Abstract
Nonadherence to medications in cancer treatment protocols may be a particular concern among adolescents and young adults (AYAs) with cancer and predictive of poor health outcomes, but data supporting this claim remain limited. The purpose of this article was to systematically review the rates, outcomes, and predictors of oral medication nonadherence among AYAs with cancer. PubMed (i.e., MEDLINE), CINAHL, and PsycINFO databases were searched in 2018 using terms related to medication adherence and cancer. A total of 37,884 records representing 34,006 unique articles were identified and reviewed. Thirteen articles representing 12 studies met inclusion criteria and examined medication adherence among AYAs with cancer. Results of these studies suggest that 21%–60% of AYAs are nonadherent to oral medications, likely placing them at increased risk for poor health outcomes (i.e., relapse, infection/fever, and death). Psychosocial factors (i.e., knowledge, beliefs about capabilities, beliefs about consequences, environmental context and resources, and emotion) were related to nonadherence and warrant future study. Of note, demographic, disease, and family composition variables did not predict nonadherence. Clinical implications as well as limitations and resulting future directions are discussed.
Keywords: adherence, self-management, systematic review, oral medication
Introduction
Cancer is the leading cause of disease-related death among adolescents and young adults (AYAs, ages 15–39 years1) due, at least in part, to the number of AYAs with cancer who experience treatment failure and/or infections.2,3 Nonadherence to medications included in cancer treatment protocols has been hypothesized to contribute to treatment failure and infection rates in this age group.4–6 Although consistent with the World Health Organization's statement that nonadherence is a primary cause of treatment failure globally,7 our understanding of the prevalence and impact of medication nonadherence among AYAs with cancer is limited.
In 2010, two reviews discussing nonadherence among AYAs with cancer were published.5,6 While these reviews have been heavily cited in the growing conversation regarding medication nonadherence among AYAs,4 it has been nearly a decade since the literature has been systematically reviewed. Since then, over 75 anticancer drugs have been approved by the FDA,8,9 roughly half of which are oral medications.10 As oral medications become a more common component of cancer treatment protocols and a growing number of medications are self-administered outside of the hospital (i.e., at home), the ability of the medical team to monitor and facilitate medication administration decreases. Understanding the current patterns and outcomes of nonadherence among AYAs with cancer, thus, is increasingly critical for informing clinical care (e.g., allocation of resources for adherence-promotion and nonadherence assessment practices).
The primary aim of this article is to provide an updated review of the rates and associated health outcomes of medication nonadherence among AYAs with cancer. This article expands on previous reviews by systematically reviewing the rates of medication nonadherence among AYAs 15–39 years of age (as opposed to a subset like 15–24 years) with cancer. In addition, this review includes health outcome data, providing the first systematic synthesis of the associations between nonadherence and health outcomes among AYAs with cancer. The secondary aim of this review is to explore predictors of medication nonadherence among AYAs with cancer. As there has only been one randomized clinical trial of an adherence-promotion intervention designed specifically for AYAs with cancer to date, a videogame with limited efficacy,11 the identification of predictors has the potential to guide future adherence-promotion efforts.
Methods
Data search
PubMed (i.e., MEDLINE), CINAHL, and PsycINFO databases were searched in April 2018 using a combination of medical subject headings (MeSH) and keywords relevant to medication adherence (i.e., “patient compliance,” “medication adherence,” and “self-management”) and cancer (i.e., “neoplasms,” “cancer,” “leukemia,” “lymphoma,” and “tumor”). No restrictions were placed on publication date or language. To increase the likelihood that all relevant articles were captured, backward reference searches (reviewing references of articles meeting inclusion criteria and reviewing references of previous reviews) and forward reference searches (reviewing articles citing articles meeting inclusion criteria and reviewing articles citing previous reviews) were conducted in December 2018.
Definition of variables
Adolescent and young adult
The National Cancer Institute's (NCI) definition of the adolescent and young adult age range of 15–39 years was used for this article.1 Because of the numerous definitions of the AYA age range used by different organizations and national clinical programs,4 it was unlikely that all articles aiming to investigate adherence among AYAs to date would use the 15–39 year age range. To allow for differences in age range, but adhere to the NCI's definition, articles were included if the mean (or median in the absence of a mean) age of the sample or subsample at baseline was between 15 and 39 years.
Medication nonadherence
Medication nonadherence is defined as the extent to which medication-taking behavior does not coincide with agreed-upon medical advice.12 Unlike the historically used concept of compliance, adherence assumes that the medical team and AYA (and their family as appropriate) engage in shared decision-making to determine the most appropriate course of treatment.13 Consistent with this conceptualization, articles defining nonadherence or noncompliance as “refusal” to initiate treatment were excluded.
No restrictions were placed on nonadherence assessment method. Included studies measured adherence using self-report methods (i.e., questionnaires and interviews), drug levels, prescription refill data, and/or electronic adherence monitoring devices. All measures of nonadherence in a given study were included.
