Supplemental Digital Content is available in the text
Keywords: injectable diacetylmorphine, injectable hydromorphone, injectable opioid agonist treatment, opioid induction protocol, opioid overdose
Objectives:
The present study aims to describe a 3-day induction protocol for injectable hydromorphone (HDM) and diacetylmorphine (DAM) used in 3 Canadian studies and examine rates of opioid-related overdose and somnolence during this induction phase.
Methods:
The induction protocol and associated data on opioid-related overdose and somnolence are derived from 2 clinical trials and one cohort study conducted in Vancouver and Montreal (2005–2008; 2011–2014; 2014–2018). In this analysis, using the Medical Dictionary for Regulatory Activities coding system we report somnolence (ie, drowsiness, sleepiness, grogginess) and opioid overdose as adverse events. Overdoses requiring intervention with naloxone are coded as severe adverse events.
Results:
Data from the 3 studies provides a total of 1175 induction injections days, with 700 induction injection days for DAM, and 475 induction injection days for HDM. There were 34 related somnolence and adverse event (AE) overdoses (4.899 per 100 injection days) in DAM and 6 (1.467 per 100 days) in HDM. Four opioid overdoses requiring naloxone (0.571 per 100 injection days) were registered in DAM and 1 in HDM (0.211 per 100 injection days), all safely mitigated onsite. The first week maximum daily dose patients received were on average 433.62 mg [standard deviation (SD) = 137.92] and 223.26 mg (SD = 68.06) for DAM and HDM, respectively.
Conclusions:
A 3-day induction protocol allowed patients to safely reach high doses of injectable hydromorphone and diacetylmorphine in a timely manner. These findings suggest that safety is not an evidence-based barrier to the implementation of treatment with injectable hydromorphone and diacetylmorphine.
Opioid use disorder (OUD) continues to be a major public health concern, exacerbated by the present opioid overdose crisis. In 2017, there were 3987 apparent opioid-related deaths in Canada corresponding to a death rate of 10.9 per 100,000 (Health Canada, 2018). Reaching and treating people with OUD is a fundamental cornerstone to managing this crisis. Over time, the low effectiveness of abstinence-based therapies (De Jong et al., 2007; Bruneau et al., 2018) has led to the recommendation that opioid agonist treatment (OAT) be the mainstay of treatment for opioid use disorder (WHO and UNODC, 2004). Long-acting oral opioids such as methadone are very effective at retaining people in care and reducing major risks associated with untreated OUD (Mattick et al., 2009; Beck et al., 2014; Mattick et al., 2014). However, those that are not attracted into OAT or who continue to use illicit opioids remain at very high risk of lethal and non-lethal harm (Wei et al., 2013; Perreault et al., 2015; Franklyn et al., 2017).
Research has shown that injectable opioid agonist treatment (iOAT) with injectable diacetylmorphine (pharmaceutical-grade heroin; DAM) or hydromorphone (a licensed opioid analgesic; HDM) is effective at engaging and retaining such individuals in care, leading to multiple improvements in physical, mental, and social health (Oviedo-Joekes et al., 2009; Strang et al., 2015; Oviedo-Joekes et al., 2016a). The rationale is that these medications can attract and retain people not currently reached by the health care system, providing structured care which improves health, while offering supervision in dedicated settings to ensure the safety of the patients (eg, by treating overdoses) (Bell, 2014). Based on the positive evidence from 8 randomized clinical trials (RCT), iOAT is currently offered in a limited number of dedicated settings in Europe and Canada under the supervision and care of health care providers (Perneger et al., 1998; van den Brink et al., 2003; March et al., 2006; Haasen et al., 2007; Oviedo-Joekes et al., 2009; Strang et al., 2010; Demaret et al., 2015; Oviedo-Joekes et al., 2016a). Expanding treatment options in these ways to better meet the diverse needs of people with OUD at different stages in their recovery process should be part of the public health response to the opioid crisis (Schottenfeld and O’Malley, 2016).
With respect to safety, injectable diacetylmorphine and hydromorphone are short-acting full mu opioid agonists whose major metabolites have high opioid agonist affinity and reach peak plasma levels immediately after injection (Vallner et al., 1981; Gyr et al., 2000). As such, they carry a higher intrinsic risk of overdose upon intake compared to oral formulations. However, since in iOAT settings these opioids are individually dosed and monitored, their related opioid overdoses and other side effects can be safely mitigated and treated by health care providers onsite (Strang et al., 2015; Oviedo-Joekes et al., 2017). This is particularly relevant during the induction phase that carries most of the risk of overdose when starting patients on OAT. For example, among patients in methadone treatment, most methadone-associated deaths have occurred during the induction phase (Baxter et al., 2013).
The need to reach adequate doses in a timely manner to engage and retain people in treatment while preventing fatal overdoses, makes induction of OAT a particularly critical phase. To our knowledge, there are no studies that present and discuss the induction phase of injectable diacetylmorphine and hydromorphone treatment within an iOAT setting. The present study aims to describe the induction protocol used in 3 Canadian studies and examine the rates of opioid-related overdose and somnolence events during the induction phase.
