Supplemental Digital Content is available in the text.
Key Words: irritable bowel syndrome with constipation, chronic idiopathic constipation, stool consistency, linaclotide, patient-reported outcomes
Goals:
This study aimed to characterize the impact of stool consistency on patient-reported bowel movement (BM) satisfaction in patients with irritable bowel syndrome with constipation (IBS-C) or chronic idiopathic constipation, with a focus on linaclotide.
Background:
As new medications for constipation become available, understanding patients’ perceptions of treatment effects may help clinicians manage patient expectations and inform clinical decision-making.
Materials and Methods:
Data were derived from the Chronic Constipation and IBS-C Treatment and Outcomes Real-world Research Platform (CONTOR) study from 2 patient-reported 7-day daily BM diaries to create a dataset of 2922 diaries representing 26,524 BMs for 1806 participants. Binary variables were created for: medication(s) used in the past 24 hours and categorization of BMs as loose or watery stools (LoWS), hard or lumpy stools (HoLS), or intermediate (neither LoWS nor HoLS). The relationship between stool consistency, medication use, and BM satisfaction was analyzed using logistic regression with SEs corrected for repeated observations.
Results:
BMs characterized as intermediate stools and LoWS were satisfactory more often (61.2% and 51.2%, respectively) than HoLS (19.4%). Participants who reported taking linaclotide rated a similar proportion of BMs as satisfactory when described as LoWS (65.6%) or intermediate (64.1%). Linaclotide use was associated with higher odds of BMs being reported as satisfactory compared with nonlinaclotide use (odds ratio: 1.23, P<0.05).
Conclusions:
Overall, CONTOR participants were more likely to report BMs classified as LoWS or intermediate as satisfactory, versus HoLS. Participants taking linaclotide were more likely to be satisfied, particularly those reporting LoWS, versus those not taking linaclotide.
Irritable bowel syndrome with constipation (IBS-C) is a functional gastrointestinal (GI) disorder characterized by recurrent abdominal pain and altered bowel habits with a predominance of constipation-related complaints.1 Estimates for the prevalence of IBS-C in the United States range from 4.3% to 16.1%.2 IBS-C is thought to reside on a continuum with chronic idiopathic constipation (CIC) or functional constipation; according to the Rome III and Rome IV criteria, a diagnosis of CIC (termed functional constipation by the Rome Foundation) is excluded by the presence of significant abdominal pain.3–5 The diagnosis of CIC is based on persistent difficult, infrequent, or incomplete defecation.4 Recent estimates for the prevalence of CIC in the United States range from 17.0% to 19.4%.6–8 The chronic symptoms of IBS-C and CIC significantly impact health-related quality of life (HRQoL), especially in the domains of general health, social functioning, and mental health.9,10
Current treatment options for IBS-C and CIC include diet and lifestyle modifications, over-the-counter or generic laxatives (such as bulking agents, stool softeners, osmotic laxatives, and stimulant laxatives), as well as prescription medications approved by the US Food and Drug Administration, such as linaclotide, lubiprostone, and plecanatide (plecanatide was not marketed in the United States when data for this study were collected).4,11 Despite the variety of medications available, many IBS-C and CIC patients remain unsatisfied with available treatment options; estimates from various studies suggest that the percentage of patients unsatisfied with treatment options range from 28% to 57%.7,12–14
There is very little insight into how IBS-C and CIC patients perceive bowel movements (BMs) and their potential changes while on medications for these conditions. In a population with BMs that may be painful, erratic, and incomplete, it is important to understand both how patients describe the consistency of their BMs and how these characteristics influence their treatment and illness experience. A better understanding of the impact of stool consistency on patient-reported BM satisfaction could provide insight into overall treatment satisfaction.
The Chronic Constipation and IBS-C Treatment and Outcomes Real-world Research Platform (CONTOR)15 is a longitudinal, observational study that combined administrative claims, patient surveys, and diary data to study symptoms, use of and experience with treatments, and patient-reported outcomes among patients with IBS-C or CIC. The primary objective of this analysis was to examine the association between stool consistency and patient-reported BM satisfaction in patients with IBS-C or CIC enrolled in CONTOR. Analyses also assessed the effect of constipation treatments, particularly linaclotide, on the association between stool consistency and patient-reported BM satisfaction to provide a greater understanding of patients’ perceptions regarding treatment effects and satisfaction.
MATERIALS AND METHODS
Study Design and Patient Selection
Patients were identified using medical and pharmacy claims and enrollment data from the Optum Research Database, a large, geographically diverse US administrative claims database. During the identification period from December 2012 to June 2015, patients who met the following claims-based identification criteria were contacted by mail and asked to complete a self-administered paper baseline survey.
