ABSTRACT
Staphylococcus aureus is implicated in disease progression in cutaneous T-cell lymphoma (CTCL). Here, we demonstrate that malignant T cell lines derived from CTCL patients as well as primary malignant CD4+ T cells from Sézary syndrome patients are considerably more resistant to alpha-toxin-induced cell death than their non-malignant counterparts. Thus, in a subset of Sézary syndrome patients the ratio between malignant and non-malignant CD4+ T cells increases significantly following exposure to alpha-toxin. Whereas toxin-induced cell death is ADAM10 dependent in healthy CD4+ T cells, resistance to alpha-toxin in malignant T cells involves both downregulation of ADAM10 as well as other resistance mechanisms. In conclusion, we provide first evidence that Staphylococcus aureus derived alpha-toxin can tilt the balance between malignant and non-malignant CD4+ T cells in CTCL patients. Consequently, alpha-toxin may promote disease progression through positive selection of malignant CD4+ T cells, identifying alpha-toxin as a putative drug target in CTCL.
KEYWORDS: Cutaneous T-cell lymphoma, Staphylococcus aureus, alpha-toxin, disintegrin and metalloproteinase domain-containing protein 10
Introduction
Cutaneous T-cell lymphoma (CTCL) represents a group of non-Hodgkin lymphomas characterized by the accumulation of clonally expanded CD4+ T cells in the skin. Mycosis fungoides (MF) is the most prevalent form and Sézary syndrome (SS) is an aggressive leukemic variant of the disease.1 CTCL patients have an increased risk of contracting severe infections compared to healthy controls,2,3 which constitute a major cause of death.4 The most frequently found pathogen is Staphylococcus aureus (S. aureus), infecting 44–76% of CTCL patients.5,6
In a murine CTCL disease model, it has been shown that bacterial infections aggravate the disease7 and a reduction of colonizing bacteria in CTCL patients with antibiotics has been associated with clinical improvement and a decrease in tumour burden.8,9 This suggests that S. aureus and its toxins fuel disease progression (as reviewed in10). However, while the link between bacterial infections and CTCL seems to persist, the underlying mechanisms are a topic of ongoing discussion.
S. aureus produces a wide range of toxins that can be subdivided into three groups: super-antigens, pore-forming toxins and exfoliative toxins.11 Previously, we have demonstrated that super-antigens released by S. aureus can exacerbate CTCL by stimulating non-malignant CD4+ T cells to produce growth factors and cytokines, which in turn trigger activation and proliferation of malignant cells.12,13 Despite the fact that the pore-forming alpha-toxin is expressed by almost all S. aureus strains (95%),11 its role in CTCL has not been investigated.
Alpha-toxin is secreted as a monomer and elicits its toxicity by forming heptameric pores in the cell membrane. Its effect depends on the toxin concentration, duration of exposure and cell type.14 The surface receptor for alpha-toxin is the disintegrin and metalloproteinase domain-containing protein 10 (ADAM10).15 Accordingly, surface expression levels of ADAM10 largely determine the toxin susceptibility of a given cell.16 However, while ADAM10 levels are important, other mechanisms can further modulate the susceptibility to alpha-toxin. For instance, multiple lineages of cells are resistant to the alpha-toxin effects by blocking pore formation, shedding or internalizing affected parts of the membrane or by closing the pore itself.17–20
Here, we show in CTCL cell lines and primary cells from SS patients that malignant CTCL cells are less sensitive to alpha-toxin than their non-malignant CD4+ T cell counterparts. Our data further show that resistance to alpha-toxin can be acquired through multiple mechanisms including downregulation of ADAM10. This is the first study to show that alpha-toxin may tilt the balance between malignant and non-malignant CD4+ T cells, favouring the persistence of malignant over non-malignant CD4+ T cells.