Nonadherence behavior can be described as a continuous (e.g., percentage or number of doses missed/number of doses prescribed) or categorical (e.g., nonadherent or adherent based on a cut point) variable. Because there is no agreed-upon cut point for categorizing adherence behavior among AYAs with cancer, the cut points used to categorize AYAs as nonadherent are those provided by the original authors and differ across studies.
Health outcomes
Health outcomes were defined as any measure of treatment outcomes (e.g., event-free survival [EFS]) or medical events (e.g., infection) potentially related to nonadherence. In the studies meeting inclusion criteria, health outcome data were obtained through patient medical records or clinical trial records.
Predictors of nonadherence
All demographic, clinical, or psychosocial variables examined as potential predictors of nonadherence in included articles were extracted by the first author. Studies included in this review assessed predictors of nonadherence using medical record data or patient or caregiver self-report. To facilitate summarization of findings across studies, predictors were classified into broader categories according to the Theoretical Domains Framework14 when appropriate.
Study selection and screening
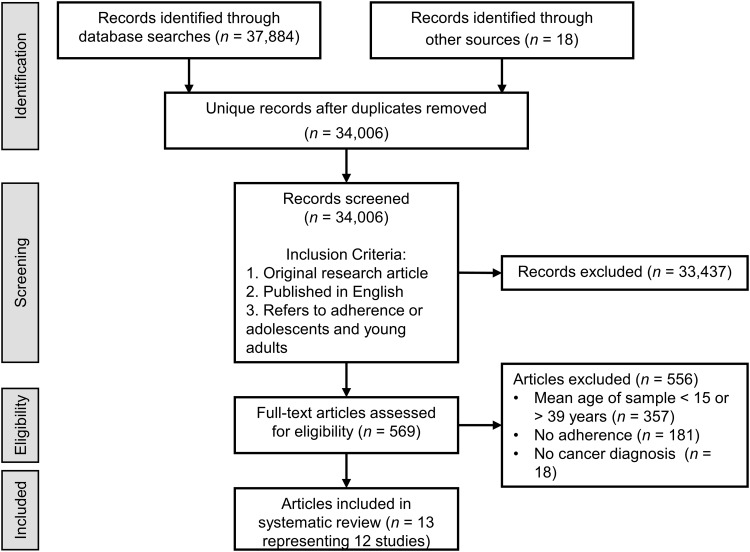
As depicted in the PRISMA flow diagram in Figure 1, the initial database search resulted in 37,884 records and backward and forward reference searches identified an additional 18 records. After duplicates were removed, 34,006 unique records remained. The titles and abstracts of these records were screened and records were retained if they met the following criteria: original research article, published in English, and includes adherence or AYAs. A total of 569 records met the inclusion criteria. The full text articles associated with these 569 records were reviewed and excluded if they included a sample or subsample with a mean age less than 15 or greater than 39 years (n = 357), did not assess adherence (n = 181), or included individuals with a diagnosis other than cancer (n = 18). All levels of screening were conducted by the first author.
FIG. 1.
Study selection flow diagram.
Risk of bias
Risk of bias was assessed using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies developed by the National Heart, Lung, and Blood Institute of the National Institutes of Health.15 Assessment tool items not relevant to the included studies were coded as “not applicable” in accordance with published guidelines.
Data extraction
Article characteristics, participant demographic and clinical characteristics, adherence data, health outcomes, and predictors of nonadherence were extracted by the first author using a data collection form with pre-defined fields. A second individual completed an independent review of the extracted data for accuracy. Inconsistencies were resolved through consultation with the original article, resulting in complete agreement on all data points.
Analysis
Descriptive statistics were used to summarize study characteristics and outcomes. Although a meta-analysis quantifying the association between nonadherence and health outcomes was originally planned, it was precluded by the heterogeneity in the definitions of nonadherence and health outcomes across studies.
Results
A total of 13 articles representing 12 studies met all inclusion criteria (Table 1) and included AYAs spanning the NCI's age range, with mean/median ages ranging from 15 to 39 years. For one study, two articles were published, one reporting on the health outcomes16 associated with nonadherence and one reporting on predictors of nonadherence.17 While data from both articles were extracted, these findings were reported within the context of one study to avoid duplicate reporting of rates of nonadherence.
Table 1.