Identification criteria:
Fully insured members of a commercial health plan aged 18 years or above, with both medical and pharmacy benefits, AND
≥1 pharmacy claim for linaclotide or lubiprostone (preauthorization required physician diagnosis of IBS-C or CIC), OR
≥1 medical claim for constipation [International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis 564.0x], OR
≥1 medical claim for irritable bowel syndrome (IBS) (ICD-9-CM diagnosis 564.1x) or abdominal pain (ICD-9-CM diagnosis 789.0x) plus ≥1 pharmacy claim for a stool softener/laxative.
Exclusion criteria (a full list of ICD codes used as exclusion criteria can be found in Table S1, Supplemental Digital Content 1, http://links.lww.com/JCG/A522):
Medical claims for diarrhea or evidence of any of the conditions or medication use commonly associated with diarrhea or diarrhea treatment, or conditions for which IBS-C or CIC treatments would not be appropriate (Table S1, Supplemental Digital Content 1, http://links.lww.com/JCG/A522).
Pharmacy claims for alosetron or repeated claims for diphenoxylate hydrochloride/atropine sulfate (≥2 claims ≥30 d apart, indicative of diarrhea).
Medical claims for profound cognitive or mental impairment that would limit their ability to participate in the study (Table S1, Supplemental Digital Content 1, http://links.lww.com/JCG/A522).
Patients were included in the CONTOR study if they completed and returned the baseline survey and met the following criteria:
≥1 pharmacy claim for linaclotide or lubiprostone (preauthorization required physician diagnosis of IBS-C or CIC), OR
Self-reported health care provider diagnosis of IBS-C, IBS, CIC, chronic constipation, or functional constipation on the baseline survey, OR
Self-reported fulfillment of modified Rome III survey criteria for IBS-C or CIC.5
Study surveys were self-administered on paper and online using a modified Dillman method.16 A combination of prepaid and postpaid incentives was used. The study was approved by the New England Institutional Review Board on September 24, 2014 (NEIRB #14-387). An informed consent statement was provided with the survey indicating that consent was implied through the return of a completed survey, which could be revoked at any time.
Patient-reported Outcomes
CONTOR participants were asked to complete a paper-based survey and 7-day daily BM diary at baseline. Quarterly surveys assessing IBS-C and CIC symptoms and HRQoL, along with monthly updates to medication use, were administered online during the 12-month follow-up period. A final 7-day daily BM diary was administered on paper at month 12.
The baseline survey captured demographic information, including age, sex, marital status, and education level, as well as an assessment of IBS-C and CIC symptoms and HRQoL. Symptom-specific questions included the number of years participants had been seeing a health care provider for their bowel or abdominal symptoms and symptom severity as assessed by the Patient Assessment of Constipation Symptoms (PAC-SYM) questionnaire.17 The PAC-SYM is a 12-item questionnaire evaluating symptoms of constipation over the past 2 weeks, with higher scores indicating worse symptoms. HRQoL was assessed via the Patient Assessment of Constipation Quality of Life questionnaire,18 a 28-item questionnaire assessing the burden of constipation on patients’ everyday functioning and well-being. Lower scores indicate better HRQoL.
The 7-day daily diary asked participants to record their daily BMs and report the following information for each BM: time of BM, whether they were satisfied with the BM, medications taken in the past 24 hours for bowel and/or abdominal symptoms, and a 1-word description of the consistency or form of the BM (see Table S2, Supplemental Digital Content 2, http://links.lww.com/JCG/A522, for the BM log). Free-text BM descriptions were reported by participants using a short, open text field in the diary, entered verbatim, and, with clinical guidance on the text provided, analytically coded into 1 of 3 mutually exclusive types: loose or watery stools (LoWS), intermediate [neither LoWS nor hard or lumpy stools (HoLS)], or HoLS. LoWS and HoLS correspond to ∼6 to 7 and ∼1 to 2 on the Bristol Stool Form Scale, respectively.
BMs were linked to patient-reported constipation medications recorded for each calendar day (ie, any medication use reported alongside a BM on a given day would be ascribed to later BMs on the same day). If no medications were reported on the calendar day in which a BM was reported, no medications were associated with that BM. Participant-reported medications, both prescription and over-the-counter, taken for bowel and/or abdominal symptoms were categorized into mutually exclusive medication categories, including osmotic laxatives; emollient laxatives; bulking agents; antibiotics; anticholinergics; antidepressants and anxiolytics; antidiarrheals; dietary supplements or other remedies such as herbal cleanses and teas; enemas and suppositories; fiber (not otherwise classified); other GI treatments; linaclotide; lubiprostone; magnesium supplements; pain treatments; probiotics; and proton pump inhibitors. A subgroup of osmotic laxatives limited to patient-reported polyethylene glycol (PEG) use was also created for BM analyses. Treatments not associated with a GI-related condition were excluded. For descriptive analyses, participants were grouped by the following categories of medication use: linaclotide, linaclotide with other medications, nonlinaclotide medications, and no medications.