Results
Malignant CTCL patient derived cell lines are resistant to alpha-toxin induced cytotoxicity
We treated different malignant and non-malignant T cell lines derived from CTCL patients with increasing concentrations of alpha-toxin. Intriguingly, lactate dehydrogenase (LDH) release and cell viability measurements revealed that all malignant cell lines consistently exhibited either low sensitivity or complete resistance to alpha-toxin-induced cell death at concentrations where non-malignant cell lines were highly sensitive (Figure 1(a,b) and Figure S2). Indeed, non-malignant T cell lines from CTCL patients displayed a similar sensitivity to alpha-toxin as CD4+ T cells isolated from healthy donors (Figure 1(c,d), and Figure S2).
Figure 1.
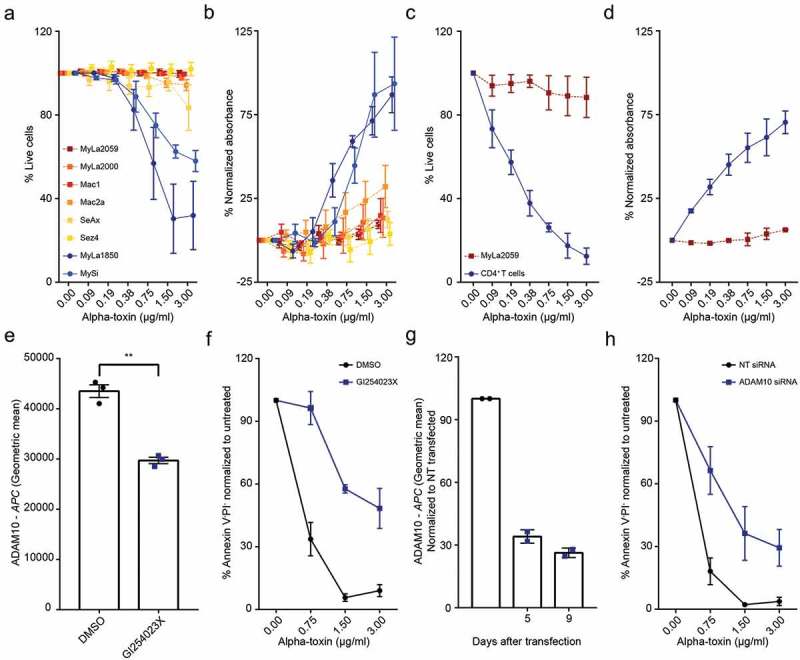
Malignant CTCL cells are less sensitive to alpha-toxin than non-malignant CD4+ T cells. Cells were exposed to alpha-toxin before LDH release was measured in the culture supernatant and/or viability was assessed by flow cytometry. (a,b) Malignant CTCL patient derived cell lines and the non-malignant CTCL cell lines MySi and MyLa1850 (n = 3–5). (c,d) Purified primary CD4+ T cells from healthy donors and the malignant CTCL cell line, MyLa2059 (n = 2–4). (e,f) ADAM10 surface expression and survival of MyLa1850 after alpha-toxin exposure following GI254023X treatment (n = 3). (g,h) Surface expression of ADAM10 of siRNA transfected CD4+ T cells from healthy donors and survival after four days of toxin exposure (n = 2). Error bars display mean ± standard error of mean.
Alpha-toxin cytotoxicity is mediated by ADAM10 in non-malignant CTCL cell lines and healthy CD4+ T cells
To determine if cell death was induced through alpha-toxin binding to ADAM10, we pre-treated the non-malignant cell line MyLa1850 with the ADAM10 inhibitor GI254023X before toxin exposure, which effectively reduced cell death (Figure 1(e,f)). ADAM10 specificity of the effect was verified by targeted RNA interference in CD4+ T cells from healthy donors prior to toxin exposure, which resulted in a similar decrease in alpha-toxin sensitivity as with the pharmacological inhibitor (Figure 1(g,h)).
Alpha-toxin selects for malignant CD4+ T cells in a subset of SS patients
After establishing the difference in alpha-toxin susceptibility between malignant and non-malignant T cell lines, we next investigated whether this difference was also apparent in primary malignant and non-malignant CD4+ T cells from SS patients. SS patients are characterized by having high numbers of circulating malignant T cells, identified by their monoclonal T-cell receptor (TCR) and/or their low expression of CD7 and CD26.21 We analysed the survival of both malignant and non-malignant CD4+ T cells from ten SS patients after treatment with alpha-toxin (patient characteristics in Supplementary Table 3). In cells from five of the ten SS patients, we observed that the malignant CD4+ T cells were more resistant to killing than the non-malignant CD4+ T cells from the same patients (Figure 2(a)). Importantly, in the patients in which the malignant cells exhibited resistance to the toxin, the ratio of malignant to non-malignant CD4+ T cells was drastically increased following alpha-toxin exposure (Figure 2(b)).
