Abstract
Gait evaluation after spinal cord injury (SCI) is an important component of determining functional status. Analysis of center of pressure (COP) provides a dynamic reflection of global locomotion and postural control and has been used to quantify various gait abnormalities. We hypothesized that COP variability would be greater for SCI versus normal dogs and that COP would be able to differentiate varying injury severity. Our objective was to investigate COP, COP variability, and body weight support percentage in dogs with chronic SCI. Eleven chronically non-ambulatory dogs after acute severe thoracolumbar SCI were enrolled. COP measurements in x (right-to-left, COPx) and y (craniocaudal, COPy) directions were captured while dogs walked on a pressure-sensitive treadmill with pelvic limb sling support. Root mean square values (RMS_COPx and RMS_COPy) were calculated to assess variability in COP. Body weight support percentage was measured using a load cell. Gait also was quantified using an open field scale (OFS) and treadmill-based stepping and coordination scores (SS, RI). Mean COPx, COPy, RMS_COPx, and RMS_COPy were compared between dogs with SCI and previously evaluated healthy controls. RMS measurements and support percentage were compared with standard gait scales (OFS, SS, RI). Mean COPy was more cranial and RMS_COPx and RMS_COPy were greater in SCI versus normal dogs (p < 0.001). Support percentage moderately correlated with SS (p = 0.019; R2 = 0.47). COP analysis and body weight support measurements offer information about post-injury locomotion. Further development is needed before consideration as an outcome measure to complement validated gait analysis methods in dogs with SCI.
Keywords: center of pressure; chronic spinal cord injury; dogs, locomotion; postural control
Introduction
Dogs frequently suffer from acute spinal cord injury (SCI) and those with severe injury may be left with chronic motor impairment.1–3 Gait analysis is an important component of neurologic evaluation and determination of functional status after SCI and allows investigation of injury and recovery mechanisms related to locomotor function.
Frankel and colleagues established a classification system separating human SCI into five grades from “complete” (A), “sensory only” (B), “motor useless” (C), “motor useful” (D), and “recovery” (E).4 This classification scheme has been variably adapted for use in veterinary medicine as the modified Frankel scale.5,6 Additional, more comprehensive ordinal gait scales have been developed for use in dogs including the Texas Spinal Cord Injury Score (TSCIS), the open field scale (OFS) and the canine Basso, Beattie, Bresnahan (cBBB) locomotor scale.5,7–9 The TSCIS gait scale incorporates proprioceptive placing and pain perception into the scoring system and can be applied to dogs with two or four limbs affected by injury as well as those with asymmetric dysfunction.5 The OFS gives a score ranging from paraplegia to normal pelvic limb motor function that subdivides recovery into stages depending on presence and frequency of different functions.7,8 The cBBB was adapted for dogs from the Basso, Beattie, Bresnahan locomotor scale used in experimental rodent models of SCI.9,10 The cBBB incorporates thoracic and pelvic limb coordination but is otherwise similar to the OFS. All of these scales offer functionally relevant information, are simple to perform, are reliable and do not require special equipment or extensive training but do not allow the assessment of more subtle locomotion patterns.
Treadmill-based scores also have been developed and validated in dogs with SCI, generating continuous data on pelvic limb stepping and coordination between thoracic and pelvic limbs.11,12 Stepping and coordination scores are calculated relative to normal thoracic limb stepping and a normal step pattern (right thoracic-left pelvic-left thoracic-right pelvic) in dogs, respectively. A walking track gait analysis also has been developed that uses paint applied to dogs' feet to produce footprints on the walking track to evaluate stride length and base of support.13 In addition to alterations in pelvic limb function after injury, this study also demonstrated potential adaptations in trunk and thoracic limb function following incomplete SCI.13 These methods can provide complementary information to the ordinal gait scales, including information not limited to pelvic limb function; however, limitations include the practicality of testing and difficulty in evaluating non-ambulatory dogs.
Gait assessment using pressure sensitive walkways and kinematic analysis also have been performed in dogs with and without SCI.14–19 Parameters of interest have included stride length, stride time and swing time, quantification of lateral paw placement, and quantification of thoracic limb and pelvic limb coordination.14–17 Studies utilizing pressure-sensitive walkways have been limited to dogs that could walk and kinematic studies have focused either on just ambulatory or non-ambulatory dogs, but not a combination of both populations.15–19 Instrumented pressure-sensitive treadmills can be utilized to analyze the center of pressure (COP) and the variability in that COP during ambulation. This method has been used to quantify normal gait and posture as well as gait abnormalities in people and horses.20–25 It has the benefit compared with other available gait analysis methods of providing a global measure of locomotor function and can be adapted for both ambulatory and non-ambulatory dogs.