METHODS
Setting and Participants
The present analysis involves data from 3 studies: (1) the North American Opiate Medication Initiative (NAOMI); (2) the Study to Assess Longer Term Opioid Medication Effectiveness (SALOME); and (3) Research on the Utilization of Therapeutic Hydromorphone (RUTH). NAOMI was an RCT comparing injectable DAM with oral methadone (2005–2008); SALOME was an RCT testing the non-inferiority of injectable HDM compared to injectable DAM (2011–2014); and RUTH was an open-label observational cohort study of individuals who received injectable HDM or DAM (2014–2018) after the trials ended. The 3 studies in this analysis were conducted at the Providence Health Care Crosstown Clinic in the Downtown Eastside of Vancouver. NAOMI also involved a site in Montreal at the Centre Hospitalier de l’Université de Montréal and at the Centre de Recherche et d’Aide aux Narcomanes (CRAN).
Participants with long-term injection of street opioids that were not benefitting sufficiently from available treatments (including oral methadone and buprenorphine), and with an OUD diagnosis, were included in the studies. Full description of recruitment, inclusion criteria, and participant profiles for the NAOMI and SALOME trials can be found elsewhere (Oviedo-Joekes et al., 2008; Oviedo-Joekes et al., 2009; Oviedo-Joekes et al., 2015a, 2015b; Oviedo-Joekes et al., 2016a). In this analysis, RUTH participants are mostly former SALOME participants that transitioned to open-label iOAT after the trial (n = 150). New patients admitted (N = 27 entered this analysis) also had long-term injection opioid use and confirmed opioid use disorder.
All participants provided written informed consent before data collection, and in the case of the NAOMI and SALOME clinical trials, before the administration of any medication. The Providence Health Care/University of British Columbia Research Ethics Board approved all studies. The NAOMI study was additionally approved by the institutional review board at the Centre Hospitalier de l’ Université de Montréal.
Treatment
Injectable medications were self-administered daily under the supervision of registered nurses (RNs). Participants underwent pre- and post-injection assessment periods, lasting 5 to 15, and 15 to 30 minutes, respectively; they could be in the injection room for up to 7 minutes. During these periods, RNs monitored participants to ensure their safety both before (eg, no signs of intoxication from opioids, alcohol or benzodiazepines), during, and after self-administration of the medications (eg, no signs of drowsiness, respiratory depression). More details on the delivery of treatment can be found elsewhere (Oviedo-Joekes et al., 2009; Oviedo-Joekes et al., 2016a, 2016c, Oviedo-Joekes et al., 2017).
During the trials, diacetylmorphine and hydromorphone were prescribed in “diacetylmorphine equivalent” doses to maintain the double-blind. In NAOMI, we used a DAM:HDM ratio of 3:1, and adjusted this to 2:1 in SALOME based on our findings (Oviedo-Joekes et al., 2011). In the RUTH study, medications were prescribed open-label. In the 3 studies, doses were individualized and adjusted to each patient's needs until a safe and effective dose was reached.
Induction Protocol
The induction protocol used in the Canadian studies was adapted from the diacetylmorphine programs pioneered in Switzerland in the early 1990s where doses were safely increased under observation, avoiding over and under dosing (Seidenberg and Honegger, 1998; Office Fédéral de la Santé Publique, 2004). Initial induction doses were determined over a 3-day period by the attending physician (Table 1). During the induction phase, nurses assessed and recorded patients’ dose tolerance and adjusted accordingly with active input from the patient and the physician. Each day, patients attended 3 sessions spaced at least 3 hours apart. Doses were split within each session, so the patients’ response to the medication (ie, tolerance) could be observed for between 15 and 30 minutes before administering the full dose.
TABLE 1.
Three-Day Induction Protocol for Injectable Hydromorphone and Diacetylmorphine in 3 Canadian Injectable Opioid Agonist Treatment (iOAT) Studies
| Induction Doses for HDM and [DAM]* in Milligrams (mg) |
| Day 1 |
| Dose 1: Give 10 mg [15 mg], wait 15 minutes. If no intoxication, add 15 mg [30 mg] more. Observe for 30 minutes†. If patient is fit to, she can leave and return at next dosing window period, in 3 hours.Dose 2: If earlier doses were well tolerated, give 25 mg [45 mg]. Wait 30 minutes. If no intoxication and patient so wishes, give 15 mg [30 mg] more. Observe for 30 minutes. Have patient leave and return at next dosing window period.Dose 3: If earlier doses were well tolerated, give 40 mg [75 mg]. Wait 30 minutes. If no intoxication and patient so wishes, give 15 mg [30 mg] more. Observe for 30 minutes. Patient then leaves is fit, and returns next day. |
| Day 2 |
| Dose 1: Administer 40% of the total daily dose at Day 1 (up to a total of 45 mg [90 mg] if tolerated all possible doses on first day) Wait 30 minutes. If no intoxication and patient so wishes, give 15 mg [30 mg] more. Observe for 30 minutes. If patient is fit to leave can do so and return at next dosing window period.Dose 2: Administer the maximum tolerated amount of Dose 1 (ie, up to 60 mg [120 mg]). Wait 30 minutes. If no intoxication and patient so wishes, give 15 mg [30 mg] more. Observe for 30 minutes. If patient is fit to leave can do so and return at next dosing window period.Dose 3: Administer the maximum tolerated amount of Dose 2 (ie, up to 75 mg [150 mg]). Wait 30 minutes. If no intoxication and patient so wishes, give 15 mg [30 mg] more. Observe for 30 minutes. If patient is fit to leave can do so and return at next dosing window period. |
| Day 3 |
| Administer the maximum tolerated amount at Day 2 (ie, up to 90 mg [180mg]) for the 3 doses on Day 3c. |
| Stabilization phase |
| On day 4 (after induction), patients continue to receive the maximum tolerated amount at Day 3 for the 3 doses. After consulting with the physician, adjust the dosage once a week until the patient feels comfortable and does not show any excessive intoxication or respiratory depression or until the maximum dose is reached (200 mg [400 mg]/dose or 500 mg [1000 mg]/day). |
| Maintenance phase |
| Doses can be adjusted upon discussing options between patient and physicians. Nurses can lower a patient's dose in any given session if there is a safety concern. |
DAM, diacetylmorphine; HDM, hydromorphone. Induction doses are presented for HDM and for DAM in brackets.