Figure 2.
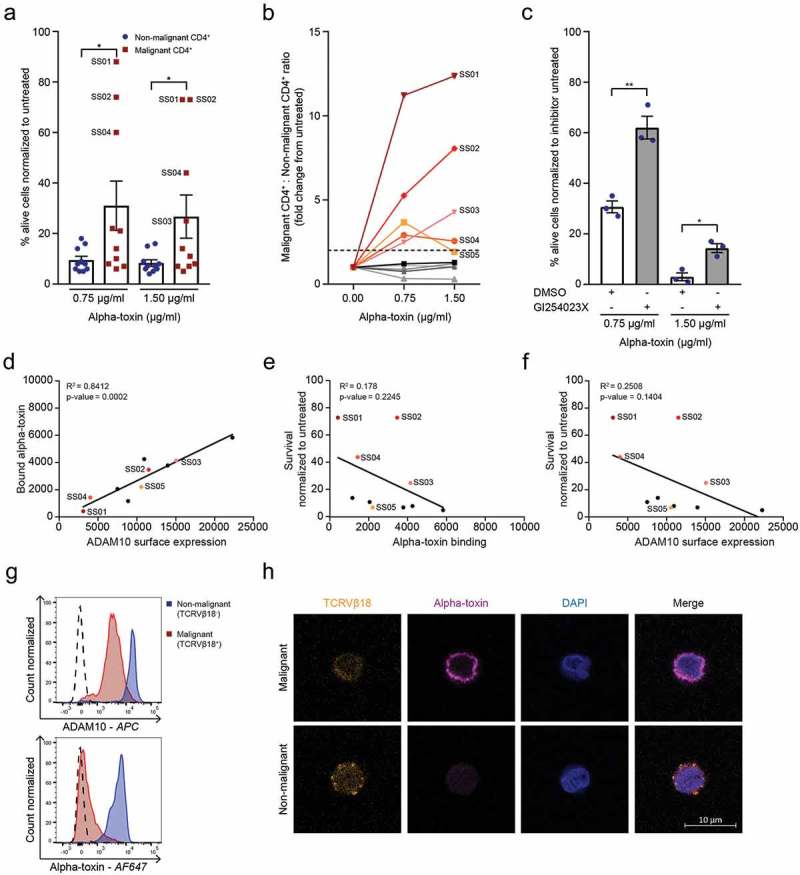
Malignant cells from SS patients are less sensitive to alpha-toxin induced death than their non-malignant CD4+ counterparts. (a) Percentage viable malignant and non-malignant CD4+ cells and (b) malignant to non-malignant CD4+ T cell ratio from SS patients normalized to untreated control (n = 10). (c) Survival of non-malignant CD4+ T cells from SS patients after GI254023X pre-treatment followed by alpha-toxin treatment (n = 3). (d–f) Correlation of (d) alpha-toxin binding versus ADAM10 receptors expression (p = .0002, R2 = 0.8412), (e) survival versus alpha-toxin binding (p = .2245, R2 = 0.178) and (f) survival versus ADAM10 surface expression (p = .1404, R2 = 0.2508) of malignant CD4+ T cells from SS patients (n = 10). (g,h) ADAM10 surface expression and alpha-toxin binding of SS01 measured with (g) flow cytometry or (h) confocal microscopy. Error bars display mean ± standard error of mean.