Statistical Analysis
The 7-day diary responses collected at baseline were pooled with the 7-day diary responses collected at 12 months to create 1 dataset for this analysis. To be included in the analysis, participants had to return at least 1 diary with data for at least 1 BM. Binary variables were created for categories of medications reported by patients and categories of stool consistency (LoWS, HoLS, or intermediate). Stool consistency categories were mutually exclusive and based on the patient-reported 1-word BM descriptions. The unit of analysis was the BM. Analyses were conducted in SAS, v9.4 or above (SAS Institute, Cary, NC). Comparisons of binary outcome measures were performed using Cochran-Mantel-Haenszel tests. Tests of significance were 2-tailed and carried out at the 5% (α=0.05) significance level.
Logistic regression multivariable models were developed to estimate the odds of patient-reported BM satisfaction for included independent variables, for example, LoWS versus HoLS. The odds ratio produced by the model tested the association between stool consistency and BM satisfaction and was adjusted for all other variables in the model. Values >1 indicated higher odds of BM satisfaction. Repeated measures were accounted for using robust SEs.
Independent variables included in the logistic models were: age, dichotomized baseline survey Patient Assessment of Constipation Quality of Life score (≥median vs. <median), dichotomized baseline survey PAC-SYM score (≥median vs. <median), dichotomized years with symptoms (≥median vs. <median), sex, race, stool consistency defined by LoWS, intermediate, or HoLS, and medication use per calendar day as reported by patients. In the multivariate analyses, medication use was captured with 2 binary variables, 1 for linaclotide use and the other for the most commonly used laxative, PEG. The reference group for each variable was nonuse of that treatment and could include either no treatment use or use of other therapies. Interaction terms were also tested between stool consistency and these 2 binary variables for medication use in the logistic regressions.
RESULTS
Patient-reported Demographic and Diary Characteristics
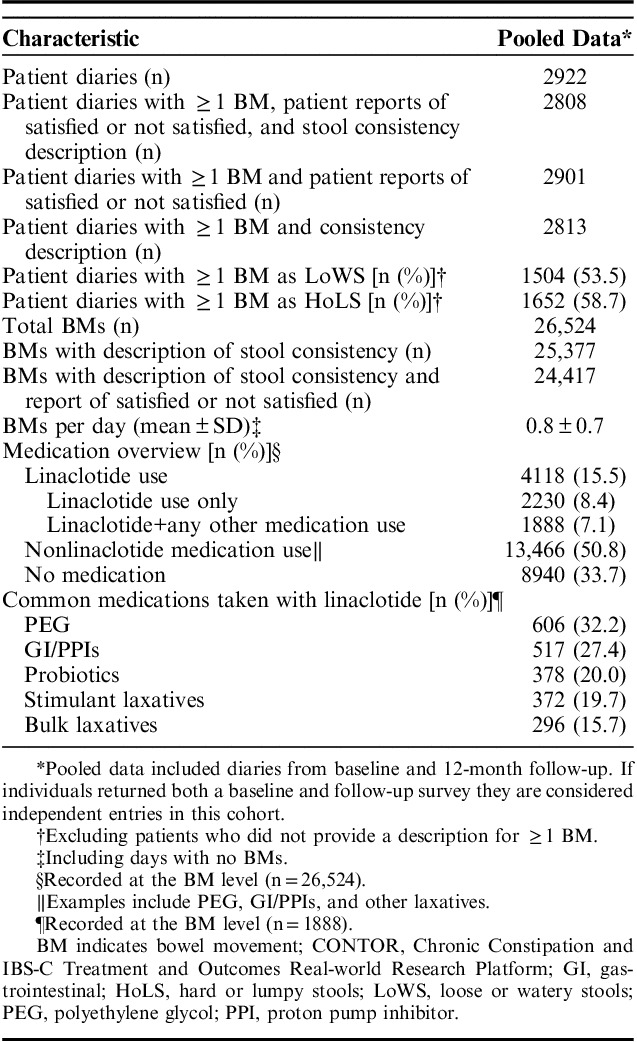
A total of 2052 eligible patients returned a baseline survey (response rate=16.8%); 1747 completed a baseline 7-day BM diary.19 Participants were predominately white (83%) and female (94%), with a mean age of 47 years (Table 1); demographics of the participants included in this analysis were similar to the demographics of the overall CONTOR study population. A total of 2922 baseline and 12-month 7-day diaries were collected (Table 2), of which 2901 contained at least 1 assessment of BM satisfaction, 2813 included at least 1 description of BM stool consistency, and 2808 included at least 1 description of BM occurrence, satisfaction, and stool consistency. Of a total of 26,524 BMs reported, almost all were accompanied by a description of stool consistency (n=25,377; 95.7%), with a subset having both a description of stool consistency and a report of satisfied or not satisfied (n=24,417; 92.1%).