Study Characteristics and Rates and Associated Outcomes of Medication Nonadherence
| Author | N | Age, M (SD), range years | Diagnosis | Medication | Nonadherence assessment | Definition of nonadherence | Prevalence of nonadherence | Health outcome |
|---|---|---|---|---|---|---|---|---|
| Festa et al.16; Tamaroff et al.17 | 21a | 15.6 (2.2), — | ALL or Hodgkin's lymphoma | Prednisone | DHEA-S levels | Lack of appropriate DHEA-S suppression | 11/21 (52%) | Relapse: NA: 5/11; A: 1/10 (χ2 = 3.23, p = 0.07) |
| Festa et al.16; Tamaroff et al.17 | 29a | 19.1 (4.1), — | Hodgkin's lymphoma | Penicillin | Bioassay with Micrococcus luteus | Lack of detectable urinary penicillin | 14/29 (48%) | |
| Ganesan et al.24 | 516 | 35b (—), 6–70 | Chronic phase CML | Imatinib | Medication refill or patient self-report | Medication interruption >1 week | 150/516 (29%) | 5-Year EFS: NA: 60%; A: 77% (p = 0.01); CCR: NA: 27%; A: 47% (p = 0.001) |
| Hullmann et al.20 | 103 | 15.8 (1.8), 13–19 | Cancer (i.e., leukemia, lymphoma, solid tumor, and brain tumor) | All medications | Medical adherence measure (patient report)c | Adolescent-reported rating of 1–9 on a scale of 1 (never take medication) to 10 (always take medication) | 59/103 (57%) | |
| Hullmann et al.20 | 103 | 15.8 (1.8), 13–19 | Cancer (i.e., leukemia, lymphoma, solid tumor, and brain tumor) | All medications | Medical adherence measure (parent report)c | Parent-reported rating of 1–9 on a scale of 1 (never take medication) to 10 (always take medication) | 48/103 (47%) | |
| Jaime-Pérez et al.25 | 57 | 17b (—), 16–20 | ALL | Maintenance medications | Direct interview with caregivers and/or patients and review of clinical files | Two or more missed doses (without medical indication) per self-report or as noted in the clinical file | 12/57 (21%) | 5-Year EFS: NA: 13%; A: 26% (HR = 1.49, p = 0.28); 5-year OS: NA: 52%, A: 44% (HR = 1.08, p = 0.87) |
| Kennard et al.28 | 44 | 15.3 (1.8), 13–17 | Cancer (i.e., ALL, Hodgkin's lymphoma, non-Hodgkin lymphoma, bone sarcoma, ependymoma, and primitive neuroectodermal tumor, CML) | Trimethoprim/sulfamethoxazole | High-performance liquid chromatographic assayc | <2.0 μg/mL of sulfamethoxazole in serum | 12/44 (27%) | 6-Year OS: NA patients more likely to be deceased than A patients (χ2 = 5.82, p < 0.003) |
| Kennard et al.28 | 44 | 15.3 (1.8), 13–17 | Cancer (i.e., ALL, Hodgkin's lymphoma, non-Hodgkin lymphoma, bone sarcoma, ependymoma, and primitive neuroectodermal tumor, CML) | Trimethoprim/sulfamethoxazole | Self-reported number of late or missed doses in the past month (patient report)c | Not applicable—average number of missed doses on a scale of 0 (no late or missed doses) to 4 (>10 late or missed doses) | M (SD) = 1.55 (1.04) | |
| Lau et al.22 | 5d | 15.2 (1.8), 13–17 | ALL | Mercaptopurine | Electronic adherence monitoring device | <90% of doses taken | 2/5 (40%) | |
| Linder et al.23 | 23 | 19b (—), 15–29 | Cancer (i.e., leukemia, lymphoma, sarcoma, brain tumor, and other solid tumor) | Oral cancer-related medication | Electronic adherence monitoring device | ≤2 Weeks (during a 4-week pre-intervention period) with ≥80% of doses taken | 9/23 (39%) | |
| Pai et al.18 | 51 | 15 (—), 12–19 | ALL | Mercaptopurine | Bioassayc | Low TGN (<343 pmole/8 × 108 RBC) and low MMP (<6535 pmole/8 × 108 RBC) | 27/51 (53%) | |
| Pai et al.18 | 51 | 15 (—), 12–19 | ALL | Mercaptopurine | Self-report of adherence to oral medication questionnaire (patient report)c | ≥1 Missed dose during maintenance | 23/51 (45%) | |
| Pizzo et al.26 | 77d | 16b (—), 1–43 | Cancer (i.e., acute leukemia, lymphoma, and solid tumor) | Trimethoprim/sulfamethoxazole | Calendar and self-report (reporter not indicated) | Missed doses at least occasionally | 77/211 (36%)e | Fever/infection: NA patients more likely to have a fever or infection than A patients (p ≤ 0.05) |
| Tebbi et al.19 | 20 | 15 (—), — | Cancerf | Oral chemotherapy | Self-report (patient and parent report) | ≥1 Missed dose in the past month | 12/20 (60%) | |
| Unnikrishnan et al.21 | 221 | 39b (—), 17–68 | CML | Imatinib | Morisky Medication Adherence Scale | Score of ≤7 | 122/221 (55%) | Undetectable BCR-ABL transcript levels: NA: 0/54; A: 29/58 (p < 0.001); MMR: NA: 27/54; A: 35/58 (p = 0.18) |
| Wu et al.27 | 143d | — (—), 18–21 | ALL | Mercaptopurine | MPR | Not applicable—MPR calculated | M (SD) = 75% (4%) of doses taken; 25% of doses not taken | |
| Wu et al.27 | 143d | — (—), 18–21 | ALL | Methotrexate | MPR | Not applicable—MPR calculated | M (SD) = 72% (4%) of doses taken; 28% of doses not taken |
Two samples from the same study.