ADAM10 mediates alpha-toxin cytotoxicity in non-malignant CD4+ T cells from SS patients
Similar to CD4+ T cells from healthy donors, cell death of non-malignant CD4+ T cells was dependent on the expression of ADAM10, as pharmacological inhibition of ADAM10 increased the survival of these cells following treatment with alpha-toxin (Figure 2(c)). In contrast, ADAM10 inhibition had no significant effect on the survival of the malignant cells (Figure S3(a)). Alpha-toxin binding correlated with ADAM10 surface expression in both malignant and non-malignant CD4+ T cells (Figure 2(d) and Figure S3(b–d)), consistent with ADAM10 being the main receptor for alpha-toxin.15 Surprisingly, neither ADAM10 expression nor alpha-toxin binding correlated with the differences in alpha-toxin sensitivity of the malignant cells (Figure 2(e,f)).
Nevertheless, in the patient with the greatest increase in the malignant to non-malignant CD4+ T cell ratio following alpha-toxin treatment (SS01), the malignant cells exhibited both reduced ADAM10 expression and alpha-toxin binding compared to the non-malignant CD4+ T cells (Figure 2(g)). This was confirmed by confocal microscopy revealing almost complete absence of alpha-toxin binding to malignant (TCRVβ18+) cells, whereas non-malignant (TCRVβ18−) CD4+ T cells from the same patient showed normal binding (Figure 2(h)).
ADAM10-independent resistance mechanisms against alpha-toxin cytotoxicity
It has previously been shown that the toxic effects of alpha-toxin can be evaded by internalisation of surface-bound toxin.17 To investigate if this evasion mechanism is active in resistant CTCL cells, we treated resistant malignant MyLa2059 and sensitive non-malignant MyLa1850 cells with fluorescent-labelled alpha-toxin and visualised its cellular localisation using confocal microscopy. At 4°C, when internalisation machinery is inactive, the toxin bound to the surface of both cell lines (Figure S4(a)). However, at 37°C incubation, malignant MyLa2059 cells exhibited fluorescence patterns consistent with internalisation of the toxin, whereas non-malignant MyLa1850 cells still only exhibited alpha-toxin binding at the cell surface (Figure S4(a)). To test if blocking internalisation could increase alpha-toxin sensitivity of the malignant cells, we incubated peripheral blood monoculear cells (PBMCs) from three SS patients with the clathrin-mediated endocytosis inhibitor, Dynasore. Pre-treatment with Dynasore led to a modest increase in toxin-induced cell death in the malignant cells and subsequent decrease in the malignant to non-malignant ratio in three patients, while the non-malignant CD4+ T cells were not affected (Figure S4(b–d)).
Discussion
Bacterial colonization of lesional skin is common in CTCL patients, with S. aureus being the most prevalent pathogen.4 Alpha-toxin is produced by essentially all S. aureus strains and is able to induce apoptosis in T cells.11,22 However, the role of alpha-toxin in CTCL has yet to be elucidated.
In this study, we were able to show that a variety of malignant CTCL cell lines derived from patients suffering from MF, SS and a CD30+ lymphoproliferative disease are resistant to alpha-toxin, while non-malignant cell lines and primary CD4+ T cells die through an ADAM10 dependent mechanism. This was confirmed in primary cells from SS patients where alpha-toxin favoured the survival of malignant T cells over non-malignant CD4+ T cells in a subset of patients by inducing death in the non-malignant CD4+ T cell population.
As in cell lines and CD4+ T cells from healthy donors, ADAM10 surface expression determined the sensitivity of non-malignant CD4+ T cells from SS patients towards the pore-forming toxin. Malignant CD4+ T cells may develop resistance to alpha-toxin through downregulation of ADAM10 expression, as the case study of patient SS01 clearly showed. However, as resistant malignant cells from other SS patients displayed normal ADAM10 expression levels and alpha-toxin binding, ADAM10 downregulation cannot be the only resistance mechanism employed by malignant CTCL cells. A possible mechanism may be internalization of affected membrane areas. Dynasore has previously been used to render resistant cells susceptible to alpha-toxin induced cell death.17,23 As pre-incubation of SS patient cells with the endocytosis inhibitor Dynasore prior to toxin challenge led to an increase in cell death in the malignant population, internalization of affected membrane parts might be an additional mechanism contributing to reduced sensitivity of the malignant cells towards the pore-forming toxin.