TABLE 1.
Demographics of Patients Who Completed the Baseline Survey and Returned at Least 1 Diary With at Least 1 Bowel Movement With a Description of Stool Consistency
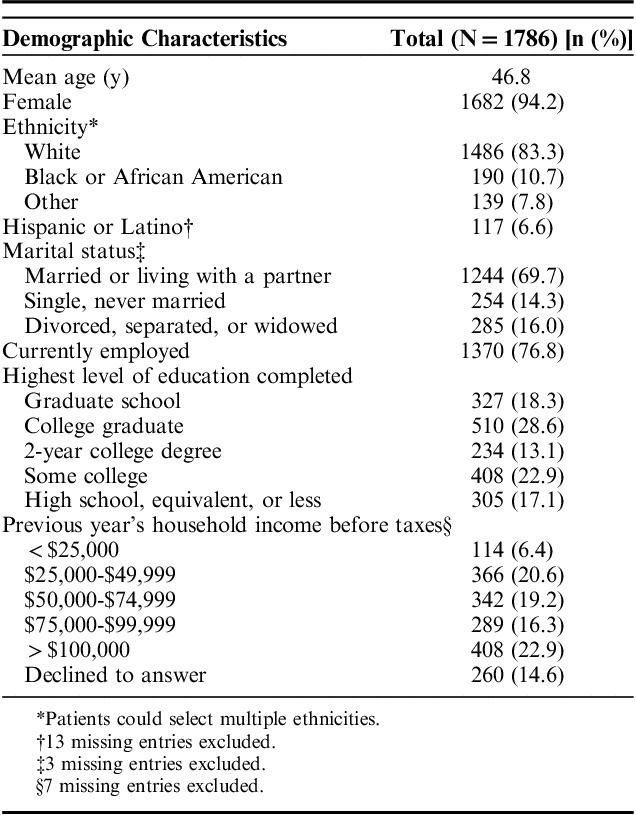
TABLE 2.
CONTOR Patient Diary Characteristics
Patient-reported Medication Use
Of the total BMs analyzed, 8940 (33.7%) BM records were associated with reports of no medication use, 13,466 (50.8%) with nonlinaclotide medication use, and 4118 (15.5%) with reported linaclotide use in the past 24 hours (Table 2). PEG was commonly reported as taken with linaclotide (32.2%); other concomitant medications included medications for GI disorders (such as cimetidine, ranitidine, and budesonide) or proton pump inhibitors, stimulant laxatives, and bulk laxatives.
Descriptors of Stool Consistency
Of the BMs accompanied by a description of stool consistency, 6415 (25.3%) were classified as LoWS and 4724 (18.6%) were classified as HoLS. The terms most frequently used to describe BMs classified as LoWS were “loose,” “watery,” “diarrhea,” “runny,” and “liquid,” whereas the terms commonly used to describe BMs classified as HoLS were “hard,” “pebbles,” “pellets,” and “lumpy.” The word “diarrhea” was used in ∼10% of BMs classified as LoWS.
BM Satisfaction by Stool Consistency
Satisfactory BMs were reported in 12,424 (50.9%) of the BM records accompanied by a description of stool consistency and report of satisfied or not satisfied. Among the BMs for each type of stool consistency, satisfactory BMs were most often reported in intermediate stools (61.2% satisfied) and LoWS (51.2% satisfied). HoLS were not predominately rated as satisfactory (19.4% satisfied) (Fig. 1). In a subanalysis, BMs where the word “diarrhea” was reported by patients to describe the BM, were rated as satisfactory 52.1% of the time.
FIGURE 1.
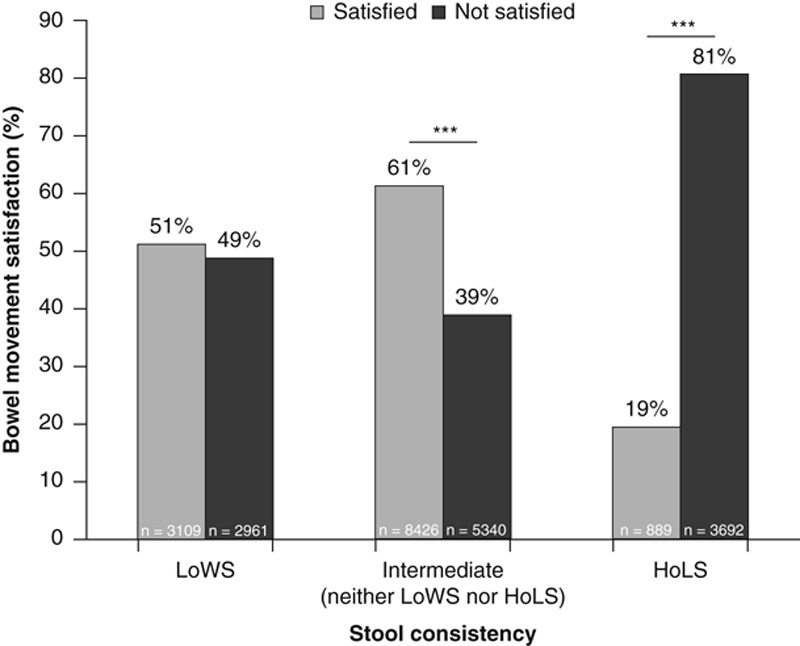
Combined baseline and 12-month BM satisfaction by stool consistency. Including only BM records accompanied by a stool consistency description and BM satisfaction rating (n=24,417). Stool consistency is based on patient-reported descriptions of each BM. ***P≤0.001. BM indicates bowel movement; HoLS, hard or lumpy stools; LoWS, loose or watery stools.