Median age, mean age not reported.
Multiple measures of adherence with same sample.
Subset of patients from larger study.
Multiple episodes per patient.
Specific diagnoses not reported.
—, Not reported; A, adherent; ALL, acute lymphoblastic leukemia; CCR, complete cytogenetic response; CML, chronic myeloid leukemia; DHEA-S, dehydroepiandrosterone sulfate; EFS, event-free survival; HR, hazard ratio; MMR, major molecular response; MPR, medication possession ratios; NA, nonadherent; OS, overall survival; RBC, red blood cell; SD, standard deviation.
Five studies included patients with one of many cancer diagnoses, with the remaining studies, including participants with one or two diagnoses (acute lymphoblastic leukemia [ALL], n = 4; chronic myeloid leukemia [CML], n = 2; and ALL or Hodgkin's lymphoma, n = 1). Study quality results were variable, with five studies providing insufficient information to evaluate multiple criteria (e.g., enrollment and retention rates) (Table 2).
Table 2.
Ratings of Study Quality
| Festa et al.16; Tamaroff et al.17 | Ganesan et al.24 | Hullmann et al.20 | Jaime-Pérez et al.25 | Kennard et al.28 | Lau et al.22 | Linder et al.23 | Pai et al.18 | Pizzo et al.26 | Tebbi et al.19 | Unnikrishnan et al.21 | Wu et al.27 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Research question or objective clearly stated | + | + | + | + | + | + | + | + | − | + | + | + |
| Study population clearly specified and defined | + | + | + | + | + | + | + | + | + | − | + | + |
| Participation rate ≥50% | NR | NR | + | NR | NR | NR | + | + | NR | NR | NR | NR |
| Subjects selected or recruited from the same or similar populations and using the same inclusion and exclusion criteria | + | + | + | + | + | + | + | + | + | + | + | + |
| Sample size justification, power description, or variance and effect estimates provided | − | − | NA | − | − | NA | NA | NA | − | NA | − | NA |
| Exposure(s) of interest measured before the outcome(s) being measured | NR | NR | NA | NR | NR | NA | NA | NA | NR | NA | NR | NA |
| Timeframe sufficient to see an association between exposure and outcome | NR | NR | NA | NR | NR | NA | NA | NA | NR | NA | NR | NA |
| Different levels of the exposure as related to the outcome examined | − | − | NA | − | + | NA | NA | NA | + | NA | − | NA |
| Exposure measures clearly defined, valid, reliable, and implemented consistently across all study participants | + | − | + | − | + | + | + | + | − | − | + | + |
| Exposure(s) assessed more than once over time | − | − | − | − | − | − | + | + | − | − | − | − |
| Outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants | − | + | NA | + | + | NA | NA | NA | + | NA | + | NA |
| Outcome assessors blinded to the exposure status of participants | NR | NR | NA | NR | NR | NA | NA | NA | NR | NA | NR | NA |
| Loss to follow-up after baseline ≤20% | NR | NR | NA | NR | NR | NA | NA | NA | NR | NA | NR | NA |
| Key potential confounding variables measured and adjusted for statistically | − | + | NA | + | − | NA | NA | NA | − | NA | − | NA |
+, Yes; −, no; NA, not applicable; NR, not reported;
Rates of nonadherence
Nonadherence to various oral medications (n = 5), mercaptopurine (n = 4), trimethoprim/sulfamethoxazole (n = 3), imatinib (n = 2), prednisone (n = 1), penicillin (n = 1), and/or methotrexate (n = 1), was assessed using self-report measures (n = 6), drug levels (n = 4), medication possession ratios (MPR, n = 2), electronic adherence monitoring devices (n = 2), or a combination of multiple measures (n = 3). Each study reporting on rates of nonadherence used a different cut point to classify patients as adherent or nonadherent. In studies assessing nonadherence through self-report measures, patients were classified as nonadherent if they reported that they missed one or more doses during the reporting period (n = 2, Rates = 45%18 and 60%19), reported that they missed doses at least sometimes (n = 2; Rates = 47%20 and 57%20), or scored a 7 or lower on the Morisky Medication Adherence Scale (n = 1, Rate = 55%21). Patients were classified as nonadherent per drug levels based on medication-specific cut points, with rates ranging from 27% to 53% (n = 4). When adherence was assessed by electronic monitoring devices, patients were classified as nonadherent if electronic monitoring data indicated they took <90% of prescribed doses (n = 1, Rate = 40%)22 or had 2 or fewer weeks (out of 4 weeks) with ≥80% of prescribed doses taken (n = 1, Rate = 39%).23 The final three studies describing rates of nonadherence used self-report data in combination with medication refill data (n = 1, Rate = 29%),24 clinical notes (n = 1, Rate = 21%),25 or medication calendars (n = 1, Rate = 36%)26 to classify patients as adherent or nonadherent using a study-specific threshold.