*During the NAOMI and SALOME clinical trials, medications were provided in DAM equivalents. HDM to DAM ration is 1:2. For open-label HDM an approximate ratio is used, with adjustments made where suited (eg, For day 1 dose 1 give 10 mg, rather than 7.5 mg of HDM as it is easier to dose).
†This observation time can be reduced to 20 min, as it is currently in the Crosstown Clinic protocols, since peak plasma levels are reached immediately after injection (See Appendix for induction protocols currently being used).
cIt is possible that some patients might need higher doses. In the first session, and after waiting 20–30 minutes, patients may be given 15 mg [30 mg] more if the patient wishes and there is no intoxication. This dose would then be given for the remaining doses.
An accelerated protocol for HDM is in place at Crosstown Clinic in Vancouver, adding +20 mg per session to cope with patients’ high opioid tolerance due to the use of potent street opioids like fentanyl. On the third day of the 3-day induction period on the accelerated protocol patients can reach a dose of up 130 mg 3 times daily, as compared to 90 mg on the regular protocol. The maximum daily dose for each day is calculated by summing each of the 3 daily doses.
Successful induction requires 6 separate injections on day 1 and on day 2. Injections could be either intravenous or intramuscular. Injection sites were often rotated for those injecting intramuscularly, given repeated injection at the same site could be painful. Those injecting intravenously may or may not rotate injection sites. Both injection site and rotation of sites was determined by patient preference.
At any time during induction, a physician or nurse could order a lower dose or more gradual induction based on the patient's response and safety concerns. In order to adjust to individual needs, the patient could also request a lower dose or a more gradual induction process. Also, during the induction phase, the nursing staff checked for persistent withdrawal symptoms to determine whether a higher dose was required.
If a patient did not tolerate a dose, their next dose was reduced to their last tolerated dose. From there, doses were increased as tolerated following the protocol and adjusted using clinical judgement (eg, considering concurrent conditions, daily events, etc) and conversation with the patient to ensure the patient reached a safe and effective dose.
In cases where a patient missed a dose or a day of medication in the induction phase, the procedure was restarted from the last dose received and tolerated at the clinic. If more than one day was missed the induction procedure was restarted from the beginning and adjusted per physician order and using clinical judgment (more details on managing missing days or doses during the induction phase can be found in the Appendix, Supplemental Digital Content 1). For patients already on oral OAT, or for those switching between injectable medications (eg, from DAM to HDM), conversion tables (Office Fédéral de la Santé Publique, 2004; Oviedo-Joekes et al., 2011) and clinical judgment as per current medical consensus (Baxter et al., 2013) were used to prevent complications and engage patients in treatment.
Accelerated Induction Protocol
On October 30, 2017, the Crosstown Clinic adopted an accelerated induction protocol in response to patients’ higher opioid tolerance given exposure to more potent street opioids like fentanyl. The accelerated 3-day hydromorphone induction protocol increased doses such that the maximum individual dose reached on the third day of induction was 130 mg compared to 90 mg in the standard protocol. The first dose was also higher, 20 mg compared to 10 (or 7.5 mg) as patients with fentanyl exposure were reporting the lower dose was not adequate to relieve withdrawal symptoms (see Appendix, Supplemental Digital Content 1).
Collection of Related Somnolence and Overdose
In both RCTs, patients were assessed for adverse events (AEs) and severe adverse events (SAEs), such as drug reactions or change in health status by nurses, coordinators, physicians, and other clinic workers. AEs and SAEs were classified by a clinical or research staff (eg, nurse, physician; SAEs were reviewed by clinical team and medical monitor or physician lead) as unrelated to the treatment or either possibly, probably, or definitely related. Details on AEs and SAEs during the trials have been published elsewhere (Oviedo-Joekes et al., 2009; Oviedo-Joekes et al., 2016a; Oviedo-Joekes et al., 2017). In this analysis, using the Medical Dictionary for Regulatory Activities (MedDRA) coding system, we report Preferred Terms “somnolence” (classifies for example drowsiness, sleepiness, groggy) as AEs and Lower Level Term “opioid overdose” as AEs or coded as SAEs when requiring the intervention of naloxone. In the RUTH cohort study, only related SAE overdoses were reported, while data on AEs were not systematically collected. Somnolence and overdoses with any relationship with the study medication are presented in this study. We also report related immediate post injection reactions (allergic reactions) and related injection site pruritus (severe itching at site of injection) using SALOME data, as these are common AEs related to the medications (Oviedo-Joekes et al., 2017).