Medication Use, Stool Consistency, and BM Satisfaction
Of the BM records that included a description of stool consistency, no medication use was associated with ∼20% of BMs categorized as LoWS; linaclotide and linaclotide with other medications were associated with greater proportions of BMs categorized as LoWS compared with nonlinaclotide and no medication use (Table 3). The proportion of satisfactory BMs was highest when linaclotide was the only medication reported in the past 24 hours (60.8%), of the BM records that included both a description of stool consistency and a rating of either satisfied or not satisfied (Fig. 2). Among these BMs, 50.5% and 49.6% of BMs accompanied by nonlinaclotide treatment or no medication use were also rated as satisfactory, respectively. When BMs were stratified by stool type and medication, the highest proportion of satisfactory BMs were those associated with linaclotide only use in the previous 24 hours and classified as LoWS (65.6%) or intermediate stools (64.1%). The proportion of satisfactory BMs classified as LoWS was higher for those with linaclotide use only (65.6%) compared with linaclotide with other medications (56.6%), nonlinaclotide (48.4%), and no medication use (45.1%). The proportion of BMs rated as satisfactory for intermediate stools was similar to those associated with linaclotide use (64.1%) compared with nonlinaclotide medication use (61.0%) and no medication use (62.9%).
TABLE 3.
Frequency of Reported Stool Consistency by Medication Use
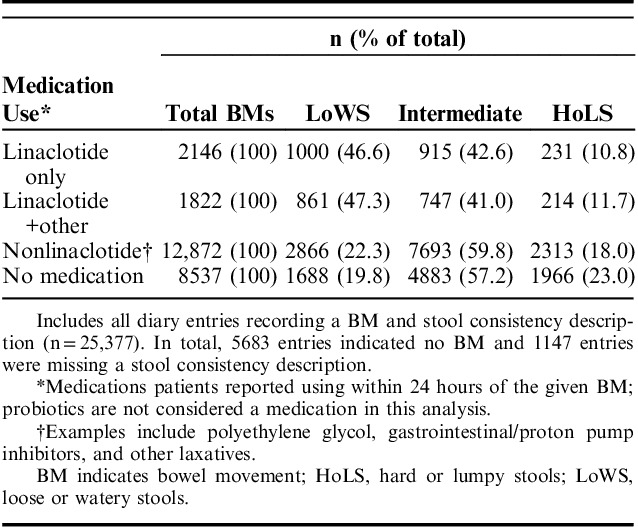
FIGURE 2.
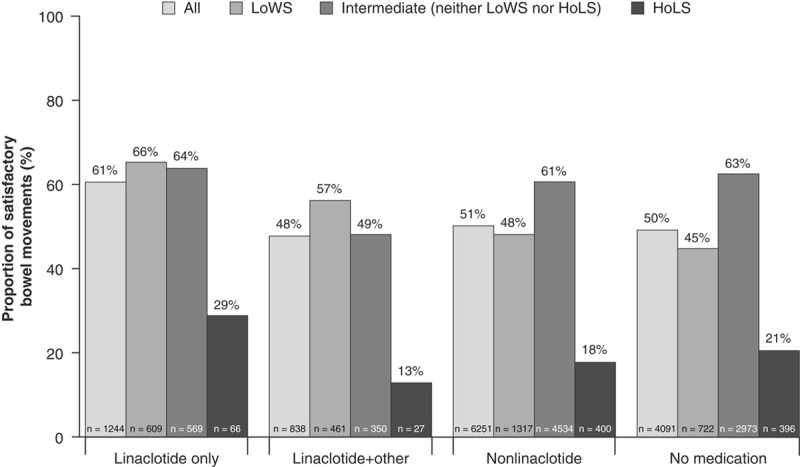
Combined baseline and 12-month BM satisfaction by stool consistency and medication use. Including only BM records accompanied by a stool consistency description and BM satisfaction rating (n=24,417). Medication uses self-reported within 24 hours of the reported BM. Common medications taken with linaclotide included polyethylene glycol, gastrointestinal/proton pump inhibitors, and antidepressants/anxiolytics. BM indicates bowel movement; HoLS, hard or lumpy stools; LoWS, loose or watery stools.