Instead of using a cut point to classify patients as adherent or nonadherent, two studies reported on the average percentage or number of missed doses. In a study of AYAs with ALL, participants missed an average of 25%–28% of medication doses as assessed by MPR.27 In the other study describing mean adherence, AYAs with cancer were asked to rate the frequency of missed or late doses on a Likert-type scale from zero (no missed doses) to four (>10 missed or late doses).28 The average score was 1.55 (standard deviation = 1.04), representing a number of missed or late doses in the past month between one or two (score of 1) and three or four (score of 2).
Associations between nonadherence and health outcomes
Six studies assessed one or more health outcomes, including molecular response (n = 3), EFS (n = 2), overall survival (OS; n = 2), relapse (n = 1), and fever/infection (n = 1). For both EFS24,25 and OS,25,28 one of two studies examining each of these outcomes found that nonadherent patients were significantly more likely to be deceased than adherent patients (EFS,24 OS28). Two studies including patients with CML found that nonadherent patients were less likely to demonstrate a molecular response to treatment (i.e., imatinib) as assessed by complete cytogenetic response24 and undetectable BCR-ABL fusion gene transcript levels,21 but not as measured by major molecular response.21 In a study of patients with multiple cancer diagnoses, nonadherence to trimethoprim/sulfamethoxazole was related to an increased risk of fever or infection.26 Finally, in a study of 21 patients with ALL or Hodgkin's lymphoma, nonadherence was not significantly related to relapse.16
Predictors of nonadherence
Seven studies included one or more potential predictors of nonadherence (Table 3). No study demonstrated a significant relationship between medication nonadherence and demographic variables (n = 0/6 studies, i.e., age, gender, race, socioeconomic status, religion, educational status, occupational status, and parental educational status), patient cognitive abilities (n = 0/2 studies), family composition (n = 0/2 studies, i.e., number of parents in household and patient marital status), or disease characteristics (n = 0/4 studies, i.e., diagnosis, stage, relapse status, time since diagnosis, and diagnosis awareness).
Table 3.
Predictors of Medication Nonadherence
| Variable | n Significant/n studies | Predictor of nonadherence |
|---|---|---|
| Demographic variables | ||
| Age17,18,20,21,24,28 | 0/6 | |
| Sex or gender17,18,20,21,24,28 | 0/6 | |
| Race20,28 | 0/2 | |
| Socioeconomic status20,21,24,28 | 0/4 | |
| Religion21 | 0/1 | |
| Educational status21 | 0/1 | |
| Occupational status21 | 0/1 | |
| Parent educational status20 | 0/1 | |
| Patient cognitive abilities | ||
| Verbal intelligence17 | 0/1 | |
| Cognitive functioning21 | 0/1 | |
| Family composition | ||
| Patient in single parent household20 | 0/1 | |
| Patient marital status21 | 0/1 | |
| Disease characteristics | ||
| Diagnosis20 | 0/1 | |
| Stage24 | 0/1 | |
| Relapse status20 | 0/1 | |
| Time since diagnosis20,28 | 0/2 | |
| Diagnosis awareness21 | 0/1 | |
| Treatment characteristics | ||
| Days in clinic20,21 | 1/2 | More frequent outpatient clinic visits20 |
| Days inpatient20 | 0/1 | |
| Treatment intensity20 | 0/1 | |
| Treatment complexity17 | 0/1 | |
| Treatment modality20 | 0/1 | |
| Treatment duration17,21 | 0/2 | |
| Treatment awareness21 | 0/1 | |
| Physician continuity17 | 0/1 | |
| Knowledge | ||
| Knowledge of cancer17 | 0/1 | |
| Knowledge of cause17 | 1/1 | Less advanced understanding of causality17 |
| Knowledge of treatment17 | 0/1 | |
| Knowledge of prognosis17 | 1/1 | Less advanced understanding of prognosis17 |
| Beliefs about capabilities | ||
| Self-efficacy20 | 0/1 | |
| Self-esteem28 | 1/1 | Lower self-esteem28 |
| Health locus of control17 | 0/1 | |
| Beliefs about consequences | ||
| Perceived vulnerability: side effects17 | 1/1 | Lower perceived vulnerability to side effects17 |
| Perceived vulnerability: symptoms17 | 1/1 | Lower perceived vulnerability to symptoms17 |
| Impact on daily life21 | 1/1 | Greater impact on daily life21 |
| Role functioning21 | 1/1 | Poorer role functioning21 |
| Body image problems21 | 1/1 | Greater body image problems21 |
| Adjustment28 | 0/1 | |
| Satisfaction with care and information21 | 1/1 | Lower satisfaction with care and information21 |
| Denial17 | 1/1 | More likely to use denial17 |
| Attribution of responsibility19 | 0/1 | |
| Future orientation17,20 | 2/2 | Lower proportion of future-oriented goals20; Less coherent organization of future events17 |