Analysis
Descriptive statistics were used to present the frequency of related somnolence (AE) and related overdose requiring the use of naloxone (SAE) for injectable HDM and injectable DAM during induction and later in the treatment process. Rates of related immediate post injection reactions (AE) and related injection site pruritus (AE) are also presented with descriptive statistics. The rates of events are presented both per 100 injections and per 100 injection days. Due to differences in rates of AEs, data for HDM and DAM are presented separately for the analysis of related events.
We conducted 2 secondary analyses of the SALOME trial data. First, to investigate the adequacy of induction, we compared the average dose received on the first day after induction was completed and during the first week of treatment with the average doses received during the subsequent 6 months of treatment. For the latter, we excluded the first 30 days of treatment to remove the effects of induction and any early dose adjustments.
Second, in the SALOME trial, urine specimens at 3 and 6 months were analyzed for the detection of opioid alkaloid impurities present with illicit heroin use but not pharmaceutical heroin (Paterson et al., 2005; Oviedo-Joekes et al., 2016b). Dose differences between patients with positive and negative urines for such impurities were tested using a logistic regression model among participants receiving injectable treatment on at least 5 out of the 7 days prior to the specimen to determine whether prescribed dose had an impact on the likelihood of using illicit heroin at 3 and 6 months. The model was estimated by generalized estimating equation (GEE) algorithm to account for dependence of observations (3 months and 6 months) from the same patients. The model was adjusted by randomized treatment (ie, DAM/HDM). Urine samples were collected by the research team independent from the clinical care team who did not have access to the urine results.
RESULTS
Data from the 3 studies are presented in Table 2. In the NAOMI and SALOME trials, a total of 216 participants provided induction data encompassing 1759 induction injections and 694 induction days involving injectable DAM. There were 34 related somnolence and AE overdose events yielding a rate of 4.899 per 100 days receiving injectable DAM in the induction phase. For 213 participants, the rate of somnolence and AE overdose events was 1.066 per 100 days of injection during the treatment period following induction. For hydromorphone, 124 participants had 1,065 induction injections and 409 induction injection days with 6 related somnolence or AE overdose events. The rate of related somnolence or AE overdose events on hydromorphone was 1.467 per 100 injection days during induction and 0.205 per 100 injection days during the treatment period following induction.
TABLE 2.
Number of Injections and Rates of Related Somnolence and Overdose During the Induction Phase in 3 Canadian iOAT Studies
| Related Somnolence and Overdose AE | Related Overdose SAE | ||||||||||
| Study | iOAT | Period | N Pt.* | Total Injections | Injection Days | Events N (per Pt.)† | Rate/100 injections | Rate/100 days | Events N (per Pt.) | Rate/100 injections | Rate/100 days |
| NAOMI and SALOME | DAM | Induction | 216 | 1,759 | 694 | 34 (29) | 1.933 | 4.899 | 4 (4) | 0.227 | 0.576 |
| DAM | After Induction | 213 | 118,807 | 47,861 | 510 (119) | 0.429 | 1.066 | 11 (10) | 0.009 | 0.023 | |
| HDM | Induction | 124 | 1,065 | 409 | 6 (6) | 0.563 | 1.467 | 1 (1) | 0.094 | 0.245 | |
| HDM | After Induction | 122 | 56,550 | 23,460 | 48 (21) | 0.085 | 0.205 | 4 (4) | 0.007 | 0.017 | |
| NAOMI, SALOME, and RUTH | DAM | Induction | 217 | 1,776 | 700 | N/A | N/A | N/A | 4 (4) | 0.225 | 0.571 |
| DAM | After Induction | 287 | 358,938 | 144,535 | N/A | N/A | N/A | 58 (27) | 0.016 | 0.040 | |
| HDM | Induction | 144 | 1,217 | 475 | N/A | N/A | N/A | 1 (1) | 0.082 | 0.211 | |
| HDM | After Induction | 220 | 180,438 | 73,887 | N/A | N/A | N/A | 10 (7) | 0.006 | 0.014 | |
AE, Adverse event; DAM, diacetylmorphine; HDM, hydromorphone; iOAT, injectable opioid agonist treatment; N, number; N/A, not applicable; Pt., patient; SAE, Severe adverse event.
MedDra Codes: Preferred Terms Somnolence (only AE); Lower Level Term Opioid Overdose can be AE or coded as SAE when requiring the intervention of naloxone.
Related refers to any adverse drug reaction classified as possibly, probably or definitely related to the study medication.
NAOMI and SALOME were randomized clinical trials, where DAM and HDM were provided double-blind. RUTH is an observational cohort study, where DAM and HDM are provided open label.
