri-Barbato D, Aquilano K. Pushing the limits of cancer therapy: the nutrient game. Front Oncol. 2018;8:148. doi: 10.3389/fonc.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693–3700. doi: 10.1172/JCI67232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrocola F, Kroemer G. Metformin: a metabolic modulator. Oncotarget. 2017;8:9017–9020. doi: 10.18632/oncotarget.14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, Mou F, Kacergis MC, Talkowski ME, Carr CE, et al. Unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell. 2016;167:1705–1718.e13. doi: 10.1016/j.cell.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuyàs E, Fernández-Arroyo S, Joven J, Menendez JA. Metformin targets histone acetylation in cancer-prone epithelial cells. Cell Cycle. 2016;15:3355–3361. doi: 10.1080/15384101.2016.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridgeman SC, Ellison GC, Melton PE, Newsholme P, Mamotte CDS. Epigenetic effects of metformin: from molecular mechanisms to clinical implications. Diabetes Obes Metab. 2018;20:1553–1562. doi: 10.1111/dom.13262. [DOI] [PubMed] [Google Scholar]
- 21.Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The “cancer immunogram”. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancerstem cell growth. Proc Natl Acad Sci U S A. 2013;110:972–977. doi: 10.1073/pnas.1221055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Wahab Z, Mert I, Tebbe C, Chhina J, Hijaz M, Morris RT, Ali-Fehmi R, Giri S, Munkarah AR, Rattan R. Metformin prevents aggressive ovarian cancer growth driven by high-energy diet: similarity with calorie restriction. Oncotarget. 2015;6:10908–10923. doi: 10.18632/oncotarget.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Yang Z, Jin P, Yang X, Li X, Wei X, Wang Y, Long S, Zhang T, Chen G, et al. Metformin suppresses tumor progression by inactivating stromal fibroblasts in ovarian cancer. Mol Cancer Ther. 2018;17:1291–1302. doi: 10.1158/1535-7163.MCT-17-0927. [DOI] [PubMed] [Google Scholar]
- 25.Oliveras-Ferraros C, Cufí S, Vazquez-Martin A, Menendez OJ, Bosch-Barrera J, Martin-Castillo B, Joven J, Menendez JA. Metformin rescues cell surface major histocompatibility complex class I (MHC-I) deficiency caused by oncogenic transformation. Cell Cycle. 2012;11:865–870. doi: 10.4161/cc.11.5.19252. [DOI] [PubMed] [Google Scholar]
- 26.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corominas-Faja B, Quirantes-Piné R, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Martin-Castillo B, Micol V, Joven J, Segura-Carretero A, Menendez JA. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging (Albany NY). 2012;4:480–498. doi: 10.18632/aging.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuyàs E, Fernández-Arroyo S, Verdura S, García RÁ, Stursa J, Werner L, Blanco-González E, Montes-Bayón M, Joven J, Viollet B, et al. Metformin regulates global DNA methylation via mitochondrial one-carbon metabolism. Oncogene. 2018;37:963–970. doi: 10.1038/onc.2017.367. [DOI] [PubMed] [Google Scholar]
- 29.Cuyàs E, Fernández-Arroyo S, Buxó M, Pernas S, Dorca J, Álvarez I, Martínez S, Pérez-Garcia JM, Batista-López N, Rodríguez-Sánchez CA, et al. Metformin induces a fasting- and antifolate-mimicking modification of systemic host metabolism in breast cancer patients. Aging (Albany NY). 2019;11:2874–2888. doi: 10.18632/aging.101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prlic M, Bevan MJ. Immunology: A metabolic switch to memory. Nature. 2009;460:41–42. doi: 10.1038/460041a. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 35.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. [DOI] [PubMed] [Google Scholar]
- 36.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira FV, Melo ACL, Low JS, de Castro ÍA, Braga TT, Almeida DC, Batista de Lima AGU, Hiyane MI, Correa-Costa M, Andrade-Oliveira V, et al. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget. 2018;9:25808–25825. doi: 10.18632/oncotarget.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Church SE, Jensen SM, Antony PA, Restifo NP, Fox BA. Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells. Eur J Immunol. 2014;44:69–79. doi: 10.1002/eji.201343718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16:871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H, Zhou Y, Wu H, Yang B, He Q. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6:36441–36455. doi: 10.18632/oncotarget.v6i34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem. 2011;117:254–258. doi: 10.3109/13813455.2011.623705. [DOI] [PubMed] [Google Scholar]
- 44.Muzik O, Burghardt P, Yi Z, Kumar A, Seyoum B. Successful metformin treatment of insulin resistance is associated with down-regulation of the kynurenine pathway. Biochem Biophys Res Commun. 2017;488:29–32. doi: 10.1016/j.bbrc.2017.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T, Kunisada T, Ozaki T, Udono H. Metformin induces CD11b+ cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and antitumor effects. Int Immunol. 2018. December 2. doi: 10.1093/intimm/dxy079 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S, Furusawa Y, Hase K, Sasaki A, Udono H. Attenuation of CD4+CD25+ regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. EBioMedicine. 2017;25:154–164. doi: 10.1016/j.ebiom.