Our group performed a prior evaluation of COP in neurologically normal chondrodystrophic dogs showed that variability in COP was low and consistent within individual dogs.26 We hypothesized that the COP variability would be greater for dogs with SCI compared with neurologically normal dogs and that COP variability would be able to discern differing motor ability among dogs with SCI. The aim of this study was to use an instrumented pressure-sensitive treadmill to investigate the COP and its variability in dogs with chronic gait deficits after severe acute SCI.
Methods
Case selection
Dogs were recruited prospectively from the patient pool of the Canine Spinal Cord Injury Program at the North Carolina State University (NCSU) College of Veterinary Medicine. All dogs were chronically non-ambulatory with absent or severely reduced pelvic limb and tail pain perception (with or without urinary and fecal incontinence). In all dogs, signs were due to an acute thoracolumbar SCI (third thoracic to third lumbar spinal cord segments) causing paralysis with loss of pain perception suffered a minimum of three months previously. Body weight between 3–30 kg was required to ensure proper measurements could be recorded by the treadmill. Below 3 kg, the dogs' steps would not register and dogs weighing greater than approximately 30 kg had trouble maintaining all four limbs on a single belt of the two-belted instrumented treadmill, which was necessary for accurate data capture. Dogs also were required to be amenable to walking on the treadmill with only verbal encouragement since tactile manipulation can affect measurements. Informed consent was obtained for all animals and examinations were conducted in accordance with the NCSU Institutional Animal Care and Use Committee (protocol #15-004-01).
Standard gait evaluation
All cases underwent a standard gait analysis consisting of walking each dog on a non-slip surface and on a treadmill for approximately 3 min, with the speed adjusted to a comfortable pace for each individual and with sling support provided for the hind quarters. All examinations were videotaped. Gait was quantified using the OFS (ranging from 0–12).7,8 OFS of ≥4 corresponds to taking at least some weight-bearing steps. Treadmill footage was scored to quantify pelvic limb steps with pelvic limb support and used to calculate a stepping score (SS) and coordination score (RI) for comparison to instrumented treadmill data.12
Instrumented treadmill gait evaluation
An instrumented force-plate treadmill (Fully Instrumented Treadmill, Bertec Corporation, Columbus, OH) designed for bipedal/human gait analysis was utilized. The set up consisted of two independent pressure-sensitive belts as well as six cameras with infrared sensors (mx-t020; Vicon) mounted on the ceiling surrounding the treadmill to track the location of specific reflective markers. All dogs were outfitted with reflective markers at the lateral aspect of each carpus and tarsus, as well as one additional marker placed on midline between the scapulae in line with the point of the elbow when the dog was standing at rest (Fig. 1). Elastic tape was used to secure the markers in place without interfering with joint flexion and extension. The interscapular marker was utilized for COP measurements while the additional markers captured kinematic data (data not presented). COP was assessed in the x (lateral) and y (craniocaudal) directions. Refer to Blau and colleagues for more detailed information on COP computations.26
FIG. 1.
(A) Set-up for dogs with spinal cord injury on the instrumented treadmill depicting sling support attached to the load cell and reflective marker placement. (B) Close-up view of load cell.
A standard sling (Walk-a-bout) was used to provide hind-quarter support. Height was adjusted such that the spine was parallel to the ground and the dog was in a biomechanically appropriate stance for locomotion. The handles of the sling were attached to a load cell in order to record the percentage body weight support provided by the sling during testing. Dogs were acclimated to the treadmill for several minutes and then walked at a steady, comfortable pace for approximately 5–10 min or until at least five “good” or “excellent” trials were recorded. Trials were subjectively designated as good to excellent if multiple (more than two) step cycles were recorded with minimal visible variation in thoracic limb gait, with all four limbs contained on a single belt of the treadmill, with all markers visible and no manual intervention by the handler. Anomalous movements such as lunging or stopping or other deviation from a steady stepping (in the thoracic limbs) were grounds to stop an individual trial. A leash was placed loosely around the neck but trials were only counted if the dog was walking willingly in response to verbal encouragement without pulling or re-direction with the leash. Treadmill speed was recorded for each dog. All trials also were videotaped using a digital video camera (HDR-CX580V; Sony) positioned to capture all four limbs of the dogs as they walked as a reference on dog behavior during an individual trial if needed.