*Lower number of patients after induction in the NAOMI and SALOME trials represent patients that completed induction but did not receive treatment afterwards (NAOMI N = 3, SALOME N = 2). In the RUTH cohort study N for “after Induction” is higher than the N for “Induction” reflecting continuation of treatment from SALOME among patients that remained at the clinic through both studies with no treatment interruptions. A total of 150 patients from SALOME transitioned to open label iOAT (with either DAM or HDM with the possibility to switch from one medication to the other). Among the RUTH participants, 27 were not participants of SALOME. Some participants in RUTH contribute to both DAM and HDM since they could have switched between iOAT medications.
†In the RUTH cohort study, only related SAE overdoses were reported. Data on AEs were not systematically collected.
Combined data from the 3 studies provides a total of 1776 induction injections and 700 induction injection days for DAM, and 1217 induction injections and 475 induction injection days for HDM. There were 4 opioid overdoses that required naloxone during DAM induction (0.571 per 100 injection days) and 58 (in 27 participants) after induction (0.040 per 100 injection days). With HDM, there was 1 related opioid overdose that required naloxone during induction (0.211 per 100 injection days) and 10 (in 7 participants) after induction (0.014 per 100 injection days). There were no related opioid overdoses in the RUTH study with either the regular or accelerated induction protocol over the 72 induction days in RUTH (16 accelerated). The 72 induction days occurred among 28 participants. This included 6 days of regular induction in 2 participants with diacetylmorphine (no accelerated inductions with DAM), 16 days of accelerated induction in 7 participants on hydromorphone and 50 days of regular induction in 19 participants on HDM.
Immediate post injection reactions and injection site pruritus related to the treatment are presented using data from the SALOME trial (Table 3). There were 7 events during DAM induction (2.115 per 100 injection days) and 70 (in 26 participants) after induction (0.427 per 100 injection days). During induction with HDM, there were 5 events (1.529 per 100 injection days) and 108 (in 16 participants) after induction (0.664 per 100 injection days).
TABLE 3.
Rates of Related Immediate Post Injection Reaction or Injection Site Pruritus During the Induction Phase in the SALOME Clinical Trial
| Related immediate post injection reaction or injection site pruritus | |||||||
| iOAT | Period | N Pt.* | Total Injections | Injection Days | Events N (per Pt.) | Rate/100 injections | Rate/100 days |
| Diacetylmorphine | Induction | 102 | 43,349 | 16,376 | 7 (7) | 0.784 | 2.115 |
| After Induction | 102 | 893 | 331 | 70 (26) | 0.161 | 0.427 | |
| Hydromorphone | Induction | 100 | 40,018 | 16,259 | 5 (5) | 0.588 | 1.529 |
| After Induction | 98 | 851 | 327 | 108 (16) | 0.270 | 0.664 | |
iOAT, (injectable opioid agonist treatment); N, number; Pt., patient; SALOME, Study to Assess Longer Term Opioid Medication Effectiveness.
Related refers to any adverse drug reaction classified as possibly, probably or definitely related to the study medication.
SALOME was a randomized non-inferiority clinical trial, where diacetylmorphine and hydromorphone were provided double-blind.
*Lower number of patients after induction represent patients that completed induction but did not receive treatment afterwards (N = 2 hydromorphone).
In the SALOME study, on the fourth day of treatment (after the 3-day induction protocol) the average daily doses received were 382.84 mg (SD = 153.18; n = 93) and 191.76 mg (SD = 80.12; n = 95) of DAM and HDM, respectively. By the end of the first week, the maximum daily dose patients had received were on average 433.62 mg (SD = 137.92) and 223.26 mg (SD = 68.06), respectively. For purposes of comparison, Figure 1A and B show the average total daily dose received as well as the range of the average doses received, over the 6-month study period excluding the first 30 days of treatment. As seen there, the average total daily dose received for DAM and HDM were 506.41 mg (SD = 205.49) and 261.18 mg (SD = 104.02).
FIGURE 1.