Multivariable Analysis of BM Satisfaction
Logistic regression was used to model the relationship between stool consistency (LoWS, HoLS, or intermediate), BM satisfaction (yes, no), and category of medication use (Table 4). In general, reports of BM satisfaction were associated with stool consistency categorized as intermediate (neither HoLS nor LoWS) or LoWS in a model without interaction terms. BMs had higher odds of being satisfactory when participants reported linaclotide use in the past 24 hours in comparison to no reported linaclotide use in the past 24 hours, after adjusting for all other variables in the model. In contrast, PEG use was associated with BMs that were not satisfactory, compared with non-PEG use.
TABLE 4.
Odds Ratio of BM Satisfaction by Independent Variables
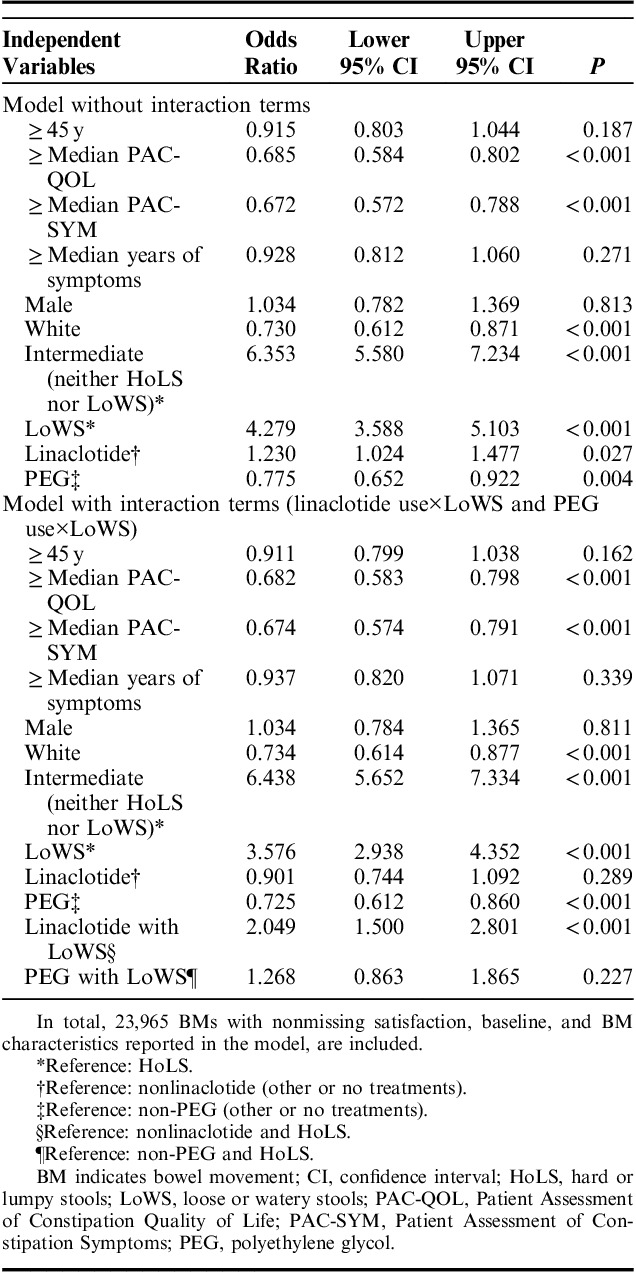
To assess whether the association between medication use and BM satisfaction (which was shown in the first model without interaction terms) depended on stool consistency, interaction terms (linaclotide use by LoWS and PEG use by LoWS) were added to the model (Table 4). BMs described as LoWS when linaclotide use was reported were more likely to be satisfactory compared with BMs when no linaclotide use was reported or when BMs were described as HoLS. There were no associations between BMs described as LoWS when PEG use was reported compared with BMs when no PEG use was reported, or BMs described as HoLS.
DISCUSSION
These analyses sought to gain a better understanding of the patient experience with BMs and constipation treatments to help inform future clinical decision-making for patients with IBS-C and CIC. In this population, linaclotide use was found to be associated with a higher proportion of BMs reported as LoWS compared with nonlinaclotide or no medication use, and LoWS were associated with an increased likelihood of BM satisfaction in comparison to HoLS. Findings from a recently reported long-term study in patients with IBS-C that rolled over from a phase 3 clinical trial of linaclotide provide support for this result, in that 70% of patients were satisfied with their linaclotide treatment, regardless of whether they had experienced self-reported diarrhea.20 LoWS was not a term used in the phase 3 trial, so a direct comparison to diarrhea is not possible. Nevertheless, stool consistency classified as LoWS from the current study would most likely be reported by patients in words that would be coded as an adverse event of diarrhea in a clinical trial, according to the Medical Dictionary for Regulatory Activities coding of preferred terms.21 Although diarrhea or LoWS may be considered an adverse event within the context of a clinical trial, our data suggest that in the real world, a substantial proportion of patients with IBS-C or CIC consider LoWS a positive treatment outcome.