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36:63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014. May 12;25(5):590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016. April 8;352(6282):227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A. 2008. December 30;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 53.Ho Y, Chen YF, Wang LH, Hsu KY, Chin YT, Yang YSH, Wang SH, Chen YR, Shih YJ, Liu LF, et al. Inhibitory effect of anoectochilus formosanus extract on hyperglycemia-related PD-L1 expression and cancer proliferation. Front Pharmacol. 2018;9:807. doi: 10.3389/fphar.2018.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, Li CW, Kim T, Chang SS, Lee HH, et al. Metformin promotes antitumor immunity via endoplasmic-reticulim-associated degradation of PD-L1. Mol Cell. 2018;71:606–620.e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dreher LS, Hoppe T. ERADicate tumor progression with metformin. Mol Cell. 2018;71:481–482. doi: 10.1016/j.molcel.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Rom-Jurek EM, Kirchhammer N, Ugocsai P, Ortmann O, Wege AK, Brockhoff G. Regulation of programmed death ligand 1 (PD-L1) expression in breast cancer cell lines in vitro and in immunodeficient and humanized tumor mice. Int J Mol Sci. 2018;19 pii: E563. doi: 10.3390/ijms19020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henle AM, Erskine CL, Benson LM, Clynes R, Knutson KL. Enzymatic discovery of a HER-2/neu epitope that generates cross-reactive T cells. J Immunol. 2013;190:479–488. doi: 10.4049/jimmunol.1201264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erskine CL, Henle AM, Knutson KL. Determining optimal cytotoxic activity of human Her2neu specific CD8 T cells by comparing the Cr51 release assay to the xCELLigence system. J Vis Exp. 2012;66:e3683. doi:10.3791/3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of ifnγ in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res. 2016;22:2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 60.Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland I, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549:106–110. doi: 10.1038/nature23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han Y, Li CW, Hsu JM, Hsu JL, Chan LC, Tan X, He GJ. Metformin reverses PARP inhibitors-induced epithelial-mesenchymal transition and PD-L1upregulation in triple-negative breast cancer. Am J Cancer Res. 2019;9:800–815. [PMC free article] [PubMed] [Google Scholar]
- 62.Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, Maeda T, Nagata Y, Kitanaka A, Mizuno S, et al. Aberrant PD-L1 expression through 3ʹ-UTR disruption in multiple cancers. Nature. 2016;534:402–406. doi: 10.1038/nature18294. [DOI] [PubMed] [Google Scholar]
- 63.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A. 2013;110:E2480–9. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271ps1. doi: 10.1126/scitranslmed.aad3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15:382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 67.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 68.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356–365. doi: 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 69.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 73.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 74.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 76.Pollak M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia. 2017;60:1662–1667. doi: 10.1007/s00125-017-4352-x. [DOI] [PubMed] [Google Scholar]
- 77.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 78.Lee H, Lee Y, Kim J, An J, Lee S, Kong H, Song Y, Lee CK, Kim K. Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes. 2018;9:155–165. doi: 10.1080/19490976.2017.1405209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kyriachenko Y, Falalyeyeva T, Korotkyi O, Molochek N, Kobyliak N. Crosstalk between gut microbiota and antidiabetic drug action. World J Diabetes. 2019;10:154–168. doi: 10.4239/wjd.v10.i3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 81.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 82.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, Bae-Jump VL. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Med. 2015;4:161–173. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5:9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haikala HM, Anttila JM, Marques E, Raatikainen T, Ilander M, Hakanen H, Ala-Hongisto H, Savelius M, Balboa D, Von Eyss B, et al. Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy. Nat Commun. 2019;10:620. doi: 10.1038/s41467-019-08541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6:64. doi: 10.1186/s40425-018-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curry JM, Johnson J, Mollaee M, Tassone P, Amin D, Knops A, Whitaker-Menezes D, Mahoney MG, South A, Rodeck U, et al. Metformin clinical trial in HPV+ and HPV- head and neck squamous cell carcinoma: impact on cancer cell apoptosis and immune infiltrate. Front Oncol. 2018;8:436. doi: 10.3389/fonc.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pietras H, Xu H, Hu X, Matheny C, Sandler A, Patel M. P1.04-33 retrospective descriptive analysis of metformin with atezolizumab in advanced non-small cell lung cancer in the OAK trial. J Thorac Oncol. 2018;13:S538–S539. doi: 10.1016/j.jtho.2018.08.748. [DOI] [Google Scholar]