All data for each trial in each dog were collected as .c3d files and visually inspected for quality and ability to accurately track marker position throughout the trial. Trials with marker loss were not included in analysis. Data processing consisted of converting .c3d files to .txt files (Visual 3D Software; C-Motion) which were subsequently imported into MATLAB (MATLAB Software, Mathworks) for analysis and calculations using purpose written code (see Supplementary Material: MATLAB Code).
Statistical analysis
All analyses were performed using Jmp 13 Pro (SAS Institute, Cary, NC). COP measurements were captured in the x (right to left, COPx) and y (craniocaudal, COPy) planes for each trial in each dog. COP summary statistics were calculated for each dog (mean and standard deviation), as well as presented collectively as a mean and standard deviation across all dogs. To evaluate the variability of COP, calculations also included the root mean square (RMS) of the COPx and COPy (RMS_COPx and RMS_COPy, respectively) for each trial in each dog. Mean RMS_COPx and mean RMS_COPy and standard deviation were also determined for each dog and collectively for all dogs. The percentage weight support was also calculated for each trial with mean and standard deviation reported for each dog and across all dogs. OFS, SS, RI were each reported as mean and standard deviation or median and range, as appropriate. Associations between age, duration of injury or limb length (greater trochanter to lateral digit) and RMS_COPx or RMS_COPy were investigated using linear regression and an analysis of variance. Limb length (distance from the front foot to intrascapular marker) was noted to be associated with RMS_COP measurements in the previously acquired data in our laboratory in neurologically normal chondrodystrophic dogs using the same protocol on the same treadmill.26 Therefore, a model was constructed incorporating limb length (measured as the distance from the greater trochanter to lateral digit for SCI dogs and from the front foot to the intrascapular marker in a standing position for normal dogs). An analysis of covariance was then performed to compare RMS_COPx or RMS_COPy between the SCI and normal dogs. Agreement between RMS_COP calculations and standard gait measures (OFS, SS, RI) was determined by calculating correlation coefficients. Support percentage was also compared with the standard gait measures by calculating correlation coefficients. For all analyses, p < 0.05 was considered significant.
Results
Seventeen dogs met the initial criteria for inclusion; however, only 11 were enrolled in the instrumented gait analysis evaluation, with six dogs eliminated due to body size, temperament issues, or unwillingness to walk without manual correction or restraint on a standard treadmill. There were four mixed breed dogs, two Dachshunds, and one each of Australian Cattle Dog, Boston Terrier, Miniature Schnauzer, Miniature Poodle, and Shih Tzu. Mean body weight was 10.63 kg (8.7); mean age was 6.1 years (2.3). Median duration of injury was 13.5 months (4 to 84 months). Seven dogs were diagnosed with intervertebral disc herniation and one dog each suffered a fibrocartilaginous embolism, traumatic intervertebral disc herniation, vertebral column fracture, and an adverse inflammatory reaction to an epaxial injection of melarsomine. Median OFS was 1 (0–6), median SS was 0 (0–72), and RI was 0 (0–41.86). The mean number of good to excellent trials per dog was 7.73 (2.3). Treadmill belt speed ranged from 0.3–0.7 mph. Mean sling support percentage of body weight was 23.67% (5.67). All dogs walked willingly on the treadmill after an acclimation period of several minutes and in response to verbal encouragement.
Mean COPx, COPy, RMS_COPx, and RMS_COPy values are presented in Table 1 with corresponding mean COP and RMS values for 11 neurologically normal chondrodystrophic dogs included for comparison.26 Individual data for the SCI dogs are included in Supplementary Table S1. For the 11 SCI dogs, study specific MATLAB code was used to extract raw data on COP, from which RMS of COP in each direction was calculated. For four dogs, this was done without directly recording the mean COP data whereas in seven dogs, both mean COP and RMS_COP data in each direction was recorded. This discrepancy was noted after the initial data capture; however, irreparable errors in the MATLAB code prevented repeat analysis and capture of mean COP values for these four dogs. This resulted in RMS_COP data for all 11 SCI dogs but mean COP data available in only seven of the 11 dogs.