It is important to understand the perceptions and expectations of patients with constipation when evaluating treatment regimens and the resulting stool consistency. With regards to the natural history, severity, and impact of symptoms, IBS-C patients tend to suffer from more persistent, severe, and impactful abdominal bowel symptoms compared with other IBS subtypes, including IBS with diarrhea.22,23 Linaclotide is a treatment option for IBS-C and CIC that is recommended for long-term use for those with continuous symptoms, rather than sporadic use.24 It is conceivable that patients taking linaclotide represent those with more severe manifestations of IBS-C and CIC, compared with patients taking other medications or no medication. Since patients with more severe IBS-C or CIC regularly experience constipation, they are more likely to experience infrequent BMs that often take the form of HoLS. Therefore, patients with increased severity may be more likely to express BM satisfaction as a result of overall symptom improvement, even if experiencing LoWS.
LoWS may also be perceived as satisfactory due to a feeling of complete evacuation or lack of straining that positively contrasts with a patient’s usual illness experience. In a 2007 survey of US residents with chronic constipation who had recently sought care for constipation symptoms, straining was the most prevalent symptom (79%), followed by abdominal discomfort (62%), infrequent BMs (57%), and bloating (57%).7 Efficacy in relieving constipation was rated as extremely or very important by 80% of participants asked to rate key attributes of medications; this was the highest rating among the available attributes and was followed by efficacy at improving the quality of BMs (79%) and tolerance (74%).7 In a similar 2015 US population-based survey, when asked to rate their symptoms, ∼65% of respondents meeting Rome III criteria for IBS-C, and ∼55% of respondents meeting Rome III criteria for CIC with abdominal symptoms, rated straining as very or extremely bothersome. In both surveys, out of the available symptoms, straining was second only to constipation in being frequently rated as very or extremely bothersome.3
Limitations
A variety of approaches were used to mitigate the limitations associated with using administrative claims data to identify patients for this study, including use of patient-reported health care provider diagnoses, modified Rome III criteria, and prior authorization requirements associated with some sample eligibility criteria. Limitations of survey data may include sampling error, coverage error, and measurement error; the analytic coding of stool types into LoWS, intermediate, or HoLS may not have captured the exact patient experience. The study population was selected from patients with commercial health plan coverage; therefore, results may not be generalizable to uninsured and older populations. Furthermore, BM satisfaction may be dependent on characteristics beyond stool consistency such as straining, urgency, or sense of complete evacuation, which were not assessed in this study.
CONCLUSIONS
The breadth and depth of the real-world patient experience captured from the CONTOR study supported the objective to characterize the impact of stool consistency on patient-reported BM satisfaction. Overall, participants with IBS-C or CIC were more likely to rate intermediate stools or LoWS as satisfactory, compared with HoLS. Results showed that BMs associated with linaclotide use and described particularly as LoWS were more likely to be satisfactory, compared with BMs associated with other treatments for constipation, particularly those described as HoLS. These results highlight that LoWS, which are commonly coded as adverse events in clinical trial reporting, may not be perceived as a negative treatment outcome by patients with IBS-C or CIC in the real-world setting.
Increased satisfaction with LoWS and dissatisfaction with HoLS should be considered during clinical decision-making in the real world, with clinicians discussing patient perceptions on BM satisfaction. The association between HoLS and BMs not rated as satisfactory, and the higher proportion of HoLS among BMs associated with no medication use, provide further evidence that treated and untreated patients differ in important ways; this difference illustrates the value of treatment in managing bowel and abdominal symptoms in patients with IBS-C and CIC.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
ACKNOWLEDGMENTS
The authors thank Paul Buzinec and Ryan Wolbeck of Optum, Eden Prairie, MN, for their contributions to the statistical analysis presented in this study. Writing and editorial assistance was provided to the authors by James Ding and Karen B. Chien, PhD of Complete HealthVizion Inc., Chicago, IL and funded by Allergan plc, Dublin, Ireland and Ironwood Pharmaceuticals Inc., Cambridge, MA.
Footnotes
Supported by Allergan plc, Dublin, Ireland and Ironwood Pharmaceuticals Inc., Cambridge, MA.