| Environmental context and resources | ||
| Peer social support20 | 0/1 | |
| Social functioning21 | 0/1 | |
| Satisfaction with social life21 | 0/1 | |
| Family incongruence28 | 1/1 | Higher incongruence28 |
| Family social support20 | 1/1 | Lower support from family20 |
| Parent overprotection20 | 1/1 | Less overprotective secondary caregiver20 |
| Parent caring20 | 0/1 | |
| Parental supervision17 | 0/1 | |
| Family functioning20 | 0/1 | |
| Financial difficulties21 | 1/1 | Greater financial difficulties21 |
| Physical functioning21 | 1/1 | Poorer physical functioning21 |
| Symptom burden21 | 1/1 | Greater symptom burden21 |
| Fatigue21 | 1/1 | Greater fatigue21 |
| Nausea and vomiting21 | 1/1 | More nausea and vomiting21 |
| Pain21 | 1/1 | Greater pain21 |
| Dyspnea21 | 1/1 | More dyspnea21 |
| Appetite loss21 | 1/1 | More appetite loss21 |
| Insomnia21 | 0/1 | |
| Constipation21 | 0/1 | |
| Diarrhea21 | 0/1 | |
| Emotion | ||
| Depressive symptoms28 | 1/1 | Greater depressive symptoms28 |
| Emotional functioning21 | 0/1 | |
| Affect20 | 0/1 | |
| Impact on worry/mood21 | 1/1 | Greater impact on worry/mood21 |
Four of seven studies identified statistically significant predictors of medication nonadherence. In a study of adolescents with ALL or Hodgkin's lymphoma, nonadherent adolescents demonstrated a less advanced understanding of the causality of their cancer diagnosis (t = 2.57, p < 0.02) and prognosis (t = 2.56, p < 0.02), lower perceived vulnerability to side effects (p < 0.005) and symptoms (p < 0.02), a more frequent use of denial (t = 4.68, p < 0.001), and a less coherent organization of future events (t = 2.54, p < 0.02) compared to adherent adolescents.17 Future orientation was also related to medication adherence in a study of 103 adolescents with cancer.20 In this study, nonadherent adolescents demonstrated a lower proportion of future-oriented goals (t = 2.39, p = 0.02) than adherent adolescents. In this same study, nonadherent patients had more frequent outpatient clinic visits (t = −2.12, p = 0.04),20 reported less support from their family (t = 2.33, p = 0.02), and described their secondary caregiver as less overprotective (t = 2.19, p = 0.04) than adherent adolescents.20 Kennard et al. also demonstrated a relationship between family functioning and nonadherence, with lower levels of agreement between AYAs and their parents regarding their home environment correlated with greater nonadherence (r = 0.39, p < 0.05).28 In addition to family incongruence, nonadherence in this sample of 44 adolescents with cancer was also correlated with greater depressive symptoms (r = 0.39, p < 0.05) and lower self-esteem (r = −0.30, p < 0.05).28 The final study to report significant predictors found that nonadherent young adults with CML reported poorer physical functioning (p = 0.006), greater symptom burden (p < 0.001), more physical symptoms (i.e., fatigue, nausea and vomiting, pain, dyspnea, and appetite loss, p = 0.002–0.028), lower satisfaction with care and information (p < 0.001), a greater impact of cancer on their life (i.e., impact on worry/mood, impact on daily life, poorer role functioning, and body image problems, p = 0.001–0.038), and more financial difficulties as a result of their cancer diagnosis (p = 0.021) than adherent young adults.21
Discussion
This systematic review identified 12 studies assessing the prevalence of medication nonadherence among AYAs with cancer. Rates of medication nonadherence ranged from 21% to 60%, with the wide range potentially due to measurement variation. Rates derived from more objective and/or psychometrically sound methods, including validated self-report measures (45%–60%), drug levels (27%–52%), or electronic monitors (39%–40%), appear to be higher than those obtained from measures of nonadherence developed for the specific study (21%–29%). Particularly, when considering rates obtained from validated and objective measures (27%–60%), findings suggest that rates of nonadherence in AYAs with cancer are comparable to or higher than those in children (10%–42%)29 and older adults (0%–54%)30,31 with cancer. These results support the previously proposed hypothesis that AYAs may be at particular risk for nonadherence.4,5 Of the six studies assessing health outcomes, four found that nonadherence to medications in cancer treatment protocols may place AYAs at an increased risk for death (n = 2/3 studies24,25,28), fever/infection (n = 1/1 study26), and/or inadequate molecular response (n = 2/2 studies24,21).