Table 1.
Summary Data for Mean and Standard Deviation COPx, COPy, RMS_COPx and RMS_COPy in Dogs with Chronic Motor Deficits Secondary to Acute Severe SCI Compared with Neurologically Normal Chondrodystophic Dogs Previously Evaluated in Our Laboratory
| Variable | SCI dogs(n = 11) | Normal dogs(n = 11) | p value |
|---|---|---|---|
| Mean COPx | −0.89cm (0.31) (n = 7) |
−0.34 cm (0.12) | 0.51 |
| Mean COPy | −3.58cm (0.39) (n = 7) |
−8.51cm (5.32) | < 0.001 |
| Mean RMS_COPx | 0.0292 (0.01) (n = 11) |
0.0138 (0.0047) | < 0.001 |
| Mean RMS_COPy | 0.0291 (0.007) (n = 11) |
0.0185 (0.0071) | < 0.001 |
Data for both groups was acquired using the same treadmill and protocol.26
p < 0.05 is significant.
COP, center of pressure in the x (left-right) or y (craniocaudal) directions; RMS, root mean square; SCI: spinal cord injury.
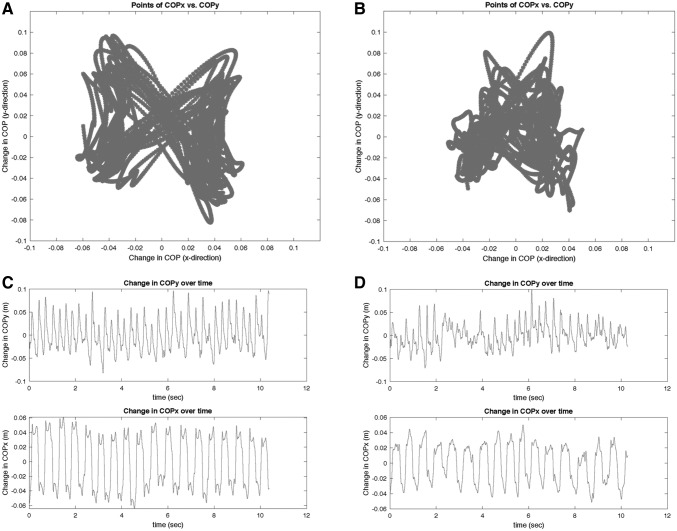
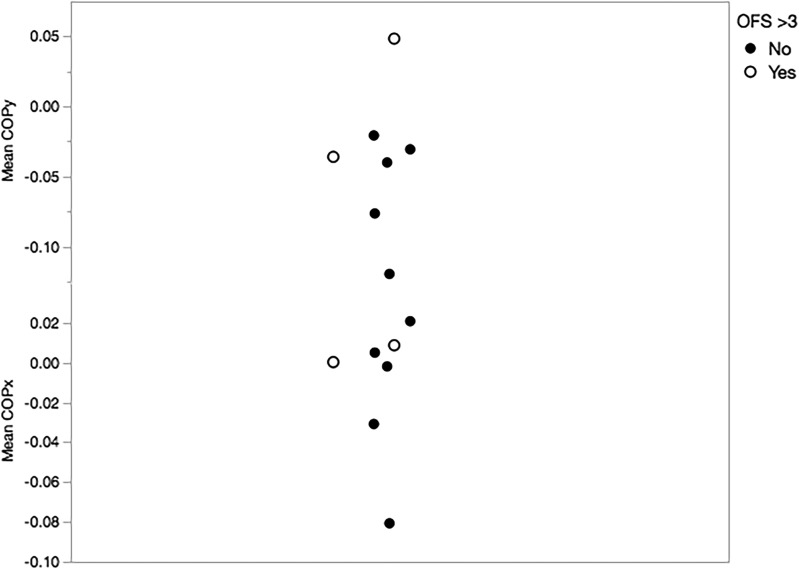
Across all dogs in whom data were available (n = 7), mean COP position relative to the interscapular reference marker was just left of midline in the x-direction and caudal to the intrascapular reference marker in the y-direction (Table 1). Representative traces depicting the change in COP in the X and Y directions relative to the interscapular marker during walking for a given trial are shown for two dogs of different levels of pelvic limb function in Figure 2A and 2B. The change in COPx and COPy over time for a single trial is shown for the same two dogs in Figure 2C and 2D. Mean COPx was not statistically different between SCI dogs and the cohort of normal dogs using the same acquisition protocol (p = 0.51). Mean COPy was located significantly more cranial in SCI dogs compared with the normal cohort (p < 0.001). Since changes to COP while walking on a treadmill might vary between dogs with different degrees of motor impairment, COP values in the x and y directions are also shown for dogs grouped by pelvic limb function (using the OFS scores; Fig. 3). Mean COPy was more cranial in dogs with OFS >3 (n = 2) compared with dogs with OFS 0–3 (n = 5), but small numbers precluded making meaningful statistical comparisons.