Here we show for the first time that malignant CTCL cells are resistant to S. aureus derived alpha-toxin at concentrations where non-malignant CD4+ T cells die. Consequently, the presence of alpha-toxin favours the persistence of malignant cells, while removing the non-malignant CD4+ T cells. As such, this study describes a new mechanism by which bacterial infections may promote CTCL disease. Multiple resistance mechanisms appear to be involved, including downregulation of ADAM10 surface expression and internalisation of surface bound alpha-toxin. Given the prevalence of S. aureus colonisation in CTCL skin lesions and their ubiquitous production of alpha-toxin, this may explain why a reduction of bacterial infections by psoralen and ultraviolet A (PUVA) and local or systemic antibiotic treatment clinically improves CTCL.8,9,24 Indeed, it is possible that the efficiency of PUVA treatment may rely on its anti-bacterial effect and an interplay between skin resident dendritic and/or malignant cells with non-malignant T cells12,13,25,26 and its modulation by alpha-toxin.
Neutralizing antibodies against alpha-toxin have passed phase 1 clinical trials and are currently in phase 2b for the treatment of ventilator-associated pneumonia.27 Our findings suggest that alpha-toxin neutralizing antibodies could be beneficial in the treatment of CTCL patients. A high incidence of bacterial infections is not restricted to CTCL,28 and future studies should address if alpha-toxin may affect the functional immune system without affecting malignant cells in other types of skin cancers.
In conclusion, this study shows that alpha-toxin may contribute to the pathology of CTCL by tilting the balance between malignant CTCL cells and non-malignant CD4+ T cells, strengthening the hypothesis that antibiotics are beneficial in the treatment of CTCL.
Methods
Cell culture and isolation of PBMCs
CTCL cell lines were derived29–33 and maintained as stated in Supplementary Table 1. PBMCs from healthy donors and SS patients were isolated by Ficoll-based density-gradient centrifugation and CD4+ T cells were enriched using LS columns (Miltenyi Biotec, #130-041-306) and CD4 MicroBeads (Miltenyi Biotec, #130-045-101) following the manufacturer`s instructions. All work was performed in accordance with the Declaration of Helsinki. Written informed consent of SS patients was obtained after approval by the Committee on Health Research Ethics (H-16025331).
Alpha-toxin treatment
Unlabelled alpha-toxin was obtained from List lab (#120), while AF647 labelled alpha-toxin was conjugated as described elsewhere.16,34 Cells were exposed to alpha-toxin for 6 hours at 37°C, unless stated otherwise.
LDH activity assay
For LDH release assays (TaKaRa, #MK401), cells were grown in RPMI 1640 (Gibco, #11835-063) or X-VIVO (Lonza, #BE02-061Q) media without phenol red. LDH measurements were normalized to maximum release induced by Triton X-100 prior to subtracting the absorbance of the untreated sample.
ADAM10 inhibition
Cells were pre-treated for 20 hours with 20 µM of the ADAM10 inhibitor GI254023X (Sigma-Aldrich, #SML0789-5MG) prior to toxin exposure.
Inhibition of ADAM10 internalisation using dynasore
Cells were pre-treated with the GTPase inhibitor Dynasore (abcam, #120192) for 1 hour at 37°C prior to toxin treatment.
siRNA transfection
Cells were transfected with short interfering RNA (siRNA) ON-TARGET plus smart pool against Human ADAM10 (Dharmacon, #L-004503-00-0005) using the Amaxa nucleofectin technology. Briefly, cells were re-suspended in IngenioR Electroporation Solution (Mirus, #MIR 50111) containing the siRNA and then transfected with an Amaxa Nucleofector 1 apparatus (Amaxa), using the pulsing parameter U-14. In all transfection experiments, the knockdown results were compared to cells that had been transfected with ON-TARGET plus non-targeting control siRNA (Dharmacon, #D-001810-01-20). Cells were exposed to alpha-toxin five days after transfection for a period of four days at 37°C.