Given the rates of nonadherence and potential link to health outcomes among AYAs with cancer, understanding how best to improve adherence is an important next step to informing clinical efforts. Seven of the 12 included studies examined predictors of nonadherence. Despite the small number of studies and the fact that each study included a different set of predictors, findings regarding the relationship between demographic, family composition, and disease characteristic variables and nonadherence are notably consistent. No study found a significant relationship between variables falling into any of these categories and nonadherence. Based on the literature to date, it is unlikely that providers will be able to accurately predict which of their patients are going to be nonadherent based on factors like age, race, socioeconomic status, education, family composition, or disease alone. Instead, this finding supports the standard of care proposed in pediatrics and endorsed by numerous professional organizations (i.e., COG and APHON) that nonadherence should be routinely assessed and monitored throughout cancer treatment in all AYAs.32 In addition, as therapeutic development efforts continue, adherence should be assessed using validated methods such as standardized self-report measures33 or electronic adherence monitoring devices34 to enable trialists to control for any effects of medication-taking behavior on trial outcomes.
Of the psychosocial predictors examined, knowledge, beliefs about capabilities (i.e., self-esteem), beliefs about consequences (i.e., perceived vulnerability), environmental context and resources (i.e., family functioning, social support, and physical symptoms/functioning), and emotion predicted nonadherence in one or more studies. Despite the relatively small number of studies supporting these predictors, it is notable that these constructs align with previously proposed theoretical models of medication adherence behavior.12,14,35 Given their empirical and theoretical support and the relatively low effect size of the only adherence-promotion intervention for AYAs with cancer tested by a randomized clinical trial,11 an important next step for the field may include adherence-promotion interventions designed to target these predictors. These findings also provide further support for promoting psychological well-being among AYAs with cancer more broadly as interventions targeting difficulties like depression and low self-esteem may have the added benefit of subsequently improving medication adherence.
The findings of this review illuminate several directions for future research. A relatively small number of studies with varied risk of bias met inclusion criteria. In these studies, each used a different measurement strategy and/or cut point to assess nonadherence. These limitations should be considered when interpreting the results of this review and highlight the need for additional research to broaden this literature base. As this research is conducted, researchers should consider the investigation of clinically relevant adherence cut points for specific medications and diseases using more objective methods of adherence measurement.36 Combining adherence electronic monitoring data with health outcome data over several years, Bhatia et al.37,38 identified clinically relevant adherence cut points in pediatric ALL. Similar research with AYAs would address two major limitations of this review by facilitating comparisons across studies and enabling a meta-analysis of the association between nonadherence and health outcomes. In addition, the identification of adherence cut points would provide clinically meaningful endpoints for future clinical trials aiming to improve adherence behavior.
In sum, this growing body of literature suggests that approximately one-third to one-half of AYAs with cancer are nonadherent to oral medications included in cancer treatment protocols. One method of reducing the risk of relapse, fever/infection, and/or mortality among AYAs with cancer may be to routinely assess medication adherence among all patients. Additional research is needed to determine the most effective methods of improving nonadherence in this population and address the limitations of the existing literature.
Acknowledgments
Gabriella A. Breen, BS, is gratefully acknowledged for her assistance with data extraction and article formatting. M.E.M. is supported by the NCI of the National Institutes of Health under Award Number K07CA200668. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Adolescents and young adults with cancer. National Cancer Institute at the National Institutes of Health; 2018. Accessed December13, 2018 from: https://www.cancer.gov/types/aya
- 2. 10 Leading causes of death by age group United States–2010. Office of Statistics and Programming, National Center for Injury Prevention and Control, Centers for Disease Control; 2010. Accessed December13, 2018 from: www.cdc.gov/injury/wisqars/pdf/10lcid_all_deaths_by_age_group_2010-a.pdf
- 3. Smits-Seemann RR, Pettit J, Li H, et al. Infection-related mortality in Hispanic and non-Hispanic children with cancer. Pediatr Blood Cancer. 2017;64(9):e26502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barr RD, Ferrari A, Ries L, et al. Cancer in adolescents and young adults: a narrative review of the current status and a view of the future. JAMA Pediatr. 2016;170(5):495–501 [DOI] [PubMed] [Google Scholar]
- 5. Butow P, Palmer S, Pai A, et al. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28(32):4800–9 [DOI] [PubMed] [Google Scholar]
- 6. Kondryn HJ, Edmondson CL, Hill J, Eden TO. Treatment non-adherence in teenage and young adult patients with cancer. Lancet Oncol. 2011;12(1):100–8 [DOI] [PubMed] [Google Scholar]