FIG. 2.
Representative traces depicting the change in center of pressure (COP) in the x and y directions relative to the interscapular marker during walking in a paraplegic dog (A) and a non-ambulatory paraparetic dog (B). The change in COPx and COPy over time for the same two dogs during the same trials is shown in (C) and (D), respectively.
FIG. 3.
Scatterplot of mean center of pressure (COP)x and mean COPy across trials for each dog measured in meters relative to the interscapular marker. Closed circles correspond to five dogs with open field scores 0–3, while open circles correspond to two dogs with open field scores >3. Scores of 4 and above reflect dogs who can take at least some weight bearing steps.
Age and duration of injury were not associated with RMS_COPx or RMS_COPy (p > 0.067). Due to previous work showing a relationship between limb length and RMS values in normal dogs, limb length was incorporated into the model to compare variability data between SCI and normal dogs. Accounting for limb length, RMS_COPx and RMS_COPy were each different between the normal dogs and dogs with SCI (p < 0.001). There was greater variability in both the x and y directions in dogs with SCI (Table 1). RMS_COPx and RMS_COPy were each poorly correlated with validated measures of pelvic limb motor function (OFS, SS, RI; R2 < 0.15, p > 0.05, all comparisons). Support percentage was poorly correlated with OFS and RI (R2 = 0.14, p = 0.26, and R2 = 0.29, p = 0.09, respectively) but was moderately correlated with SS (R2 = 0.47, p = 0.019).
Discussion
Using a pressure-sensitive instrumented treadmill, we demonstrated that COP and corresponding variability in COP can be evaluated in dogs with chronic motor impairment secondary to prior acute severe SCI. We developed a sling support mechanism that permitted evaluation in non-ambulatory dogs and quantified the amount of weight support provided by the harness. Results showed that the average COP was more cranial and the variability in COP in both the left to right and craniocaudal directions was greater in dogs with SCI compared with historical, neurologically normal control dogs.26 These measures provide global information on locomotion and postural control post-injury and might complement currently available means of specialized gait analysis in clinical trials utilizing dogs as a model of SCI.
COP data provide a global assessment of the pattern and variability during locomotion and our results suggest that this method can discern the chronically impaired following prior, severe injury from neurologically normal canine populations. The COP in healthy dogs walking on a treadmill is characterized by craniocaudal movement twice and left to right movement once each step cycle creating a “butterfly” pattern relative to the inter-scapular reference marker.26 In the SCI dogs, the general butterfly pattern persisted but the RMS of the COP was greater in both directions, reflecting less predictable changes in the COP during ambulation after injury. These findings support greater variability in the movement of the body in dogs with SCI walking with pelvic limb support.
This is consistent with prior studies using pressure sensitive walkway or kinematic analysis in which the variations in several different gait parameters were greater for dogs with thoracolumbar SCI compared with non-neurologic controls.14,17 Similarly, increased COP variability has been demonstrated in stroke patients compared with healthy geriatric controls.24 COP patterns can also differentiate between neuropathic patients with foot drop and healthy controls.21 Since the control group we used for comparisons consisted of chondrodystrophic dogs (because of their high prevalence of acute spinal cord injury secondary to acute intervertebral disc extrusions) and our SCI study population included a variety of chondrodystrophic and non-chondrodystrophic breeds and body sizes, at least some of the difference in the RMS_COPx and RMS_COPy values can be explained by the SCI dogs being a more diverse group including dogs with longer limb length. However, accounting for differences in limb length between the two groups, RMS values in both the x and y directions remained significantly higher in SCI dogs compared with dogs without gait deficits.