- 89.Kubo T, Ninomiya T, Hotta K, Kozuki T, Toyooka S, Okada H, Fujiwara T, Udono H, Kiura K. Study protocol: phase-ib trial of nivolumab combined with metformin for refractory/recurrent solid tumors. Clin Lung Cancer. 2018;19:e861–e864. doi: 10.1016/j.cllc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 90.Zhou H, Liu J, Zhang Y, Zhang L. Inflammatory bowel disease associated with the combination treatment of nivolumab and metformin: data from the FDA adverse event reporting system. Cancer Chemother Pharmacol. 2019;83:599–601. doi: 10.1007/s00280-018-03763-5. [DOI] [PubMed] [Google Scholar]
- 91.Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, Arranz R, Lorenzo A, Gullón P, Donnay O, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–27. doi: 10.1016/j.ejca.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 92.Changizzadeh PN, Mukkamalla SKR, Armenio VA. Combined checkpoint inhibitor therapy causing diabetic ketoacidosis in metastatic melanoma. J Immunother Cancer. 2017;5:97. doi: 10.1186/s40425-017-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, Da Meda L, Madelaine-Chambrin I, Bagot M, Basset-Seguin N, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother. 2017;66:1399–1410. doi: 10.1007/s00262-017-2033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Godwin JL, Jaggi S, Sirisena I, Sharda P, Rao AD, Mehra R, Veloski C. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer. 2017;5:40. doi: 10.1186/s40425-017-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maamari J, Yeung SJ, Chaftari PS. Diabetic ketoacidosis induced by a single dose of pembrolizumab. Am J Emerg Med. 2019;37:376.e1–376.e2. doi: 10.1016/j.ajem.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Wang H, Yao H, Li C, Fang JY, Xu J. Regulation of PD-L1: emerging routes for targeting tumor immune evasion. Front Pharmacol. 2018;9:536. doi: 10.3389/fphar.2018.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gu W, Wang L, Wu Y, Liu JP. Undo the brake of tumour immune tolerance with antibodies, peptide mimetics and small molecule compounds targeting PD-1/PD-L1 checkpoint at different locations for acceleration of cytotoxic immunity to cancer cells. Clin Exp Pharmacol Physiol. 2019;46:105–115. doi: 10.1111/1440-1681.13056. [DOI] [PubMed] [Google Scholar]
- 98.Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 100.Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135:639–646. doi: 10.1007/s10549-012-2170-x. [DOI] [PubMed] [Google Scholar]
- 101.Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, Hershman DL, Hobday TJ, Ligibel JA, Mayer IA, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126:215–220. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- 102.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 103.Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 104.Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora P, et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase II trial. Clin Cancer Res. 2016;22:1076–1085. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PubMed] [Google Scholar]
- 105.Martin-Castillo B, Pernas S, Dorca J, Álvarez I, Martínez S, Pérez-Garcia JM, Batista-López N, Rodríguez-Sánchez CA, Amillano K, Domínguez S, et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: the METTEN study. Oncotarget. 2018;9:35687–35704. doi: 10.18632/oncotarget.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chae YK, Oh MS, Giles FJ. Molecular biomarkers of primary and acquired resistance to T-cell-mediated immunotherapy in cancer: landscape, clinical implications, and future directions. Oncologist. 2018;23:410–421. doi: 10.1634/theoncologist.2017-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qorraj M, Böttcher M, Mougiakakos D. PD-L1/PD-1: new kid on the “immune metabolic” block. Oncotarget. 2017;8:73364–73365. doi: 10.18632/oncotarget.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Trabacca MS, Galimberti V, Veronesi P, et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 112.DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M, Pruneri G, Serrano D, Schwab M, Hofmann U, et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat. 2014;148:81–90. doi: 10.1007/s10549-014-3141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spencer CN, Wells DK, LaVallee TM. It is a capital mistake to theorize who to treat with checkpoint inhibitors before one has data. Trends Cancer. 2019;5:79–82. doi: 10.1016/j.trecan.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Karasaki T, Nagayama K, Kuwano H, Nitadori JI, Sato M, Anraku M, Hosoi A, Matsushita H, Morishita Y, Kashiwabara K, et al. An immunogram for the cancer-immunity cycle: towards personalized immunotherapy of lungCancer. J Thorac Oncol. 2017;12:791–803. doi: 10.1016/j.jtho.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Zahoor H, Grivas P. The cancer immunogram: a pledge for a comprehensive biomarker approach for personalized immunotherapy in urothelial cancer. Eur Urol. 2019;75:445–447. doi: 10.1016/j.eururo.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 116.van Dijk N, Funt SA, Blank CU, Powles T, Rosenberg JE, van der Heijden MS. The cancer immunogram as a framework for personalized immunotherapy in urothelial cancer. Eur Urol. 2019;75:435–444. doi: 10.1016/j.eururo.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tarantino P, Curigliano G. Defining the immunogram of breast cancer: a focus on clinical trials. Expert Opin Biol Ther. 2019;19:383–385. doi: 10.1080/14712598.2019.1598372. [DOI] [PubMed] [Google Scholar]