- 7. Sabate E. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003 [Google Scholar]
- 8. Sun J, Wei Q, Zhou Y, et al. A systematic analysis of FDA-approved anticancer drugs. BMC Syst Biol. 2017;11(Suppl 5):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinch MS. An analysis of FDA-approved drugs for oncology. Drug Discov Today. 2014;19(12):1831–5 [DOI] [PubMed] [Google Scholar]
- 10. FDA approved drugs for oncology. Centerwatch; 2018. Accessed December13, 2018 from: www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology
- 11. Kato PM, Cole SW, Bradlyn AS, Pollock BH. A video game improves behavioral outcomes in adolescents and young adults with cancer: a randomized trial. Pediatrics. 2008;122(2):e305–17 [DOI] [PubMed] [Google Scholar]
- 12. Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tilson HH. Adherence or compliance? Changes in terminology. Ann Pharmacother. 2004;38(1):161–2 [DOI] [PubMed] [Google Scholar]
- 14. Allemann SS, Nieuwlaat R, van den Bernt BJF, et al. Matching adherence interventions to patient determinants using the Theoretical Domains Framework. Front Pharmacol. 2016;7:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Study quality assessment tools. National Heart Lung and Blood Institute of the National Institutes of Health. Accessed December13, 2018 from: www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 16. Festa RS, Tamaroff MH, Chasalow F, Lanzkowsky P. Therapeutic adherence to oral medication regimens by adolescents with cancer. I. Laboratory assessment. J Pediatr. 1992;120(5):807–11 [DOI] [PubMed] [Google Scholar]
- 17. Tamaroff MH, Festa RS, Adesman AR, Walco GA. Therapeutic adherence to oral medication regimens by adolescents with cancer. II. Clinical and psychologic correlates. J Pediatr. 1992;120(5):812–7 [DOI] [PubMed] [Google Scholar]
- 18. Pai ALH, Drotar D, Kodish E. Correspondence between objective and subjective reports of adherence among adolescents with acute lymphoblastic leukemia. Child Health Care. 2008;37(3):225–35 [Google Scholar]
- 19. Tebbi CK, Zevon MA, Richards ME, Cummings KM. Attributions of responsibility in adolescent cancer patients and their parents. J Cancer Educ. 1989;4(2):135–42 [DOI] [PubMed] [Google Scholar]
- 20. Hullmann SE, Brumley LD, Schwartz LA. Medical and psychosocial associates of nonadherence in adolescents with cancer. J Pediatr Oncol Nurs. 2015;32(2):103–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Unnikrishnan R, Veeraiah S, Mani S, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk. 2016;16(6):366–71.e363 [DOI] [PubMed] [Google Scholar]
- 22. Lau RC, Matsui D, Greenberg M, Koren G. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30(2):85–90 [DOI] [PubMed] [Google Scholar]
- 23. Linder LA, Wu YP, Macpherson CF, et al. Oral medication adherence among adolescents and young adults with cancer before and following use of a smartphone-based medication reminder app. J Adolesc Young Adult Oncol. 2019;8(2):122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86(6):471–4 [DOI] [PubMed] [Google Scholar]
- 25. Jaime-Pérez JC, Jiménez-Castillo RA, Pinzón-Uresti MA, et al. Real-world outcomes of treatment for acute lymphoblastic leukemia during adolescence in a financially restricted environment: results at a single center in Latin America. Pediatr Blood Cancer. 2017;64(7):e26396. [DOI] [PubMed] [Google Scholar]
- 26. Pizzo PA, Robichaud KJ, Edwards BK, et al. Oral antibiotic prophylaxis in patients with cancer: a double-blind randomized placebo-controlled trial. J Pediatr. 1983;102(1):125–33 [DOI] [PubMed] [Google Scholar]
- 27. Wu YP, Stenehjem DD, Linder LA, et al. Adherence to oral medications during maintenance therapy among children and adolescents with acute lymphoblastic leukemia: a medication refill analysis. J Pediatr Oncol Nurs. 2018;35(2):86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennard BD, Stewart SM, Olvera R, et al. Nonadherence in adolescent oncology patients: preliminary data on psychological risk factors and relationships to outcome. J Clin Psychol Med Settings. 2004;11(1):30–9 [Google Scholar]
- 29. Davies HA, Lilleyman JS. Compliance with oral chemotherapy in childhood lymphoblastic leukaemia. Cancer Treat Rev. 1995;21(2):93–103 [DOI] [PubMed] [Google Scholar]
- 30. Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016;21(3):354–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall AE, Paul C, Bryant J, et al. To adhere or not to adhere: rates and reasons of medication adherence in hematological cancer patients. Crit Rev Oncol Hematol. 2016;97:247–62 [DOI] [PubMed] [Google Scholar]
- 32. Pai ALH, McGrady ME. Assessing medication adherence as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62(Suppl 5):S818–28 [DOI] [PubMed] [Google Scholar]
- 33. Zelikovsky N, Schast AP. Eliciting accurate reports of adherence in a clinical interview: development of the Medical Adherence Measure. Pediatr Nurs. 2008;34(2):141–6 [PubMed] [Google Scholar]
- 34. McGrady ME, Holbein CE, Smith AW, et al. An independent evaluation of the accuracy and usability of electronic adherence monitoring devices. Ann Intern Med. 2018;169(6):419–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGrady ME, Brown GA, Pai AL. Medication adherence decision-making among adolescents and young adults with cancer. Eur J Oncol Nurs. 2016;20:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quittner AL, Modi AC, Lemanek KL, et al. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. 2008;33(9):916–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2014;124(15):2345–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in hispanic and non-hispanic white children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(17):2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]