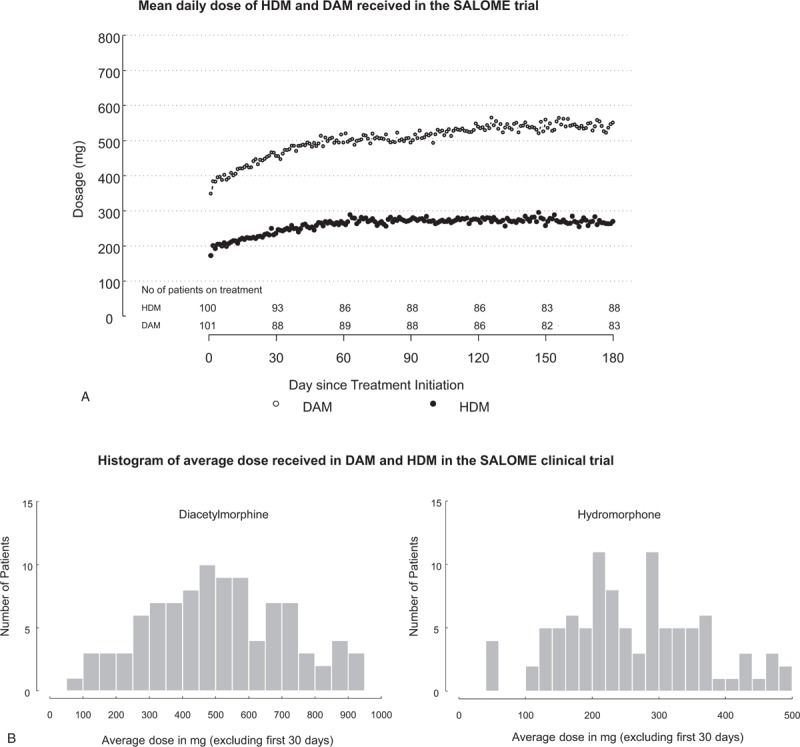
Doses reflect the average daily dose. Patients received up to 3 doses per day, maximum daily dose allowed was 1000 mg of DAM or 500 mg of HDM. Treatments were delivered double blind and prescribed in DAM equivalents in a 2:1 ratio DAM:HDM. (A) Mean daily dose of injectable hydromorphone (HDM) and diacetylmorphine (DAM) received in the SALOME trial. Data reflect the first 6 months of treatment in the SALOME trial. (B) Histogram of average dose received in HDM and DAM in the SALOME clinical trial. Data reflect the first 6 months of treatment in the SALOME trial, excluding the first 30 days (dose adjustment period).
Figure 2 shows the average dose (in DAM equivalents) received in the prior week by urine test result among those retained at least 5 out of the 7 days in the prior week at 3 and at 6 months in the SALOME study. Differences in the dose received between those with positive (520.9 mg; SD = 230.5) and negative (534.1 mg; SD = 220.8) urine tests for street heroin markers were not significant (P = 0.379).
FIGURE 2.
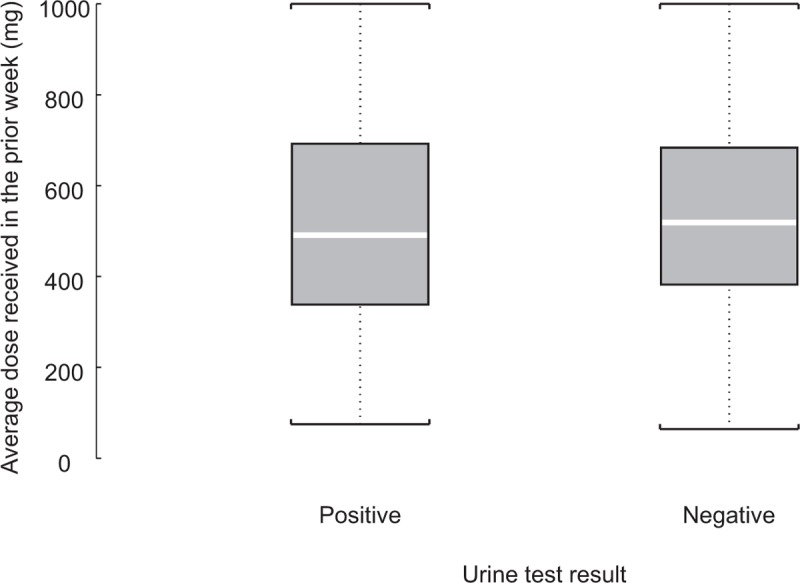
Average dose received in the prior week by urine test result among those retained 5 out of 7 days in the prior week in SALOME. Median: line inside box; mean: symbol marker. Logistic regression model estimated by GEE algorithm to account for dependence of observations (3 months and 6 months) from the same patients was fitted to assess the relationship between urine result and average dose received in the prior 7 days (adjusted by randomization treatment, ie, DAM and HDM). Relationship between dose and positive urine test was not significant (P = 0.379). Total events 459 = 110 positive (520.9 mg.; SD = 230.5) and 349 negative (534.1 mg.; SD = 220.8) urine test for street heroin markers. Urine samples were collected by an independent team; results were not accessed by the clinical care team. Average dose received in diacetylmorphine equivalents (200 mg of diacetylmorphine = 100 mg of hydromorphone). A total of 176 (87%) in and 174 (86%) participants were retained 5 out of 7 days at the week of the 3 and 6 months of the outcomes evaluations measures (out of the total sample of 202 in the SALOME trial). Among those retained, total percentage of positive urine analysis = 23% (29% in DAM and 16% in HDM). DAM, diacetylmorphine; HDM, hydromorphone.
DISCUSSION
The present study is the first to investigate the safety of an induction protocol for injectable diacetylmorphine and hydromorphone in the context of iOAT for opioid use disorder. With data from 3 Canadian studies, encompassing a total of 2993 induction injections, there were only 5 related opioid overdoses that required naloxone (4 with DAM, 1 with HDM). As expected, the rate per injection day was higher for AEs and SAEs during induction compared to after induction. However, all overdose events were safely treated onsite and there were no deaths or other lasting sequelae of these events. These data confirm the overall safety of iOAT during both induction and later treatment.