Flow cytometry
Cell surface staining was performed in FACS-PBS (PBS + 1% FBS + 0.02% NaN3) or Brilliant Stain Buffer (BD Bioscience, #563794), using primary conjugated antibodies as listed in Supplementary Table 2. Malignant cells were identified as CD3+CD4+CD7− and CD26− and/or TCRVβ#+, as previously described;35 representative examples are depicted in Figure S1. Dead cells were excluded using Propidium iodide (eBioscience, #MBS500PI) and/or Annexin V – FITC or PE (BioLegend, #640945/#640908). To improve Annexin V binding, cells were stained in Annexin V Binding Buffer (BD Bioscience, #51-66121E). All flow cytometric analyses were performed using a 3- or 5-laser BD LSR-Fortessa and analysed using FlowJo (TreeStar) software.
Immunofluorescent microscopy
CTCL cell lines were incubated on ice for 30 minutes prior to 2 hours treatment with 1.5 µg/ml AF647 labelled alpha-toxin. Cells were fixed in 2% PFA for 10 minutes at room temperature and permeabilised with ice-cold methanol. Nuclei were stained with DAPI (ThermoFisher, #62248) prior to mounting in SlowFade Diamond Antifade mountant (ThermoFisher, #S36967).
Primary SS patient cells were incubated for 1 hour on ice prior to 6 hours treatment with 1.5 µg/ml AF647 labelled alpha-toxin on ice or at 37°C. Cells were stained with anti-TCRVβ18 (Beckman Coulter, #IM2049) and fixed with Fix Perm (BD, #554714) for 15 minutes at room temperature. After nuclei staining with Hoechst-33342 (ThermoFisher, #62249) for 10 minutes at room temperature, cells were mounted in ProLong Diamond Antifade mounting media (ThermoFisher, #P36965). All experiments were visualized on a ZEISS LSM 710 and analysed using the ZEN 2.5 lite software.
Funding Statement
This work was supported by a donation from the LEO Foundation and grants from The Novo Nordisk Research Foundation (NNF14OC0012345), the Danish Cancer Society (Kræftens Bekæmpelse), the Fight Cancer Program (Knæk Cancer), and the Lundbeck Foundation.
Abbreviations
| ADAM10 | a disintegrin and metalloproteinase domain-containing protein 10 |
| CTCL | Cutaneous T-cell lymphoma |
| LDH | lactate dehydrogenase |
| MF | Mycosis fungoides |
| PBMCs | peripheral blood mononuclear cells |
| PUVA | psoralen and ultraviolet A |
| S. aureus | Staphylococcus aureus |
| siRNA | short interfering RNA |
| SS | Sézary syndrome |
| TCR | T-cell receptor |
Acknowledgments
We thank the SS patients that donated blood for this study as well as the photophoresis team at Copenhagen University Hospital Bispebjerg for obtaining the samples.
Author contribution
E.B performed experiments; E.B., T.B.B. analysed the data and made the figures; E.B., A.W-O., M.G., L.M.L., S.F., C.N., T.K., B.G.J.S, S.B.K, T.H., J.L.P., C.M.B., C.G., L.I., J.C.B., M.H.A., A.W., T.B.B., and N.Ø. designed the research. E.B., T.B.B. and N.Ø. wrote the original draft of the paper. E.B., A.W-O., M.G., L.M.L., S.F., C.N., T.K., B.G.J.S, S.B.K, T.H., J.L.P., C.M.B., C.G., L.I., J.C.B., M.H.A., A.W., T.B.B., and N.Ø. reviewed and edited the manuscript. T.B.B., and N.Ø. supervised the project. N.Ø. acquired the funding.
Disclosure of potential conflicts of interest
The authors declare no competing financial interest.