The small number of SCI dogs included in this study, all of whom suffered from an initial severe injury, precludes making broad generalizations on the relationship between COP and severity of injury, including the ability of this method to detect differences between normal dogs and a population of more mildly affected SCI dogs. However, we did detect differences in the variability when SCI dogs were segregated into higher and lower functioning populations. Specifically, dogs with greater pelvic limb motor function (i.e., those able to some weight-bearing steps) had increased variability in the x direction whereas they had comparable or slightly less variability in the y direction compared with dogs with worse pelvic limb motor function (i.e., those with minimal to absent pelvic limb movement). Since COP analysis in normal dogs showed low inter-individual variability, the variability noted among the SCI dogs reported here might reflect the impact of severity of impairment on stability and postural control during locomotion.15–17,26
Consistent with this, COP measurements were noted to be useful to measure the severity of gait disturbance among human neuropathic patients.22 However, a contributing factor to these findings might have been the sling support and load cell set up. While marked swinging in any direction or visible changes in speed caused a given trial to be rejected, the effect of the sling and possibly other subtle changes in movement could have contributed to these differences in variability between dogs of differing levels of function. Prior kinematic evaluation of thoracic limb-pelvic limb coordination and lateral paw placement in non-ambulatory dogs with thoracolumbar SCI found no quantifiable impact of weight support but this was tested using an abdominal band in a subset of normal, control dogs.16,17 Moreover, the impact of support on coordination and foot placement is likely to be different to its impact on COP. Evaluation of a larger number of dogs would be useful to further explore the association between the degree of neurologic impairment and COP patterns.
The mean COP in the y direction (craniocaudal) in SCI dogs was located more cranially compared with values in neurologically normal dogs using the same protocol.26 While the two groups were not size or breed matched, this difference likely reflects a compensatory forward loading of weight in dogs with chronic pelvic limb weakness. Interestingly, among dogs with SCI, a greater degree of forward shifting towards the inter-scapular marker (as reflected by a less negative mean COPy) was present in higher functioning compared with lower functioning dogs. The latter finding is based on a small number of dogs; however, it suggests increased forward loading of body weight occurs even among dogs who regain a greater degree of motor ability and can take some weight-bearing steps. It is possible that the less pronounced forward shifting of body weight in dogs with minimal to absent motor function (relative to those with higher OFS scores) could be attributed to the greater degree of sling support provided for their pelvic limbs. This decreased the need to bear more weight on the thoracic limbs and the sling was also connected to the load cell which was attached to the rigid frame of the treadmill above the level of their hips, perhaps skewing body mass more caudally. While the clinical significance of a more cranially located COP in dogs with chronic SCI is not known, increases in thoracic limb weight bearing and other alterations in thoracic limb function after thoracolumbar SCI have been previously demonstrated in rodents and dogs.13–15,27,28
Additionally, in dogs with prior amputation of a thoracic or pelvic limb, it has been suggested that changes in weight distribution and forces applied during locomotion might predispose dogs to future orthopedic abnormalities of the remaining limbs.29–31 It is possible that more cranial body weight distribution in dogs with chronic pelvic limb weakness also could have long-term functional consequences. Quantifying COP values longitudinally in dogs with SCI of varying severity might allow recognition of the degree and timing of development of this forward shifting tendency after injury and provide an objective rehabilitation target. COP measurements have been noted to be beneficial in evaluating the effects of rehabilitation in human patients recovering from stroke.24 With further validation, COP might be useful as an outcome measure in clinical trials exploring the role of rehabilitation or other therapeutic intervention on normalization of weight distribution and locomotor patterns in dogs recovering from SCI.
Despite challenges with interpreting the effect of sling support on COP data, support percentage was significantly associated with one validated gait score, the treadmill-based SS. This suggests that standardized quantification of percentage body weight support provided during ambulation might offer complementary data to pelvic limb gait scoring methods in this population and also be worthy of development as an independent outcome measure. Further refinement of technique for both the sling and load cell mechanism as well as validation amongst a larger number of non-ambulatory dogs with both acute and chronic SCI would be necessary to determine its reliability and reproducibility. It is also currently unknown if this method is capable of tracking changes in weight support over time prior to independent ambulation (such as during an initial recovery period from acute SCI) or in response to a therapeutic intervention.