During the induction phase, consistent daily and documented assessment of the patient's response is the only reliable guide to determining a patient's subsequent dose (Baxter et al., 2013). Such monitoring at the time of peak effect (within 20 minutes for DAM and HDM) is necessary to determine whether the patient continues to experience withdrawal symptoms or experiences any signs of intoxication. Patients may attribute new symptoms or discomforts to their dose, and as such, nurses and physicians must be prepared to reassess the patient (Baxter et al., 2013). In such cases, meeting with the patient, along with input from nursing staff, is helpful to determine appropriate modifications that the patient will accept.
It has been established that a key goal of the induction phase is to safely reach the highest tolerated dose in a timely manner in order to retain the patient in treatment and diminish street opioid use as rapidly as possible (Leavitt et al., 2000; Maremmani et al., 2003; Donny et al., 2005; Trafton et al., 2006). As seen in the secondary analyses of SALOME data, patients in the first week could reach over 85% the average daily dose received during their last five months of the study. This indicates that patients reached an adequate dose during induction that was further adjusted to meet their individual needs during treatment. Further analysis of SALOME urinalysis data demonstrated no relationship between iOAT dose and street heroin use at 3 and 6 months among those receiving treatment.
It should be acknowledged that the 3 studies that provided data for the present analysis were not designed to uncover a pre-specified research question regarding the induction phase. Nevertheless, we are presenting unique data collected during 2 clinical trials and one cohort study that provide evidence of a very low rate of opioid-related overdoses during thousands of induction injections. Also, data can only be descriptive, since comparisons (eg, between study periods) cannot account for unknown confounders. Nevertheless, these data provide a starting point to discussing induction in a clinical setting with iOAT, which has never been published before.
CONCLUSIONS
In conclusion, the present study offers the first opportunity to explore and discuss an induction protocol with injectable diacetylmorphine and hydromorphone in the context of iOAT for opioid use disorder. This study suggests that a 3-day protocol allowed patients to safely reach high doses in a timely manner. Moreover, the few overdoses that occurred in the context of thousands of injections were safely treated onsite. Reaching and treating patients that have been injecting opioids for a long period of time requires approaches that ensure patient engagement by adjusting to their needs. As noted earlier, despite strong and compelling evidence, iOAT remains inaccessible outside of a few select settings in Canada and Europe. The findings of this study suggest that safety is not an evidence-based barrier to the implementation of treatment with injectable hydromorphone and diacetylmorphine.
Supplementary Material
Footnotes
The RUTH Study was funded by a Canadian Institutes of Health Research Project Grant. The SALOME trial was funded through an operating grant from the Canadian Institutes of Health Research in partnership with Providence Health Care with additional financial support from the InnerChange Foundation, Providence Health Care Research Institute, St. Paul's Hospital Foundation and Vancouver Coastal Health. The NAOMI study was supported by grants from the Canadian Institutes of Health Research, the Canada Foundation for Innovation, the Canada Research Chairs Program, the University of British Columbia, Providence Health Care, the University of Montreal, Centre de Recherche et Aide aux Narcomanes, the Government of Quebec, Vancouver Coastal Health, and the BC Centre for Disease Control. Further financial support was provided by the Michael Smith Foundation for Health Research Career Award and the Canada Institutes of Health Research New Investigator Award (EOJ) and the Canada Research Chairs Program (MTS). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
The authors have no competing interests to declare.
REFERENCES
- Baxter LE, Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med 2013; 7:377–386. [DOI] [PubMed] [Google Scholar]
- Beck T, Haasen C, Verthein U, et al. Maintenance treatment for opioid dependence with slow-release oral morphine: a randomized cross-over, non-inferiority study versus methadone. Addiction (Abingdon, England) 2014; 109:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. Pharmacological maintenance treatments of opiate addiction. Br J Clin Pharmacol 2014; 77:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau J, Ahamad K, Goyer ME, et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ 2018; 190:E247–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong CA, Roozen HG, van Rossum LG, et al. High abstinence rates in heroin addicts by a new comprehensive treatment approach. Am J Addict 2007; 16:124–130. [DOI] [PubMed] [Google Scholar]
- Demaret I, Quertemont E, Litran G, et al. Efficacy of heroin-assisted treatment in belgium: a randomised controlled trial. Eur Addict Res 2015; 21:179–187. [DOI] [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, et al. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction (Abingdon, England) 2005; 100:1496–1509. [DOI] [PubMed] [Google Scholar]
- Franklyn AM, Eibl JK, Gauthier GJ, et al. The impact of cocaine use in patients enrolled in opioid agonist therapy in Ontario, Canada. Int J Drug Policy 2017; 48:1–8. [DOI] [PubMed] [Google Scholar]
- Gyr E, Brenneisen R, Bourquin D, et al. Pharmacodynamics and pharmacokinetics of intravenously, orally and rectally administered diacetylmorphine in opioid dependents, a two-patient pilot study within a heroin-assisted treatment program. Int J Clin Pharmacol Ther 2000; 38:486–491. [DOI] [PubMed] [Google Scholar]