Supplementary materials
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Blaizot R, Ouattara E, Fauconneau A, Beylot-Barry M, Pham-Ledard A.. Infectious events and associated risk factors in Mycosis Fungoides/Sézary syndrome: a retrospective cohort study. Br J Dermatol. 2018. doi: 10.1111/bjd.17073. [DOI] [PubMed] [Google Scholar]
- 3.Odum N, Lindahl LM, Wod M, Krejsgaard T, Skytthe A, Woetmann A, Iversen L, Christensen K.. Investigating heredity in cutaneous T-cell lymphoma in a unique cohort of Danish twins. Blood Cancer J. 2017;7:e517–e517. doi: 10.1038/bcj.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and sézary syndrome. JAMA. 1992;267:1354–1358. doi: 10.1001/jama.267.4.507b. [DOI] [PubMed] [Google Scholar]
- 5.Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. 1997;89:32–40. [PubMed] [Google Scholar]
- 6.Nguyen V, Huggins RH, Lertsburapa T, Bauer K, Rademaker A, Gerami P, Guitart J. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol. 2008;59:949–952. doi: 10.1016/j.jaad.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Fanok MH, Sun A, Fogli LK, Narendran V, Echstein M, Kannan K, Dolgalev I, Lazaris C, Heguy A, Laird ME, et al. Role of dysregulated cytokine signaling and bacterial triggers in the pathogenesis of cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:1116–1125. doi: 10.1016/j.jid.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tokura Y, Yagi H, Ohshima A, Kurokawa S, Wakita H, Yokote R, Shirahama S, Flirukawa F, Takigawa M. Cutaneous colonization with staphylococci influences the disease activity of Sézary syndrome: a potential role for bacterial superantigens. Br J Dermatol. 1995;133:6–12. doi: 10.1111/j.1365-2133.1995.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 9.Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008;159:105–112. doi: 10.1111/j.1365-2133.2008.08612.x. [DOI] [PubMed] [Google Scholar]
- 10.Willerslev-Olsen A, Krejsgaard T, Lindahl L, Bonefeld C, Wasik M, Koralov S, Geisler C, Kilian M, Iversen L, Woetmann A, et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins. 2013;5:1402–1421. doi: 10.3390/toxins5081402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grumann D, Nübel U, Bröker BM. Staphylococcus aureus toxins – their functions and genetics. Infect Genet Evol. 2014;21:583–592. doi: 10.1016/j.meegid.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Krejsgaard T, Willerslev-Olsen A, Lindahl LM, Bonefeld CM, Koralov SB, Geisler C, Wasik MA, Gniadecki R, Kilian M, Iversen L, et al. Staphylococcal enterotoxins stimulate lymphoma-associated immune dysregulation. Blood. 2014;124:761–770. doi: 10.1182/blood-2014-01-551184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, Litvinov IV, Fredholm S, Petersen DL, Nastasi C, Gniadecki R, Mongan NP, Sasseville D, et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. 2016;127:1287–1296. doi: 10.1182/blood-2015-08-662353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins. 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilke GA, Wardenburg JB. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin–mediated cellular injury. Proc Natl Acad Sci. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virreira Winter S, Zychlinsky A, Bardoel BW. Genome-wide CRISPR screen reveals novel host factors required for Staphylococcus aureus α-hemolysin-mediated toxicity. Sci Rep. 2016;6:24242. doi: 10.1038/srep24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 2009;583:337–344. doi: 10.1016/j.febslet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Valeva A, Walev I, Pinkernell M, Walker B, Bayley H, Palmer M, Bhakdi S. Transmembrane β-barrel of staphylococcal α-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci. 1997;94:11607–11611. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walev I, Palmer M, Martin E, Jonas D, Weller U, Höhn-Bentz H, Husmann M, Bhakdi S. Recovery of human fibroblasts from attack by the pore-forming alpha-toxin of Staphylococcus aureus. Microb Pathog. 1994;17:187–201. doi: 10.1006/mpat.1994.1065. [DOI] [PubMed] [Google Scholar]
- 20.Russo MJ, Bayley H, Toner M. Reversible permeabilization of plasma membranes with an engineered switchable pore. Nat Biotechnol. 1997;15:5.doi: 10.1038/nbt0397-278. [DOI] [PubMed] [Google Scholar]