We determined that COP using instrumented treadmill analysis was feasible as a supplementary means by which to evaluate locomotion in non-ambulatory dogs with chronic SCI; however, there were substantial limitations. Data collection and analysis requires specialized, expensive equipment, is labor intensive, and time consuming. This is further complicated by patient willingness and size limitations (big and small) for dogs using a treadmill designed for human (bipedal) locomotion. This technique also requires relatively clean data and obtaining high quality trials in ataxic and weak animals proved challenging. Further, while using a load cell attached to a commercially available hind end harness provided useful information on support percentage, a more standardized harness and attachment system would facilitate ease of adjustment for dogs of differing body size and ensure greater accuracy and consistency of measurements. Data analysis also requires purpose-designed code, specific software, and expertise limiting the widespread applicability of this technique to evaluate gait.
Overall, our findings demonstrate that COP measurements can be obtained in dogs with chronic deficits after acute severe SCI and might be useful to discern changes in limb and trunk strength, stability, coordination, and weight distribution after injury. This information could provide insight into the plasticity of motor networks and the underlying mechanisms involved in compensation, recovery or a favorable treatment response. Further refinement and adaptation to dogs with severe SCI is needed before this technique can be utilized as a reliable outcome measure in clinical trials to monitor response to a particular therapeutic intervention.
Supplementary Material
Acknowledgments
T32 OD011130—Comparative Medicine and Translational Research Training Program; North Carolina State University Research and Innovation Seed Funding.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Granger N. and Carwardine D. (2014). Acute spinal cord injury tetraplegia and paraplegia in small animals. Vet. Clin. Small Anim. 44, 1131–1156 [DOI] [PubMed] [Google Scholar]
- 2. Olby N.J. (2010). The pathogenesis and treatment of acute spinal cord injuries in dogs. Vet. Clin Small. Anim. 791–807 [DOI] [PubMed] [Google Scholar]
- 3. Olby N.J., Levine J., Harris T., Muñana K., Skeen T., and Sharp N. (2003). Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996–2001). J. Am. Vet. Med. Assoc. 222, 762–769 [DOI] [PubMed] [Google Scholar]
- 4. Frankel H.L., Hancock D.O., Hyslop G., Melzak J., Michaelis S., and Ungar G.H. (1969). The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 7, 179–192 [DOI] [PubMed] [Google Scholar]
- 5. Levine G.J., Levine J.M., Budke C.M., Kerwin S.C., Au J., Vinayak A., Hettlich B.F., and Slater M.R. (2009). Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Vet. Med. 89, 121–127 [DOI] [PubMed] [Google Scholar]
- 6. Van Wie E.Y., Fosgate G.T., Mankin J.M., Jeffery N.D., Kerwin S.C., Levine G.J., Greatting H.H., Chen A.V., Barker A.K., and Levine J.M. (2013). Prospectively recorded versus medical record-derived spinal cord injury scores in dogs with intervertebral disk herniation. J. Vet. Intern. Med. 27, 1273–1277 [DOI] [PubMed] [Google Scholar]
- 7. Olby N.J, De Risio L., Munana K.R., Wosar M.A., Skeen T.M., Sharp N., and Keene B.W. (2001). Development of a functional scoring system in dogs with acute spinal cord injuries. Am. J. Vet. Res. 62, 1624–1628 [DOI] [PubMed] [Google Scholar]
- 8. Olby N.J., Muguet-Chanoit A.C., Lim J.H., Davidian M., Mariani C.L., Freeman A.C., Platt S.R., Humphrey J., Kent M., Giovanella C., Longshore R., Early P.J., and Munana K.R. (2016). A placebo-controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate in dogs with intervertebral disk herniation. J. Vet. Intern. Med. 30, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song R.B., Basso D.M., da Costa R.C., Fisher L.C., Mo X., and Moore S.A. (2016). Adaptation of the Basso-Beattie-Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J. Neurosci. Methods 268, 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 11. Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., and Joosten E.A. (2005). The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma 22, 214–225 [DOI] [PubMed] [Google Scholar]