All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
D.C.A.T. and D.S.R. are employees of Ironwood Pharmaceuticals Inc. and own stock and stock options. D.S.R. is a member of Albemarle Scientific Consulting LLC and is a former consultant for Ritter Pharmaceuticals. J.L.A. and R.T.C. are employees of Allergan plc and own stock and stock options. J.A.D. has received research funding from AbbVie, Biogen, Janssen, Pfizer, PhRMA, Regeneron, and Sanofi; she has served as an advisory board member or consultant to Allergan plc, Sanofi, Shire, and Vertex. C.M., A.G.H., B.E., and S.K. are employees of Optum; Optum was paid by Allergan plc and Ironwood Pharmaceuticals Inc. to conduct the study. W.D.C. has received grant support from Ironwood Pharmaceuticals Inc., Nestlé, and Prometheus Laboratories; has served as an advisor or consultant for Allergan plc, Biomerica, IM Health, Ironwood Pharmaceuticals Inc., Outpost, Prometheus Laboratories, QOL Medical, Ritter, and Salix Pharmaceuticals; and has served as a speaker for the Gi Health Foundation.
REFERENCES
- 1.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hungin APS, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. [DOI] [PubMed] [Google Scholar]
- 3.Heidelbaugh JJ, Stelwagon M, Miller SA, et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015;110:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407. [DOI] [PubMed] [Google Scholar]
- 5.Rome Foundation. Rome III diagnostic criteria for functional gastrointestinal disorders. 2006. Available at: www.theromefoundation.org/assets/pdf/19_RomeIII_apA_885-898.pdf. Accessed December 11, 2017.
- 6.Locke GR, III, Zinsmeister AR, Fett SL, et al. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. [DOI] [PubMed] [Google Scholar]
- 7.Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Locke GR, Schleck CD, et al. Risk factors for chronic constipation and a possible role of analgesics. Neurogastroenterol Motil. 2007;19:905–911. [DOI] [PubMed] [Google Scholar]
- 9.Belsey J, Greenfield S, Candy D, et al. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31:938–949. [DOI] [PubMed] [Google Scholar]
- 10.DiBonaventura M, Sun SX, Bolge SC, et al. Health-related quality of life, work productivity and health care resource use associated with constipation predominant irritable bowel syndrome. Curr Med Res Opin. 2011;27:2213–2222. [DOI] [PubMed] [Google Scholar]
- 11.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–1712. [DOI] [PubMed] [Google Scholar]
- 12.Emmanuel A, Quigley EMM, Simrén M, et al. Factors affecting satisfaction with treatment in European women with chronic constipation: an internet survey. United European Gastroenterol J. 2013;1:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrazzi S, Thompson GW, Irvine EJ, et al. Diagnosis of constipation in family practice. Can J Gastroenterol. 2002;16:159–164. [DOI] [PubMed] [Google Scholar]
- 14.Müller-Lissner S, Tack J, Feng Y, et al. Levels of satisfaction with current chronic constipation treatment options in Europe—an internet survey. Aliment Pharmacol Ther. 2013;37:137–145. [DOI] [PubMed] [Google Scholar]
- 15.Abel JA, Taylor DCA, Doshi JA, et al. Chronic Constipation and IBS-C Treatment and Outcomes Real World Research Platform (CONTOR): a large, longitudinal observational study. Am J Gastroenterol. 2016;11:S236–S260. [Google Scholar]
- 16.Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. Hoboken, NJ: Wiley Publishing; 2014. [Google Scholar]
- 17.Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34:870–877. [DOI] [PubMed] [Google Scholar]
- 18.Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40:540–551. [DOI] [PubMed] [Google Scholar]
- 19.Essoi B, Taylor DCA, Abel JL, et al. An innovative approach to mixed-mode longitudinal data collection: methods and response rates from the chronic constipation & IBS-C treatment and outcomes real world research platform (CONTOR). Poster presented at ISPOR 22nd Annual International Meeting. Boston, MA, May 20–24, 2017.
- 20.Chey WD, Shiff S, Schneier H, et al. Two years on linaclotide: tolerability and treatment satisfaction in IBS-C patients with and without diarrhea. Am J Gastroenterol. 2014;109:S530. [Google Scholar]
- 21.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109–117. [DOI] [PubMed] [Google Scholar]
- 22.Schmulson M, Lee OY, Chang L, et al. Symptom differences in moderate to severe IBS patients based on predominant bowel habit. Am J Gastroenterol. 1999;94:2929–2935. [DOI] [PubMed] [Google Scholar]
- 23.Shah ED, Almario CV, Spiegel B, et al. Patients with IBS-C report more severe, bothersome, frequent and diffuse abdominal pain vs. IBS-D: results of a nationwide population-based study. Gastroenterology. 2017;152:S712–S713. [Google Scholar]
- 24.Rey E, Mearin F, Alcedo J, et al. Optimizing the use of linaclotide in patients with constipation-predominant irritable bowel syndrome: an expert consensus report. Adv Ther. 2017;34:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.