- 21.Gibson JF, Huang J, Liu KJ, Carlson KR, Foss F, Choi J, Edelson R, Hussong JW, Mohl R, Hill S, et al. Cutaneous T cell lymphoma: current practices in blood assessment and the utility of T-cell receptor Vβ chain restriction. J Am Acad Dermatol. 2016;74:870–877. doi: 10.1016/j.jaad.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, et al. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7:e36532. doi: 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura M, Namiira S, Akamatsu H, Horio T. Antimicrobial effects of phototherapy and photochemotherapy in vivo and in vitro. Br J Dermatol. 1996;135:528–532. doi: 10.1111/j.1365-2133.1996.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 25.Vieyra-Garcia P, Fink-Puches R, Porkert S, Lang R, Pöchlauer S, Ratzinger G, Tanew A, Selhofer S, Paul-Gunther S, Hofer A, et al. Evaluation of low-dose, low-frequency oral psoralen–UV-A treatment with or without maintenance on early-stage mycosis fungoides: a randomized clinical trial. JAMA Dermatol. 2019;155:538. doi: 10.1001/jamadermatol.2018.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woetmann A, Lovato P, Eriksen KW, Krejsgaard T, Labuda T, Zhang Q, Mathiesen AM, Geisler C, Svejgaard A, Wasik MA, et al. Nonmalignant T cells stimulate growth of T-cell lymphoma cells in the presence of bacterial toxins. Blood. 2007;109:3325–3332. doi: 10.1182/blood-2006-02-004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruzin A, Wu Y, Yu L, Yu X-Q, Tabor DE, Mok H, Tkaczyk C, Jensen K, Bellamy T, Roskos L, et al. Characterisation of anti-alpha toxin antibody levels and colonisation status after administration of an investigational human monoclonal antibody, MEDI4893, against Staphylococcus aureus alpha toxin. Clin Transl Immunol. 2018;7:e1009. doi: 10.1002/cti2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kullander J, Forslund O, Dillner J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009;18:472–478. doi: 10.1158/1055-9965.EPI-08-0905. [DOI] [PubMed] [Google Scholar]
- 29.Kaltoft K, Bisballe S, Rasmussen HF, Thestrup-Pedersen K, Thomsen K, Sterry W. A continuous T-cell line from a patient with Sézary syndrome. Arch Dermatol Res. 1987;279:293–298. doi: 10.1007/BF00431220. [DOI] [PubMed] [Google Scholar]
- 30.Abrams JT, Lessin S, Ghosh SK, Ju W, Vonderheid EC, Nowell P, Murphy G, Elfenbein B, DeFreitas E. A clonal CD4-positive T-cell line established from the blood of a patient with sézary syndrome. J Invest Dermatol. 1991;96:31–37. doi: 10.1111/1523-1747.ep12514693. [DOI] [PubMed] [Google Scholar]
- 31.Davis TH, Morton CC, Miller-Cassman R, Balk SP, Kadin ME. Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med. 1992;326:1115–1122. doi: 10.1056/NEJM199204233261704. [DOI] [PubMed] [Google Scholar]
- 32.Netchiporouk E, Gantchev J, Tsang M, Thibault P, Watters AK, Hughes J-DM, Ghazawi FM, Woetmann A, Ødum N, Sasseville D, et al. Analysis of CTCL cell lines reveals important differences between mycosis fungoides/Sézary syndrome vs. HTLV-1+ leukemic cell lines. Oncotarget. 2017;8:95981–95998. doi: 10.18632/oncotarget.v8i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surewaard BGJ, Thanabalasuriar A, Zeng Z, Tkaczyk C, Cohen TS, Bardoel BW, Jorch SK, Deppermann C, Bubeck Wardenburg J, Davis RP, et al. α-toxin induces platelet aggregation and liver injury during staphylococcus aureus sepsis. Cell Host Microbe. 2018;24:271–284.e3. doi: 10.1016/j.chom.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buus TB, Willerslev-Olsen A, Fredholm S, Blümel E, Nastasi C, Gluud M, Hu T, Lindahl LM, Iversen L, Fogh H, et al. Single-cell heterogeneity in Sézary syndrome. Blood advances. 2018;2:12.doi: 10.1182/bloodadvances.2018022608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaltoft K, Bisballe S, Dyrberg T, Boel E, Rasmussen PB, Thestrup-Pedersen K. Establishment of two continuous T-cell strains from a single plaque of a patient with mycosis fungoides. Vitro Cell Dev Biol J Tissue Cult Assoc. 1992;28A:161–167. doi: 10.1007/BF02631086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