- Haasen C, Verthein U, Degkwitz P, et al. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry 2007; 191:55–62. [DOI] [PubMed] [Google Scholar]
- Health Canada. National report: Apparent opioid-related deaths in Canada. Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/national-report-apparent-opioid-related-deaths-released-june-2018.html Accessed June 26, 2018. [Google Scholar]
- Leavitt SB, Shinderman M, Maxwell S, et al. When “enough” is not enough: new perspectives on optimal methadone maintenance dose. Mt Sinai J Med 2000; 67:404–411. [PubMed] [Google Scholar]
- March JC, Oviedo-Joekes E, Perea-Milla E, et al. Controlled trial of prescribed heroin in the treatment of opioid addiction. J Subst Abuse Treat 2006; 31:203–211. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pacini M, Lubrano S, et al. When “enough” is still not “enough”. Effectiveness of high-dose methadone in the treatment of heroin addiction. Heroin Addict Relat Clin Probl 2003; 5:17–32. [Google Scholar]
- Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; CD002207. [DOI] [PubMed] [Google Scholar]
- Office Fédéral de la Santé Publique. Manuel Traitement avec prescription d’héroine. Directives, recommandations, informations. Berne, Swiss: Office Fédéral de la Santé Publique; 2004. [Google Scholar]
- Oviedo-Joekes E, Brissette S, MacDonald S, et al. Safety profile of injectable hydromorphone and diacetylmorphine for long-term severe opioid use disorder. Drug Alcohol Depend 2017; 176:55–62. [DOI] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Brissette S, Marsh DC, et al. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 2009; 361:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Guh D, Brissette S, et al. Hydromorphone compared with diacetylmorphine for long-term opioid dependence: a randomized clinical trial. JAMA Psychiatry 2016; 73:1–9. [DOI] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Guh D, Brissette S, et al. Hydromorphone compared with diacetylmorphine for long-term opioid dependence: a randomized clinical trial. JAMA Psychiatry 2016; 73:447–455. [DOI] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Guh D, Brissette S, et al. Hydromorphone Compared With Diacetylmorphine for Long-term Opioid Dependence: A Randomized Clinical Trial—Supplement to Trial Protocol. 2016c. Available at: http://jamanetwork.com/data/Journals/PSYCH/935259/YOI160008supp2_prod.pdf Accessed January 28, 2019. [DOI] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Marchand K, Guh D, et al. History of treatment access and drug use among participants in a trial testing injectable opioids under supervision for long-term heroin injectors. J Addict Med Ther 2015; 3:1015. [Google Scholar]
- Oviedo-Joekes E, Marchand K, Lock K, et al. The SALOME study: recruitment experiences in a clinical trial offering injectable diacetylmorphine and hydromorphone for opioid dependency. Subst Abuse Treat Prev Policy 2015; 10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Marsh DC, Guh D, et al. Potency ratio of hydromorphone and diacetylmorphine in substitution treatment for long-term opioid dependency. J Opioid Manag 2011; 7:371–376. [DOI] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Nosyk B, Brissette S, et al. The North American Opiate Medication Initiative (NAOMI): profile of participants in North America's First Trial of Heroin-Assisted Treatment. J Urban Health 2008; 85:812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson S, Lintzeris N, Mitchell TB, et al. Validation of techniques to detect illicit heroin use in patients prescribed pharmaceutical heroin for the management of opioid dependence. Addiction (Abingdon, England) 2005; 100:1832–1839. [DOI] [PubMed] [Google Scholar]
- Perneger TV, Giner F, del Rio M, et al. Randomised trial of heroin maintenance programme for addicts who fail in conventional drug treatments. BMJ 1998; 317:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M, Julien D, White ND, et al. Psychological predictors of retention in a low-threshold methadone maintenance treatment for opioid addicts: a 1-year follow-up study. Subst Use Misuse 2015; 50:24–31. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, O’Malley SS. Meeting the growing need for heroin addiction treatment. JAMA Psychiatry 2016; 73:437–438. [DOI] [PubMed] [Google Scholar]
- Bern, Seidenberg A, Honegger U. Methadone, Heroin and other Opiods—Medical Manual for Outpatient Opioid—Assisted Treatment. 1998. [Google Scholar]
- Strang J, Groshkova T, Uchtenhagen A, et al. Heroin on trial: systematic review and meta-analysis of randomised trials of diamorphine-prescribing as treatment for refractory heroin addictiondagger. Br J Psychiatry 2015; 207:5–14. [DOI] [PubMed] [Google Scholar]
- Strang J, Metrebian N, Lintzeris N, et al. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet 2010; 375:1885–1895. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Minkel J, Humphreys K. Determining effective methadone doses for individual opioid-dependent patients. PLoS Med 2006; 3:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallner JJ, Stewart JT, Kotzan JA, et al. Pharmacokinetics and bioavailability of hydromorphone following intravenous and oral administration to human subjects. J Clin Pharmacol 1981; 21:152–156. [DOI] [PubMed] [Google Scholar]
- van den Brink W, Hendriks VM, Blanken P, et al. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ 2003; 327:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Wang L, Wang X, et al. A study of 6-year retention in methadone maintenance treatment among opioid-dependent patients in Xi’an. J Addict Med 2013; 7:342–348. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO, UNODC, UNAIDS Position Paper: Substitution Maintenance Therapy in the Management of Opioid Dependence and HIV/AIDS Prevention. World Health Organization; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