- 12. Olby N.J., Lim J.H., Babb K., Bach K., Domaracki C., Williams K., Griffith E, Harris T., and Muguet-Chanoit A. (2014). Gait scoring in dogs with thoracolumbar spinal cord injuries when walking on a treadmill. BMC Vet. Res. 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song R.B., Oldach M.S., Basso D.M., da Costa R.C., Fisher L.C., Mo X., and Moore S.A. (2016). A simplified method of walking track analysis to assess short-term locomotor recovery after acute spinal cord injury caused by thoracolumbar intervertebral disc extrusions in dogs. Vet. J. 210, 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon-Evans W.J., Evans R.B., Knap K.E., Hildreth J.M., Pinel C.B., Imhoff D.J., and Conzemius M.G. (2009). Characterization of spatiotemporal gait characteristics in clinically normal dogs and dogs with spinal cord disease. Am. J. Vet. Res. 70, 1444–1449 [DOI] [PubMed] [Google Scholar]
- 15. Gordon-Evans W.J., Evans R.B., and Conzemius M.G. (2009). Accuracy of spatiotemporal variables in gait analysis of neurologic dogs. J. Neurotrauma 26, 1055–1060 [DOI] [PubMed] [Google Scholar]
- 16. Hamilton L., Franklin R., and Jeffery N.D. (2007). Development of a universal measure of quadrupedal forelimb-hindlimb coordination using digital motion capture and computerized analysis. BMC Neurosci. 8, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamilton L., Franklin R., and Jeffery N.D. (2008). Quantification of deficits in lateral paw positioning after spinal cord injury in dogs. BMC Vet. Res. 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foss K., da Costa R.C., Rajala-Schultz P.J., and Allen M.J. (2013). Force plate gait analysis in Doberman Pinchers with and without cervical spondylomyelopathy. J. Vet. Intern. Med. 27, 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foss K., da Costa R.C., and Moore S. (2013). Three-dimensional kinematic gait analysis of Doberman Pinschers with and without cervical spondylomyelopathy. J. Vet. Intern. Med. 27, 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hobbs S.J. and Clayton H.M. (2013). Sagittal plane ground reaction forces, centre of pressure and centre of mass in trotting horses. Vet. J. 198, e14–e19 [DOI] [PubMed] [Google Scholar]
- 21. Jamshidi N., Rostami M., Naiarian S., Menhai M.B., and Saadatnia M. (2010). Differences in center of pressure trajectory between normal and steppage gait. J. Res. Med. Sci 15, 33–40 [PMC free article] [PubMed] [Google Scholar]
- 22. Jamshidi N., Esfahani M.H., Farzad A., and Jamshidi M. (2012). Design and testing of statistical methods to classify the seveity of steppage gait based on center of pressure data. Med. Hypotheses 79, 334–337 [DOI] [PubMed] [Google Scholar]
- 23. Nauwelaerts S., Hobbs S.J., and Back W. (2017). A horse's locomotor signature: COP path determined by individual limb. PLOS One 12, e0167477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roerdink M., De Haart M., Daffertshofer A., Donker S.F., Geurts A., and Beek P.J. (2006). Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp. Brain Res. 174, 256–269 [DOI] [PubMed] [Google Scholar]
- 25. Svoboda Z., Bizovska L., Janura M., Kubonova E., Janurova K., and Vuillerme N. (2017). Variability in spatial temporal gait parameters and center of pressure displacements during gait in elderly fallers and nonfallers: a 6-month prospective study. PLOS One 12, e0171997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blau S.R., Davis L.M., Gorney A.M., Dohse C.S., Williams K.D., Lim J.H., Pfitzner W.G. Laber E., Sawicki G.S., and Olby N.J. (2017). Quantifying center of pressure variability in chondrodystrophic dogs. Vet. J. 226, 26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ballermann M., Tse A.D., Misiaszek J.E., and Fouad K. (2006). Adaptations in the walking pattern of spinal cord injured rats. J. Neurotrauma 23, 897–907 [DOI] [PubMed] [Google Scholar]
- 28. Webb A.A. and Muir G.D. (2002). Compensatory locomotor adjustments of rats with cervical or thoracic spinal cord hemisections. J. Neurotrauma 19, 239–256 [DOI] [PubMed] [Google Scholar]
- 29. Cole G.L. and Millis D. (2017). The effect of limb amputation on standing weight distribution in the remaining three limbs in dogs. Vet. Comp. Orthop. Traumatol. 30, 59–61 [DOI] [PubMed] [Google Scholar]
- 30. Fuchs A., Goldner B., Nolte I., and Schilling N. (2014). Ground reaction force adaptations to tripedal locomotion in dogs. Vet. J. 201, 307–315 [DOI] [PubMed] [Google Scholar]
- 31. Goldner B., Fuchs A., Nolte I., and Schilling N. (2015). Kinematic adaptations to tripedal locomotion in dogs. Vet. J. 204, 192–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.